Abstract
The antimicrobial compound produced by Bacillus paralicheniformis UBBLi30 showed UV spectra absorption at 208 nm. Fourier transform infrared (FTIR) revealed characteristic bands for aliphatic chain related to hydrophobic amino acids (l-isoleucine/l-leucine) (3068, 2965 and 2871 cm−1) and peptide bonds (1538, 1667 and 3312 cm−1). The proton nuclear magnetic resonance (1H NMR) showed signals for aromatic amino acid (6.5–9.5 ppm) and alkyl amines (3–4 ppm). The results of carbon (13C) NMR showed signals for aromatic, nitro and amide compounds. Besides this, the mass fragments (1422.576 [M+H]+, 711.912 [M+2H]2+ and 475.174 [M+3H]3+ m/z) observed in electrospray ionization mass spectrometry (ESI-MS) were coordinated well to the fragments of polypeptide antibiotic bacitracin. The presence of bacA gene further confirmed the production of bacitracin. Bacitracin inhibited the growth of a range of Gram-positive bacteria such as Micrococcus luteus, methicillin-resistant Staphylococcus aureus (MRSA), S. aureus, Streptococcus pyogenes and Propionibacterium acnes, and biofilm formation of M. luteus and MRSA. Moreover, this polypeptide reduced the zeta potential of M. luteus and MRSA, indicating the electrostatic sorption on bacterial surface and concentration-dependent cell membrane damages. Besides this, polypeptide showed stability in the presence of proteases (proteinase K, trypsin and pepsin), pH (1, 3, 5, 7, 9 and 11) and temperature up to 100 °C. B. paralicheniformis UBBLi30 therefore has the potential to be utilized as a bio-preservative to control the growth of spoilage and pathogenic bacteria.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-2109-6) contains supplementary material, which is available to authorized users.
Keywords: Bacillus paralicheniformis UBBLi30, Bacitracin, Zeta potential, Biofilm, MRSA
Introduction
Bacillus paralicheniformis is a spore-forming, facultatively anaerobic, saprophytic Gram-positive bacteria present ubiquitously in the environment. It has been reported that, phylogenetically B. paralicheniformis is most closely related to B. licheniformis (Dunlap et al. 2015; Du et al. 2019). Being a saprophyte, these bacteria have an extraordinary ability to produce a range of metabolites including amino acids, vitamins, antibiotics and enzymes (Dunlap et al. 2015; Elshaghabee et al. 2017; Harwood et al. 2018). Due to immense potential, B. paralicheniformis and B. licheniformis were recognized as industrially important strains for the production of a wide range of enzymes and biomolecules (de Boer et al. 1994; Dunlap et al. 2015; Harwood et al. 2018). Besides this, these strains were selectively employed as probiotic/feed additives in poultry, aquaculture and livestock industries (Elshaghabee et al. 2017; Mingmongkolchai and Panbangred 2018; Makled et al. 2019). Jia et al. (2018) have shown that the feed supplementation of B. licheniformis and Saccharomyces cerevisiae improved levels of nutrient in ruminants and could be the alternatives of antibiotic monensin. Makled et al. (2019) have shown the beneficial effects of probiotic B. paralicheniformis SO‐1 on growth performance, digestive enzyme activities and immune response of Nile tilapia. Moreover, both the strains were recognized for immunity, growth enhancement and disease resistance (Deng et al. 2012; Elshaghabee et al. 2017; Xu et al. 2018; Makled et al. 2019).
Members of the genus Bacillus are well known for the production of antimicrobial compounds (AMCs) such as bacteriocins, peptide, lipopeptide and non-peptide-based antibiotics (polyketides) (Abriouel et al. 2011; Sumi et al. 2014; Arbsuwan et al. 2018). B. paralicheniformis, in particular has rarely been investigated for the production of AMCs (Collins et al. 2016; Zhao and Kuipers 2016). Collins et al. (2016) identified a novel two peptide (α = 3254.34 and β = 2472.06 Da) lantibiotic formicin from B. paralicheniformis APC 1576 with broad spectrum of activity against Gram-positive strains (Staphylococcus aureus, Clostridium difficile and Listeria monocytogenes). Al-Amoudi et al. (2016) showed pronounced antimicrobial activity of B. paralicheniformis Bac84 against S. aureus, Salmonella typhimurium and Pseudomonas syringae; however, the identification of AMC remained undisclosed. In the present study, for the first time we describe screening, production, characterization and identification of cyclic polypeptide bacitracin from indigenously isolated B. paralicheniformis UBBLi30 strain.
Materials and methods
Microorganisms
Bacillus paralicheniformis UBBLi30 (MTCC5377) was isolated from traditional fermented food and preserved in culture collection of Unique Biotech Limited, India. The complete genome sequence of UBBLi30 was sequenced and deposited at GenBank (accession number NZ_SULF01000020). Micrococcus luteus MTCC 106T, methicillin-resistant Staphylococcus aureus (MRSA) ATCC BAA 1720™, Staphylococcus aureus MTCC 737, Streptococcus pyogenes MTCC 442, Propionibacterium acnes MTCC 1951T, Pseudomonas aeruginosa MTCC 1688, Escherichia coli MTCC 1687 and Candia albicans ATCC 14053 were used as indicator strains in antimicrobial testing.
Screening of bacteria for antimicrobial production
A single colony of UBBLi30 was inoculated in 10 ml nutrient broth (NB, pH 7.4 ± 0.2) (HiMedia, India) and incubated on orbital shaker (160 rpm; Scigenics, India) at 37 °C for 24 h. 1% (v/v) of the above culture was transferred to 100 ml of NB and incubated further under the same conditions described above for up to 24 h. Cells-free supernatant (CFS) was obtained by centrifugation at 11,000×g for 20 min at 4 °C (Sorvall Legend XTR, Thermo Scientific, USA) and pH was measured (DPH 504, Global, India) and adjusted to 7.0 with 1 N NaOH/HCl (HiMedia/Thermo Fisher Scientific, India). The CFS of original pH and pH 7.0 were filter sterilized through 0.2 µm cellulose acetate filter (Minisart® NML, Sartorius, Germany) and evaluated for antimicrobial activity against M. luteus.
The single colony of M. luteus was grown overnight at 30 °C (120 rpm) in 10 ml NB. The 100 µl of culture was inoculated in 20 ml molten Mueller–Hinton (MH) agar (HiMedia, India) and poured in sterile plates (90 × 15 mm, Genaxy® Scientific, India). The wells (9 mm) were created into the agar and 25 µl of sample dispensed into a well. The plates were kept for 15 min at 4 °C to facilitate diffusion of sample and then transferred to 30 °C for 24 h. The zone of clearance around the well indicated positive activity and measured in millimeters (mm). Sterile NB was used as a control.
Production and isolation of antimicrobial compound (AMC)
The production of AMC was performed in clarified NB (pH 7.4 ± 0.2) to avoid interference of media ingredient (up to 40 kDa) during the purification process. In brief, Amberlite® XAD16N (Sigma-Aldrich, USA) beads were activated with 80% isopropanol (Thermo Fisher Scientific, India) containing 0.1% trifluoroacetic acid (HiMedia, India) (IPA-TFA, v/v/v) for 30 min at 28 °C (120 rpm). Activated beads were collected by using Whatman™ filter paper 1 (diameter 125 mm; GE Healthcare Life Sciences, UK) and rinsed several times with ultrapure water. The freshly prepared NB was added with 10 g l−1 activated beads and incubated at 28 °C for 30 min (120 rpm) (van Staden 2015). After incubation, NB was separated from the beads and autoclaved as per the routine procedure.
As described earlier, a single colony of overnight grown UBBLi30 was inoculated in 10 ml clarified NB and incubated at 37 °C for 24 h (160 rpm). After incubation, 1% (v/v) of this culture was further transferred into 1 l clarified NB (6.6 ± 0.05 log10 cfu ml−1) and incubated under the same conditions for 24 h. CFS was collected by centrifugation at 11,000×g for 20 min at 4 °C and pH was measured. 20 g of activated XAD16N beads was added to 1 l CFS and incubated on an orbital shaker (80 rpm: 1 h; 50 rpm: 1 h) at 4 °C for 2 h. The beads were collected, rinsed with ultrapure water followed by 30% (v/v) ethanol (CH Fine Chemical, China) and several steps of ultrapure water wash (van Staden 2015). The bead-bound antimicrobial compound was eluted with 80% IPA–0.1% TFA (v/v/v) and filtered through a 0.2 µm cellulose acetate filter. The isopropanol of the filtrate was removed by using Rotavapor (Rotavapor® R-300, Buchi, Switzerland) and antimicrobial activity of the concentrate was determined against M. luteus as described earlier.
Purification of AMC
The Rotavapor concentrate of AMC was purified further using reversed-phase Sep-Pak® Vac 35 cc (10 g) C18 cartridge (Waters, USA). In brief, C18 cartridge was preconditioned as per the procedure described by the manufacturer and loaded with the sample. The cartridge was washed with ultrapure water and eluted using isopropanol gradient (10–90% containing 0.1% TFA, v/v/v) with 10% increments at a flow of 1 ml min−1 (Peristaltic pump, Scigenics, India). All the collected fractions were evaluated for antimicrobial activity against M. luteus and profiling on thin-layer chromatography (TLC). Fractions showing positive antimicrobial activity and similar profile on TLC were mixed together, concentrated using Rotavapor and freeze-dried (Lyo lab, USA). The freeze-dried samples were stored at − 20 until further use.
Characterization of AMC
Protein concentration was estimated by using Pierce™ bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, USA). Molisch, Sakaguchi and ninhydrin reactions were performed as per the standard procedures and oil spreading test as per Morikawa et al. (2000).
Spectrophotometric analysis
Spectrophotometric analysis was performed by using Evolution 201 UV visible spectrophotometer (Thermo Scientific, USA). A 1 mg of sample was dissolved in 10 ml ultrapure water containing 0.1% TFA (v/v) and filtered through a 0.2 µm cellulose acetate filter. The scanning (1100–199 nm; scan speed 200 nm) of sample was performed after baseline adjustment with ultrapure water containing 0.1% TFA (v/v).
Thin-layer chromatography (TLC)
One milligram sample was dissolved in 1 ml 50% IPA-0.1% TFA (v/v/v) and 25 µl loaded onto TLC silica gel 60 F254 plates (7 × 10 cm, Merck, Germany). After air drying, the plates were kept to develop in pre-saturated chamber containing n-butanol:ethanol:acetic acid:water (v/v, 6:12:1:6) as a mobile phase (Arbsuwan et al. 2018). The plates were dried at 80 °C, visualized under UV at 254 nm and simultaneously stained with 0.3% (w/v) ninhydrin. The Rf values were calculated and bands were cut into sections and subjected to antimicrobial activity against M. luteus.
High-performance liquid chromatography (HPLC)
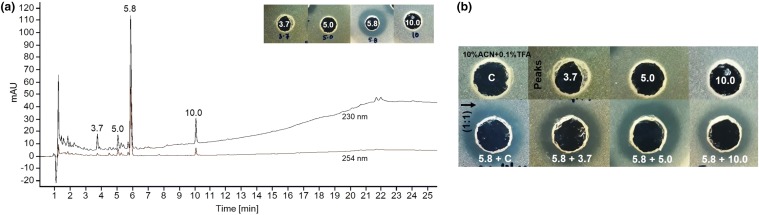
One milligram sample was dissolved in 1 ml 10% acetonitrile (Thermo Fisher Scientific, India) containing 0.1% TFA (v/v/v) and filtered through a 0.2 µm cellulose acetate filter. The filtrate was loaded to a Poroshell 120, EC-C18 HPLC column (4 μm, 46 × 150 mm; Agilent, USA) attached to the Agilent Technologies 1260 Infinity II LC system (Agilent, USA). The antimicrobial compounds were eluted in an increasing gradient (B: 25% to 60%) of acetonitrile containing 0.1% TFA (v/v) over 25 min (A: ultrapure water containing 0.1% TFA, v/v). The peaks detected at 230 and 254 nm were collected manually and evaluated for antimicrobial activity against M. luteus.
Fourier transform infrared (FTIR) analysis
FTIR analysis was performed on Thermo Nicolet Nexus 670 spectrometer (Thermo Scientific, USA) at 4000–400 cm−1 wavenumber regions with scan resolution of 4 cm−1. KBr was used as beam splitter and DTGS KB as detector.
Nuclear magnetic resonance (NMR) and mass analysis
Proton (1H) and carbon (13C) NMR spectra were collected on Bruker Avance III-400 MHz spectrometer (Bruker, USA). The samples were prepared in deuterated dimethyl sulfoxide (DMSO-d6). The mass analysis was performed by using TSQ Altis™ Triple Quadrupole Mass Spectrometer (Thermo Scientific, USA) operated in positive electrospray ionization (ESI+) mode with scan filter + c ESI Q1MS (50,000–1600,000), m/z (Dalton).
Analysis of molecular weight
Molecular weight was determined using tricine–sodium dodecyl sulfate-polyacrylamide gel electrophoresis (tricine SDS-PAGE) as described by Schägger (2006). The half, unstained gel was washed three to four times with ultrapure water and overlaid with M. luteus cells seeded in MH agar as described earlier. The zone of inhibition was observed to locate the position of active AMC.
Molecular phylogeny for 16S rRNA and bacA gene
The evolutionary analysis of gene sequences pertaining to 16S rRNA and bacA (bacitracin) were conducted in comparison to already published gene sequence data of B. licheniformis and paralicheniformis strains in MEGA(X) (Kumar et al. 2018) by using the maximum likelihood (ML) method based on the Tamura–Nei model (Tamura and Nei 1993).
Stability at pH, temperature and proteases
The pH of the ultrapure water was adjusted to 1, 3, 5, 7, 9, 11 and 14 by using 1 N NaOH/HCl and AMC was inoculated at a final concentration of 1 mg ml−1. After 30 min incubation at 37 °C, the pH was neutralized to 7 and antimicrobial activity was determined against M. luteus.
AMC was dissolved in 0.1% TFA water (v/v) to the final concentration of 1 mg ml−1. 10 ml aliquots were each incubated at 40, 60, 80 and 100 °C in water bath. Approximately, 1 ml sample was removed from each tube at the interval of 10, 30 and 60 min and tested for antimicrobial activity against M. luteus. Simultaneously, a 10 ml aliquot was subjected to autoclaving at 121 °C for 15 min and tested for antimicrobial activity.
AMC (1 mg each) was incubated separately with 1 ml of (1 mg ml−1) proteinase K (Sigma-Aldrich, USA) and trypsin (HiMedia, India) dissolved in 10 mM Tris–HCl (pH 8.0; Roche, Germany) and pepsin (prepared in 0.1% TFA water) at 37 °C for 3 h. After incubation, the enzymes were heat inactivated at 95 °C for 5 min and tested for antimicrobial activity against M. luteus.
Antimicrobial activity
Micrococcus luteus, MRSA, S. aureus, Strp. pyogenes, Prop. acnes, P. aeruginosa, E. coli and C. albicans were each cultivated overnight in their respective growth media. Approximately, 105 cells of each strain were seeded separately in molten MH agar and poured into sterile plates. The wells were prepared as described previously and 25 µl AMC (1 and 5 mg ml−1 prepared in 0.1% TFA water, v/v) was dispensed into a well. The plates were kept at cool and incubated as described earlier. The zone of clearance around the well was measured in millimeters.
Determination of minimum inhibitory concentration (MIC)
The MIC was determined by the agar dilution plate method as described by Andrews (2001) and Ahire et al. (2015). The freeze-dried AMC was diluted in the range of 16–0.015625 mg l−1. The surface-dried MH agar plates containing the respective dilution were spot inoculated (2 µl) with 1 × 106 cfu ml−1 cells of M. luteus and MRSA. The experiment was performed in six replicates. MIC was defined as the lowest concentration in the agar medium that prevented growth.
Effect of AMC on zeta potential of bacteria
Micrococcus luteus and MRSA cells were employed to investigate the effect of AMC on zeta potential (surface potential) of bacteria. A 2 ml of overnight-grown bacteria was centrifuged at 11,000×g for 20 min at 4 °C. The pellets were washed thrice with phosphate-buffered saline (PBS, pH 7.4) and ultrapure water (pH adjusted to 7.0). The washed pellets were resuspended in 5 ml ultrapure water (pH 7.0) containing 1 mg ml−1 AMC. Cells suspended in ultrapure water (pH 7.0) served as control. The zeta potential was measured by using DTS1070 capillary cell on Zetasizer Nano-ZS (Malvern, UK) at 0, 2 and 4 h time interval. Bacterial viability was assessed on agar plates and expressed as log10 cfu ml−1.
Scanning electron microscopy (SEM) analysis
Bacteria (M. luteus and MRSA) were treated with 5 mg ml−1 antimicrobial compound in PBS at 37 °C for 4 h and prepared for SEM as described by Hartmann et al. (2010). The fixed samples were sputter-coated with gold and visualized on SEM (Carl Zeiss, Germany). The images were processed with ImageJ software (Scion Corporation, USA).
Inhibition of biofilm formation
Micrococcus luteus and MRSA were each inoculated in 10 ml NB and incubated at their respective growth conditions for 24 h. The bacteria were diluted (OD600: 0.02 ± 0.001) in NB and dispensed (200 µl per well) into 96-well plates (Nunclon TM Delta Surface, Thermo Scientific, Denmark). The AMC (M. luteus: 0.5, 1, 4 µg and 1 mg; MRSA: 4, 8, 16 µg and 5 mg) was added to the designated wells and incubated at the respective growth conditions for 24 h. The wells containing the cell suspension without antimicrobial compound served as control. The plates were sealed with parafilm (Bemis, USA) to avoid media evaporation and incubated at the respective growth conditions for 24 h (Ahire and Dicks 2014, 2015; Ahire et al. 2017). After incubation, the free cell densities and biofilm formation were estimated as per the procedure described by Ahire and Dicks (2014).
Statistical analysis
GraphPad Prism (USA) was used to perform statistical analysis. The p value < 0.05 was considered statistically significant. The data are presented as mean ± standard deviation (SD).
Results
Screening, production and purification of AMC
The supernatant of 24 h-grown Bacillus paralicheniformis UBBLi30 showed inhibition of indicator organism M. luteus. The zone of inhibition recorded for pH 6.9 ± 0.02 and 7.0 ± 0.2 was 23.3 ± 0.5 and 23.6 ± 0.5 mm, respectively. The difference analyzed between inhibition zones was non-significant (ns, p > 0.05). The incubation of 6.6 ± 0.05 log10 cfu ml−1 UBBLi30 in clarified NB for 24 h changed the pH from 7.4 ± 0.2 to 7.7 ± 0.05 with cell density of 10.2 ± 0.2 log10 cfu ml−1. The CFS showed 25.6 ± 0.5 mm zone of inhibition against M. luteus. Similarly, the IPA (80% containing 0.1% TFA, v/v/v) extracted Rotavapor concentrate of AMC had an activity of 39.6 ± 0.6 mm. The further purification on C18 cartridge showed that elution with 40, 50, 60 and 70% IPA containing 0.1% TFA (v/v/v) retained antimicrobial activity, viz,. 19.6 ± 0.6, 45.0 ± 0.0, 42.3 ± 0.5 and 17.6 ± 0.6 mm, respectively. On TLC, antimicrobial fractions showed matching profile (data not shown). Overall, C18 purification yielded ~ 130 mg l−1 of compound with 31.0 ± 1 mm activity per 25 µg against M. luteus.
Characterization of AMC
The BCA reaction showed 378.1 ± 2.8 µg of protein per 1000 µg of sample. The absence of purple color formation in Molisch test indicated negative reaction for carbohydrates. No pink/red color developed when the compound reacted with Sakaguchi reagents as compared with control (1% arginine, w/v). A slight yellow tint was observed when the compound was boiled with ninhydrin. Moreover, the sample failed to spread oil as compared to triton X-100.
The spectrophotometric analysis of the compound showed characteristic intense peak at λ max 208 nm (Fig. S1). On TLC, under UV, a single band was visualized with Rf = 0.47 (Fig. 1b), which showed intense dark color when interacted with ninhydrin. Simultaneously, with ninhydrin two other bands at Rf = 0.55 and 0.65 (Fig. 1a) were visualized. Further investigation revealed that Rf = 0.47 band was highly active against M. luteus (inset in Fig. 1), whereas Rf = 0.55 showed less activity with blur inhibition. No antimicrobial activity was detected with band Rf = 0.65. In HPLC, four peaks were detected; out of those, peak at 5.8 min was intense and biologically active against M. luteus (Fig. 2a, insert). The remaining peaks (3.7, 5.0 and 10 min) were small and non-antimicrobial (Fig. 2a, insert). Furthermore, the antimicrobial activity determined by mixing (1:1) 5.8 min peak with 3.7, 5.0 and 10 was identical (Fig. 2b). No significant activity change was noticed.
Fig. 1.

Thin-layer chromatography (TLC) analysis of C18 purified antimicrobial compound. a Stained with ninhydrin, b under UV at 254 nm. The inset shows the activity of the TLC band (Rf = 0.47) against Micrococcus luteus MTCC 106T. Rf retardation factor
Fig. 2.
a High-performance liquid chromatography (HPLC) analysis of the purified compound. The inset shows the antimicrobial activity of the collected peaks against Micrococcus luteus MTCC 106T. b Antimicrobial activity of collected HPLC peaks, control (C: 10% acetonitrile containing 0.1% 0.1% trifluoroacetic acid, v/v) and 1:1 mixture against M. luteus
In FTIR analysis, the −NH or –OH stretch was recorded at 3312.42 cm−1, the = C−H stretch at 3068.49 and −C−H stretch at 2965.40 and 2871.23 cm−1, the C=O, C=C and C−O stretch at 1667.37, 1538.73, and 1201.55 and 1135.51 cm−1, the −C−H bending at 1454.25 and 721.23 cm−1 and −C−H out-of-plane bending at 835.19 and 800.41 cm−1 (Fig. S2). The 1H and 13C NMR showed the following signals: 1H NMR (400 MHz, DMSO-d6) δ ppm 0.45–0.65 (m, 1 H), 0.76–0.93 (m, 5 H), 1.00–1.07 (m, 1 H), 1.12–1.30 (m, 1 H), 1.36 (br s, 1 H), 1.40–1.68 (m, 3 H), 1.70–1.85 (m, 1 H), 1.85–1.97 (m, 1 H), 2.32–2.47 (m, 1 H), 2.76 (br s, 1 H), 3.03 (br d, J = 10.76 Hz, 1 H), 3.25–3.49 (m, 3 H), 3.53–3.64 (m, 4 H), 3.68–3.86 (m, 2 H), 4.13–4.32 (m, 1 H), 4.32–4.59 (m, 1 H), 7.08–7.34 (m, 2 H), 7.70 (br s, 1 H), 8.03 (br d, J = 8.56 Hz, 1 H), 8.33–8.53 (m, 1 H) (Fig. S3). 13C NMR (101 MHz, DMSO-d6) δ ppm 10.79 (s, 1 C), 11.17 (s, 1 C), 11.67 (s, 1 C), 15.10 (s, 1 C), 15.38 (s, 1 C), 17.16 (s, 1 C), 21.53 (s, 1 C), 22.93 (s, 1 C), 23.21 (s, 1 C), 24.25 (s, 1 C), 30.09 (s, 1 C), 36.94 (s, 1 C), 38.43 (s, 1 C), 67.82 (s, 1 C), 69.82 (s, 1 C), 70.06 (s, 1 C), 70.16 (s, 1 C), 74.11 (s, 1C), 74.27 (s, 1 C), 127.86 (s, 1 C), 129.18 (s, 1 C), 158.28 (s, 1 C), 158.60 (s, 1 C), 171.34 (s, 1 C), 171.54 (s, 1 C), 173.88 (s, 1 C) (Fig. S4).
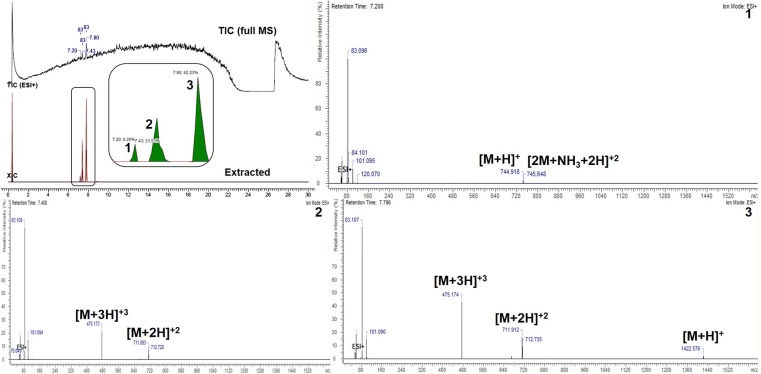
The extracted ion chromatogram (XIC) showed intense peak at 7.8 min with fragmentation pattern of 1422.576 [M+H]+, 711.912 [M + 2H]2+ and 475.174 [M+3H]3+ m/z (Fig. 3). The peak at 7.4 min corresponds to 711.893 [M+2H]2+ and 475.173 [M+3H]3+ m/z (Fig. 3). The base peak of 744.918 [M+H]+ and ammonium ion form 745.648 [2M + NH3 + 2H]2+ m/z were estimated for 7.2 min retention time (Fig. 3). In tricine SDS-PAGE analysis, the single band of ~ 5 kDa molecular mass was visible, which was active against M. luteus as shown in Fig. S5.
Fig. 3.
ESI-MS of purified antimicrobial compound of B. paralicheniformis UBBLi30. TIC total ion chromatogram, XIC extracted ion chromatogram
Molecular phylogeny for 16S rRNA and bacA gene
As anticipated, sequencing and phylogenetic analysis of the 16S rRNA gene of strain UBBLi30 revealed high relatedness with the respective gene sequences of B. paralicheniformis strains (CBMAI1303, KJ16 and MDJK30) reported in NCBI GenBank (Fig. 4a). In case of bacA gene, the sequences were found to be phylogenetically closer to B. paralicheniformis (strain CBMAI1303) and B. licheniformis strain (Fig. 4b). Also, the bacA gene sequence of UBBLi30 was found to be 99.99% similar to B. paralicheniformis (strains ATCC9945a and CBMAI1303), when aligned using BLAST program (https://blast.ncbi.nlm.nih.gov).
Fig. 4.
Molecular phylogenetic relatedness among isolated strain UBBLi30 and other B. licheniformis and paralicheniformis strains for a 16S rRNA, b bacA gene sequences (partial or complete) by maximum likelihood approach. Trees in panels (a: 16S rRNA gene; b: for bacA gene) are arbitrarily rooted. Bars (0.050) shown in panels indicate sequence divergence. Numbers at branch points are bootstrap values (based on 500 samplings expressed in percentage)
Stability at pH, temperature and proteases
The antimicrobial activity remained 100% when the compound was incubated at 37 °C for 30 min in solutions of different pH values (1, 3, 5, 7, 9 and 11) as compared to the control. At pH 14, no antimicrobial activity was detected. In heat stability studies, the compound was stable and showed 100% antimicrobial activity when incubated up to 60 min at 40, 60, 80 and 100 °C. However, after autoclaving (121 °C for 15 min), the compound retained 80% of antimicrobial activity. Besides this, none of the proteases (proteinase K, trypsin and pepsin) were effective to degrade AMC. The 100% antimicrobial activity was detected after enzyme treatment as compared to the control.
Antimicrobial activity
The zones of inhibition were detected for 1 and 5 mg ml−1 concentration of AMC against M. luteus (30.6 ± 0.5; 35.6 ± 0.6), MRSA (9.6 ± 0.6; 12.3 ± 0.5), S. aureus (10.6 ± 0.5; 13.6 ± 0.5), Strp. pyogenes (10.3 ± 0.6; 14.3 ± 0.5) and Prop. acnes (11 ± 1; 15 ± 1) (Fig. 5). No inhibitory activity was recorded against P. aeruginosa, E. coli and C. albicans (Fig. 5).
Fig. 5.

Antimicrobial activity of peptide (1 and 5 mg ml−1) against indicator strains. Data points presented are the average of three independent experiments (mean ± standard deviation)
MIC of AMC
The MIC of AMC was 0.015625 mg l−1 against M. luteus MTCC 106T and 8 mg l−1 against methicillin-resistant S. aureus (MRSA).
Effect of AMC on zeta potential of bacteria
Micrococcus luteus cells when mixed with AMC showed change in zeta potential from − 36.5 ± 4.5 to − 9.47 ± 3.1 mV (0 h). The further incubation of cells with AMC changed the zeta potential from − 39.6 ± 9.4 to − 10.2 ± 3.4 mV at 2 h and − 38.8 ± 13 to − 11.3 ± 4.1 mV at 4 h. The changes recorded in zeta potential at 0 and 2 h between control and treated cells were significant, except at 4 h (Fig. 6a). However, the zeta potential changes within a group remained non-significant (Fig. 6a). 11.6 ± 0.05 log10 cfu ml−1 of M. luteus cells was significantly reduced to zero at two and subsequent hours of incubation with AMC. This reduction was significant as compared to the control (5.5 ± 0.05 at 2 h and 4.8 ± 0.02 log10 cfu ml−1 at 4 h) (Fig. 6b). Similarly, the CFU reduction observed in the control group from 0 to 4 h was significant (Fig. 6b).
Fig. 6.
Effect of antimicrobial peptide (1 mg ml−1) on a zeta potential, b viability (log10 cfu ml−1) of Micrococcus luteus MTCC 106 T and methicillin-resistant Staphylococcus aureus (MRSA) ATCC BAA 1720™ at 0, 2 and 4 h. Control: cells in ultrapure water (pH 7.0). p = ****0.0001, ***0.001, **0.01, *0.05
In case of MRSA, the zeta potential of cells was significantly changed from 3.3 ± 2.8 to − 49.8 ± 4.7 after addition of AMC. During the subsequent incubation, the changes recorded in zeta potential were significant as compared to the control (2 h: 3.2 ± 2.9 to − 46.4 ± 4.8; 4 h: 3.3 ± 4.1 to − 46.7 ± 4.8); however, the changes within a group remained non-significant (Fig. 6a). Moreover, the cells of MRSA were significantly decreased (2 h: 9.8 ± 0.02; 4 h: 9.4 ± 0.04 log10 cfu ml−1) during the incubation with AMC as compared to the control (2 h: 10.1 ± 0.06; 4 h: 10.9 ± 0.02 log10 cfu ml−1) (Fig. 6b). The changes recorded within a group were significant.
SEM analysis
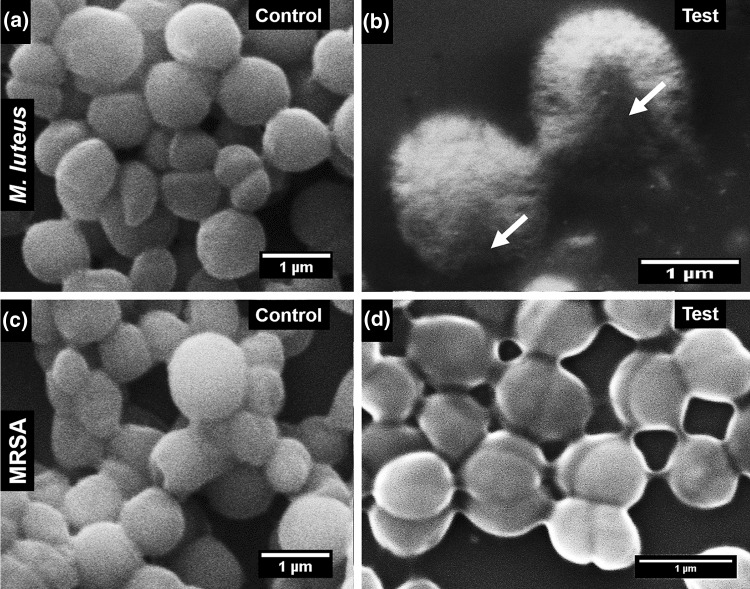
SEM image of M. luteus cells treated with AMC showed visible damage (Fig. 7b) as compared to the control (Fig. 7a). However, no visible changes were observed when MRSA cells were treated with AMC and the control (Fig. 7c, d).
Fig. 7.
Scanning electron microscopy (SEM) images of cells treated with 5 mg ml−1 peptide. a Micrococcus luteus MTCC 106T control cells. b M. luteus treated with peptide. c Methicillin-resistant Staphylococcus aureus (MRSA) ATCC BAA 1720™ control cells. d MRSA treated with peptide. The arrow indicates damage
Inhibition of biofilm formation
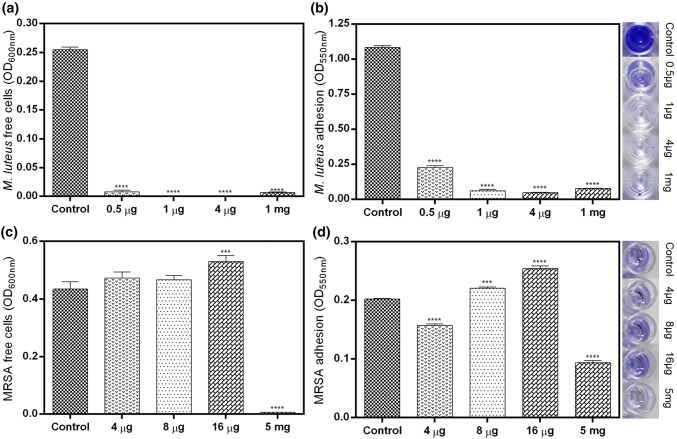
Adhesion of M. luteus cells to the hydrophilic surface of the 96-well plate was prevented by different concentrations of AMC. After 24 h, the free cell density readings at 600 nm for 0.5 µg (0.008 ± 0.003), 1 µg (0.001 ± 0.0), 4 µg (0.0006 ± 0.0005) and 1 mg (0.006 ± 0.001) of AMC were significantly less as compared to the control (0.25 ± 0.005) (Fig. 8a). Furthermore, the total biofilm formation estimated by crystal violet staining showed significant decrease as per the increasing concentrations of AMC (0.5 µg: 0.23 ± 0.01; 1 µg: 0.06 ± 0.01; 4 µg: 0.05 ± 0.001 and 1 mg: 0.07 ± 0.001) as compared to the control (1.08 ± 0.01) (Fig. 8b).
Fig. 8.
Biofilm formation after 24 h peptide exposure. a Optical density readings of free cells, b crystal violet staining of adhered biomass of Micrococcus luteus MTCC 106T. c Optical density readings of free cells, d crystal violet staining of adhered biomass of methicillin-resistant Staphylococcus aureus (MRSA) ATCC BAA 1720™. The insets are the images of crystal violet biomass of the respective bacteria. p = ****0.0001, ***0.001
The optical density readings of MRSA treated with 4 µg (0.47 ± 0.02) and 8 µg (0.47 ± 0.01) of AMC were insignificantly increased as compared to the control (0.43 ± 0.02), except 16 µg (0.53 ± 0.02), which was significant (Fig. 8c). However, the cell density readings recorded with 5 mg (0.006 ± 0.0006) were significantly decreased as compared with the control. In crystal violet staining, 8 µg (0.22 ± 0.002) and 16 µg (0.25 ± 0.004) showed significantly higher readings as compared to the control (0.20 ± 0.001), except 4 µg (0.16 ± 0.003) and 5 mg (0.09 ± 0.004) which were significantly decreased from the control (Fig. 8d).
Discussion
This study aimed to investigate the antimicrobial potential of indigenously isolated Bacillus paralicheniformis UBBLi30 strain. The 24 h old supernatant of UBBLi30 showed inhibition of M. luteus, indicating strain ability to produce AMCs. It was noticed that the strain produced maximum AMC at 37 °C for 24 h, which was higher as compared to B. paralicheniformis Bac84 reported at 37 °C for 24 h (Al-Amoudi et al. 2016). Overall, the hydrophobic poly-aromatic resin XAD16N and reversed-phase C18 cartridge purification yielded ~ 130 mg l−1 of AMC with 31.0 ± 1 mm activity per 25 µg against M. luteus.
On characterization, the AMC showed positive reaction with BCA and ninhydrin indicating its proteinaceous nature. This finding was further supported by negative results of Molisch and oil drop spreading test for carbohydrate and cyclic lipopeptide surfactin. The proteins which contain tryptophan and or tyrosine are known to absorb UV at 280 nm; however, proteins lacking both absorb UV at 205 nm for peptide bonds (Aitken and Learmonth 2009). In the present study, the compound showed intense absorption peak at 208 nm which may be attributed to the presence of conjugation compounds, aromatic ring of amino acids and peptide bonds (Field et al. 2007; Aitken and Learmonth 2009). This suggests that the compound is protein, but without tryptophan and or tyrosine. In further investigation, the negative Sakaguchi test to detect arginine, which is one of the components of most of the bacteriocins (Dicks et al. 2018), suggested the absence of arginine in protein and hence the nonbacteriocin nature of AMC.
TLC analysis showed a single highly active antimicrobial band (Rf = 0.47) under UV, suggesting the presence of conjugation compounds, and color reaction with ninhydrin indicated the absence of macrolactins and amicoumacins group of antimicrobials (Arbsuwan et al. 2018). The other bands (Rf = 0.55 and 0.65) visualized after ninhydrin stain may be due to the free amino acids (Arbsuwan et al. 2018). In HPLC, the antimicrobial component was eluted at 5.8 min and had no significant activity enhancement when mixed with other collected peaks, indicating the absence of other active antimicrobials or activity-enhancing sub-components.
FTIR analysis suggested that bands detected at 3068, 2965 and 2871 cm−1 were for aliphatic chain related to hydrophobic amino acids (l-isoleucine/l-leucine) or fatty acids in structure (Li et al. 2017) and 1538, 1667 and 3312 cm−1 indicated peptide bonds (Bechard et al. 1998). Similarly, the 1H NMR showed signals for aromatic amino acid (6.5–9.5 ppm), alkyl amines (3–4 ppm) and –CH3 resonance and −C−CH2−C− (sp3 hydrogen; 1–2 ppm), suggesting the polypeptide nature of AMC (Field et al. 2007). Furthermore, the results of 13C NMR showed carbon signals for aromatic, nitro and amide compounds, and are in agreement with proton NMR (Field et al. 2007).
The mass spectral analysis yielded fragments of compound (1422.576, 711.912, 475.174 Da), which are similar to the fragments of polypeptide antibiotic bacitracin (Choi et al. 2017). These results suggested that antimicrobial peptide produced by B. paralicheniformis UBBLi30 is a bacitracin and this could be the first time it has been detected in B. paralicheniformis. Bacitracin is a cyclic polypeptide mostly produced by certain species of B. licheniformis and B. subtilis (Johnson et al. 1945; Choi et al. 2017). Furthermore, the single band of ~ 5 kDa detected in tricine SDS-PAGE was coordinated well with tricine SDS-PAGE of polypeptide (bacitracin) produced by B. licheniformis CS32 (Choi et al. 2017). To confirm further, we analyzed the whole genome sequence of UBBLi30 (GenBank accession number NZ_SULF01000020) and detected the bacitracin gene bacA (length: 14,472 nucleotides; scaffold 20: NZ_SULF01000020.1), suggesting the production of bacitracin. Moreover, the molecular phylogenetic analysis of bacA gene interestingly revealed the close relatedness of tested sequence within species (B. paralicheniformis strain CMAI1303 & ATCC9945a) and out of the species (B. licheniformis strain), suggesting that this gene may probably be conserved in the genus Bacillus. The 744.918 Da fragment remained unknown and not identified in this study.
The antimicrobial peptide produced by UBBLi30 was resistant to protease activity (proteinase K, trypsin and pepsin), pH (1, 3, 5, 7, 9 and 11) and temperature (40, 60, 80 and 100 °C) like cyclic peptides produced by Bacillus spp. (Bera and Mondal 2019). The presence of unusual amino acids and their cyclic arrangement in peptide are mainly responsible for resistance (Boto et al. 2018). Choi et al. (2017) observed similar results for cyclic polypeptide (bacitracin) isolated from B. licheniformis CS32. In antimicrobial evaluation, the peptide showed inhibitory activity against Gram-positive bacteria, suggesting that the peptide isolated from UBBLi30 is bacitracin. These results are in agreement with earlier studies that polypeptide bacitracin inhibits the growth of only Gram-positive bacteria (O’Donnell et al. 2015).
Zeta potential (surface charge) is a potential at the shear plane of the electrical double layer encompassing a cell in solution (Halder et al. 2015). It is essential for the growth and metabolic activity of bacterial cells. In this study, we observed significant decrease in negative zeta potential of M. luteus after addition of peptide; however, the positive potential of MRSA was significantly decreased to negative value, indicating the electrostatic sorption of peptides on the bacterial surface. Furthermore, the results of no viability of M. luteus and gradual decrease of MRSA suggested that peptide (1 mg ml−1) binding changed the zeta potential, but did not directly damage the membrane. Cell death may be caused due to the sequestering of undecaprenyl pyrophosphate, a lipid carrier that shuttles cell wall biosynthetic intermediates from the cell’s cytoplasm to its exterior (Economou et al. 2013). On the contrary, SEM analysis of M. luteus showed visible cell damage when higher concentration of peptide (5 mg ml−1) was employed, which is in agreement with the previous finding that higher concentration of bacitracin damages the membrane of Gram-positive bacteria (Economou et al. 2013). The MRSA cells remained visually undamaged to the 5 mg ml−1 peptide, suggesting requirement of higher concentrations of peptide to damage the cell membrane. Biofilms are complex microbial communities encased in the sticky polymeric matrix attached to biotic and abiotic surfaces (Achinas et al. 2019). They are difficult to eradicate due to their resistance to environment and antibiotics (Ahire et al. 2015). The biofilms of pathogenic and multidrug-resistance bacteria on indwelling medical devices leads to serious life-threatening infections (Achinas et al. 2019). Every year, methicillin-resistant S. aureus (MRSA) claims 80,000 life-threatening infections in the USA (Hetem et al., 2017; Oh et al. 2018). Besides this, M. luteus has occasionally been reported for pneumonia, septic arthritis, meningitis and endocarditis (Hetem et al., 2017). In this study, we observed that the biofilm formation of M. luteus and MRSA was significantly inhibited by UBBLi30 peptide. This inhibition was dependent on the concentration of the peptide and sensitivity of the strain. Moreover, higher peptide levels were required to inhibit biofilm formation of MRSA as compared with M. luteus. These results are in agreement that increasing concentrations of peptide decreased the biofilm formation of MRSA (Oh et al. 2018).
Conclusion
The indigenously isolated Bacillus paralicheniformis UBBLi30 strain has the ability to produce antimicrobial peptide bacitracin (1422.576 Da), reported to show biological activity against a range of Gram-positive bacteria and inhibition of biofilm formation of M. luteus and methicillin-resistant S. aureus. The reduction of zeta potential of M. luteus and MRSA may be due to the electrostatic sorption of peptide on bacterial surface. This peptide is resistant to the action of proteases (proteinase K, trypsin and pepsin), pH (1, 3, 5, 7, 9 and 11) and temperature up to 100 °C. Bacillus paralicheniformis UBBLi30 therefore has the potential to be utilized as a bio-preservative to control the growth of spoilage and pathogenic bacteria.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
JJA, LSG and RSM contributed to the study conception. JJA designed the experiments. Material preparation, data collection and analysis were performed by JJA and MSK. The first draft of the manuscript was written by JJA and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors disclose that financial support for the research described in the manuscript was provided by Unique Biotech Limited, Hyderabad, India. Dr. Ratna Sudha Madempudi is the Managing Director of Unique Biotech Limited. This does not alter our adherence to journal policies on sharing data and materials.
Human participants and animal studies
The research conducted for this article did not involve studies on humans or animals.
References
- Abriouel H, Franz CM, Omar NB, Gálvez A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev. 2011;35(1):201–232. doi: 10.1111/j.1574-6976.2010.00244.x. [DOI] [PubMed] [Google Scholar]
- Achinas S, Charalampogiannis N, Euverink GJW. A brief recap of microbial adhesion and biofilms. Appl Sci. 2019;9:2801. doi: 10.3390/app9142801. [DOI] [Google Scholar]
- Ahire JJ, Dicks LM. 2, 3-Dihydroxybenzoic acid-containing nanofiber wound dressings inhibit biofilm formation by Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2014;58(4):2098–2104. doi: 10.1128/AAC.02397-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahire JJ, Dicks LM. Nisin incorporated with 2, 3-dihydroxybenzoic acid in nanofibers inhibits biofilm formation by a methicillin-resistant strain of Staphylococcus aureus. Probiotics Antimicrob Prot. 2015;7(1):52–59. doi: 10.1007/s12602-014-9171-5. [DOI] [PubMed] [Google Scholar]
- Ahire JJ, Neveling DP, Hattingh M, Dicks LM. Ciprofloxacin-eluting nanofibers inhibits biofilm formation by Pseudomonas aeruginosa and a methicillin-resistant Staphylococcus aureus. PLoS ONE ONE. 2015;10(4):e0123648. doi: 10.1371/journal.pone.0123648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahire JJ, Robertson DD, Van Reenen AJ, Dicks LM. Surfactin-loaded polyvinyl alcohol (PVA) nanofibers alters adhesion of Listeria monocytogenes to polystyrene. Mater Sci Eng C. 2017;77:27–33. doi: 10.1016/j.msec.2017.03.248. [DOI] [PubMed] [Google Scholar]
- Aitken A, Learmonth MP. Protein determination by UV absorption. In: Walker J, editor. The protein protocols handbook. Totowa: Humana Press; 2009. pp. 3–6. [Google Scholar]
- Al-Amoudi S, Essack M, Simões MF, Bougouffa S, Soloviev I, Archer JA, Lafi FF, Bajic VB. Bioprospecting red sea coastal ecosystems for culturable microorganisms and their antimicrobial potential. Mar Drugs. 2016;14(9):165. doi: 10.3390/md14090165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(S1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Arbsuwan N, Payoungkiattikun W, Sirithorn P, Daduang S, Jangpromma N, Dhiravisit A, Hahm YT, Neubert LK, Klaynongsruang S. Purification and characterization of macrolactins and amicoumacins from Bacillus licheniformis BFP011: a new source of food antimicrobial substances. CyTA J Food. 2018;16(1):50–60. doi: 10.1080/19476337.2017.1337047. [DOI] [Google Scholar]
- Bechard J, Eastwell KC, Sholberg PL, Mazza G, Skura B. Isolation and partial chemical characterization of an antimicrobial peptide produced by a strain of Bacillus subtilis. J Agri Food Chem. 1998;46(12):5355–5361. doi: 10.1021/jf9803987. [DOI] [Google Scholar]
- Bera S, Mondal D. Natural cyclic peptides as clinical and future therapeutics. Curr Org Chem. 2019;23(1):38–75. doi: 10.2174/1385272823666190110103558. [DOI] [Google Scholar]
- Boto A, Pérez de la Lastra J, González C. The road from host-defense peptides to a new generation of antimicrobial drugs. Molecules. 2018;23(2):311. doi: 10.3390/molecules23020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, Cho SS, Simkhada JR, Rahman MS, Choi YS, Kim CS, Yoo JC. A novel multifunctional peptide oligomer of bacitracin with possible bioindustrial and therapeutic applications from a Korean food-source Bacillus strain. PLoS ONE ONE. 2017;12(5):e0176971. doi: 10.1371/journal.pone.0176971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FW, O’Connor PM, O'Sullivan O, Rea MC, Hill C, Ross RP. Formicin–a novel broad-spectrum two-component lantibiotic produced by Bacillus paralicheniformis APC 1576. Microbiol. 2016;162(9):1662–1671. doi: 10.1099/mic.0.000340. [DOI] [PubMed] [Google Scholar]
- de Boer AS, Priest F, Diderichsen B. On the industrial use of Bacillus licheniformis: a review. Appl Microbiol Biotechnol. 1994;40(5):595–598. doi: 10.1007/BF00173313. [DOI] [Google Scholar]
- Deng W, Dong XF, Tong JM, Zhang Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult Sci. 2012;91(3):575–582. doi: 10.3382/ps.2010-01293. [DOI] [PubMed] [Google Scholar]
- Dicks LM, Dreyer L, Smith C, Van Staden AD. A review: the fate of bacteriocins in the human gastro-intestinal tract: do they cross the gut–blood barrier? Front Microbiol. 2018;9:2297. doi: 10.3389/fmicb.2018.02297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ma J, Yin Z, Liu K, Yao G, Xu W, Fan L, Du B, Ding Y, Wang C. Comparative genomic analysis of Bacillus paralicheniformis MDJK30 with its closely related species reveals an evolutionary relationship between B. paralicheniformis and B. licheniformis. BMC Genomics. 2019;20(1):283. doi: 10.1186/s12864-019-5646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap CA, Kwon SW, Rooney AP, Kim SJ. Bacillus paralicheniformis sp. nov., isolated from fermented soybean paste. Int J Syst Evol Microbiol. 2015;65(10):3487–3492. doi: 10.1099/ijsem.0.000441. [DOI] [PubMed] [Google Scholar]
- Economou NJ, Cocklin S, Loll PJ. High-resolution crystal structure reveals molecular details of target recognition by bacitracin. Proc Natl Acad Sci USA. 2013;110(35):14207–14212. doi: 10.1073/pnas.1308268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshaghabee FM, Rokana N, Gulhane RD, Sharma C, Panwar H. Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol. 2017;8:1490. doi: 10.3389/fmicb.2017.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field LD, Sternhell S, Kalman JR. Organic structures from spectra. 4. New York: Wiley; 2007. [Google Scholar]
- Halder S, Yadav KK, Sarkar R, Mukherjee S, Saha P, Haldar S, Karmakar S, Sen T. Alteration of zeta potential and membrane permeability in bacteria: a study with cationic agents. SpringerPlus. 2015;4:672. doi: 10.1186/s40064-015-1476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, Ulrich AS. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother. 2010;54(8):3132–3142. doi: 10.1128/AAC.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood CR, Mouillon JM, Pohl S, Arnau J. Secondary metabolite production and the safety of industrially important members of the Bacillus subtilis group. FEMS Microbiol Rev. 2018;42(6):721–738. doi: 10.1093/femsre/fuy028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetem DJ, Rooijakkers HM, Ekkelenkamp MB. Staphylococcus and micrococci. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. ) Elsevier, Amsterdam: Infectious diseases; 2017. pp. 1509–1522.e2. [Google Scholar]
- Jia P, Cui K, Ma T, Wan F, Wang W, Yang D, Wang Y, Guo B, Zhao L, Diao Q. Influence of dietary supplementation with Bacillus licheniformis and Saccharomyces cerevisiae as alternatives to monensin on growth performance, antioxidant, immunity, ruminal fermentation and microbial diversity of fattening lambs. Sci Rep. 2018;8(1):16712. doi: 10.1038/s41598-018-35081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Anker H, Meleney FL. Bacitracin: a new antibiotic produced by a member of the Bacillus subtilis group. Science. 1945;102(2650):376–377. doi: 10.1126/science.102.2650.376. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang Z, Li X, Yin T, Bian K, Gao F, Gao D. Facile synthesis of bacitracin-templated palladium nanoparticles with superior electrocatalytic activity. J Power Sources. 2017;341:183–191. doi: 10.1016/j.jpowsour.2016.12.006. [DOI] [Google Scholar]
- Makled SO, Hamdan AM, El-Sayed AF. Effects of dietary supplementation of a marine thermotolerant bacterium, Bacillus paralicheniformis SO-1, on growth performance and immune responses of Nile tilapia, Oreochromis niloticus. Aquacult Nutr. 2019;24(4):817–827. doi: 10.1111/anu.12899. [DOI] [Google Scholar]
- Mingmongkolchai S, Panbangred W. Bacillus probiotics: an alternative to antibiotics for livestock production. J Appl Microbiol. 2018;124(6):1334–1346. doi: 10.1111/jam.13690. [DOI] [PubMed] [Google Scholar]
- Morikawa M, Hirata Y, Imanaka T. A study on the structure–function relationship of lipopeptide biosurfactants. Biochim Biophys Acta Mol Cell Biol Lipids. 2000;1488(3):211–218. doi: 10.1016/S1388-1981(00)00124-4. [DOI] [PubMed] [Google Scholar]
- Odonnell JA, Gelone SP, Safdar A. Topical antibacterials. In: Dixon N, editor. Mandell, Douglas, and Bennetts principles and practice of infectious diseases. Amsterdam: Elsevier; 2015. [Google Scholar]
- Oh E, Bae J, Kumar A, Choi HJ, Jeon B. Antioxidant-based synergistic eradication of methicillin-resistant Staphylococcus aureus (MRSA) biofilms with bacitracin. Int J Antimicrob Agents. 2018;52(1):96–99. doi: 10.1016/j.ijantimicag.2018.03.006. [DOI] [PubMed] [Google Scholar]
- Schägger H. Tricine–sds-page. Nat Protoc. 2006;1(1):16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- Sumi CD, Yang BW, Yeo IC, Hahm YT. Antimicrobial peptides of the genus Bacillus: a new era for antibiotics. Can J Microbiol. 2014;61(2):93–103. doi: 10.1139/cjm-2014-0613. [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- van Staden AD (2015) In vitro and in vivo characterization of amyloliquecidin, a novel two-component lantibiotic produced by Bacillus amyloliquefaciens (Doctoral dissertation, Stellenbosch: Stellenbosch University).
- Xu S, Lin Y, Zeng D, Zhou M, Zeng Y, Wang H, Zhou Y, Zhu H, Pan K, Jing B, Ni X. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci Rep. 2018;8(1):1744. doi: 10.1038/s41598-018-20059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Kuipers OP. Identification and classification of known and putative antimicrobial compounds produced by a wide variety of Bacillales species. BMC Genomics. 2016;17(1):882. doi: 10.1186/s12864-016-3224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.