Abstract
Objective
Our objective was to assess healthcare resource utilization (HRU) and costs among patients with major depressive disorder (MDD) with and without treatment-resistant depression (TRD) and those without MDD in US Integrated Delivery Networks (IDNs).
Methods
This was a retrospective matched-cohort study. The Optum© Integrated Claims Electronic Health Record de-identified database was used to identify adult patients with TRD (January 2011–June 2017) across US IDNs. TRD patients were propensity score matched 1:1 with non-TRD MDD and non-MDD patients on demographics. Rates of HRU and costs were compared up to 2 years following the first antidepressant pharmacy claim (or randomly imputed date for non-MDD patients) using negative binomial and ordinary least squares regressions, respectively, with 95% confidence intervals (CIs) from nonparametric bootstraps (costs only) adjusted for baseline comorbidity index and costs.
Results
All 1582 TRD patients were matched to non-TRD MDD and non-MDD patients and evaluated. TRD patients were on average 46 years old, and 67% were female. Mean duration of observation was 20.1, 19.6, and 17.9 months in the TRD, non-TRD MDD, and non-MDD cohorts, respectively. Patients with TRD had significantly higher rates of HRU than did non-TRD MDD patients (inpatient visits 0.35 vs. 0.16 per patient per year [PPPY]; adjusted incidence rate ratio [IRR] 2.04 [95% CI 1.74–2.39]) and non-MDD patients (0.35 vs. 0.09 PPPY, adjusted IRR 3.05 [95% CI 2.54–3.66]). TRD patients incurred significantly higher costs PPPY than did non-TRD MDD patients ($US25,807 vs. 13,701, adjusted cost difference $US9479 [95% CI 7071–11,621]) and non-MDD patients ($US25,807 vs. 8500, adjusted cost difference $US11,433 [95% CI 8668–13,876]).
Conclusions
HRU and costs associated with TRD are significant in US IDNs.
Electronic supplementary material
The online version of this article (10.1007/s41669-019-0154-z) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Patients with major depressive disorder (MDD) and treatment-resistant depression (TRD) had higher healthcare resource utilization (HRU) and costs than did patients with MDD who were not resistant to treatment and those without MDD in US Integrated Delivery Networks (IDNs). |
| Patients with TRD had longer durations of antidepressant therapies and greater variation in antidepressants; effective treatment strategies are urgently needed for this patient population. |
| The burden of TRD was mainly driven by medical-related HRU and costs. |
Introduction
Major depressive disorder (MDD) is a debilitating psychiatric disorder characterized by a loss of interest in previously enjoyable activities, chronic depression, and suicidal ideations [1]. MDD is one of the most prevalent behavioral health disorders in the United States (US) [2, 3], representing 6.7% (16.2 million adults) of the overall population [2]. The economic burden associated with MDD was estimated at $US210.5 billion in 2010, with 45–47% of this burden attributed to direct healthcare costs and 48–50% due to workplace costs [4].
Antidepressant therapy represents the standard of care for patients with MDD; however, a considerable portion of patients (approximately 30%) develop treatment-resistant depression (TRD) [5], which is commonly defined as the failure to respond to at least two different antidepressants that are of adequate dose and duration [6, 7]. Although the health and economic burdens associated with MDD are considerable, recent studies have shown that patients with TRD experience an economic burden that is typically two-to-threefold higher than patients who respond to treatment [8, 9]. A recent study examining direct healthcare costs associated with TRD found that TRD patients had greater healthcare resource utilization (HRU) that resulted in considerably higher direct costs per patient per year (TRD $US17,261; non-TRD MDD $US9790; non-MDD $US4781) [8].
Modification of therapeutic approaches with each failed response requires TRD patients to seek ongoing care from healthcare providers across multiple settings [6, 10, 11]. However, fragmented care resulting from the lack of coordination among different healthcare providers and organizations can interfere with the continuity of measurement-based care required to treat these patients [12, 13]. To overcome this challenge, collaborative care models such as integrated delivery networks (IDNs) have been proposed [11, 14]. Despite variations in the structure of IDNs, they are broadly defined as healthcare organizations that consist of multiple physicians, hospitals, and other sources of patient care [15–18]. IDNs are increasingly recognized for their capacity to provide quality care across a continuum of settings (e.g., hospitals, health clinics, community health centers) within a single system [11, 19–21].
As organizations dedicated to coordinated patient care, IDNs typically assume financial accountability for patient outcomes, which is often associated with increased efficiency and reduced healthcare costs [11]. Although care in IDNs is expected to decrease costs, conclusive evidence is lacking [15]. IDNs are commonly discussed within the context of improved quality of care [22], yet evidence regarding the costs among MDD and TRD patients treated within US IDNs is sparse. The limited number of reports assessing behavioral health services in collaborative care models [15, 23, 24] have focused on heterogeneous populations of depressed patients who often have other comorbidities. As a result, these reports may not accurately capture the costs of care among TRD and non-TRD MDD patients. Therefore, this study was conducted to describe the HRU and costs among three cohorts of patients—those with TRD, those with non-TRD MDD, and those with no evidence of MDD—who were treated within IDNs in the US.
Methods
Data Source
Optum Integrated Claims Electronic Health Records (EHRs) from 1 January 2011 to 30 June 2017 was used for this study. This database included information on > 10 million individuals insured by UnitedHealth Group with their adjudicated claims linked to Humedica’s EHR. Data were de-identified and compliant with the requirements outlined in the Health Insurance Portability and Accountability Act.
Study Design
This retrospective matched-cohort study comprised three mutually exclusive TRD, non-TRD MDD, and non-MDD cohorts. The first antidepressant pharmacy claim (on or after 1 July 2011 for TRD and non-TRD MDD patients, and randomly imputed dates on or after 1 July 2011 for non-MDD patients) was defined as the index date. Patient characteristics were evaluated in the 6 months that preceded the index date (baseline period), and study outcomes (treatment patterns, HRU, and costs) were evaluated from the index date up to 2 years post-index date, the end of continuous enrollment in a health insurance plan, or end of data availability (30 June 2017), whichever occurred first. Since the average time to become TRD was reported to be 1.3 years [6], a 2-year period was used to allow MDD patients the opportunity to be classified as TRD.
Patients with one or more visits to an IDN were considered IDN patients. Once patients were seen in an IDN, they remain flagged as IDN patients even if they later received care in a non-IDN setting. The IDN indicator was not determined at the patient level and was therefore not time or visit specific. Although a consensus definition of an IDN is lacking [15], an IDN was defined as a coordinated hospital inpatient and outpatient system that offers healthcare services in a defined geographic area in the Optum database. Multiple coordinated hospitals or IDNs could be included. IDNs that were included are primarily hospital based in Optum, but they provide more comprehensive services than solely inpatient care. Moreover, Optum defined an IDN as a provider network-level variable; providers self-identified whether they were IDNs and were required to have inpatient data to support their status as IDNs. In this study, 80.1% of the MDD cohort were associated with an IDN, whereas 75.7% were associated in the non-MDD cohort (Tables S1–2).
Study Cohorts
Patients with TRD (TRD cohort) were compared with two control cohorts: patients with MDD who did not have TRD (non-TRD MDD cohort) and patients without MDD (non-MDD cohort). Patients in both control cohorts were propensity score (PS) matched 1:1 with patients in the TRD cohort.
To be included in both the TRD and the non-TRD MDD cohorts, patients were required to meet the following criteria: (1) one or more diagnoses for MDD (International Classification of Diseases, Ninth Edition, Clinical Modification [ICD-9-CM]: 296.2x [MDD – single episode], 296.3x [MDD – recurrent episode]; ICD, Tenth Edition, CM [ICD-10-CM]: F32.x [excluding F32.8], F33.x [excluding F33.8]); (2) one or more claims for an antidepressant between 1 July 2011 and 30 June 2017 (MDD diagnosis could occur before or after the first antidepressant pharmacy claim); (3) one or more diagnoses of depression (ICD-9-CM: 296.2x, 296.3x, 300.4x, 311.x, 309.0x, or 309.1x; ICD-10-CM: F32.x, F33.x, F34.1, or F43.21) during the baseline or observation period. Both the baseline and follow-up periods were used to identify patients with depression due to the episodic nature of the disease [25].
Treatment-Resistant Depression (TRD) Cohort
In the TRD cohort, a pharmacy claims-based algorithm was used to identify MDD patients who were likely to have TRD within 2 years after the index date (i.e., follow-up). MDD patients were considered likely to have TRD if their MDD did not respond to two antidepressant treatment regimens, including augmentation therapy with an anticonvulsant, anxiolytic, antipsychotic, lithium, psychostimulant, or thyroid hormone medication, with adequate dose and duration (> 6 weeks) as per the American Psychiatric Association guidelines [7]. Failure of a treatment regimen was defined as a switch of antidepressant (i.e., < 180 days after the end of the previous treatment), the addition of an antidepressant, or the initiation of an augmentation therapy. The initiation of the third treatment regimen had to occur > 6 weeks after the start of the first antidepressant treatment.
Non-TRD Major Depressive Disorder (MDD) Cohort
Patients in the non-TRD MDD cohort included MDD patients who did not meet the criteria for TRD, as previously defined, within 2 years of the index date.
Non-MDD Cohort
Patients in the non-MDD cohort consisted of a randomly selected sample of patients without MDD (ICD-9-CM: 296.2x, 296.3x; ICD-10-CM: F32.x, F33.x [excluding F33.8]) from 1 January 2011 to 30 June 2017. Since a retrospective matched-cohort study design was used, the random selection of 500,000 non-MDD patients was considered sufficient to provide a pool of controls eligible for matching.
All Cohorts
All patients included in this study were required to meet the following conditions: (1) no diagnosis for specific psychiatric comorbidities (i.e., psychosis [ICD-9-CM: 298.xx; ICD-10-CM: F23.x, F25.x, F44.89], schizophrenia [ICD-9-CM: 295.xx; ICD-10-CM: F20.x, F25.x], bipolar disorder/manic depression [ICD-9-CM: 296.0x, 296.1x, 296.4x, 296.5x, 296.6x, 296.7x, 296.8x; ICD-10-CM: F30.x, F31.x], dementia [ICD-9-CM: 290.xx, 294.1x; ICD-10-CM: F01.x, F02.x, F03.x]) between 1 January 2011 and 30 June 2017; (2) ≥ 18 years at the index date; (3) ≥ 6 months of continuous enrollment in a health insurance plan before and after the index date; (4) no antidepressant claims during the baseline period.
Study Measures
Baseline characteristics included demographics, physical and mental comorbidities, medication use, HRU, and costs were used to assess the distribution of the TRD, non-TRD, and non-MDD cohorts.
For each cohort, the proportion of patients receiving antidepressant medications during the observation period was reported. Duration of antidepressant therapy was also presented and defined as the number of days with medication available between the first antidepressant claim (i.e., index date) and the last day of supply of antidepressant with no gaps > 14 days (gaps were excluded in duration of therapy).
HRU and costs were categorized as all-cause, behavioral health-related, depression-related, and suicide-related. For each category, HRU and costs were divided into inpatient visits, inpatient days, emergency department (ED) visits, outpatient visits, and other visits. Behavioral health-related medical HRU and costs (including depression-related but not suicide-related costs) were identified using primary or secondary ICD-9-CM diagnostic codes 290.xx–319.xx and ICD-10-CM diagnostic codes F01.xxx–F99.xxx. Psychiatric pharmacy costs included the following classes of agents: anxiolytics, antidepressants, anticonvulsants/mood stabilizers, antipsychotics, and other mood stabilizers (e.g., lithium). Depression-related HRU and costs were identified using primary or secondary ICD-9-CM diagnostic codes 296.2x, 296.3x, 300.4x, 309.0x, 309.1x, 311.xx and ICD-10-CM diagnostic codes F32.x, F33.x, F34.1, or F43.21. Antidepressant pharmacy costs included the following classes of agents, selective serotonin reuptake inhibitors (SSRIs), norepinephrine-dopamine reuptake inhibitors (NDRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), serotonin modulators (i.e., nefazodone, trazodone, vilazodone, venlafaxine), tricyclics and tetracyclics, norepinephrine-serotonin modulators, monoamine oxidase inhibitors (MAOIs), and other (i.e., olanzapine-fluoxetine). Suicide-related HRU and costs were defined using primary or secondary ICD-9-CM diagnostic codes E95x or V62.84 and ICD-10-CM diagnostic codes T14.91x, X71.x-X83.x, T36.x- T65.x with a suffix of ‘2’ indicating ‘intentional self-harm’, T71.x with a suffix of ‘2’ indicating ‘intentional self-harm’ or R45.851.
Statistical Analysis
Patients with TRD were matched 1:1 to non-TRD MDD and non-MDD patients using PS defined as the conditional probability of having TRD based on observable characteristics [26, 27]. The PS across patients were classified into strata, each containing an equal proportion of patients (5%). One TRD patient was matched to one non-TRD MDD and one non-MDD patient within the same stratum. The logistic regression model was used to estimate the PS, where having TRD (yes/no) was the binary dependent variable and patient characteristics were the predictors and included age, sex, race, year of the index date, geographical region, and type of healthcare plan. Baseline patient characteristics were compared using standardized differences; a covariate with a standardized difference < 10% was considered well-balanced [26]. Rates of HRU were compared between matched cohorts using multivariable negative binomial regression (i.e., incidence rate ratios [IRRs]). Costs were expressed as per patient per year (PPPY) in $US, year 2017 values, using the Consumer Price Index for Medical care using means, standard deviations (SDs), and medians, and adjusted cost differences were calculated using multivariable ordinary least squares regression with 95% confidence intervals (CIs) and p-values obtained from nonparametric bootstraps with 499 replications. To account for censoring in the cost data, a phase-based approach has been recommended in studying cumulative costs [28]. Specifically, mean costs per patient per month (PPPM) in the 6 months before and up to 2 years after the index date and stratified by 1-month periods were calculated as a sensitivity analysis. Since the matching was limited to demographic variables, baseline Quan-Charlson comorbidity index (Quan-CCI) [29] and all-cause healthcare costs were adjusted for in the multivariable models due to the remaining imbalances observed after matching. Given that TRD is complex, with multifactorial causes and effects with respect to other comorbidities, minimally matching on and controlling for potential confounders to avoid conditioning on mediators was deemed appropriate in this context. For the parameter estimates, standard errors, and Akaike information criterion from the regression models, see Tables S3–7.
Results
Baseline Demographic and Clinical Characteristics
Among 12,730 treated patients with MDD, 1582 (12%) met the criteria for TRD (Table S1). A total of 63,828 patients without MDD who met the inclusion criteria were identified (Table S2). Before matching, TRD patients were younger than non-TRD MDD and non-MDD patients (Table S8). After matching, cohorts were well-balanced (standardized difference < 10%) on demographic factors (Table 1). TRD patients were more likely to have other behavioral health-related medication use during the baseline period, even after matching. See Figs. S1–S2 for PS distributions. The mean duration of the observation period was 20.1, 19.6, and 17.9 months in the TRD, non-TRD MDD, and non-MDD cohorts, respectively.
Table 1.
Baseline characteristics, healthcare resource utilization, and all-cause healthcare costs evaluated 6 months prior to the index date
| Variables | Matched populationa | ||||
|---|---|---|---|---|---|
| TRD cohort | Non-TRD MDD cohort | Std. diff.b (%) | Non-MDD control cohort | Std. diff.b (%) | |
| N = 1582 | N = 1582 | N = 1582 | |||
| Age at index date, years | 45.6 ± 16.6 [45] | 45.4 ± 16.7 [44] | 0.7 | 44.9 ± 16.8 [44] | 4.1 |
| Female | 1052 (66.5) | 1067 (67.4) | 2.0 | 1088 (68.8) | 5.0 |
| Race | |||||
| White | 1345 (85.0) | 1339 (84.6) | 1.1 | 1313 (83.0) | 5.4 |
| Black | 68 (4.3) | 69 (4.4) | 0.3 | 58 (3.7) | 3.2 |
| Asian | 16 (1.0) | 15 (0.9) | 0.6 | 15 (0.9) | 0.6 |
| Hispanic | 51 (3.2) | 68 (4.3) | 5.7 | 76 (4.8) | 8.1 |
| Other/unknown | 102 (6.4) | 91 (5.8) | 2.9 | 120 (7.6) | 4.5 |
| Year of index datec | |||||
| 2011 | 215 (13.6) | 203 (12.8) | 2.2 | 224 (14.2) | 1.6 |
| 2012 | 266 (16.8) | 256 (16.2) | 1.7 | 273 (17.3) | 1.2 |
| 2013 | 260 (16.4) | 262 (16.6) | 0.3 | 286 (18.1) | 4.4 |
| 2014 | 250 (15.8) | 243 (15.4) | 1.2 | 259 (16.4) | 1.6 |
| 2015 | 292 (18.5) | 294 (18.6) | 0.3 | 291 (18.4) | 0.2 |
| 2016 | 246 (15.5) | 274 (17.3) | 4.8 | 249 (15.7) | 0.5 |
| 2017 | 53 (3.4) | 50 (3.2) | 1.1 | 0 (0.0) | 25.9 |
| Geographical regiond | |||||
| Northeast | 160 (10.1) | 163 (10.3) | 0.6 | 163 (10.3) | 0.6 |
| Midwest | 792 (50.1) | 768 (48.5) | 3.0 | 807 (51.0) | 1.9 |
| South | 396 (25.0) | 419 (26.5) | 3.3 | 378 (23.9) | 2.6 |
| West | 191 (12.1) | 178 (11.3) | 2.6 | 185 (11.7) | 1.2 |
| Unknown | 43 (2.7) | 54 (3.4) | 4.0 | 49 (3.1) | 2.3 |
| Type of healthcare plan | |||||
| Medicare | 283 (17.9) | 259 (16.4) | 4.0 | 254 (16.1) | 4.9 |
| Commercial | 1299 (82.1) | 1323 (83.6) | 4.1 | 1328 (83.9) | 5.0 |
| Preferred provider organization | 78 (4.9) | 85 (5.4) | 2.0 | 84 (5.3) | 1.7 |
| Point of service plan | 907 (57.3) | 908 (57.4) | 0.1 | 955 (60.4) | 6.3 |
| Health management organization | 189 (11.9) | 187 (11.8) | 0.4 | 176 (11.1) | 2.6 |
| Exclusive provider organization | 84 (5.3) | 93 (5.9) | 2.5 | 71 (4.5) | 3.8 |
| Other healthcare plan | 41 (2.6) | 50 (3.2) | 3.4 | 42 (2.7) | 0.4 |
| Quan-CCI [29] | 0.6 ± 1.3 [0] | 0.5 ± 1.1 [0] | 12.1 | 0.3 ± 0.9 [0] | 29.0 |
| Number of unique mental health diagnoses | 1.2 ± 1.6 [1] | 0.9 ± 1.3 [0] | 20.8 | 0.2 ± 0.5 [1] | 84.3 |
| Other mental health-related medication use [42, 43]e | 614 (38.8) | 343 (21.7) | 35.2 | 152 (9.6) | 62.4 |
| Top five most frequent physical comorbidities [44]f | |||||
| Hypertension | 405 (25.6) | 338 (21.4) | 9.9 | 243 (15.4) | 24.6 |
| Diabetes | 196 (12.4) | 147 (9.3) | 9.9 | 92 (5.8) | 22.5 |
| Chronic pulmonary disease | 167 (10.6) | 157 (9.9) | 2.1 | 94 (5.9) | 16.6 |
| Obesity | 127 (8.0) | 128 (8.1) | 0.2 | 78 (4.9) | 12.5 |
| Hypothyroidism | 140 (8.8) | 121 (7.6) | 4.4 | 100 (6.3) | 9.5 |
| Top five most frequent mental comorbiditiesg | |||||
| Depressionh | 556 (35.1) | 478 (30.2) | 10.3 | 37 (2.3) | 76.7 |
| Anxiety disorders | 345 (21.8) | 245 (15.5) | 15.9 | 70 (4.4) | 49.1 |
| Sleep–wake disorders | 220 (13.9) | 154 (9.7) | 12.8 | 65 (4.1) | 33.3 |
| Substance-related and addictive disorders | 188 (11.9) | 116 (7.3) | 15.3 | 55 (3.5) | 30.9 |
| Other conditions that may be a focus of clinical attention | 121 (7.6) | 110 (7.0) | 2.7 | 65 (4.1) | 14.9 |
| Had ≥ 1 healthcare visit/service | |||||
| Inpatient | 217 (13.7) | 144 (9.1) | 14.3 | 57 (3.6) | 35.0 |
| ED | 203 (12.8) | 178 (11.3) | 4.8 | 112 (7.1) | 18.9 |
| Outpatient | 1398 (88.4) | 1342 (84.8) | 9.8 | 1180 (74.6) | 30.3 |
| Other | 366 (23.1) | 306 (19.3) | 9.2 | 193 (12.2) | 27.8 |
| All-cause healthcare costs ($US, year 2017 values) | 21,872 ± 61,869 [4937] | 13,696 ± 44,271 [2643] | 15.2 | 7160 ± 24,755 [1350] | 31.2 |
| Medical costs | 19,802 ± 60,601 [3363] | 12,357 ± 43,421 [1740] | 14.1 | 5918 ± 23,754 [868] | 30.2 |
| Pharmacy costs | 2070 ± 6719 [420] | 1339 ± 4201 [230] | 13.0 | 1242 ± 5943 [105] | 13.1 |
Data are presented as n (%) or mean ± standard deviation [median] unless otherwise indicated
ED emergency department, ICD-x-CM International Classification of Diseases, xth edition, Clinical Modification, MDD major depressive disorder, Quan-CCI Quan-Charlson comorbidity index, SD standard deviation, Std. diff. standardized difference, TRD treatment-resistant depression
aPatients were matched on propensity score (the probability of being in the TRD cohort vs. the non-TRD MDD or non-MDD cohort), modelled using a logistic regression model adjusted for categorical age, sex, race, year of the index date, geographical region, and type of healthcare plan
bFor continuous variables, the std. diff. was calculated by dividing the absolute difference in means of the control and the TRD cohorts by the pooled SD of both groups. The pooled SD is the square root of the average of the squared SDs. For dichotomous variables, the std. diff. was calculated using the following equation, where P is the respective proportion of participants in each group: (PTRD − Pcontrol)/√[(PTRD(1 − PTRD) + Pcontrol(1 − Pcontrol))/2]
cFor TRD and non-TRD MDD patients, the index date was defined as the date of the first prescription fill for an antidepressant. For non-MDD patients, the index date was randomly generated
dBased on US census regions (http://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf)
eIncludes anxiolytics, anticonvulsants/mood stabilizers, antipsychotics, psychostimulants, thyroid hormone (T3), and lithium. Agents were grouped according to their generic name
fThe top five most frequent Elixhauser comorbidities identified in the TRD cohort were reported
gThe top five most frequent mental disorders identified in the TRD cohort were reported
hDepression diagnoses included the following diagnoses ICD-9-CM: 296.2x (MDD—single episode), 296.3x (MDD—recurrent episode), 300.4x (dysthymic disorder), 309.0x (adjustment disorder with depressed mood), 309.1x (prolonged depressive reaction), and 311.x (depressive disorder, not elsewhere classified) or ICD-10-CM: F32x (MDD—single episode), F33x (MDD—recurrent episode), F341 (dysthymic disorder) and F4321 (adjustment disorder with depressed mood). All patients had to have a diagnosis of MDD during the study period, but only a portion had an MDD diagnosis during the baseline period
Treatment Patterns
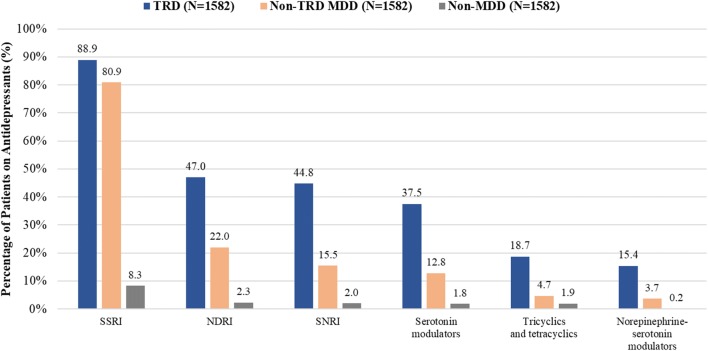
Patients with TRD used a greater variety of antidepressants than did patients in the other cohorts. In the TRD and non-TRD MDD cohorts, the three most frequently used antidepressants included SSRIs (89 vs. 81%), NDRIs (47 vs. 22%), and SNRIs (45 vs. 16%; Fig. 1). The use of antidepressants was low in the non-MDD cohort (SSRIs 8%, NDRIs 2%, and SNRIs 2%). The duration of antidepressant therapy was longer among TRD patients than among non-TRD MDD and non-MDD patients (Table S9).
Fig. 1.
Medication treatment patterns during the observation period among TRD, non-TRD MDD, and non-MDD cohorts. MDD major depressive disorder, NDRIs norepinephrine-dopamine reuptake inhibitors, SNRIs serotonin-norepinephrine reuptake inhibitors, SSRIs selective serotonin reuptake inhibitors, TRD treatment-resistant depression
Healthcare Resource Utilization (HRU) and Costs
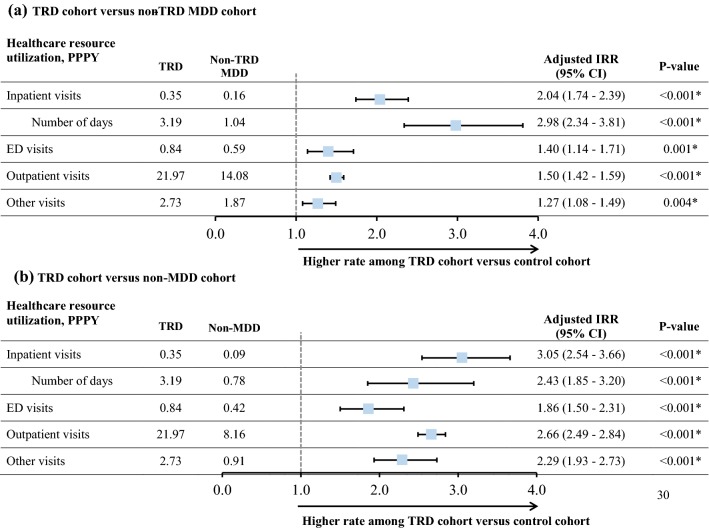
Patients with TRD had higher rates of HRU during the observation period than did the non-TRD MDD and non-MDD patients (Fig. 2). TRD patients had 2.04 (95% CI 1.74–2.39) and 3.05 (95% CI 2.54–3.66) times the rate of inpatient visits for non-TRD MDD and non-MDD patients, respectively. All-cause inpatient length of stay was higher for TRD patients (3.19 days) than for non-TRD (1.04 days) and non-MDD (0.78 days) patients (adjusted IRR 2.98 [95% CI 2.34–3.81] and 2.43 [95% CI 1.85–3.20], respectively). Similarly, TRD patients had higher rates of behavioral health-related HRU (e.g., 2.61 [95% CI 2.23–3.06] and 17.44 [95% CI 11.62–26.16] times the rate of inpatient visits for TRD patients vs. non-TRD MDD and non-MDD patients, respectively); the rate of depression- and suicide-related inpatient visits was 2.48 (95% CI 1.98–3.11) and 3.26 (95% CI 2.28–4.68), respectively, times higher for the TRD than for the non-TRD MDD patients (Table S10).
Fig. 2.
Healthcare resource utilization measured from the index date up to 2 years post-index datea. aMultivariable negative binomial regression included baseline all-cause healthcare costs and Quan-CCI. *Significant at the 5% level. CI confidence interval, ED emergency department, HRU healthcare resource utilization, IRR incidence rate ratio, MDD major depressive disorder, PPPY per patient per year, Quan-CCI Quan–Charlson comorbidity index, TRD treatment-resistant depression
During the baseline period, the mean all-cause healthcare cost PPPY was $US21,872, $US13,696, and $US7160 for TRD, non-TRD MDD, and non-MDD patients, respectively (Table 1). For the TRD and non-TRD MDD cohorts, all-cause medical costs were $US19,802 and 12,357, respectively, which accounted for > 90% of all-cause healthcare costs. During the follow-up period, the unadjusted cost differences PPPY illustrated that all-cause healthcare costs for the TRD cohort were 88% and 304% higher than for the non-TRD MDD and non-MDD cohorts ($US25,807 [TRD] vs. 13,701 [non-TRD MDD] or 8500 [non-MDD]; Table 2). After adjusting for baseline Quan-CCI and all-cause healthcare costs, TRD patients had significantly higher PPPY all-cause healthcare costs than did non-TRD MDD and non-MDD patients ($US9479 [95% CI 7071–11,621] more than the non-TRD MDD cohort and $US11,433 [95% CI 8668–13,876] more than the non-MDD cohort; Fig. 3). Similarly, TRD patients had the highest all-cause inpatient costs ($US3805 [95% CI 2289–5268] more than the non-TRD MDD cohort and $US4199 [95% CI 2566–5484] more than the non-MDD cohort), which were the main drivers of the adjusted cost difference (Table 2). Additionally, mean costs PPPM in the 6 months before and up to 2 years after the index date and stratified by a 1-month period are presented in Fig. S3. Although there was some variability, mean costs PPPM were on average higher in TRD than non-TRD MDD cohorts ($US1762–2863 vs. 839–1757) each month.
Table 2.
Healthcare costs per patient per year by type of visit, measured from the index date up to 2 years post-index date
| Healthcare cost PPPY | TRD cohort [A] | Non-TRD MDD cohort [B] | Non-MDD cohort [C] | [A vs. B] | [A vs. C] |
|---|---|---|---|---|---|
| Overall population | 1582 | 1582 | 1582 | ||
| All-cause medical and pharmacy costs | 25,807 ± 42,549 [11,931] | 13,701 ± 27,545 [5394] | 8500 ± 28,085 [2189] | 9479 (7071–11,621); < 0.001* | 11,433 (8668–13,876); < 0.001* |
| All-cause medical costs | 21,908 ± 39,844 [8896] | 11,752 ± 26,061 [4027] | 7249 ± 26,921 [1574] | 7818 (5422–9855); < 0.001* | 9468 (6785–11,722); < 0.001* |
| Inpatient costs | 7897 ± 25,551 [0] | 3304 ± 14,630 [0] | 2102 ± 15,222 [0] | 3805 (2289–5268); < 0.001* | 4199 (2566–5484); < 0.001* |
| ED costs | 1090 ± 5742 [0] | 409 ± 1744 [0] | 267 ± 1483 [0] | 528 (263–839); < 0.001* | 493 (221–770); < 0.001* |
| Outpatient costs | 11,277 ± 21,406 [5352] | 7025 ± 15,417 [3007] | 4382 ± 17,958 [1156] | 3011 (1751–4013); < 0.001* | 4075 (2252–5598); < 0.001* |
| Other costs | 1644 ± 6462 [0] | 1014 ± 7966 [0] | 498 ± 4214 [0] | 474 (− 43 to 925); 0.072 | 701 (59–1256); 0.024* |
| All-cause pharmacy costs | 3899 ± 8024 [1754] | 1949 ± 4433 [650] | 1251 ± 5094 [150] | 1661 (1278–2073); < 0.001* | 1964 (1544–2406); < 0.001* |
| Behavioral health-related medical and pharmacy costs | 3673 ± 6246 [1855] | 1496 ± 2742 [627] | 242 ± 1225 [0] | 2137 (1805–2485); < 0.001* | 3355 (3072–3699); < 0.001* |
| Behavioral health-related medical costsa | 2675 ± 5927 [956] | 1180 ± 2630 [345] | 200 ± 1163 [0] | 1460 (1160–1781); < 0.001* | 2406 (2135–2732); < 0.001* |
| Inpatient costs | 657 ± 3985 [0] | 214 ± 1553 [0] | 57 ± 833 [0] | 433 (253–674); < 0.001* | 578 (398–797); < 0.001* |
| ED costs | 74 ± 511 [0] | 30 ± 286 [0] | 16 ± 234 [0] | 40 (8–71); 0.004* | 49 (24–78); < 0.001* |
| Outpatient costs | 1841 ± 3463 [779] | 899 ± 1913 [289] | 118 ± 666 [0] | 928 (737–1112); < 0.001* | 1702 (1530–1883); < 0.001* |
| Other costs | 104 ± 1178 [0] | 37 ± 387 [0] | 10 ± 193 [0] | 59 (11–116); 0.024* | 77 (32–130); < 0.001* |
| Psychiatric pharmacy costsb | 998 ± 1501 [450] | 316 ± 701 [109] | 42 ± 333 [0] | 677 (598–765); < 0.001* | 948 (876–1032); < 0.001* |
| Depression-related medical and pharmacy costs | 1810 ± 3246 [825] | 830 ± 1556 [335] | 72 ± 708 [0] | 973 (785–1162); < 0.001* | 1725 (1566–1898); < 0.001* |
| Depression-related medical costsc | 1270 ± 3162 [341] | 593 ± 1482 [156] | 43 ± 625 [0] | 672 (489–863); < 0.001* | 1218 (1064–1389); < 0.001* |
| Inpatient visits | 380 ± 2072 [0] | 119 ± 996 [0] | 13 ± 504 [0] | 261 (152–384); < 0.001* | 367 (272–484); < 0.001* |
| ED visits | 27 ± 350 [0] | 14 ± 139 [0] | 5 ± 132 [0] | 12 (− 4 to 34); 0.172 | 22 (5–44); 0.004* |
| Outpatient costs | 816 ± 2084 [274] | 439 ± 1058 [135] | 22 ± 283 [0] | 376 (270–502); < 0.001* | 794 (688–908); < 0.001* |
| Other costs | 47 ± 619 [0] | 20 ± 158 [0] | 4 ± 127 [0] | 23 (− 4 to 54); 0.092 | 35 (13–63); < 0.001* |
| Antidepressant pharmacy costs | 540 ± 781 [259] | 237 ± 477 [84] | 28 ± 309 [0] | 300 (260–347); < 0.001* | 507 (463–553); < 0.001* |
| Suicide-related medical costsd | 177 ± 1541 [0] | 52 ± 617 [0] | 2 ± 66 [0] | 128 (53–210); < 0.001* | 182 (112–258); < 0.001* |
| Inpatient visits | 156 ± 1525 [0] | 44 ± 608 [0] | 0 ± 0 [0] | 116 (40–195); < 0.001* | 164 (94–241); < 0.001* |
| ED visits | 1 ± 21 [0] | 1 ± 14 [0] | 0 ± 2 [0] | 0 (− 1 to 2); 0.481 | 1 (0–2); 0.004* |
| Outpatient costs | 17 ± 149 [0] | 8 ± 109 [0] | 2 ± 66 [0] | 10 (1–20); 0.024* | 16 (9–26); < 0.001* |
| Other costs | 2 ± 68 [0] | 0 ± 0 [0] | 0 ± 2 [0] | 2 (0–4); < 0.001* | 1 (− 1 to 2); 0.313 |
Data are presented as mean ± standard deviation [median] or as adjusted cost difference (95% CI); multivariable ordinary least squares regression included baseline all-cause healthcare costs and Quan-CCI. Costs are presented as $US, year 2017 values
CI confidence interval, ED emergency department, ICD-x-CM International Classification of Diseases, xth revision, Clinical Modification, MDD major depressive disorder, PPPY per patient per year, Quan-CCI Quan-Charlson comorbidity index, TRD treatment-resistant depression
aBehavioral health-related costs were defined as all costs during a visit with any of the following ICD-9-CM diagnostic codes: 290.xx-319.xx and ICD-10-CM diagnostic codes: F01.xxx-F99.xxx
bPsychiatric pharmacy costs include the following classes of agents (generic product identifier prefix): anxiolytics (‘57’), antidepressants (‘58’), antipsychotics/antimanics (‘59’), anticonvulsants (‘7299’, ‘721’, ‘726’), and other mood stabilizers (e.g., lithium)
cDepression-related costs were defined as all costs during a visit with any of the following ICD-9-CM diagnostic codes: 296.2x, 296.3x, 300.4x, 309.0x, 309.1x, 311.xx and ICD-10-CM diagnostic codes F32.x, F33.x, F34.1, or F43.21
dSuicide-related costs were defined as all costs during a visit with any of the following ICD-9-CM diagnostic codes: E95x, V62.84 and ICD-10-CM diagnostic codes T14.91x, X71.x-X83.x, T36.x- T65.x with a suffix of ‘2’ indicating ‘intentional self-harm’, T71.x with a suffix of ‘2’ indicating ‘intentional self-harm’, R45.851
*Significant at the 5% level
Fig. 3.
All-cause healthcare costs per patient per year measured from the index date up to 2 years post-index datea,b. aMultivariable ordinary least squares regression included baseline all-cause healthcare costs and Quan-CCI; bTotal costs are presented in the black text. Medical and pharmacy costs are presented in the green and blue texts, respectively. *Significant at the 5% level. CI confidence interval, ED emergency department, MDD major depressive disorder, PPPY per patient per year, Quan-CCI Quan-Charlson comorbidity index, TRD treatment-resistant depression
Discussion
The results of this retrospective matched-cohort analysis demonstrated that patients with TRD in IDN settings across the US had a longer duration of antidepressant therapy and a greater number of unique antidepressant medications than patients with MDD that were not resistant to treatment and than patients without MDD. From baseline to follow-up, TRD patients had higher costs and rates of HRU than did non-TRD MDD and non-MDD patients. All-cause medical and pharmacy costs were also higher, on average, among TRD patients. During the baseline period, TRD patients had higher rates of HRU, particularly behavioral health-related HRU than did non-TRD MDD patients and non-MDD patients. TRD patients also had higher healthcare costs, with inpatient costs serving as the main driver of this increased adjusted cost difference.
In this study, healthcare costs were considerably high and contrary to expectations that IDNs are associated with lower costs [11, 15, 30]. Instead, the higher HRU and costs among TRD patients relative to non-TRD MDD or non-MDD patients in this study aligned with previous commercial claims-based studies of patients treated in non-IDN settings [6, 8, 9, 31, 32]. A recent study, by Amos et al. [8], evaluated HRU and costs among commercially insured TRD patients using a similar study design as the present study, which allows for indirect comparisons. Results of that analysis demonstrated that the rates of HRU for TRD patients were higher than rates for patients in the non-TRD MDD and non-MDD cohorts (2.0 and 4.7 times the rate of inpatient visits versus the non-TRD MDD and non-MDD cohorts, respectively; all p < 0.001). As in our study, TRD patients also incurred significantly higher healthcare costs PPPY than non-TRD MDD patients and non-MDD patients ($US6709 more than the non-TRD MDD cohort and $US9917 more than the non-MDD cohort; all p < 0.001). Interestingly, the adjusted all-cause healthcare cost difference for TRD versus non-TRD MDD patients in this study were higher than the cost difference reported by Amos et al. [8] ($US9479 vs. 6709). Given our IDN definition, the difference in costs may result from linking outpatient and inpatient institutions, which can increase the costs of both outpatient and inpatient care, as suggested by previous literature on IDNs [33]. Another potential explanation to account for this difference could be the continuity of care provided by healthcare providers in IDNs resulting in an increased awareness of patients’ worsening symptoms, which would require additional care and ultimately contribute to greater HRU and costs.
To the best of our knowledge, this is the first study to highlight treatment patterns, HRU, and costs associated with TRD, non-TRD MDD, and non-MDD patients treated in US IDNs. As a result, direct comparisons with other studies are a challenge because of the differences in study parameters and patient populations evaluated. However, one study published in 2003, by Liu et al. [34], examined the cost effectiveness of collaborative care for veterans with MDD and reported an increase in costs associated with collaborative care. As expected, their explanation for the higher costs were the greater resources needed to adequately treat MDD patients. Despite methodological differences between that study and the present work, it is reasonable to conclude that the high HRU and costs pertain to the greater needs of the TRD patient population. The HRU and costs among the cohorts in this study provide a useful benchmark to help direct efforts in reducing the health and economic burdens associated with MDD, particularly TRD. Consequently, this study fills an important knowledge gap for IDN decision makers regarding real-world treatment patterns and cost differences for TRD patients, which significantly contributes to the overall burden of MDD.
Although the results of this study do not support lower costs among TRD or non-TRD MDD patients treated in IDNs, it is possible that the true benefit of IDNs among these patient populations is in the quality of care received. Given the care coordination among providers across a continuum of settings, IDNs are particularly relevant for patients with behavioral health disorders [19, 35]. Several studies have documented improved patient outcomes within IDNs for patients with depressive disorders [36–39]. For example, one study based on longitudinal data found that patients treated in highly integrated programs had greater improvement in self-reported physical health and mental health recovery assessed by their clinician [40]. Another study that compared outcomes among patients with depression who received collaborative care and those who received usual care found that collaborative care was associated with significant improvement in adherence to therapy (75 vs. 50%), patient perception of quality of care (93 vs. 75%), and patient-reported improvement in efficacy of antidepressant therapy (88 vs. 63%) [36]. The fact that several studies highlight improved quality of care among heterogeneous patient populations underscores the value of IDNs. It is possible that the higher quality of care may result in reduced costs long term; however, additional studies are warranted to explore this possibility among TRD, non-TRD MDD, and non-MDD patients treated across different types of IDNs versus non-IDN settings.
Limitations
This study should be interpreted within the context of certain limitations. First, the treatment failure algorithm used to identify patients with TRD relied solely on pharmacy claims; clinical considerations to specifically assess treatment failure, response, and remission could not be incorporated. Furthermore, the algorithm used to identify the TRD study population may not be representative of all TRD patients as only patients with an adequate dose and duration of antidepressant were selected. Second, because patients with a single IDN visit were included in the analysis, it is possible that some of the patients evaluated did not experience the care associated with multiple visits to an IDN. Additionally, once patients are seen in an IDN, they remain flagged as IDN patients even if they later receive care in a non-IDN setting. Although this may prevent us from accurately identifying patients who are truly seen in an IDN setting, we hypothesized that patients seen in an IDN typically prefer to continue their care within the same IDN in which they were initially seen based on available literature on patient experience in IDNs [33, 41]. Third, PS matching based on demographic variables was conducted to minimize the confounding between the TRD and non-TRD MDD or non-MDD cohorts. However, since imbalances remained after matching, we further adjusted for baseline Quan-CCI and all-cause healthcare costs. Although PS matching and adjustment techniques were used to minimize the potential confounding, these comparisons may still be subject to residual confounding from unmeasured confounders. Fourth, to evaluate baseline characteristics and study outcomes, a minimum of 6 months of continuous enrollment in a health insurance plan before and after the index date was imposed on the study population. However, patients who do not have ≥ 6 months of continuous enrollment before or after the index date may have differed in the continuity of care received. This may limit the generalizability of the HRU and cost burden reported in this study. Lastly, there is currently no industry standard to define an IDN [15]. In this study, an IDN was defined as a coordinated hospital inpatient and outpatient system offering healthcare services in a defined geographical region. Therefore, the results may not be generalizable to all US IDNs.
Conclusions
Patients with TRD who received care in IDNs had higher HRU and incremental costs than patients with MDD who were not resistant to treatment and those without MDD. The HRU and economic burden associated with TRD were substantial in the US IDN setting and was mainly driven by non-behavioral health-related HRU and costs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing assistance was provided by Gloria DeWalt, an employee of Analysis Group, Inc. Support for this assistance was provided by Janssen Scientific Affairs, LLC.
Author Contributions
HS, KJ, DS, and JJS contributed to the conception and design of the study and data analysis and interpretation. DP, JWW, PL, and PG contributed to the conception and design of the study, acquisition of data, and data analysis and interpretation.
Data Availability
The data supporting the findings of this study are available from Optum. Restrictions apply to the availability of these data. Data were used under license for the current study and are not publicly available.
Compliance with Ethical Standards
Funding
This study was funded by Janssen Scientific Affairs, LLC.
Conflict of interest
DP, JWW, PL, and PG are employees of Analysis Group, Inc, which received consultancy fees from Janssen Scientific Affairs, LLC for the conduct of this study. HS, KJ, and JJS are employees of Janssen Scientific Affairs, LLC. KJ is also a stockholder of Johnson & Johnson. DS was a post-doctoral fellow at Thomas Jefferson University.
Contributor Information
Dominic Pilon, Email: dominic.pilon@analysisgroup.com.
Holly Szukis, Email: HSzukis1@its.jnj.com.
Kruti Joshi, Email: KJoshi33@its.jnj.com.
David Singer, Email: dasinger137@gmail.com.
John J. Sheehan, Email: Jsheeha2@its.jnj.com
Jennifer W. Wu, Email: Jennifer.Wu@analysisgroup.com
Patrick Lefebvre, Email: Patrick.Lefebvre@analysisgroup.com.
Paul Greenberg, Email: Paul.Greenberg@analysisgroup.com.
References
- 1.Mayo Clinic. Depression (major depressive disorder). 2018. https://www.mayoclinic.org/diseases-conditions/depression/symptoms-causes/syc-20356007. Accessed 06 Aug 2018.
- 2.Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute of Mental Health (NIMH). Major depression. 2017. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Accessed 06 Jul 2018.
- 4.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76(2):155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 5.Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin N Am. 1996;19(2):179–200. doi: 10.1016/S0193-953X(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 6.Kubitz N, Mehra M, Potluri RC, Garg N, Cossrow N. Characterization of treatment resistant depression episodes in a cohort of patients from a US commercial claims database. PLoS One. 2013;8(10):e76882. doi: 10.1371/journal.pone.0076882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder third Edition. 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed 27 Jun 2018.
- 8.Amos TB, Tandon N, Lefebvre P, Pilon D, Kamstra RL, Pivneva I, et al. Direct and indirect cost burden and change of employment status in treatment-resistant depression: a matched-cohort study using a US commercial claims database. J Clin Psychiatry. 2018 doi: 10.4088/jcp.17m11725. [DOI] [PubMed] [Google Scholar]
- 9.Olfson M, Amos TB, Benson C, McRae J, Marcus SC. Prospective service use and health care costs of medicaid beneficiaries with treatment-resistant depression. J Manag Care Spec Pharm. 2018;24(3):226–236. doi: 10.18553/jmcp.2018.24.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanova JI, Birnbaum HG, Kidolezi Y, Subramanian G, Khan SA, Stensland MD. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr Med Res Opin. 2010;26(10):2475–2484. doi: 10.1185/03007995.2010.517716. [DOI] [PubMed] [Google Scholar]
- 11.Enthoven AC. Integrated delivery systems: the cure for fragmentation. Am J Manag Care. 2009;15(10 Suppl):S284–S290. [PubMed] [Google Scholar]
- 12.Peek CJ, Cohen DJ, deGruy FV., 3rd Research and evaluation in the transformation of primary care. Am Psychol. 2014;69(4):430–442. doi: 10.1037/a0036223. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(Suppl 2):S61–S71. doi: 10.1016/j.drugalcdep.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lake J, Turner MS. Urgent need for improved mental health care and a more collaborative model of care. Perm J. 2017 doi: 10.7812/tpp/17-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang W, Chang J, Laclair M, Paz H. Effects of integrated delivery system on cost and quality. Am J Manag Care. 2013;19(5):e175–e184. [PubMed] [Google Scholar]
- 16.Bao Y, Casalino LP, Pincus HA. Behavioral health and health care reform models: patient-centered medical home, health home, and accountable care organization. J Behav Health Serv Res. 2013;40(1):121–132. doi: 10.1007/s11414-012-9306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rittenhouse DR, Casalino LP, Gillies RR, Shortell SM, Lau B. Measuring the medical home infrastructure in large medical groups. Health Aff (Millwood). 2008;27(5):1246–1258. doi: 10.1377/hlthaff.27.5.1246. [DOI] [PubMed] [Google Scholar]
- 18.Alakeson V, Frank RG, Katz RE. Specialty care medical homes for people with severe, persistent mental disorders. Health Aff (Millwood). 2010;29(5):867–873. doi: 10.1377/hlthaff.2010.0080. [DOI] [PubMed] [Google Scholar]
- 19.Jones AL, Mor MK, Haas GL, Gordon AJ, Cashy JP, Schaefer JH, Jr, et al. The role of primary care experiences in obtaining treatment for depression. J Gen Intern Med. 2018;33(8):1366–1373. doi: 10.1007/s11606-018-4522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LexisNexis Risk Solutions. The ABCs of IDNs: the new keys to commercial success for Life Sciences Organizations. 2016. https://risk.lexisnexis.com/-/media/files/healthcare/white-paper/abcs-idn-wp-pdf.pdf. Accessed 30 Aug 2018.
- 21.Datamonitor Healthcare. Integrated Delivery Networks in the US—Stakeholder interest in integrated delivery networks is growing. 2016. https://pharmaintelligence.informa.com/~/media/Informa-Shop-Window/Pharma/Files/Reports/Integrated-Delivery-Networks-in-the-US–TOC-June-2017-1.pdf. Accessed 30 Aug 2018.
- 22.Whitebird RR, Solberg LI, Jaeckels NA, Pietruszewski PB, Hadzic S, Unutzer J, et al. Effective implementation of collaborative care for depression: what is needed? Am J Manag Care. 2014;20(9):699–707. [PMC free article] [PubMed] [Google Scholar]
- 23.Dickinson LM, Rost K, Nutting PA, Elliott CE, Keeley RD, Pincus H. RCT of a care manager intervention for major depression in primary care: 2-year costs for patients with physical vs psychological complaints. Ann Fam Med. 2005;3(1):15–22. doi: 10.1370/afm.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grochtdreis T, Brettschneider C, Wegener A, Watzke B, Riedel-Heller S, Harter M, et al. Cost-effectiveness of collaborative care for the treatment of depressive disorders in primary care: a systematic review. PLoS One. 2015;10(5):e0123078. doi: 10.1371/journal.pone.0123078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gartlehner G, Gaynes BN, Amick HR, Asher G, Morgan LC, Coker-Schwimmer E et al. Nonpharmacological versus pharmacological treatments for adult patients with major depressive disorder. AHRQ Comp Effect Rev. 2015. [PubMed]
- 26.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan W, Bai H. Propensity score analysis: concepts and issues. Propensity score analysis: fundamentals and developments. 2015;3–19.
- 28.Wijeysundera HC, Wang X, Tomlinson G, Ko DT, Krahn MD. Techniques for estimating health care costs with censored data: an overview for the health services researcher. Clinicoecon Outcomes Res. 2012;4:145–155. doi: 10.2147/CEOR.S31552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 30.Melek SP, Norris DT, Paulus J, Matthews K, Weaver A, Davenport S. Potential economic impact of integrated medical-behavioral healthcare: updated projections for 2017. Milliman, Inc. Prepared for American Psychiatric Association. 2017. http://www.milliman.com/insight/2018/Potential-economic-impact-of-integrated-medical-behavioral-healthcare-Updated-projections-for-2017/. Accessed 08 Apr 2019.
- 31.Olchanski N, McInnis Myers M, Halseth M, Cyr PL, Bockstedt L, Goss TF, et al. The economic burden of treatment-resistant depression. Clin Ther. 2013;35(4):512–522. doi: 10.1016/j.clinthera.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Pilon D, Sheehan JJ, Szukis H, Singer D, Jacques P, Lejeune D, et al. Medicaid spending burden among beneficiaries with treatment-resistant depression. J Comp Eff Res. 2019;8(6):381–392. doi: 10.2217/cer-2018-0140. [DOI] [PubMed] [Google Scholar]
- 33.Burns LR, Pauly MV. Integrated delivery networks: a detour on the road to integrated health care? Health Aff (Millwood). 2002;21(4):128–143. doi: 10.1377/hlthaff.21.4.128. [DOI] [PubMed] [Google Scholar]
- 34.Liu CF, Hedrick SC, Chaney EF, Heagerty P, Felker B, Hasenberg N, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatr Serv. 2003;54(5):698–704. doi: 10.1176/appi.ps.54.5.698. [DOI] [PubMed] [Google Scholar]
- 35.Unutzer J, Park M. Strategies to improve the management of depression in primary care. Prim Care. 2012;39(2):415–431. doi: 10.1016/j.pop.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katon W, Von Korff M, Lin E, Walker E, Simon GE, Bush T, et al. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA. 1995;273(13):1026–1031. doi: 10.1001/jama.1995.03520370068039. [DOI] [PubMed] [Google Scholar]
- 37.Katon W, Von Korff M, Lin E, Simon G, Walker E, Unutzer J, et al. Stepped collaborative care for primary care patients with persistent symptoms of depression: a randomized trial. Arch Gen Psychiatry. 1999;56(12):1109–1115. doi: 10.1001/archpsyc.56.12.1109. [DOI] [PubMed] [Google Scholar]
- 38.Hunkeler EM, Katon W, Tang L, Williams JW, Jr, Kroenke K, Lin EH, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ. 2006;332(7536):259–263. doi: 10.1136/bmj.38683.710255.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler M, Kane RL, McAlpine D, Kathol RG, Fu SS, Hagedorn H, et al. Integration of mental health/substance abuse and primary care. Evid Rep Technol Assess (Full Rep). 2008;173:1–362. [PMC free article] [PubMed] [Google Scholar]
- 40.Gilmer TP, Henwood BF, Goode M, Sarkin AJ, Innes-Gomberg D. Implementation of integrated health homes and health outcomes for persons with serious mental illness in Los Angeles County. Psychiatr Serv. 2016;67(10):1062–1067. doi: 10.1176/appi.ps.201500092. [DOI] [PubMed] [Google Scholar]
- 41.O’Malley AS, Cunningham PJ. Patient experiences with coordination of care: the benefit of continuity and primary care physician as referral source. J Gen Intern Med. 2009;24(2):170–177. doi: 10.1007/s11606-008-0885-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elixhauser A, Steiner C, Kruzikas. D. HCUP Methods Series Report # 2004-1. 06 February 2004.
- 43.US Agency for Healthcare Research and Quality. Comorbidity Software Documentation. Rockville, MD, USA; 2004. p. 12–5. http://www.hcup-us.ahrq.gov/reports/ComorbiditySoftwareDocumentationFinal.pdf. Accessed 2013.
- 44.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-V. Amer Psychiatric Pub Inc; 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from Optum. Restrictions apply to the availability of these data. Data were used under license for the current study and are not publicly available.