Abstract
Background
Infectious Diseases Society of America/Society for Healthcare Epidemiology of America (IDSA/SHEA) guidelines describe recommended therapy for Clostridioides difficile infection (CDI).
Objective
To describe CDI treatment and, among those with severe CDI, determine predictors of adherence to the 2010 IDSA/SHEA treatment guidelines.
Design
We analyzed 2013–2015 CDI treatment data collected through the Centers for Disease Control and Prevention’s Emerging Infections Program. Generalized linear mixed models were used to identify predictors of guideline-adherent therapy.
Patients
A CDI case was defined as a positive stool specimen in a person aged ≥ 18 years without a positive test in the prior 8 weeks; severe CDI cases were defined as having a white blood cell count ≥ 15,000 cells/μl.
Main Measures
Prescribing and predictors of guideline-adherent CDI therapy for severe disease.
Key Results
Of 18,243 cases, 14,257 (78%) were treated with metronidazole, 7683 (42%) with vancomycin, and 313 (2%) with fidaxomicin. The median duration of therapy was 14 (interquartile range, 11–15) days. Severe CDI was identified in 3250 (18%) cases; of 3121 with treatment data available, 1480 (47%) were prescribed guideline-adherent therapy. Among severe CDI cases, hospital admission (adjusted odds ratio [aOR] 2.48; 95% confidence interval [CI] 1.90, 3.24), age ≥ 65 years (aOR 1.37; 95% CI 1.10, 1.71), Charlson comorbidity index ≥ 3 (aOR 1.27; 95% CI 1.04, 1.55), immunosuppressive therapy (aOR 1.21; 95% CI 1.02, 1.42), and inflammatory bowel disease (aOR 1.56; 95% CI 1.13, 2.17) were associated with being prescribed guideline-adherent therapy.
Conclusions
Provider adherence to the 2010 treatment guidelines for severe CDI was low. Although the updated 2017 CDI guidelines, which expand the use of oral vancomycin for all CDI, might improve adherence by removing the need to apply severity criteria, other efforts to improve adherence are likely needed, including educating providers and addressing barriers to prescribing guideline-adherent therapy, particularly in outpatient settings.
KEY WORDS: infectious disease, decision-making, epidemiology, Clostridioides difficile
INTRODUCTION
Clostridioides difficile is a leading cause of US healthcare–associated infections,1 causing an estimated 453,000 infections and 29,000 associated deaths in 2011.2C. difficile infection (CDI) increases 30-day mortality by 2.5-fold3,4 and is associated with higher healthcare expenditures5,6 and longer hospital length of stay.7
CDI symptoms range in severity from mild diarrhea to pseudomembranous colitis and can result in toxic megacolon and death. The Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) published CDI treatment guidelines in 2010.8 Metronidazole was recommended for an initial episode of mild to moderate disease, and oral vancomycin was recommended as first-line therapy for an initial episode of severe disease. These guidelines were updated in 2017 and now recommend oral vancomycin or fidaxomicin for all initial CDI, regardless of severity9 (Table 1).
Table 1.
Clostridioides difficile Treatment Recommendations for Initial Episodes in Adults According to 2010 and 2017 Infectious Diseases Society of America/Society for Healthcare Epidemiology of America Guidelines
| Guideline year | Treatment recommendations by disease severity | |
|---|---|---|
| 2010 8 | Mild/moderate* | • Metronidazole 500 mg 3 times per day by mouth for 10–14 days |
| Severe† | • Vancomycin, 125 mg 4 times per day by mouth for 10–14 days | |
| Severe-complicated‡ | • Vancomycin, 500 mg 4 times per day by mouth or nasogastric tube, plus metronidazole, 500 mg every 8 h intravenously. If complete ileus, consider adding rectal instillation of vancomycin. | |
| 2017 9 | Non-severe§ |
• Vancomycin 125 mg 4 times per day by mouth for 10 days OR • Fidaxomicin 200 mg by mouth 2 times per day for 10 days • If above agents are unavailable, metronidazole 500 mg by mouth 3 times per day for 10 days |
| Severeǁ |
• Vancomycin 125 mg 4 times per day by mouth for 10 days OR • Fidaxomicin 200 mg by mouth 2 times per day for 10 days |
|
| Fulminant‡ | • Vancomycin 500 mg 4 times per day by mouth or by nasogastric tube. If ileus, consider adding rectal instillation of vancomycin. Intravenously administered metronidazole (500 mg every 8 h) should be administered together with oral or rectal vancomycin particularly if ileus is present. | |
*White blood cell count < 15,000 cells/μl and a serum creatinine level < 1.5 times the premorbid level
†White blood cell count ≥ 15,000 cells/μl or serum creatinine level ≥ 1.5 times the premorbid level
‡Hypotension or shock, ileus, or megacolon
§White blood cell count < 15,000 cells/μl and a serum creatinine level < 1.5 mg/dl
ǁWhite blood cell count ≥ 15,000 cells/μl or serum creatinine level > 1.5 mg/dl
mg milligrams
Use of guideline-adherent therapy has been associated with improved clinical response, such as shorter time to symptom resolution.10,11 Despite the benefits of appropriate therapy, previous studies indicated low provider adherence to the 2010 treatment guidelines, particularly in patients with severe disease12–16; however, these were single-center studies and did not determine factors associated with prescribing guideline-adherent treatment. We sought to describe CDI treatment, regardless of severity, across geographically diverse areas. We then examined a subset with severe CDI to determine provider adherence to and predictors of prescribing guideline-adherent CDI therapy.
METHODS
CDI Surveillance
The Centers for Disease Control and Prevention’s (CDC) Emerging Infections Program (EIP) conducts population-based CDI surveillance in 35 counties in 10 states (California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee), comprising a total surveillance population of > 11 million persons.17 This surveillance activity underwent ethical review by CDC and all EIP sites and either was deemed non-research or received an institutional review board approval with a waiver of informed consent.
Case Identification and Data Collection
Laboratories serving the catchment areas reported all positive C. difficile tests to EIP staff. A CDI case was defined as a positive stool specimen (C. difficile toxin or molecular assay) obtained during 2013–2015 in a person aged ≥ 18 years without a positive test in the prior 8 weeks. An initial chart review was performed on all cases in 8 EIP sites and a random sample of cases in 2 EIP sites with the largest surveillance populations.18 Cases were classified as community-onset if a positive specimen was collected in an outpatient setting or within 3 days after hospital admission, or they were classified as healthcare facility-onset if a positive specimen was collected in a long-term care facility (LTCF), from a LTCF resident, or > 3 days after hospital admission. All community-onset cases and a 10% random sample of healthcare facility–onset cases underwent additional chart review using a standardized form to collect comorbidities, healthcare and medication exposures, clinical course, and outcomes. Community-onset cases were further classified as community-associated if there was no documented overnight stay in a healthcare facility in the preceding 12 weeks. All other cases were classified as healthcare-associated.
Severe Disease and Guideline-Adherent Therapy
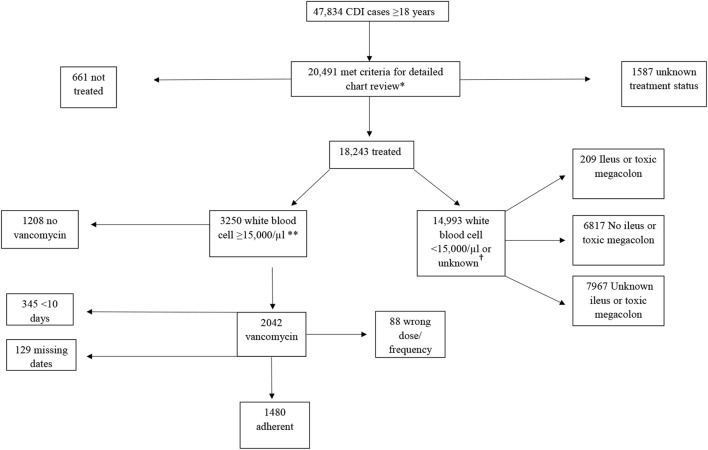
The 2010 IDSA/SHEA guidelines defined severe CDI as white blood cell (WBC) ≥ 15,000/μl or serum creatinine level ≥ 1.5 times the premorbid level.8 Accordingly, we defined cases as severe if the WBC count was ≥ 15,000 cells/μl within 1 calendar day before or after stool collection. In 2015, the case abstraction form was modified to include WBC ≥ 15,000 cells/μl within 7 days before or after stool collection (Fig. 1). We were unable to use serum creatinine to define severe CDI as this information was not collected during chart review.
Figure 1.
Clostridioides difficile infection cases, 2013–2015. Single asterisk symbol indicates community-onset and 10% of healthcare facility–onset CDI cases. Dagger symbol indicates white blood cell ≥ 15,000 cells/μl within 1 calendar day of stool specimen collection in 2013–2014, and within 7 calendar days of stool specimen collection starting in 2015. CDI, Clostridioides difficile infection
Cases that underwent treatment for severe CDI were classified as being prescribed guideline-adherent or non-guideline-adherent therapy based on the 2010 IDSA/SHEA treatment guidelines (Table 1). If a case was prescribed vancomycin at a dose of at least 125 milligrams (mg) 4 times per day for 10 days, treatment was considered adherent. Since no cases were prescribed intravenous vancomycin, we did not include route of administration in the definition of adherent therapy. A gap in therapy > 48 h was considered cessation of therapy. If a patient died before receiving 10 days of therapy and if the case was started on vancomycin at the right dose and frequency within 48 h of specimen collection, treatment was considered adherent.
Statistical Analysis
Only cases with a full chart review were included in the analyses. Descriptive analyses were performed to summarize relevant variables and treatment data for all treated cases and for the subset with severe disease. Multiple imputation was performed for missing race (20% of cases) based on the distribution of known race by age, sex, and surveillance site.
Generalized linear mixed models were used to evaluate factors associated with prescription of guideline-adherent therapy. Unadjusted analyses were performed on demographics and other relevant case characteristics, including epidemiologic classification, location of stool collection, hospital admission, medications, and selected healthcare exposures in the prior 12 weeks. Charlson comorbidity index was calculated and included as an ordinal variable.19
All variables with a p value less than 0.20 in unadjusted analyses were included in the initial multivariable model. Backward selection was used to construct the final multivariable model, which included all variables with a p value < 0.05. Crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A 2-tailed p value < 0.05 was considered statistically significant. We performed a sensitivity analysis to evaluate factors associated with prescription of guideline-adherent therapy using an expanded definition of disease severity that included a CDI-associated complication (ileus, toxic megacolon, or colectomy) or intensive care unit (ICU) admission on the day of or following the C. difficile–positive specimen collection. All analyses were performed with SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Description of CDI Cases
Of 47,834 adult CDI cases, 20,491 (43%) cases had undergone full chart review. Among these cases, 18,243 (89%) were treated for CDI and 661 (3%) were not treated (Fig. 1); treatment data were not available for 1587 (8%) cases. Among those treated, the median age was 63 (interquartile range (IQR) 48–76) years, 62% (n = 11,292) were female, and 52% (n = 9424) were admitted to a hospital at the time of or within 7 days after stool collection (Table 2). Sixty-nine percent (n = 12,542) and 25% (n = 4502) of the treated cases had received antibiotics or immunosuppressive medications, respectively, in the 12 weeks prior to CDI diagnosis. Eighteen percent (n = 3250) of the treated cases met the severe CDI definition; 159 (5%) of these had ileus or toxic megacolon within 5 days of specimen collection. Two hundred nine treated cases (1%) that did not meet the severe CDI definition had ileus or toxic megacolon. Those treated and not treated were similar regarding age, sex, race, and comorbidities. Treated cases were more likely to have been admitted to a hospital, had stool collected at a hospital, or had taken antibiotics in the prior 12 weeks.
Table 2.
Description of Clostridioides difficile Infection Treated Cases (n = 18,243)
| Treated (n = 18,243) N (%) or median (IQR) |
|
|---|---|
| Age | |
| 18–44 | 3953 (22) |
| 45–64 | 5843 (32) |
| ≥ 65 years | 8447 (46) |
| Sex | |
| Male | 6951 (38) |
| Female | 11,292 (62) |
| Charlson comorbidity index | |
| 0 | 6876 (38) |
| 1–2 | 5430 (30) |
| ≥ 3 | 5937 (32) |
| Inflammatory bowel disease | 1230 (7) |
| Diverticular disease | 1985 (11) |
| Hospital admission* | 9424 (52) |
| Epidemiologic class | |
| Healthcare-associated | 7309 (40) |
| Community-associated | 10,932 (60) |
| Unknown 2 (<1) | |
| Location of stool collection | |
| Hospital/LTACH† | 8682 (48) |
| Outpatient | 7551 (41) |
| Emergency room/observation unit | 1528 (8) |
| Long-term care facility | 459 (3) |
| Other or unknown | 23 (< 1) |
| Medications in prior 12 weeks | |
| Antibiotics | 12,542 (69) |
| Immunosuppressive | 4502 (25) |
| unknown | 23 (< 1) |
| Healthcare exposures in prior 12 weeks‡ | 9884 (54) |
| CDI recurrence | 2883 (16) |
| Colectomy | 49 (< 1) |
| All-cause 30-day mortality | 417 (2) |
| ICU admission | 642 (4) |
| Length of stay§ | 4 (2, 7) |
| WBC count ≤ 1000 cells/μl | 134 (< 1) |
| WBC count ≥ 15,000 cells/μl | 3250 (18) |
| Ileus or toxic megacolon | 368 (2) |
| Pseudomembranous colitis | 55 (< 1) |
*Admitted to acute care hospital at time of or within 7 days after stool collection
†Includes samples collected in emergency room or observation unit on the same day as acute care admission
‡Includes chronic hemodialysis, surgery, emergency room visit, hospital admission, residence in long-term care facility or skilled nursing facility, and observation unit admission in prior 12 weeks
§Length of stay indicates the time from CDI diagnosis to discharge
CDI Clostridioides difficile infection,ICU intensive care unit, LTACH long-term acute care hospital, WBC white blood cell
Treatment
All CDI Cases
Metronidazole was the most common CDI treatment medication among all cases (n = 14,257, 78%) (Table 3). Five hundred milligrams was the most common starting dose (n = 13,088, 92%), and 83% (n = 11,778) were prescribed metronidazole 3 times per day. Metronidazole monotherapy was used in 56% (n = 10,173) of cases. Sixty-nine percent (n = 7046) of those prescribed only metronidazole were prescribed 500 mg 3 times per day for at least 10 days. Of the 31% of patients prescribed only metronidazole and who were not prescribed 500 mg 3 times per day for 10 days, 39% (n = 1207) were prescribed a different dose or frequency, 28% (880) were prescribed < 10 days of therapy, and 33% (1040) were missing dates to calculate duration of treatment.
Table 3.
Description of Clostridioides difficile Infection Treatment Among All Treated Cases (n = 18,243) and a Subset with Severe Disease (n = 3250)
| All cases | Severe cases | |
|---|---|---|
| N (%) | N (%) | |
| Any metronidazole | N = 14,257 (78) | N = 2576 (79) |
| Dose | ||
| 500 mg | 13,088 (92) | 2473 (96) |
| Other | 535 (4) | 61 (2) |
| Unknown | 634 (4) | 42 (2) |
| Frequency | ||
| Three times a day | 11,778 (83) | 2130 (83) |
| Other | 1797 (13) | 403 (15) |
| Unknown | 682 (4) | 43 (2) |
| Route | ||
| Oral | 11,728 (82) | 1508 (58) |
| Intravenous | 2443 (17) | 1060 (41) |
| Unknown | 86 (< 1) | 8 (< 1) |
| Any vancomycin | N = 7683 (42) | N = 2042 (63) |
| Doses | ||
| 125 mg | 5077 (66) | 1305 (64) |
| 250 mg | 1795 (23) | 506 (25) |
| 500 mg | 444 (6) | 194 (9) |
| Other | 32 (< 1) | 9 (< 1) |
| Unknown | 335 (4) | 28 (1) |
| Frequency | ||
| Four times a day | 6874 (89) | 1895 (93) |
| Other | 427 (6) | 108 (5) |
| Unknown | 382 (5) | 39 (2) |
| Route | ||
| Oral | 7612 (99) | 2013 (99) |
| Rectal | 42 (< 1) | 24 (1) |
| Unknown | 29 (< 1) | 5 (< 1) |
| Any fidaxomicin | N = 313 (2) | N = 64 (2) |
| Doses | ||
| 200 mg | 284 (91) | 61 (95) |
| Other | 4 (1) | 0 |
| Unknown | 25 (8) | 3 (5) |
| Frequency | ||
| Two times a day | 281 (90) | 60 (94) |
| Three times a day | 2 (< 1) | 0 |
| Unknown | 30 (10) | 4 (6) |
| Route | ||
| Oral | 313 (100) | 64 (100) |
| Any treatment | N = 18,243 | N = 3250 |
| Monotherapy | ||
| Metronidazole | 10,173 (56) | 1162 (36) |
| Vancomycin | 3576 (20) | 626 (19) |
| Fidaxomicin | 117 (< 1) | 10 (< 1) |
| Combination therapy with metronidazole and vancomycin | ||
| Any | 4037 (22) | 1405 (43) |
| Sequential | 851 (5) | 176 (5) |
| Metronidazole first | 581 (3) | 113 (3) |
| Vancomycin first | 270 (1) | 63 (2) |
| Overlap | 3186 (17) | 1229 (38) |
| ≥ 2 days | 2925 (16) | 1141 (35) |
| Vancomycin taper | 866 (5) | 168 (5) |
| Probiotics | 3223 (18) | 701 (22) |
| Fecal microbiota transplant | 89 (< 1) | 10 (< 1) |
| Days from stool collection to therapy | ||
| ± 7 days | 15,811 (87) | 3019 (93) |
| ± 2 days | 13,117 (72) | 2798 (86) |
| Days of therapy, median (IQR) | 14 (11–15) | 14 (11–17) |
IQR interquartile range, mg milligrams
Forty-two percent (n = 7683) of all treated cases were prescribed vancomycin. The most common starting doses were 125 mg in 66% (n = 5077) and 250 mg in 23% (n = 1795) of cases. Eighty-nine percent (n = 6874) were prescribed vancomycin 4 times per day. Twenty percent (n = 3576) were prescribed vancomycin only and 5% (n = 866) were treated with vancomycin tapers. Combination therapy (metronidazole and vancomycin) was used in 22% (n = 4037) of all treated cases.
Only 2% (n = 313) of treated cases were prescribed fidaxomicin, with < 1% (n = 117) prescribed fidaxomicin only. Ninety percent (n = 281) received 200 mg twice per day.
Most (n = 13,117, 72%) cases started therapy within 2 days of stool collection. The median length of all therapy was 14 (IQR 11–15) days. Probiotics were used in 3223 (18%) cases. Less than 1% (n = 89) of cases received a fecal microbiota transplant (FMT).
Severe CDI Cases
Of 3355 cases with severe disease, 3250 (97%) were treated for CDI, 53 (2%) were not treated, and 52 (1%) had unknown CDI treatment status. Forty-three percent of treated severe cases were prescribed a combination of medications; 626 (19%) were treated with vancomycin alone, and 1162 (36%) were treated with metronidazole only (Table 3). Of those treated for severe CDI, 129 (4%) were missing prescription dates to calculate duration of therapy. Of the 3121 treated cases with data to calculate duration, 1480 (47%) received guideline-adherent therapy. Of those who did not receive guideline-adherent therapy, 1208 (74%) were never started on vancomycin, 88 (5%) did not receive the recommended dose or frequency, and 345 (21%) received < 10 days of therapy (Fig. 1). Prescription of guideline-adherent therapy for severe cases was 44%, 45%, and 52% in 2013, 2014, and 2015, respectively.
Among 3355 severe cases, 2692 (80%) were community-onset, of whom 90% were hospitalized. Community-onset severe CDI patients who were hospitalized were more likely than those not hospitalized to receive guideline-adherent therapy (50% versus 25%; p = 0.0001).
Predictors of Guideline-Adherent Therapy Among Severe CDI Cases
In unadjusted analysis, age ≥ 65 years, higher Charlson comorbidity index, inflammatory bowel disease (IBD), healthcare-associated epidemiologic classification, antibiotics, and immunosuppressive therapy were significantly associated with prescription of guideline-adherent therapy (Table 4). In addition, hospital interactions, including hospital admission at the time of or within 7 days of stool collection and having stool collected in the hospital, were significantly associated with prescription of guideline-adherent therapy.
Table 4.
Unadjusted Analysis for Predictors of Being Prescribed Guideline-Adherent Therapy Among Severe Clostridioides difficile Infection Cases (n = 3121)*
| Non-guideline-adherent therapy (n = 1641) N (%) |
Guideline-adherent therapy (n = 1480) N (%) |
Unadjusted odds ratio (95% confidence interval) | p value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 655 (40) | 579 (39) | Reference | |
| Female | 986 (60) | 901 (61) | 1.04 (0.90, 1.20) | 0.63 |
| Age (years) | ||||
| 18–44 | 292 (18) | 208 (14) | Reference | |
| 45–64 | 484 (29) | 382 (26) | 1.12 (0.90, 1.41) | 0.31 |
| ≥ 65 | 865 (53) | 890 (60) | 1.33 (1.21, 1.82) | < 0.001 |
| Race | ||||
| White | 1342 (82) | 1240 (84) | Reference | |
| Non-white | 299 (18) | 240 (16) | 0.87 (0.72, 1.06) | 0.17 |
| Charlson comorbidity index | ||||
| 0 | 483 (29) | 328 (22) | Reference | |
| 1–2 | 536 (33) | 502 (34) | 1.37 (1.13, 1.65) | 0.001 |
| ≥ 3 | 622 (38) | 650 (44) | 1.54 (1.29, 1.85) | < 0.001 |
| Inflammatory bowel disease | 80 (5) | 98 (7) | 1.39 (1.03, 1.89) | 0.03 |
| Diverticular disease | 196 (12) | 186 (13) | 1.04 (0.84, 1.29) | 0.72 |
| Epidemiologic class | ||||
| Healthcare-associated | 857 (52) | 854 (58) | Reference | |
| Community-associated | 784 (48) | 626 (42) | 0.80 (0.68, 0.91) | 0.001 |
| Location of stool collection | ||||
| Hospital/LTACH† | 1329 (81) | 1300 (88) | Reference | |
| Ambulatory and other locations‡ | 312 (19) | 180 (12) | 0.56 (0.45, 0.68) | < 0.001 |
| Hospital admission§ | 1414 (86) | 1395 (94) | 2.73 (2.10, 3.55) | < 0.001 |
| Admitted from home | 1384 (84) | 1242 (84) | 0.96 (0.79, 1.16) | 0.64 |
| Medications in prior 12 weeks | ||||
| Antibiotics | 1263 (77) | 1185 (80) | 1.24 (1.04, 1.48) | 0.02 |
| Immunosuppressive therapy | 438 (27) | 481 (33) | 1.30 (1.11, 1.52) | 0.001 |
| Healthcare exposures in prior 12 weeksǁ | 1069 (65) | 1016 (69) | 1.19 (1.02, 1.38) | 0.03 |
*Excludes patients with unknown duration of treatment, unknown treatment status, or who did not receive treatment
†Includes samples collected in emergency room or observation unit on the same day as acute care admission
‡Other locations include long-term care facilities
§Admitted to hospital at time of or within 7 days after stool collection
ǁIncludes chronic hemodialysis, surgery, ER visit, hospital admission, residence in long-term care facility or skilled nursing facility, or observation unit admission in prior 12 weeks
LTACH long-term acute care hospital
In multivariable analysis, hospital admission (aOR 2.48; 95% CI 1.90, 3.24), age ≥ 65 years (aOR 1.37; 95% CI 1.10, 1.71), Charlson comorbidity index ≥ 3 (aOR 1.27; 95% CI 1.04, 1.55), immunosuppressive therapy (aOR 1.21; 95% CI 1.02, 1.42), and IBD (aOR 1.56; 95% CI 1.13, 2.17) were independently associated with prescription of guideline-adherent therapy among those with severe disease (Table 5).
Table 5.
Multivariable Analysis for Predictors of Being Prescribed Guideline-Adherent Therapy Among Severe Clostridium difficile Infection Cases (n = 3121)*
| Adjusted odds ratio (95% confidence interval) | p value | |
|---|---|---|
| Age (years) | ||
| 18–44 | Reference | |
| 45–64 | 1.08 (0.86, 1.37) | 0.51 |
| ≥ 65 | 1.37 (1.10, 1.71) | 0.01 |
| Charlson comorbidity index | ||
| 0 | Reference | |
| 1–2 | 1.19 (0.98, 1.45) | 0.08 |
| ≥ 3 | 1.27 (1.04, 1.55) | 0.02 |
| Hospital admission† | 2.48 (1.90, 3.24) | < 0.001 |
| Inflammatory bowel disease | 1.56 (1.13, 2.17) | 0.01 |
| Immunosuppressive therapy in prior 12 weeks | 1.21 (1.02, 1.42) | 0.03 |
*Excludes patients with unknown duration of treatment, unknown treatment status, or who did not receive treatment
†Admitted to hospital at time of or within 7 days after stool collection
Sensitivity Analysis
In unadjusted analysis, significant predictors of guideline-adherent therapy did not change when the definition of severity was expanded to include a CDI-associated complication or ICU admission. In multivariable analysis, antibiotic use in the prior 12 weeks was significantly associated with prescription of guideline-adherent therapy (aOR 1.19; 95% CI 1.01, 1.40) while age was no longer significantly associated with prescription of guideline-adherent therapy.
DISCUSSION
In this analysis of adult CDI patients from 10 different states during 2013–2015, more than 95% were treated, most commonly with metronidazole. Vancomycin, either alone or in combination with another medication, was used in 42% of patients overall and 63% of patients with severe CDI. Fidaxomicin was rarely used. In severe CDI patients, adherence to guideline-recommended therapy was low (47%). The most common type of provider non-adherence was not prescribing vancomycin, followed by not prescribing 10 days of vancomycin. Hospital admission was the most significant predictor for receiving guideline-adherent therapy for severe disease. Our findings of low adherence to guideline-recommended therapy for severe CDI have important implications given the recent publication of updated IDSA/SHEA CDI treatment guidelines that recommend either oral vancomycin or fidaxomicin for all initial non-severe and severe CDI episodes and raise concerns that adherence could continue to be poor in those with severe CDI.9
Metronidazole was the most commonly prescribed therapy among all CDI patients, with 78% prescribed metronidazole. We found a higher percentage of non-severe CDI patients were prescribed metronidazole alone (60%) than vancomycin alone (20%). Early studies demonstrated improved response to treatment with vancomycin in severe CDI, but were not definitive for non-severe CDI leading to the 2010 guideline recommendation for treating mild-moderate disease with metronidazole and severe disease with vancomycin.8 For example, in a 2007 study that compared oral metronidazole and oral vancomycin, 97% of patients with severe CDI were cured with oral vancomycin versus 76% with oral metronidazole (p = 0.02), but in those with mild CDI, cure rates were not statistically different: 90% with oral metronidazole and 98% with oral vancomycin (p = 0.36).10
However, oral vancomycin is now considered to be superior to oral metronidazole for CDI treatment, regardless of disease severity, based on studies published after the 2010 guidelines. These studies have shown lower response rates to oral metronidazole than oral vancomycin for mild-moderate and severe CDI.11,20 The largest randomized, blinded comparison of metronidazole and vancomycin for CDI treatment showed vancomycin to be superior to metronidazole for all CDI patients with 81% vs 73% clinical success (p = 0.02).11
In contrast to prior studies, which were smaller and largely single center,12–16 we were able to examine CDI treatment across multiple sites. Consistent with prior studies, we found that provider adherence to the 2010 treatment guidelines for severe CDI was low, with fewer than half of patients prescribed guideline-adherent therapy. Brown et al. found that approximately 35% of patients with severe CDI did not receive guideline-adherent therapy at a tertiary care county teaching hospital.12 Similarly, at another institution, adherence to the 2010 treatment guidelines was 43% for all patients with CDI, 66% for those with mild-moderate disease, 25% for severe disease, and 36% for severe-complicated disease.14 In our analysis, more than two-thirds of patients with severe CDI who were not prescribed adherent therapy were not prescribed vancomycin and a quarter were prescribed less than the recommended 10 days of therapy. Educating providers on starting vancomycin for the recommended duration of therapy may improve adherence with the new guidelines.
Probiotics were prescribed to a minority of all CDI cases (18%). Although a few studies have suggested that probiotics may be effective in CDI prevention,21,22 probiotics are not recommended for CDI treatment in either the 2010 or updated 2017 guidelines.8,9 Similarly, FMT was used in < 1% of all CDI cases; this is actually higher than expected given this was an analysis of only incident CDI, and FMT is a recommended therapy for only recurrent CDI.8,9
Another medication used in only a few patients was fidaxomicin, with only 2% of patients prescribed any fidaxomicin and < 1% only fidaxomicin. A similarly low fidaxomicin usage rate of 1.98% was reported in Veteran Affairs Medical Centers during 2011–2015.23 Fidaxomicin was approved by the US Food and Drug Administration for treatment of CDI in 2011,24 and some providers may not have initially been familiar with this drug. However, we found no increase in its use from 2013 to 2015, whereas fidaxomicin use increased from 2011 to 2015 among Veteran Affairs Medical Centers,23 suggesting provider adherence or awareness may be partly influenced by institutional policies. Fidaxomicin is more expensive than some of the other recommended medications and this may have limited its uptake by individual providers, institutions, and insurance companies and acceptability by patients.9,25
Our findings have important implications for adherence to the new 2017 guidelines.9 We found inadequate use of oral vancomycin for severe disease despite it being guideline-recommended therapy as well as infrequent use of fidaxomicin and vancomycin monotherapy in general. These practices may reflect a lack of knowledge of guideline-recommended therapy or lack of provider confidence in the improved efficacy of vancomycin and fidaxomicin over metronidazole. It is possible that the new CDI treatment guidelines will actually improve provider adherence by simplifying treatment and removing the need for a provider to consider disease severity. However, our findings of low provider adherence to the 2010 treatment guidelines raise concerns about adherence to the updated guidelines. To ensure optimal patient outcomes, ongoing assessments of adherence and additional efforts to improve compliance may be needed.
Hospital admission was most strongly associated with receiving guideline-adherent therapy for severe disease even after controlling for age and comorbidities. It is unclear if this reflects the perception of providers that hospitalized patients are more ill or a lack of education of outpatient providers regarding CDI treatment guidelines. Barriers to obtaining oral vancomycin in the outpatient setting might also contribute to decreased use, including increased cost and limited availability at some pharmacies.9,26 Efforts to eliminate these barriers as well as targeted education of providers may improve outpatient CDI management.
This analysis has some limitations. Incomplete documentation in the medical records and limited access to some outpatient records might have limited our ability to identify all relevant predictors of guideline-adherent therapy. When prescription information was available, we were unable to ascertain if patients took the medication as prescribed. We were only able to use WBC count to categorize severe disease (since serum creatinine was not collected), which could have underestimated the burden of severe CDI. However, we did perform a sensitivity analysis using an expanded definition of severity and results were similar to the primary analysis. Further, because we did not have creatinine data, we were unable to determine which patients had mild-moderate disease and therefore could not determine provider adherence to guideline-recommended therapy in this group of patients. In addition, we were not able to assess the association between prescription of guideline-adherent therapy and outcomes in patients with severe CDI, since mortality data was not collected on all patients. However, observational studies examining the effect of guideline-adherent CDI therapy on outcomes have shown mixed results,12,14,16,27 while randomized trials have primarily shown improved clinical response in those treated with vancomycin and no effect on recurrence and mortality.10,11 Lastly, we did not account for CDI overtreatment, which could also be associated with adverse outcomes.
In conclusion, we found that providers during 2013–2015 most commonly used metronidazole to treat CDI, whereas they infrequently used vancomycin monotherapy and fidaxomicin. Provider adherence to the 2010 guideline-recommended therapy for severe CDI was low. Targeted education, particularly of outpatient providers, and efforts to reduce barriers to prescribing and obtaining recommended medications may help increase awareness of and adherence to the updated CDI treatment guidelines.
Acknowledgments
We would like to acknowledge Lucy Fike for her assistance in data analysis and Deborah Nelson, Rebecca Tsay, Olivia Almendares, Zirka Smith, Catherine Espinosa, Michelle Wiles, and Wendy Bamberg for their assistance in collecting and managing EIP data.
Conflict of Interest
D.N.G. is a member of the Scientific Advisory Boards of Merck, Rebiotix, Actelion, DaVolterra, and Summit; is a consultant for Pfizer, MGB Pharma, and Sanofi Pasteur; holds a research grant from Seres Therapeutics; and holds patents and technology for the prevention of CDI. G.K.D. serves on the Drug Safety Monitoring Board for a C. difficile treatment study by Seres. All other authors have no known conflicts of interest.
Disclaimer
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Compliance with Ethical Standards
This surveillance activity underwent ethical review by CDC and all EIP sites and either was deemed non-research or received an institutional review board approval with a waiver of informed consent.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Magill SS, O'Leary E, Janelle SJ, et al. Changes in prevalance of health care-associated infections in U.S. Hospitals. The New England journal of medicine. 2018 Nov 1;379(18):1732-44. [DOI] [PMC free article] [PubMed]
- 2.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. The New England journal of medicine. 2015;372(9):825–34. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hensgens MP, Goorhuis A, Dekkers OM, van Benthem BH, Kuijper EJ. All-cause and disease-specific mortality in hospitalized patients with Clostridium difficile infection: a multicenter cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;56(8):1108–16. doi: 10.1093/cid/cis1209. [DOI] [PubMed] [Google Scholar]
- 4.Oake N, Taljaard M, van Walraven C, Wilson K, Roth V, Forster AJ. The effect of hospital-acquired Clostridium difficile infection on in-hospital mortality. Archives of internal medicine. 2010;170(20):1804–10. doi: 10.1001/archinternmed.2010.405. [DOI] [PubMed] [Google Scholar]
- 5.Desai K, Gupta SB, Dubberke ER, Prabhu VS, Browne C, Mast TC. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC infectious diseases. 2016;16:303. doi: 10.1186/s12879-016-1610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55(Suppl 2):S88–92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forster AJ, Taljaard M, Oake N, Wilson K, Roth V, van Walraven C. The effect of hospital-acquired infection with Clostridium difficile on length of stay in hospital. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2012;184(1):37–42. doi: 10.1503/cmaj.110543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infection control and hospital epidemiology. 2010;31(5):431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 9.McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clinical Infectious Diseases. 2018;66(7):e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(3):302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- 11.Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(3):345–54. doi: 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- 12.Brown AT, Seifert CF. Effect of treatment variation on outcomes in patients with Clostridium difficile. The American journal of medicine. 2014;127(9):865–70. doi: 10.1016/j.amjmed.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 13.Crowell KT, Julian KG, Katzman M, et al. Compliance with Clostridium difficile treatment guidelines: effect on patient outcomes. Epidemiology and infection. 2017;145(11):2185–92. doi: 10.1017/S0950268817000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtin BF, Zarbalian Y, Flasar MH, von Rosenvinge E. Clostridium difficile-associated disease: adherence with current guidelines at a tertiary medical center. World journal of gastroenterology. 2013;19(46):8647–51. doi: 10.3748/wjg.v19.i46.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knaus SJ, Saum L, Cochard E, Prichard W, Skinner B, Medas R. Impact of Evidence-Based Guidelines on Outcomes of Hospitalized Patients With Clostridium difficile Infection. Southern medical journal. 2016;109(3):144–50. doi: 10.14423/SMJ.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 16.Patel I, Wungjiranirun M, Theethira T, et al. Lack of adherence to SHEA-IDSA treatment guidelines for Clostridium difficile infection is associated with increased mortality. The Journal of antimicrobial chemotherapy. 2017;72(2):574–81. doi: 10.1093/jac/dkw423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emerging Infections Program. [cited 2019 July 23]; Available from: https://www.cdc.gov/ncezid/dpei/eip/index.html.
- 18.Clostridium difficile Infection (CDI) Tracking. [cited 2019 July 23]; Available from: https://www.cdc.gov/hai/eip/clostridium-difficile.html.
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Siegfried JD, Yanina; Flagiello, Thomas, Scipione, Marco R.; Phillips, Michael; Papadopoulos, John; Chen, Donald; Safdar, Amar Initial Therapy for Mild to Moderate Clostridium difficile Infection: Exploring the Role of Oral Metronidazole Versus Vancomycin in 168 Hospitalized Patients. Infectious Diseases in Clinical Practice. 2016;24(4):210-6.
- 21.Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. The Cochrane database of systematic reviews. 2017;12:Cd006095. doi: 10.1002/14651858.CD006095.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pattani R, Palda VA, Hwang SW, Shah PS. Probiotics for the prevention of antibiotic-associated diarrhea and Clostridium difficile infection among hospitalized patients: systematic review and meta-analysis. Open medicine : a peer-reviewed, independent, open-access journal. 2013;7(2):e56–67. [PMC free article] [PubMed] [Google Scholar]
- 23.Giancola SE, Williams RJ, 2nd, Gentry CA. Evaluation of fidaxomicin usage patterns and outcomes for Clostridium difficile infection across the United States Veterans Health Administration. Journal of clinical pharmacy and therapeutics. 2018 Jan 21. [DOI] [PubMed]
- 24.Fidaxomicin, DIFICID - FDA. [cited 2019 July 23]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/201699s000lbl.pdf.
- 25.Cruz MP. Fidaxomicin (Dificid), a Novel Oral Macrocyclic Antibacterial Agent For the Treatment of Clostridium difficile-Associated Diarrhea in Adults. P & T : a peer-reviewed journal for formulary management. 2012;37(5):278–81. [PMC free article] [PubMed] [Google Scholar]
- 26.Bunnell KL, Danziger LH, Johnson S. Economic Barriers in the Treatment of Clostridium difficile Infection With Oral Vancomycin. Open forum infectious diseases. 2017;4(2):ofx078. doi: 10.1093/ofid/ofx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens VW, Nelson RE, Schwab-Daugherty EM, et al. Comparative Effectiveness of Vancomycin and Metronidazole for the Prevention of Recurrence and Death in Patients With Clostridium difficile Infection. JAMA internal medicine. 2017;177(4):546–53. doi: 10.1001/jamainternmed.2016.9045. [DOI] [PubMed] [Google Scholar]