Abstract
Objective
Our objective was to review, compare and gain insight into economic evaluations in primary open-angle glaucoma (POAG) with a focus on existing decision analytic models.
Methods
A literature review was performed using clinical and specialized databases following best practices. Relevant inclusion criteria included the development of a decision analytic model, the assessment of POAG interventions, and a full economic evaluation in terms of costs and health-related outcomes. Model inputs and settings were extracted, compared and analyzed. Main study incremental outcomes were also reported.
Results
The literature review identified 22 full articles in alignment with the eligibility criteria for a total of 15 countries and a wide range of years from 1983 to 2018. Interventions included as competing alternatives in the eligible studies were topical medications (33%), screening or diagnosis (33%), surgical interventions (10%), laser trabeculoplasty (10%) and minimally invasive surgeries (3%). Markov models using transition states were the most common type of modeling approach. Cost-utility models using a mid- to long-term time horizon with a national payer perspective were the most frequent type of economic evaluation identified. Model states commonly included disease severity levels, as defined by glaucoma staging systems, and other relevant events such as blindness and death. Authors did not sufficiently justify key modeling assumptions, inputs or the robustness of their findings.
Conclusions
Decision analytic models in POAG can reasonably guide future modeling research by revealing common practices, inputs and assumptions. Furthermore, this review revealed evidence gaps in terms of unexplored interventions and treatment sequences.
Electronic supplementary material
The online version of this article (10.1007/s41669-019-0141-4) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Decision analytic modeling for primary open-angle glaucoma (POAG) has been undertaken for more than 35 years in 15 different countries, providing enough background and useful insights to guide future modeling research. |
| Existing economic assessments include the study of topical medications, screening or diagnosis alternatives, surgery, laser trabeculoplasty and minimally invasive surgeries. |
| The structure of a decision analytic model for POAG can be justified by using published glaucoma staging systems from a clinical standpoint. |
| Evidence gaps in the literature are associated with a lack of competing alternatives, inclusion of treatment sequences and sufficient justification of key modeling assumptions, inputs and outcomes robustness. |
Introduction
Primary open-angle glaucoma (POAG) is a chronic and progressive optic neuropathy that causes an increase in intraocular eye pressure that can lead, if left untreated, to damage in the optic nerve and ultimately to severe or complete vision loss [1]. The worldwide prevalence of glaucoma is estimated at 3.54%; it primarily affects adults aged > 40 years and represents the second most common cause of blindness after cataract [2, 3]. Among the two dominant types of glaucoma, namely open-angle and angle-closure, the first one alone accounts for about 90% of all cases. Unlike close-angle glaucoma, the development of POAG is generally slower and asymptomatic until serious vision loss occurs [4]. Interventional treatments aim to delay or stop progression of the disease by regulating intraocular pressure (IOP) [1]. Medical drugs, laser trabeculoplasty and incisional surgery have proven to be effective interventions in lowering IOP, but a stepwise change in the intervention type according to disease severity has been suggested to maximize cost effectiveness [3, 5].
Early detection and care in POAG are relevant as advanced disease stages are critical from both a clinical and economic perspective. Indeed, clinical interventions become increasingly specialized and costly [3]. Minimally invasive glaucoma surgery (MIGS) claims to be less intrusive and have an improved safety profile compared with standard surgery (filtration surgery), while simultaneously reducing topical medication dependency [2].
Given the progressive nature of the disease, its clinical burden as well as the wide range of stepwise treatment options, the use of long-term economic models represents an opportunity to overcome the lack of head-to-head comparisons and insufficient patient follow-up beyond clinical trial horizon. Economic models allow an estimation of the additional costs, effectiveness and cost effectiveness for optimal resource allocation and decision making. The current review aims to provide insights into existing decision analytic models for POAG to guide future research in the subject.
Methods
General
A systematic literature review of published economic models for POAG was carried out in January 2019, aiming to identify published articles where authors used a decision analytic model. No time restrictions were included in the search. Both clinical and specialized sources were consulted; selected databases were MEDLINE® and the National Health Services Economic Evaluation Database (NHS-EED). In consistency with the eligibility criteria, a search strategy was developed with an exhaustive list of keywords and phrases identified with medical subject headings (MeSH) in English. The full search strategy and number of hits per keywords can be found in the Appendix in the Electronic Supplementary Material (ESM). Following the PRISMA (Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses) statement [6], JBH and LBD independently conducted the search from the identification phase up to the inclusion phase; discrepancies in the findings were jointly discussed and resolved. A self-developed data extraction grid was used for retrieving relevant model inputs, settings and outcomes. Relevant modeling insights were discussed as a result of the comparison and analysis of different identified models. For each type of POAG intervention, some of the most relevant study findings in terms of incremental cost-effectiveness ratios (ICERs) are presented, but more detail can be found in the Appendix in the ESM.
Eligibility Criteria
Inclusion and exclusion criteria were determined following an operationalized population, intervention, comparators, outcomes and study design (PICOS) scheme as shown in Table 1. The selected population were adult patients (aged ≥ 18 years) with a confirmed diagnosis of POAG. Other types of glaucoma were excluded if they were clearly stated, but papers referring to an undefined type of glaucoma were kept for screening. For the intervention and comparators of interest, all forms of medical interventions were eligible, including but not limited to common POAG therapies such as topical medications, laser trabeculoplasty and any type of surgery as well as screening and observational strategies. Along with a full treatment cost estimation, studies of interest were those that equally reported health gains either as quality- or disability-adjusted life-years (QALYs or DALYs, respectively), life-years only or any other natural measurement of effectiveness as part of their outcomes. For the purpose of this research, only cost-effectiveness analyses (CEA) or cost-utility analyses (CUA) where a decision analytic model was used were of interest. The sole estimation of cost effectiveness or cost utility as part of a prospective or retrospective analysis without a decision analytic model was an exclusion criterion. Conference abstracts and articles adopting other types of economic evaluations were also discarded.
Table 1.
Study eligibility criteria in PICOS format
| PICOS | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Adult (aged ≥ 18 years) patients with primary open-angle glaucoma |
Other types of glaucoma, including but not limited to: Primary congenital forms or childhood glaucomas Secondary glaucomas Primary angle closure Secondary angle closure |
| Intervention/comparators | Medical interventions including screening, observation and diagnosis | Non-medical interventions |
| Outcomes |
Alongside a full treatment cost estimation, studies must include one of the following outcomes: quality-adjusted life-years life-years any natural measurement of effectiveness related to the disease |
Projections about clinical or economic outcomes as standalone |
| Study design |
Decision analytic models evaluating: Cost effectiveness Cost utility |
1. No decision analytic model developed 2. Other types of economic evaluations, including but not limited to: Cost–benefit Cost minimization Budget impact model |
PICOS population, intervention, comparators, outcomes and study design
Data Extraction
Variables of interest were extracted following an operationalized PICOS scheme, but other relevant items such as study perspective, time horizon, cost year and currency, discounting, base-case outcomes, main sensitivity analysis findings (as reported by the author) and sources of information for costs and utility values were also extracted.
Comparators were further classified between screening or diagnostic strategies, topical medications, laser trabeculoplasty and two types of surgery: standard invasive and MIGS [2, 7]. Decision analytic models were divided into decision trees, Markov models and microsimulations. The full extracted data from each single eligible article can be found in the Appendix in the ESM.
Results
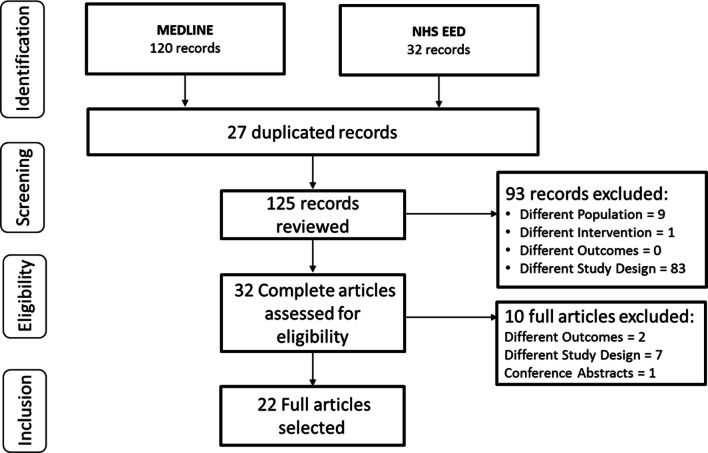
A total of 125 records were reviewed after duplicates were removed. In the final inclusion phase, 22 full-text articles fulfilled the eligibility criteria and were selected for detailed screening. Figure 1 documents the full identification, screening, eligibility and inclusion process as a PRISMA diagram.
Fig. 1.
PRISMA flowchart presenting the process of identification, screening, confirming eligibility and final inclusion from the preselected databases. NHS EED National Health Services Economic Evaluation Database, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Of the retrieved decision analytic models, 14 were Markov, four were decision trees and four were microsimulations. The publication dates of the identified papers ranged from 1983 to 2018, with 15 different country perspectives. CUAs were the most frequent type of analyses, over CEAs (74 vs. 26%, respectively), and QALYs were the most common type of effectiveness measure. Topical medications and screening or diagnostic strategies were the most frequent interventions analyzed in those papers (38% each), followed by both surgical interventions and laser trabeculoplasty (9% each); a single article studied one type of MIGS (3%). Table 2 presents a detailed summary of study characteristics.
Table 2.
Characteristics and modeling insights from the eligible studies
| Study, country (cost year; currencya) | Perspective | Evaluation type | Comparator type | Model type | Max. time horizon (years) | Discounting (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | S | O | CEA | CUA | TM | LT | SI | MIGS | D/S | MK | DT | MS | NA/O | Costs | HO | ||
|
Ordóñez et al. [20] Colombia (2017; USD) |
x | x | x | x | x | x | Life | 5.0 | 5.0 | ||||||||
|
CADTH [23] Canada (2016; CAD) |
x | x | x | x | Life | 1.5 | 1.5 | ||||||||||
|
John and Parikh [8] India (2016; INR) |
x | x | x | x | 10 | 3.0 | 3.0 | ||||||||||
|
John and Parikh [9] India (2015; INR) |
x | x | x | x | 10 | 3.0 | 3.0 | ||||||||||
|
Paletta Guedes et al. [5] Brazil (2014; BRL) |
x | x | x | x | x | x | x | Life | 5.0 | 5.0 | |||||||
|
Boodhna and Crabb [13] UK (2015; GBP) |
x | x | x | x | 25 | NA | NA | ||||||||||
|
Kaplan et al. [19] USA (2013; USD) |
x | x | x | x | 5 | 3.0 | 3.0 | ||||||||||
|
Orme et al. [15] UK (2008–09; GBP) |
x | x | x | x | 10 | 3.5 | 3.5 | ||||||||||
|
Stein et al. [17] USA (2010; USD) |
x | x | x | x | x | x | 25 | 3.5 | NA | ||||||||
|
van Gestel et al. [14] Netherlands (2006; EUR) |
x | x | x | x | x | Life | 4 | 1.5 | |||||||||
|
Paletta Guedes et al. [18] Brazil (2010; BRL) |
x | x | x | x | 5 | 3 | NA | ||||||||||
|
Wittenborn and Rein [10] Barbados, Ghana (2005; USD) |
x | x | x | x | Life | 3 | 3 | ||||||||||
|
Rein et al. [24] USA (2005; USD) |
x | x | x | x | x | Life | 3 | 3 | |||||||||
|
Stewart et al. [16]; NOR, SWE, DEN, UK (2005; USD) |
x | x | x | x | 5 | NA | NA | ||||||||||
|
Hernández et al. [12] UK (2006; GBP) |
x | x | x | x | Life | 3.5 | NA | ||||||||||
|
Peeters et al. [25] Netherlands (2001; EUR) |
x | x | x | x | 20 | 4 | 4 | ||||||||||
|
Payet et al. [26] France (2005; EUR) |
x | x | x | x | 5 | 3.5 | NA | ||||||||||
|
Vaahtoranta-Lehtonen et al. [11] Finland (2003; EUR) |
x | x | x | x | x | 20 | 5 | 5 | |||||||||
|
Bernard et al. [27] France (2002; EUR) |
x | x | x | x | 3 | 3 | 0 | ||||||||||
|
Eandi et al. [28] Italy (1998–99; ITL) |
x | x | x | x | NA | NA | NA | ||||||||||
|
Rochi and Tingey [29] Canada (1996; CAD) |
x | x | x | x | 10 | 5 | 5 | ||||||||||
|
Gottlieb et al. [30] USA (1980; USD) |
x | x | x | x | NA | 5 | 5 | ||||||||||
CEA cost-effectiveness analysis, CUA cost-utility analysis, DEN Denmark, D/S diagnosis/screening, DT decision tree, LT laser trabeculoplasty, HO health outcomes, max maximum, MIGS minimally invasive glaucoma surgery, MK Markov, MS microsimulation, NA not available, NOR Norway, O other, P payer, S societal, SI surgical intervention, SWE Sweden, TM topical medications, y years
aCurrency is listed as per the International Organization for Standardization code
The following sections describe some of the main findings of the identified papers in terms of ICERs for each type of POAG intervention. Further details of all identified studies and their outcomes can be found in the ESM. Finally, we highlight a list of key modeling lessons and evidence gaps in the literature.
Screening for Glaucoma
Ten of the identified articles included one type of screening as a comparative intervention. The common study objectives were either the frequency with which the procedure was conducted or the type of screening or diagnostic strategy deemed most cost effective.
In the comparison versus opportunistic case finding, usually referred to as the country reference practice, regular community screening was commonly associated with increased health gains and higher costs [8–11]. Particularly relevant is the 2008 article by Hernandez et al. [12], who adopted a UK payer perspective to study the cost effectiveness of screening according to the age of the cohort (40, 60 and 75 years) for six different levels of glaucoma prevalence. Their analysis demonstrated that screening, whether performed by a technician or an optometrist, becomes increasingly cost effective as the age of the cohort and prevalence increases [12].
Authors have likewise studied the frequency upon which visual field testing should be performed (identified options were annually, every 6 months, every 24 months or three times after diagnosis), with positive ICERs in all cases when compared with current practice [13, 14]. In the 2012 analysis carried out in the Netherlands by van Gestel et al. [14] using a societal perspective, an increased frequency of screening was not necessarily associated with improved ICER outcomes (€173,486 vs. 21,516 per QALY for testing every 6 or every 24 months, respectively).
Topical Medications
The study of topical medications in CEA or CUA was also common; in particular, seven papers identified the inclusion of prostaglandin analogues as a competitor (alone or in combination). The 2012 CUA by Orme et al. [15] conducted from the UK payer perspective found latanoprost was a dominant option versus bimatoprost and travoprost. In another analysis published in 2009, Stewart et al. [16] studied the cost utility of latanoprost versus timolol in the UK, Norway, Sweden and Denmark, presenting timolol in all cases as a cost-effective alternative: reported ICERs were $US51,831 (Norway), $US124,270 (Sweden), $US55,722 (Denmark) and $US8175 (UK) per QALY [16].
Laser Trabeculoplasty
Three relevant papers studied laser trabeculoplasty as an alternative. A CUA published by Stein et al. [17] in 2012 studied the use of laser trabeculoplasty versus observation in the USA, estimating that trabeculoplasty was cost effective compared with observation only, with an estimated ICER of $US16,824 per QALY. Two papers compared laser trabeculoplasty and prostaglandin analogues and consistently found that laser trabeculoplasty was a less costly alternative but also presented contrasting health outcomes in terms of the most effective intervention as measured by QALYs [17, 20].
Surgical Treatments
The study of filtration surgery in economic models was seen in cohorts of patients with progressive disease. Three CUAs compared surgery and topical medications. In a 2012 study adopting a Brazilian healthcare perspective, Paletta Guedes et al. [18] estimated that surgery (nonpenetrating deep sclerectomy) was a dominant option versus three adjunct therapies of prostaglandin analogues with timolol and dorzolamide in patients reaching the maximum medication dose. In 2015, Kaplan et al. [19] explored the cost effectiveness of the Baerveldt implant versus trabeculectomy with mitomycin from a USA societal perspective and presented a positive ICER of $US29,055 per QALY for the Baerveldt implant.
Minimally Invasive Glaucoma Surgery
The study of MIGS in economic models was identified in a single article published in 2018 by Ordóñez et al. [20] from a Colombian public health payer perspective. Compared with laser trabeculoplasty and three different prostaglandin analogues adjunct to timolol and dorzolamide, the micro-bypass stent with timolol was estimated to be a dominated option as it had a higher cost with a lower effectiveness in terms of QALYs [20].
Modeling Lessons and Evidence Gaps
By identifying common modeling practices and challenges in decision analytic models for POAG, four main lessons for future research in the subject were drawn from the findings:
Type of decision analytic model: Authors neglected to justify their choice of decision analytic model. A Markov transition model was commonly used to capture the full progression of the disease from early to advanced stages. The use of decision trees was not encountered in analyses with a time horizon > 10 years, suggesting an insufficient projection of long-term costs and health gains. The use of microsimulations was associated with models incorporating individual-level patient characteristics that could ultimately play a role in detailed risk adjustments.
Defining model stages: Models aiming to capture the full disease progression in the form of transition states looked into different glaucoma staging systems documented in the literature [21, 22]. Fundamental discrepancies mainly concerned the number of possible POAG stages before uni- or bilateral blindness. Authors frequently used three POAG severity states: mild, moderate and severe, but the use of five POAG severity states also bears clinical justification [22]. An exemplary Markov structure based on the Hodapp Classification System and incorporating other relevant states is detailed in Fig. 2.
Temporality: Authors most commonly used a long-term to lifetime horizon. Analyses adopting shorter time horizons (< 20 years) failed to properly justify their temporality and poorly represented the expected long-term outcomes.
Handling treatment switches: Changes in treatment strategy are common as the disease progresses [5]. Nevertheless, existing economic evaluations insufficiently captured the nature of treatment switches or sequence strategies, with few exceptions. van Gestel et al. [14] and Paletta Guedes et al. [5] approached the challenge of building a long-term economic model based both on disease stages and on comparative treatment sequences.
Fig. 2.
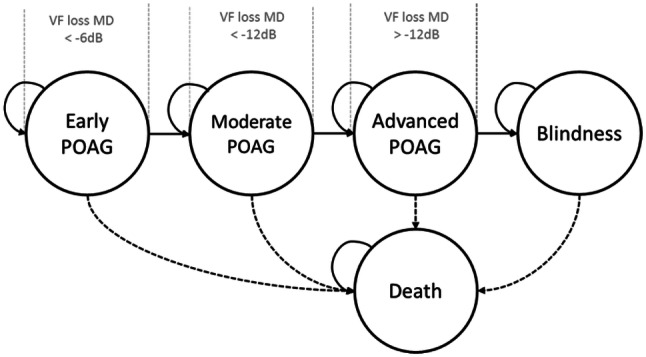
Exemplary Markov structure following the Hoddap Parish and Anderson criteria for POAG disease-severity states [7]. dB decibel, MD mean deviation, POAG primary open-angle glaucoma, VF visual field
Discussion
Developing decision analytic models for POAG, a chronic and progressively degenerative disease, has been commonly approached with mid- to long-term Markov state-transition models using different glaucoma staging systems. This “tunnel” approach has been justified from a clinical point of view as it correctly represents the stepwise changes in costs and utilities as the disease progresses.
Measuring effectiveness in terms of QALYs remained within the scope of usual practice, but alternative natural measurements of effectiveness were not uncommon. By order of frequency, topical medications, screening or diagnostic strategies and standard and laser surgeries were included as comparative alternatives, but the study of MIGS remains a gap in the literature, with only one study. The joint challenge of building a model that can capture both long-term outcomes and treatment sequences according to disease severity has been insufficiently explored.
One of the limitations of this review was that the eligibility criteria led to an underestimation of the available economic evaluations in POAG, since (1) the review was limited to English publications, (2) only full economic analyses assessing both costs and health outcomes were considered and (3) articles estimating cost effectiveness with the use of simple prospective or retrospective data (e.g., clinical trials) but that did not have a decision analytic model were excluded.
Given the evidence, existing decision analytic models for POAG can reasonably guide similar research, but focus on justifying assumptions and choice of model and temporality should be increased.
Data Availability
The data extracted and analyzed in this literature review are available in the main article or the Appendix in the ESM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contributions
Conception and design: JB-H, SF. Collection and assembly of data: JB-H, LB-D. Interpretation: JB-H, SF. Manuscript preparation: JB-H. Manuscript review: JB-H, LB-D, SF.
Compliance with Ethical Standards
Funding
No sources of funding were used to conduct this review or prepare this manuscript.
Conflicts of interest
J. Bartelt-Hofer, L. Ben-Debba, and S. Flessa have no conflicts of interest that are directly relevant to the content of this article.
References
- 1.Deutsche Ophthalologische Gesellschaft, “Stellungnahme zur Glaukomfrüherkennung,” 2015. [Online]. https://www.dog.org/wp-content/uploads/2015/11/SN-Glaukom-August-2015.pdf. Accessed 28 Sep 2018.
- 2.Richter G, Coleman A. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206. doi: 10.2147/OPTH.S80490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Ophthalmology Primary open-angle glaucoma preferred practice pattern guidelines. Ophthalmology. 2015;123(1):41–111. [Google Scholar]
- 4.Schellak N, Schellak G, Bezuidenhout S. Glaucoma: a brief review. South Afr Pharm J. 2015;82(5):18–22. [Google Scholar]
- 5.Paletta Guedes R, Paletta Guedes V, de Mello Gomes CE, Chaoubah A. Maximizing cost-effectiveness by adjusting treatment strategy according to glaucoma severity. Medicine. 2016;95(52):1–8. doi: 10.1097/MD.0000000000005745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tezlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 7.European Glaucoma Society Terminology and guidelines for glaucoma. Br J Ophthalmol. 2017;101(4):1–72. doi: 10.1136/bjophthalmol-2016-EGSguideline.001. [DOI] [PubMed] [Google Scholar]
- 8.John D, Parikh R. Cost-effectiveness of community screening for glaucoma in rural India: a decision analytical model. Public Health. 2018;155:142–151. doi: 10.1016/j.puhe.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 9.John D, Parikh R. Cost-effectiveness and cost utility of community screening for glaucoma in urban India. Public Health. 2017;148:37–48. doi: 10.1016/j.puhe.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Wittenborn JS, Rein DB. The cost-effectiveness of glaucoma interventions in Barbados and Ghana. Optom Vis Sci. 2011;88(1):155–163. doi: 10.1097/OPX.0b013e3181fc30f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaahtoranta-Lehtonen H, Tuulonen A, Aronen P, Sintonen H, Suoranta L, Kovanen N, Linna M, Läärä E, Malmivaara A. Cost effectiveness and cost utility of an organized screening programme for glaucoma. Acta Ophthalmol Scand. 2007;85:508–518. doi: 10.1111/j.1755-3768.2007.00947.x. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez RA, Burr JM, Vale LD. Economic evaluation of screening for open-angle glaucoma. Int J Technol Assess Health Care. 2008;24(2):203–2011. doi: 10.1017/S0266462308080288. [DOI] [PubMed] [Google Scholar]
- 13.Boodhna T, Crabb DP. More frequent, more costly? Health economic modelling aspects of monitoring glaucoma patients in England. Health Serv Res. 2016;16(611):2–13. doi: 10.1186/s12913-016-1849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Gestel A, Webers CA, Severens JL, Beckers HJ, Jansonius N, Hendrikse F, Schouten J. The long-term outcomes of four alternative treatment strategies for primary open-angle glaucoma. Acta Ophthalmol. 2012;90(1):20–31. doi: 10.1111/j.1755-3768.2011.02318.x. [DOI] [PubMed] [Google Scholar]
- 15.Orme M, Collins S, Loftus J. Long-term medical management of primary open-angle glaucoma and ocular hypertension in the UK: optimizing cost-effectiveness and clinic resources by minimizing therapy switches. J Glaucoma. 2012;21(7):433–449. doi: 10.1097/IJG.0b013e31821dac2a. [DOI] [PubMed] [Google Scholar]
- 16.Stewart W, Stewart J, Mychaskiw M. Cost-effectiveness of latanoprost and timolol maleate for the treatment of glaucoma in Scandinavia and the United Kingdom, using a decision-analytic health economic model. Eye. 2009;23(1):132–140. doi: 10.1038/sj.eye.6702964. [DOI] [PubMed] [Google Scholar]
- 17.Stein J, Kim D, Peck W, Giannetti S, Hutton D. Cost effectiveness of medications compared with laser trabeculoplasty in patients with newly-diagnosed open-angle glaucoma. Arch Ophthalmol. 2012;130(4):497–505. doi: 10.1001/archophthalmol.2011.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paletta Guedes RA, Paletta Guedes VM, Chaoubah A. Cost-effectiveness comparison between non-penetrating deep sclerectomy and maximum-tolerated medical therapy for glaucoma within the Brazilian National Health System. Arq Bras Oftalmol. 2012;75(1):11–15. doi: 10.1590/S0004-27492012000100002. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan RI, de Morales G, Cioffi GA, Al-Aswad LA, Blumberg D. Comparative Cost-effectiveness of the Baerveldt Implant, Trabeculectomy With Mitomycin, and Medical Treatment. JAMA Ophthalmol. 2015;133(5):560–567. doi: 10.1001/jamaophthalmol.2015.44. [DOI] [PubMed] [Google Scholar]
- 20.Ordóñez J, Ordóñez A, Osorio U. Cost-effectiveness analysis of iStent trabecular micro-bypass stent for patients with open-angle glaucoma in Colombia. Curr Med Res Opin. 2019;35(2):329–340. doi: 10.1080/03007995.2018.1506022. [DOI] [PubMed] [Google Scholar]
- 21.Ng M, Sample P, Pascual J, Zangwill L, Girkin C, Liebmann J, Weinreb R, Racette L. Comparison of visual field severity classification systems for glaucoma. J Glaucoma. 2012;21(8):551–561. doi: 10.1097/IJG.0b013e31821dac66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills RP, Budenz D, Lee PP, Noecker RJ, Walt JG, Siegartel LR, Evans SJ, Doyle JJ. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Opthalmol. 2006;141(1):24–30. doi: 10.1016/j.ajo.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 23.CADTH. Pharmacoeconomic review report for monoprost. May 2018. [Online]. https://www.cadth.ca/sites/default/files/cdr/pharmacoeconomic/SR0541_Monoprost_PE_Report.pdf. Accessed 07 Feb 2019.
- 24.Rein DB, Wittenborn JS, Lee PP, Wirth KE, Sorensen SW, Hoerger TJ, Saaddine JB. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open-angle glaucoma in the United States. Ophthalmology. 2009;116(1):823–832. doi: 10.1016/j.ophtha.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 25.Peeters A, Schouten J, Webers C, Prins M, Hendrikse F, Severens J. Cost-effectiveness of early detection and treatment of ocular hypertension and primary open-angle glaucoma by the ophthalmologist. Eye. 2008;22(1):354–362. doi: 10.1038/sj.eye.6702637. [DOI] [PubMed] [Google Scholar]
- 26.Payet S, Denis P, Berdeaux G, Launois R. Assessment of the cost effectiveness of travoprost versus latanoprost as single agents for treatment of glaucoma in France. Clin Drug Invest. 2008;28(3):183–198. doi: 10.2165/00044011-200828030-00005. [DOI] [PubMed] [Google Scholar]
- 27.Bernard L, Althin R, Dhawan R, Grima D, Lam A, Aballéa S. Clinical and economic impacts of latanoprost 0.005% in first-line treatment of open-angle glaucoma and ocular hypertension in France. Eur J Ophthalmol. 2003;13:S30–S43. doi: 10.1177/112067210301304S04. [DOI] [PubMed] [Google Scholar]
- 28.Eandi C, Brogliatti B, Micieli M, Grignolo F. Cost -effective analysis by Markov Chains of open-angle glaucoma therapy. Preliminary results. Acta Ophthalmol. 1999;77(S229):56–57. doi: 10.1111/j.1600-0420.1999.tb01154.x. [DOI] [Google Scholar]
- 29.Rochi A, Tingey D. Economic evaluation of dorzolamide vs. pilocarpine for primary open-angle glaucoma. Can J Ophthalmol. 1997;32(6):414–418. [PubMed] [Google Scholar]
- 30.Gottlieb L, Schwartz B, Pauker S. Glaucoma screening. A cost-effectiveness analysis. Surv Opthalmol. 1983;28(3):206–226. doi: 10.1016/0039-6257(83)90098-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data extracted and analyzed in this literature review are available in the main article or the Appendix in the ESM.