Abstract
Aging is associated with declines in physical and cognitive performance. While there is no doubt about beneficial effects of physical exercise on proxies of strength and balance, the overall evidence for positive effects of resistance and balance training on executive functions is rather inconsistent. Whether the simultaneous exercising of strength and balance, i.e., instability resistance training, promotes executive functions in older adults is unknown. In the present trial, we tested the effects of unstable vs. stable resistance training on executive functions. Sixty-eight healthy older adults aged 65–79 years were randomly assigned to either an instability free-weight resistance training or one of two stable machine-based resistance training programs. Each group exercised twice a week on non-consecutive days for 10 weeks. Four tests to evaluate specific domains of executive functions were administered prior and following training: working memory, processing speed, response inhibition and set-shifting. The instability resistance training group improved working memory, processing speed and response inhibition from pre to post-test. In contrast, we found no improvements in executive functions for both stable resistance training groups. Our results demonstrate that 10 weeks of instability resistance training suffice to improve executive functions in older adults.
Subject terms: Ageing, Human behaviour, Randomized controlled trials
Introduction
The decline of neuromuscular control, motor performance and cognition with aging and adverse health outcomes such as functional limitations and possible falls are major health care issues of the 21st century1–3. Impaired executive functioning, as part of cognition, has been associated with reduced physical functioning and impaired locomotion in particular3. Therefore, improving executive functions and/or slowing age-related decline, is of great interest. While, based on a recent Cochrane review, the evidence for computerised cognitive training is somewhat inconclusive4, physical exercise interventions appear to be beneficial for executive functions based on single studies5–11. However, the overall evidence for beneficial effects of physical exercise interventions on executive functions is rather inconsistent12,13. Accordingly, we aimed to systematically investigate challenging and cognitively demanding vs. less demanding physical exercises11–13. Consequently, the effects of resistance training modalities with different cognitive and physical demands on executive functions in older adults were tested in this study.
The conflicting findings can mainly be explained by the abundance of different cognitive outcomes, tests and the variety of different study designs6 making comparisons difficult. Furthermore, Diamond and Ling12,13 argue that the reason why simple “mindless” exercise interventions like aerobic training (e.g., running on a treadmill) or pure balance training have little or no effect on executive functions is that they lack any cognitive challenge, attention or social component. Studies employing resistance training show that it may improve cognition in older adults6,8,9, yet consistent evidence of positive effects are still amiss6,12,13. All in all, it appears, that resistance training is more beneficial when it is challenging (e.g., progressive increase of load and sets)6,9. Similar to aerobic training, the abundance of cognitive outcomes, tests and exercise designs of resistance training interventions makes it difficult to compare existing study outcomes.
Unlike aerobic and resistance training, balance training is a less frequently practised exercise modality. However, in contrast to aerobic and resistance training, demanding balance training shows more consistent positive effects on executive functions5,14. We have to distinguish demanding balance training from ‘simple balance exercises’ or toning routines. The latter often require only one-legged balance tasks with displacements in the transversal plane and no displacement in the sagittal and frontal plane. In contrast, demanding balance exercises may include challenging eye–hand coordination, leg–arm coordination as well as spatial orientation and reaction demands to moving objects or persons in all planes14. It may as well include responses to perturbations, forcing participants to permanently re-stabilise within a metastable state of equilibrium5,15. It is not surprising that physical exercise interventions incorporating several training modalities and, therefore, providing higher demands and challenges, procure a superiority to basic aerobic and resistance training with regard to more consistent effects6,7,16. A recent randomised controlled trial (RCT)17 showed the feasibility and effectiveness of a multicomponent training, so called instability resistance training or resistance training on unstable surfaces (concurrent balance and resistance training), on proxies of strength, power and balance in older adults. Participants of the instability resistance group increased measures of balance, strength and power similar to the stable machine-based resistance training. Importantly, the instability resistance training group exercised with less than half the load during the major squat exercise (e.g., 52 kg vs. 20 kg). However, the trial did not test for cognitive effects. We know from previous research that both, resistance6,8,9 and balance5,14 training, may benefit cognition in older adults, particularly when it is challenging, raising the question whether the combination of both, i.e. balance and resistance training may enhance cognition in older adults, given the higher challenges and the need of increased attention.
Therefore, the present RCT aimed to determine whether physically and mentally challenging instability free-weight resistance training (I-FRT) affects cognitive performance in healthy older adults differently compared to traditional “less cognitive challenging” stable machine-based resistance training (S-MRT & S-MRTHIP). To assure, that the modality (unstable/stable) is the decisive factor, we implemented two stable groups to exclude any potential effect of a particular stable training program.
We hypothesised that the combined challenge of resistance training and balance training (instability resistance training) would result in increased executive functions after 10 weeks in comparison to stable resistance training. Secondly, we hypothesised that there would be no difference between the two different stable training modalities.
Results
The exercise compliance for all participants over 10 weeks was on average 95.3 ± 0.86%. Demographic and baseline descriptors of the 68 participants who completed the 10-week trial are presented in Table 1. Baseline values at pre-test showed no differences between groups with respect to all investigated cognitive outcome variables (ps ≥ 0.122). Three participants were not able to conduct the Stroop-Colour-Word Test, due to colour-blindness. Furthermore, because of technical issues, post-test results for the Stroop-Colour-Word Test for one participant were not recorded. We found violations of normal distribution and homogeneity within the Trail Making Test and the Digit Symbol Substitution Test. The analysis with non-parametric tests revealed no changes in the outcomes. Accordingly, we only reported parametric results.
Table 1.
Demographics and characteristics of participants at baseline.
| Characteristics | Unstable | Stable | Baseline difference | ||||
|---|---|---|---|---|---|---|---|
| I-FRT (n = 21) | S-MRT (n = 24) | S-MRTHIP (n = 23) | |||||
| M | SD | M | SD | M | SD | p-value | |
| Age (years) | 71.3 | 3.9 | 69.5 | 3.8 | 69.9 | 3.9 | 0.288 |
| Body height (cm) | 171 | 9 | 169 | 7 | 1.69 | 9 | 0.820 |
| Body mass (kg) | 76.9 | 15.7 | 73.8 | 12.4 | 76.6 | 13.6 | 0.691 |
| Sex (f/m) | 12/9 | 16/8 | 13/10 | − | |||
| Physical activity (h/w) | 9.4 | 9.2 | 11.9 | 8.6 | 12.2 | 7.2 | 0.215* |
| MMSE | 27.9 | 1.6 | 27.8 | 1.8 | 28.0 | 1.6 | 0.914* |
| CDT | all participants were classified as non-pathological | ||||||
| GDS | 0.9 | 1.0 | 1.1 | 1.6 | 1.0 | 1.4 | 0.957* |
| FAB_D | 15.1 | 2.1 | 15.3 | 2.0 | 15.7 | 2.2 | 0.521* |
Note: I-FRT = instability free-weight resistance training, S-MRT = stable machine-based resistance training, S-MRTHIP = stable machine-based adductor/abductor training; M = mean; SD = standard deviation; f = female; m = male; MMSE = Mini Mental State Examination; CDT = Clock Drawing Test; GDS = Geriatric Depression Scale; FAB_D = Frontal Assessment Battery, German Version. P-values denoted with an * violated distribution assumptions and non-parametric differ from parametric results and are displayed.
All outcome measures are displayed in Table 2.
Table 2.
Outcomes of the exercise intervention for pre- and post-testing.
| Variables | Unstable | Stable | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-FRT (n = 21) | S-MRT (n = 24) | S-MRTHIP (n = 23) | ||||||||||
| pre | post | pre | post | pre | post | |||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| DSST | ||||||||||||
| Number | 37.8 | 8.11 | 45.0 | 8.2 | 42.9 | 8.2 | 44.7 | 8.5 | 40.9 | 8.1 | 42.9 | 9.7 |
| DMT | ||||||||||||
| Score | 97.0 | 14.9 | 107.3 | 14.9 | 99.7 | 13.0 | 99.8 | 16.4 | 98.4 | 11.3 | 98.3 | 13.2 |
| Stroop | ||||||||||||
| Circle (ms) | 623.3 | 109.4 | 620.3 | 115.0 | 602.7 | 91.5 | 622.3 | 69.8 | 629.9 | 82.6 | 631.1 | 69.4 |
| Neural (ms) | 754.9 | 124.4 | 714.5 | 125.6 | 706.0 | 142.6 | 718.8 | 104.7 | 757.5 | 98.9 | 721.3 | 94.1 |
| Incongruent (ms) | 903.2 | 146.6 | 829.2 | 139.1 | 863.1 | 167.7 | 862.5 | 144.4 | 886.6 | 126.2 | 885.8 | 138.12 |
| Score | 1.45 | 0.14 | 1.35 | 0.12 | 1.43 | 0.18 | 1.39 | 0.18 | 1.41 | 0.19 | 1.40 | 0.14 |
| TMT | ||||||||||||
| A (s) | 41.9 | 16.6 | 31.6 | 8.2 | 36.6 | 9.4 | 31.4 | 7.7 | 42.0 | 12.3 | 31.5 | 10.6 |
| B (s) | 96.0 | 42.9 | 76.2 | 30.1 | 95.7 | 42.6 | 81.0 | 51.9 | 108.1 | 43.0 | 80.9 | 33.9 |
| Ratio | 54.1 | 32.8 | 44.7 | 24.2 | 59.1 | 38.7 | 49.6 | 48.7 | 66.1 | 40.1 | 46.5 | 35.0 |
Note: I-FRT = instability free-weight resistance training, S-MRT = stable machine-based resistance training, S-MRTHIP = stable machine-based adductor/abductor training; M = mean; SD = standard deviation. DSST = Digit Symbol Substitution Test; DMT = Digit Memory Test; Stroop = Stroop Colour-Word Test; TMT = Trail Making Test.
Falls efficacy scale
The fear of falling was overall reduced by 3–7%. However, there were no differences between groups.
Executive functions
Digit memory test
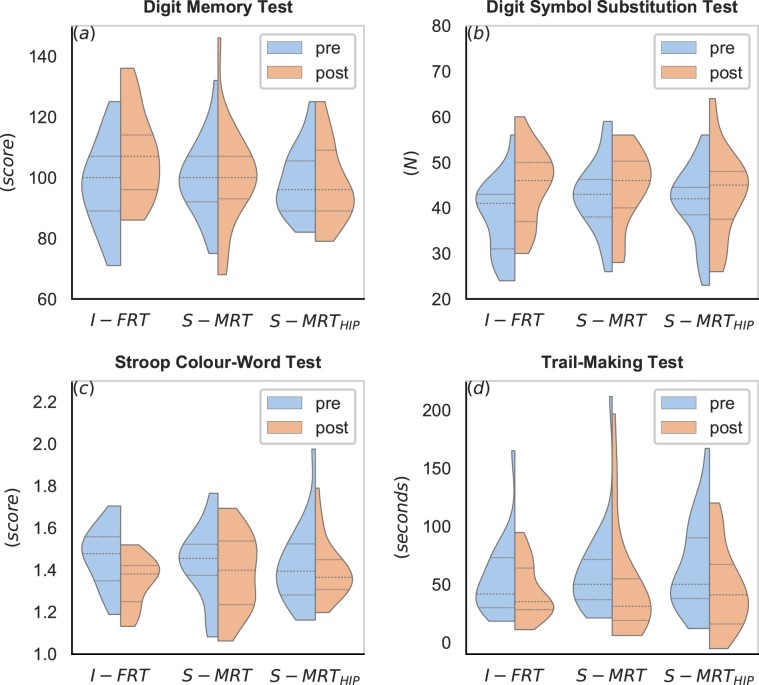
There was a significant effect between groups (d = 0.32). The planned contrasts revealed that the condition “unstable” improved working memory significantly by 11% compared to the “stable” condition (d = 0.32), which did not change at all (0%). See Fig. 1a and Table 3.
Figure 1.
Violinplots showing the results for the executive functions pre (blue) and post (orange) intervention. I-FRT = instability free-weight resistance training, S-MRT = stable machine-based resistance training, S-MRTHIP = stable machine-based adductor/abductor training. dashed line = median; dotted line = upper/lower quartile. The width of the plots is scaled to data distribution.
Table 3.
Statistical results. Note: I-FRT = instability free-weight resistance training, S-MRT = stable machine-based resistance training, S-MRTHIP = stable machine-based adductor/abductor training; FES-I = Fall Efficacy Scale. DSST = Digit Symbol Substitution Test; DMT = Digit Memory Test; Stroop = Stroop Colour-Word Test; TMT = Trail Making Test; TuT = Time under Tension; *no pre-post measures, thus only t-tests were calculated; BF = Bayes Factor; p ≤ 0.05.
| ANOVA | planned contrasts | 95%-CI (dunb) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | d | df | t | p | dunb | |||
| FES-I | 2,65 | 0.84 | 0.436 | 0.32 | ||||||
| Unstable vs. Stable | 65 | 0.92 | 0.364 | −0.24 | −0.75, 0.28 | |||||
| S-MRT vs. S-MRTHIP | 65 | 0.90 | 0.373 | 0.27 | −0.30, 0.85 | |||||
| DSST | 2,65 | 3.95 | 0.024 | 0.53 | ||||||
| Unstable vs. Stable | 65 | 2.81 | 0.007 | 0.73 | 0.21, 1.27 | |||||
| S-MRT vs. S-MRTHIP | 65 | 0.08 | 0.937 | −0.02 | −0.60, 0.55 | |||||
| DMT | 2,65 | 6.05 | 0.004 | 0.66 | ||||||
| Unstable vs. Stable | 65 | 3.48 | < 0.001 | 0.91 | 0.38, 1.45 | |||||
| S-MRT vs. S-MRTHIP | 65 | 0.09 | 0.928 | 0.02 | −0.55, 0.60 | |||||
| Stroop | 2,61 | 2.36 | 0.103 | 0.42 | ||||||
| Unstable vs. Stable | 61 | 2.09 | 0.041 | 0.55 | 0.01, 1.09 | |||||
| S-MRT vs. S-MRTHIP | 61 | 0.66 | 0.510 | −0.20 | −0.79, 0.40 | |||||
| TMT | 2,65 | 0.94 | 0.394 | 0.34 | ||||||
| Unstable vs. Stable | 43 | 0.68 | 0.502 | 0.17 | −0.34, 0.69 | |||||
| S-MRT vs. S-MRTHIP | 45 | 1.20 | 0.233 | 0.33 | −0.24, 0.91 | |||||
| t-tests | ||||||||||
| Load* | S-MRT vs. I-FRT | 43 | 15.24 | < 0.001 | 4.34 | 3.34, 5.46 | ||||
| S-MRT vs. S-MRTHIP | 45 | 9.19 | < 0.001 | 2.64 | 1.88, 3.48 | |||||
| I-FRT vs. S-MRTHIP | 42 | 9.86 | < 0.001 | 2.72 | 1.98, 3.52 | |||||
| TuT* | S-MRT vs. I-FRT | 43 | 2.98 | 0.006 | 1.11 | 0.32, 1.96 | ||||
| S-MRT vs. S-MRTHIP | 45 | 6.29 | < 0.001 | 2.31 | 3.35, 1.39 | |||||
| I-FRT vs. S-MRTHIP | 42 | 9.05 | < 0.001 | 3.27 | 4.50, 2.20 | |||||
Digit symbol substitution test
We found a medium effect between groups (d = 0.53). While I-FRT improved by 19%, the “stable” modality improved only by 4.5%. The effect is also supported by the planned contrast analysis, revealing a medium effect (d = 0.73) in favour of the “unstable” condition compared to the “stable” condition. See Fig. 1b and Table 3.
Stroop-colour-word test
There was no significant effect of the pre-post difference between groups (d = 0.42). However, we found a medium effect (d = 0.55), when comparing the “unstable” condition with the “stable” condition in favour of higher pre-post differences for I-FRT (8% for I-FRT and less than 3% for the stable groups) (see Fig. 1c and Table 3). Individual errors, like uttering a wrong colour, were rare (<5%) and evenly distributed across pre- and post-testing and groups. Therefore, errors were not used for any further analysis.
Trail making test
The analysis for the Trail Making Test remained non-significant, the ANOVA (d = 0.34) and the planned contrasts (d = 0.17) revealed only small effects, indicating no difference between groups (see Fig. 1d and Table 3).
Training intensity
Given the inter-individual differences of the participants, load and TuT were not normally distributed, therefore we used non-parametric statistics.
Load
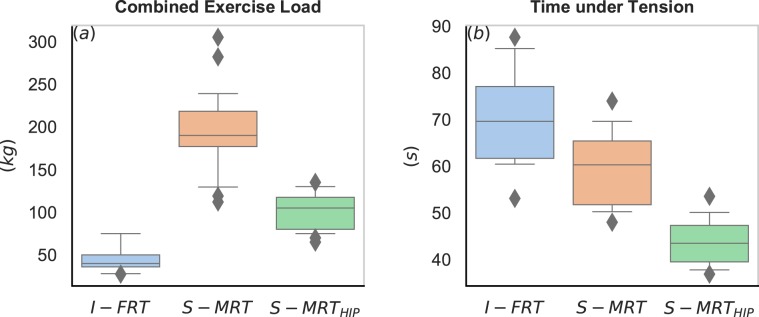
On average, I-FRT exercised in total with ~150 kg less than S-MRT and with ~56 kg less than S-MRTHIP, implicating that I-FRT exercised with considerably lower loads than the other groups. The numeric differences were reflected in the statistical analysis revealing very large effects (d ≥ 2.72) for higher loads of the stable modalities (see Fig. 2a and Table 3).
Figure 2.
Boxplots showing the maximal training load (a) and Time under Tension (b) during the last training phase. I-FRT = free-weight instability resistance training (blue), S-MRT = machine based stable resistance training (orange), S-MRTHIP = machine-based adductor/abductor training (green). The box shows the quartiles of the dataset while the whiskers extend to show the rest of the distribution. Outliers are plotted as individual diamond shaped points.
Time under Tension (TuT)
The t-tests revealed that I-FRT exhibited greater overall TuT in comparison to S-MRT (~13 s) and even greater TuT compared to S-MRTHIP (~26 s) per set across both main exercises (see Fig. 2b and Table 3).
Discussion
The goal of the present three-arm double-blinded RCT was to compare the effects of instability resistance training vs. stable resistance training on executive functions in older adults. Executive functions were assessed by performing four established tests: the Digit Memory Test, the Digit Symbol Substitution Test, a computerised Stroop-Colour-Word Test (Victoria Version) and the Trail Making Test. We first hypothesised that a multicomponent challenging exercise modality (i.e., instability resistance training) would exceed the effects of the less challenging stable machine-based resistance training in healthy older adults. In agreement with our first hypothesis we found meaningful improvements from pre- to post-testing for I-FRT compared to the stable resistance training groups in the Digit Memory Test, the Digit Symbol Substitution Test and the Stroop-Colour-Word Test. We detected similar improvements across all groups in the Trail Making Test, with no particular advantage for one group or modality. Our second hypothesis, that there would be no difference between stable training modalities was confirmed by the planned contrast analysis, indicating that the feature “unstable”, which imposes higher challenges, physically as well as mentally, might play the pivotal role in improving executive functions in healthy older adults. The overall outcome of our RCT suggests that free-weight instability resistance training is capable to improve executive functions in healthy older adults within 10-weeks. These findings extend previous study results of multicomponent exercise interventions on executive functions in older adults6,7.
Our previous research advocates the feasibility and effectiveness of instability resistance training17. Feasibility (high compliance and no training-related drop outs) was confirmed by this study. The novelty of the current RCT compared to our last RCT17 is the effect on executive functioning, given that no tests of executive functions were administered the last time. In contrast to previous investigations8,14 showing that exercise interventions affected only selective domains, our results suggest that instability free-weight resistance training appears to affect all tested executive functions. Indeed, we found medium effects for an improvement in working memory, processing speed and response inhibition for the I-FRT group compared to the stable modalities, while all groups demonstrated improvements over time for the Trail Making Test. In comparison, previous research (e.g. Forte and colleagues18) found pre-test to post-test improvements of 29% in the Trail Making Test after four weeks of training while no meaningful increase was reached after three months. They argued that these changes were probably due to a learning effect. Accordingly, such a learning effect should be present in all non-exercising control groups. However, looking thoroughly at control groups in other studies, conflicting results can be detected19–21. While Klusmann and collegues20 stated a decline in the Trail Making Test B/A by 10% after six months of training, Napoli et al.19 found no change at all (0.8–1.7%) after one year of exercise intervention. Contrary to these results, Vaughan and colleagues21 found improvements in the Trail Making Test B within the control group by 7%, although the effect was small (d = 0.25). Furthermore, a recent review6 reported meaningful improvements in Trail Making Tests when comparing exercise interventions with passive controls. We used a modified version of the Trail Making Test with a different arrangement of numbers and letters for post-testing, therefore, we cannot exclude the possibility that improvements are due to an easier post-test. Thus, given the inconclusive study results in the Trail Making Test, we follow the argument by Forte and colleagues18 assuming pre- to post-differences to originate from a learning effect.
It seems that that the combined demands of resistance training and balance training promote and accelerate beneficial effects of physical activity on executive functions. The participants in this study exercised for 10-weeks. The duration of most studies covered longer periods of time (more details in the review by de Asteasu et al. 2017)6. Only a few RCTs lasted three to four months with mixed results. For example, while Forte and colleagues18 found positive effects in executive functioning for the resistance training group, Kimura et al.22 and Barnes et al.23 found no positive impact on cognition for this type of training. Notably, a resistance training program conducted by Liu-Ambrose and colleagues8 promoted positive effects on cognition after 12 but not after 6 months of intervention. A recent study by Rogge and colleagues5 found meaningful improvements in memory and spatial cognition after a 12 week balance training. However, no effects on executive functions were found. This might be due to the fact that the tested population consisted of young and healthy adults possibly not yet facing age-related negative declines of executive functioning. Therefore, younger participants may not be as susceptible to improvements through physical activity as older adults are.
Cortical plasticity24,25 and neurogenesis26 were identified as potential mechanisms which may increase cognitive performance in consequence of balance training. Increased production of neurotrophic growth factors like insulin growth factor 1 (IGF-1), brain-derived neurotrophic factor (BDNF) and reduced levels of homocysteine27–29 are assumed to play a role in preventing cognitive decline through resistance training. Indeed, increased levels of IGF-1 and BDNF seem to promote neural growth and improved cognitive performance27,28. Opposed to increased levels of IGF-1 and BDNF, increased levels of homocysteine are associated with impaired cognitive performance29 due to its neurotoxicity30. In addition, resistance training results in positive cortical adaptations similar to those of aerobic training31. Aerobic training can induce structural changes in the brain volume of older adults, more specifically in grey matter (anterior cingulate cortex, supplementary motor areas, posterior middle frontal gyrus and left superior temporal lobe) and in white matter (anterior third of corpus callosum)32. However, Voelcker-Rehage and colleagues14 recently pointed out that aerobic and coordination training improve cognitive performance in older adults in different ways. They found unchanged activity patterns in the superior, middle and medial frontal cortex as well as the superior and middle temporal cortical areas as a result of coordination training. Still, cognitive performance increased overall as compared to a control group. The authors argued that coordination training economises cognitive processes and facilitates automatization. Thus less compensatory activation is needed to counteract degenerative aging effects especially as higher prefrontal activation seems to be associated with age-related structural and functional decline14. The increased activation in untrained older adults may indicate compensatory mechanisms of executive control to counteract neural processing impairments14,33. Therefore, lower brain activation levels in line with exercise related improvements in cognitive performance would indicate a more efficient processing and automatization. In addition, Niemann and colleagues34 pointed out that balance and coordination training engages the (pre)frontal and parietal cortex and the basal ganglia in a similar way which can be associated with early stages of motor learning. While performing a balance task, we are faced with constant, yet unique challenges and subsequent adaptive processes comparable to early motor learning stages. Thus, balance appears to trigger neuroplasticity on multiple levels, structurally by increasing grey and white matter and by optimising signal processing and freeing cognitive resources. Interestingly, we found that instability resistance training affected motor signal processing by reducing motor noise during challenging locomotion35. The uncontrolled manifold control analysis (UCM) was used to calculate multijoint-covariation related to the stabilization of a particular performance variable. The UCM analysis tests the extent to which all available degrees of freedom (DoF) that contribute to a task-relevant performance variable co-vary so as to stabilize, i.e., reduce the variance of, that performance variable. Within the UCM analysis, variability is partitioned into two components: “good” variance that has no effect on the performance variable and “bad” variance, that results in a variable performance. The unstable environment provided by an instability resistance training apparently stimulates exploration of motor solutions in a manner that appears to transfer to challenging locomotor tasks. A recent consensus paper pointed out the cerebellum’s role in movement and cognition, particularly the interaction of prefrontal structures and the function of the cerebellum in movement automatization, as well as mediating executive functions36. Given the effect of instability resistance training as a tool to reduce the motor noise through a better state estimation within the anticipatory control-loop35, the here reported improvements in executive functions, and the possible structural and functional connections, it appears obvious that instability resistance training benefits cognitive processes which in turn may positively affect motor control.
Without doubt, instability resistance training is challenging, both physically and mentally. The continuous combination of movements to counteract perturbations, challenges the vestibular system5, cognition, as well as neuromuscular activation37–39. In fact, this combination of modalities appears to facilitate and augment complementary effects of physical exercise training on executive functions in older adults. Physical exercise promotes the release of neurotrophic factors such as IGF-1 and BDNF and thus may prepare the central nervous system to process the cognitive demands of instability resistance training more efficiently and enduring40,41. This is in line with an argument by Moreau and Conway (2013) stating that programs which are characterised by complexity, novelty and variety would be the most efficient at promoting executive functions42.
The reasons why we did not detect any meaningful effect within both stable machine-based resistance training groups may be versatile. On the one hand, the duration of our RCT was substantially shorter when compared to other RCTs in which significant effects were found8. Therefore, we cannot exclude the possibility that longer intervention periods may, in fact, provide positive effects on executive functions through stable machine-based resistance training. On the other hand, our participants were cognitively healthy older adults based on the Mini-Mental-State-Examination and the Frontal Assessment Battery. Thus, the higher effort of the additional balance challenges and/or longer TuT of the instability resistance training group may have been necessary to elicit changes within three months of intervention in this cognitively healthy population.
A limitation that warrants discussion is that our results cannot be generalized to less healthy or frail older adults. Even though, frail older adults are capable to adapt to physical exercise similar to healthy older adults43. Following the argumentation of Diamond and Ling12, attention, reduction of stress, and loneliness can be driving forces in improved executive functions in older adults. Given the blinding of the instructors and assessors, it appears unlikely that one group received more attention than the others. However, we cannot rule out that instability resistance training might have affected stress-reduction more than stable resistance training. In addition, it might be possible that participants of the I-FRT group bonded more than participants of the stable RT groups over the more challenging RT and mastering this challenge. Nonetheless, further investigation is needed. In addition, we can only speculate about neuroplasticity since we did not use any functional imaging techniques in our study. Furthermore, improvements of I-FRT might only be due to longer TuT, given that this parameter was not controlled for. However, there were no differences between both stable groups, although TuT differed considerably. Therefore, we’d suggest that TuT is not a decisive factor for improvements of unstable resistance training, nonetheless, this issue needs further investigation.
In conclusion, given the importance of cognition and, more specifically, executive functions with aging, this RCT provided evidence about the feasibility and effectiveness of multicomponent instability resistance training in older adults after 10 weeks of progressive training. This extends existing findings on exercise interventions to promote executive function in older adults. High exercise loads do not seem to be mandatory to induce improvements in older adults neither on a physical level9,17 nor a cognitive level as was shown here. Free-weight instability resistance training possibly affects the central nervous system on different organisational levels, ranging from neuroplasticity to neurotrophins. From an applied point of view, this seems reasonable, given that biological systems are highly interlinked and interdependent. Thus, we suggest that free-weight instability resistance training may not only serve as an alternative to traditional machine-based resistance training. The effects on executive functions and the instability-dependent decrease of the absolute training load make it especially suitable for those individuals restricted by high strain, like older adults.
Methods
This investigation is part of the Kassel Fall Prevention Study II exploring the effects of three different resistance training modalities on locomotor control and proxies of strength and balance in older adults as stated within the trial registration. Due to the relevance of cognition in fall prevention research and the distinctive nature of the field of research, we decided to publish and discuss the results for the cognitive tasks separately. The effects on locomotor control and proxies of strength and balance are published and discussed elsewhere35. Sample size calculations where based on different outcome variables (see Eckardt & Rosenblatt (2019)35 trial registration).
Study design
The study is part of a registered three-arm, double-blinded (assessors as well as participants were blinded) RCT (ClinicalTrials.gov: NCT03017365 on 01/04/2017) examining the effects of primarily unstable vs. unstable resistance training on executive functions in older adults. Participants were naïve to the study hypothesis. The local ethics committee of the University of Kassel gave their approval (E052016058) and we complied with the relevant ethical standards of the latest Declaration of Helsinki (WMA, Oct. 2013). All participants provided written informed consent prior to enrolment.
Participants
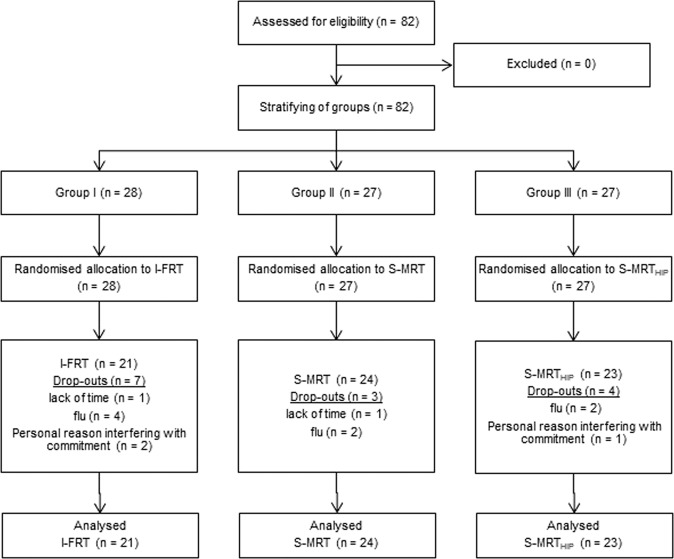
We recruited 82 participants (range: 65–80 years) via public advertisement in a local newspaper. Inclusion criteria were determined as the ability to walk independently without any walking aid and normal or corrected-to-normal vision. To account for possible cognitive and mental health conditions, participants were excluded based on pathological ratings of the Clock Drawing Test (CDT)44, the Mini-Mental-State-Examination (MMSE,<24 points)45, the Falls Efficacy Scale – International (FES-I, >24 points)46,47, the Geriatric Depression Scale (GDS, >9 points)48, the Freiburg Questionnaire of Physical Activity (FQoPA, <1 h)49 and the Frontal Assessment Battery (FAB-D, <13 points)50. Sixty-eight participants completed the trial. Figure 3 shows the CONSORT flow diagram and the number of participants in the treatment arms at each stage of the trial.
Figure 3.
CONSORT diagram with participant flow.
Randomisation
We stratified participants (1:1:1) into one of three groups based on age and sex. An independent assessor randomly assigned the groups to one of three training modalities: S-MRT, I-FRT, or S-MRTHIP. The randomisation sequence was generated using www.randomizer.org and concealed until groups were stratified.
Assessments
Data was collected in the biomechanics laboratory of the University of Kassel, Germany. Four participants were tested simultaneously by different assessors assigned to specific tests, respectively. The allocation of participants to the assessors was carried out randomly.
Global cognitive functioning was assessed using the Mini-Mental-State-Examination, a screening tool for mild cognitive impairment45. The Frontal Assessment Battery consists of six neuropsychological tasks evaluating cognitive and behavioural frontal lobe functions51. Physical activity was assessed using the Freiburg Questionnaire of Physical Activity49. Individual concerns about falling were evaluated using the Falls Efficacy Scale International46.
A recent systematic review identified processing speed, memory, set-shifting and response inhibition as key domains of executive functions related to the risk of falls52. Therefore, we choose to assess these key domains by using the following tests:
Executive function assessment
To test working memory, we used the Digit Memory Test53. This test consists of two parts, A: digit forwards and B: digit backwards. The assessor read out aloud random sequences of numbers (one per second), beginning with three digits and ending with nine digits. Participants had to repeat each sequence exactly in the given order of numbers. Part A consisted of repeating the numbers in a forward direction and Part B in a reversed backward direction. The total number of correct responses backwards and forwards were added up and converted into a standard score54 as an index for working memory. We used a different arrangement of digits for the post test (e.g.; pre: 296; post: 548). The test-retest reliability for our age groups (65–80 years) is considered from acceptable to good (ICC’s 0.71–0.88)53.
We administered the Digit Symbol Substitution Test55,56, which requires response speed, sustained attention, visual spatial skills and set shifting. Participants were asked to fill in a series of symbols correctly coded within 120 seconds. The first seven digit-symbol combinations where used as test-trial and neither measured nor included in the analysis. We arranged a different combination of digit-symbols for the post test. The test-retest reliability for the Digit Symbol Substitution Test is acceptable to excellent, ranging from 0.79 to 0.9756.
To assess selective attention and conflict resolution, we administered a computerised Stroop-Colour-Word Test (Victoria Version)57,58. The stimuli (24 per condition, 3 conditions) were presented in a random order on a computer screen. Participants were asked to verbally utter the presented colour as fast as possible. During condition I, coloured circles in green, blue, yellow, or red were shown. In condition II, neutral but coloured words (loud, above, hard or strong [in German]) were presented. Lastly, condition III consisted of incongruently coloured words (green, blue, yellow or red [in German]). We used E-Prime 2.0 (Psychology Software Tools, Inc., Sharpsburg, USA) to control stimulus presentation and record reaction times. Reaction times <250 ms and >3*interquartile range were dismissed. The ability to selectively attend and control response output was calculated as the time ratio (i.e., Stroop score) of colour-word interference and colour only tasks (condition III / condition I). If the participant’s voice did not trigger the microphone or the participant made a noise other than a response directed at naming the colour (i.e., vocalised pause: “um,” “uh”), the experimenter coded an error. Wrong responses were also coded as an error. The test-retest reliability for the Stroop-Colour-Word Test was previously reported to be good (ICC’s 0.71–0.79)57,59. It is noteworthy that the reliability is based on the use of response times59.
We used the paper & pencil version of the Trail Making Test (A + B)60,61 to assess set shifting and processing speed. To complete Part A, participants had to draw a line from 1 to 2, 2 to 3, 3 to 4, etc. Part B included additional letters, so that participants had to draw a line from 1 to A, A to 2, 2 to B, B to 3, etc., while the numbers were printed spatially distributed across the sheet. The time (in seconds) to complete the task was recorded with an ordinary stop watch. To quantify set shifting, we calculated the difference between Part B and Part A. The lower the scores the better the set shifting ability8. We used a different arrangement of letters and numbers for the post test. The test-retest reliability of the Trail Making Test across alternate versions was reported to be from acceptable to good (ICC’s 0.76–0.89)62.
Exercise intervention
All three groups began training one week after the baseline assessments were completed. Training was supervised by two skilled instructors at all times (participant-to-instructor ratio of 5:1). The instructors recorded attendance and compliance (percentage of the total classes attended) was calculated using the attendance records. All intervention groups trained for 10 weeks, twice per week on non-consecutive days for, at most, 60 min each. The 10-week intervention period consisted of a one-week introductory phase and three major training blocks lasting three weeks each. Training intensity was progressively and individually increased from block to block over the 10-week training programme by modulating load and sets for all groups and the level of instability for group I-FRT. After week one, four and seven the training load (weight) was increased following one repetition maximum (1-RM) testing using the prediction equation provided by Epley63 for each major exercise. The 1-RM was performed under stable conditions for every group.
S-MRT
This group executed a) squats at the Smith machine and worked out at the b) leg-press. Secondary exercise were core exercises.
I-FRT
The main exercises of this group were squats too, however they were conducted using free weights and instability devices. In addition, the participants of this groups conducted front-lunges on instability devices. The secondary exercises were core exercises incorporating instability devices.
S-MRTHIP
The focus of this training group were exercises targeting the thigh/hip adductors and abductors at resistance machines (see Fig. 4E). Secondary exercises were adduction and abduction exercises using elastic rubber straps. In addition, lateral core exercises were introduced.
Figure 4.
Photographs of the main exercises. (A) Squats using instability devices and dumbbells; (B) front lunges; (C) Squats at the Smith machine, placing the barbell at the hip, (D) Leg Press (E) thigh/hip adductor- and abductor resistance machine.
A detailed description of the training programme, machines, equipment and progression is outlined in Table 4 and in Eckardt & Rosenblatt (2019)35.
Table 4.
Detailed intervention program for all groups and phases. Note: I-FRT = free-weight instability resistance training; S-MRT = stable machine-based resistance training, S-MRTHIP = stable machine-based adductor/abductor training. bw = body weight; 1-RM = one repetition maximum; BOSU = BOth Sides Utilized; ROM = Range of Motion; ML = mediolateral.
| Intro-phase (1 week) | Block I (3 weeks) | Block II (3 weeks) | Block III (3 weeks) | |
|---|---|---|---|---|
| ~2 × 12 reps (with low weights) | 3 × 15 reps (50% of the 1-RM) | 3–4 × 15 reps (60% of the 1-RM) | 4 × 15 reps (60% of the 1-RM) | |
| I-FRT | ||||
| Cross-Trainer | 10 min | 10 min | 10 min | 10 min |
| Squats | 150° knee flex/ext angle on AIREX coordination rocker board round | 120° knee flex/ext angle on Thera-Band balance pads placed on AIREX coordination rocker board angled | 100° knee flex/ext angle on AIREX balance pad placed on AIREX coordination rocker board angled | 100° knee flex/ext angle on BOSU ball or Variosensa board |
| Front lunges | Thera-Band Balance Pads (front foot) | AIREX coordination rocker board round (front foot) and Thera-Band Balance Pads (rear foot) | AIREX balance pad (front foot) and Thera-Band Balance Pads (rear foot) | AIREX balance pad (front foot) and AIREX balance spinner soft (rear foot) |
| Core Exercise (Bridge Exercise) | No additional device | TOGU DYNAIR (under feet) | TOGU DYNAIR (under shoulder) & BOSU (under feet) | Swiss ball (under feet) |
| Walking with dumbbells | 2 min without dumbbells on terrasensa flats | 3 min with 5% of bw on terrasensa flats | 4 min with 10% of bw on terrasensa classics | 5 min with 15% of bw on terrasensa |
| Walking with dumbbells | 2 min without dumbbells | 3 min with 5% of BW | 4 min with 10% of BW | 5 min with 15% of BW |
| S-MRT | ||||
| Cross-Trainer | 10 min | 10 min | 10 min | 10 min |
| Smith-Machine | 150° knee flex/ext angle | 120° knee flex/ext angle | 100° knee flex/ext angle | 100° knee flex/ext angle |
| Leg-Press | 90° knee flex/ext angle | 90° knee flex/ext angle | 90° knee flex/ext angle | 90° knee flex/ext angle |
| Core Exercise | Bridge exercise (2 × 15 reps) | Bridge exercise (3 × 20 reps) | Crunches (4 × 20 reps) | Air Bike Crunches (4 × 20 reps) |
| Walking with dumbbells | 2 min without dumbbells | 3 min with 5% of BW | 4 min with 10% of BW | 5 min with 15% of BW |
| S-MRTHIP | ||||
| Cross-Trainer | 10 min | 10 min | 10 min | 10 min |
| Adductor | Habituation | Full ROM | Full ROM | Full ROM |
| Abductor | Habituation | Full ROM | Full ROM | Full ROM |
| Adductor Thera-Band | Habituation | Full ROM | Full ROM | Full ROM |
| Abductor Thera-Band | Habituation | Full ROM | Full ROM | Full ROM |
| Core Exercise | Side plank on knees | Side Crunches | Standing Oblique Crunch | Russian Sitting twist with dumbbell 5% bw |
| treadmill walking on robowalk | 2 min habituation | 3 min with ML pull above knee joint with 5% of BW | 4 min with ML pull at ankles with 5% of BW | 5 min with ML pull above knee joint and at ankles with 5% of BW, respectively |
Training intensity
The combined load (weight) for the two main exercises during the last training phase, transcribed from the participants’ training sheets. In addition, we assessed the mean Time under Tension (TuT) for the prescribed 15 repetitions.
Data analysis
Prior to the main statistical analysis, normal distribution was checked by visual inspection and tested with the Kolmogorov-Smirnov test for each dependent variable. In addition, Levene’s test for homogeneity of variance was conducted. Baseline differences were tested between groups with a one-way ANOVA or a Kruskal-Wallis test depending on data distribution and homogeneity. We calculated pre-post differences for all variables of interest. To test our hypothesis, we ran several one-way analyses of variance with planned contrasts between ‘unstable and stable’ to test our first hypothesis and planned contrasts between the two stable groups to test our second hypothesis.
In addition, differences in the absolute training intensity within the last training block were analysed. Therefore, the absolute training load, defined as the added weight of the two main exercises, for each group and the TuT of the main exercises were investigated. We used pre-planned independent two-sided t-tests (or non-parametrical alternatives) to investigate differences between groups. Ryan-Holm-Bonferroni64 corrected p-values for the t-tests are reported.
To improve readability, we calculated the effect size Cohen’s d for ANOVAs. Exploratory Software for Confidence Intervals was used for the calculation of Cohen’s dunb (an unbiased estimate of the population effect size δ), associated 95% confidence intervals (see Cumming 2012 for details)65 for planned contrasts and t-tests. Following Cohen (1988)66, d-values ≤ 0.49 indicate small effects, 0.50 ≤ d ≤ 0.79 indicate medium effects, and d ≥ 0.80 indicate large effects. However, given that we did not implement a passive control group, even small effect sizes can be regarded as meaningful. Alpha level was set at 5%. The effect size serves as a measure of how much the results deviate from the null hypothesis65–67. For the other tests we used SPSS version 26.0 (SPSS Inc., Chicago, IL, USA).
Supplementary information
Acknowledgements
The trial was supported by a grant of the Regional Government of Hessen (Germany). We thank Jan W. for helping to code the Stroop-test, Meagen L., Kai V., Ingo K., Elisabeth H. for the data acquisition and Jonas L., Yanneck N., Simon D., Heiko W. for carrying out the training programs. We like to thank Teresa Liu-Ambrose for her critical review and her comments on this manuscript. Further, we would like to thank sensa by Huebner Group for providing terrasensa plates and h/p/cosmos for providing the treadmill.
Author contributions
N.E. designed the study, analysed the data and wrote the manuscript; C.B. contributed to the study design, coded the Stroop experiment and critically reviewed the manuscript; A.K. contributed to the study design and data analysis and critically reviewed the manuscript. All authors approved the final versions of the manuscript.
Data availability
All data analysed during this study are included in this published article (and its Supplementary Information files).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59105-0.
References
- 1.Papegaaij S, Taube W, Hogenhout M, Baudry S, Hortobágyi T. Age-related decrease in motor cortical inhibition during standing under different sensory conditions. Front. Aging Neurosci. 2014;6:1–8. doi: 10.3389/fnagi.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidler R, et al. Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. Neuroimage. 2015;108:47–59. doi: 10.1016/j.neuroimage.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: A complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gates NJ, et al. Computerised cognitive training for maintaining cognitive function in cognitively healthy people in late life. Cochrane database Syst. Rev. 2019;3:CD012277. doi: 10.1002/14651858.CD012277.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogge A-K, et al. Balance training improves memory and spatial cognition in healthy adults. Sci. Rep. 2017;7:5661. doi: 10.1038/s41598-017-06071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Asteasu MLS, Martínez-Velilla N, Zambom-Ferraresi F, Casas-Herrero Á, Izquierdo M. Role of physical exercise on cognitive function in healthy older adults: A systematic review of randomized clinical trials. Ageing Res. Rev. 2017;37:117–134. doi: 10.1016/j.arr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Voelcker-Rehage C, Niemann C. Structural and functional brain changes related to different types of physical activity across the life span. Neurosci. Biobehav. Rev. 2013;37:2268–2295. doi: 10.1016/j.neubiorev.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Liu-Ambrose, T. et al. Resistance training and executive functions. Arch. Intern. Med. (2010). [DOI] [PMC free article] [PubMed]
- 9.Northey Joseph Michael, Cherbuin Nicolas, Pumpa Kate Louise, Smee Disa Jane, Rattray Ben. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. British Journal of Sports Medicine. 2017;52(3):154–160. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 10.Soga Keishi, Masaki Hiroaki, Gerber Markus, Ludyga Sebastian. Acute and Long-term Effects of Resistance Training on Executive Function. Journal of Cognitive Enhancement. 2018;2(2):200–207. doi: 10.1007/s41465-018-0079-y. [DOI] [Google Scholar]
- 11.Hillman, C. H., McAuley, E., Erickson, K. I., Liu-Ambrose, T. & Kramer, A. F. On mindful and mindless physical activity and executive function: A response to Diamond and Ling (2016). Dev. Cogn. Neurosci. 37, (2019). [DOI] [PMC free article] [PubMed]
- 12.Diamond A, Ling DS. Conclusions about interventions, programs, and approaches for improving executive functions that appear justified and those that, despite much hype, do not. Dev. Cogn. Neurosci. 2016;18:34–48. doi: 10.1016/j.dcn.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond A, Ling DS. Aerobic-Exercise and resistance-training interventions have been among the least effective ways to improve executive functions of any method tried thus far. Dev. Cogn. Neurosci. 2019;37:100572. doi: 10.1016/j.dcn.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voelcker-Rehage C, Godde B, Staudinger UM. Cardiovascular and coordination training differentially improve cognitive performance and neural processing in older adults. Front. Hum. Neurosci. 2011;5:26. doi: 10.3389/fnhum.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kibele A, Granacher U, Mühlbauer T, Behm DG. Stable, Unstable, and Metastable States of Equilibrium: Definitions and Applications to Human Movement. J. Sport. Sci. Med. 2015;14:885–887. [PMC free article] [PubMed] [Google Scholar]
- 16.Williams P, Lord SR. Effects of group exercise on cognitive functioning and mood in older women. Aust. N. Z. J. Public Health. 1997;21:45–52. doi: 10.1111/j.1467-842X.1997.tb01653.x. [DOI] [PubMed] [Google Scholar]
- 17.Eckardt N. Lower-extremity resistance training on unstable surfaces improves proxies of muscle strength, power and balance in healthy older adults: a randomised control trial. BMC Geriatr. 2016;16:191. doi: 10.1186/s12877-016-0366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forte R, et al. Enhancing cognitive functioning in the elderly: multicomponent vs resistance training. Clin. Interv. Aging. 2013;8:19–27. doi: 10.2147/CIA.S36514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Napoli N, et al. Effect of weight loss, exercise, or both on cognition and quality of life in obese older adults. Am. J. Clin. Nutr. 2014;100:189–198. doi: 10.3945/ajcn.113.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klusmann V, et al. Complex Mental and Physical Activity in Older Women and Cognitive Performance: A 6-month Randomized Controlled Trial. Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 2010;65A:680–688. doi: 10.1093/gerona/glq053. [DOI] [PubMed] [Google Scholar]
- 21.Vaughan S, et al. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: a randomised controlled trial. Age Ageing. 2014;43:623–629. doi: 10.1093/ageing/afu010. [DOI] [PubMed] [Google Scholar]
- 22.Kimura K, et al. The influence of short-term strength training on health-related quality of life and executive cognitive function. J. Physiol. Anthropol. 2010;29:95–101. doi: 10.2114/jpa2.29.95. [DOI] [PubMed] [Google Scholar]
- 23.Barnes DE, et al. The Mental Activity and eXercise (MAX) Trial. JAMA Intern. Med. 2013;173:797. doi: 10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taubert M, et al. Dynamic Properties of Human Brain Structure: Learning-Related Changes in Cortical Areas and Associated Fiber Connections. J. Neurosci. 2010;30:11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seidler RD, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34:721–33. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc. Natl. Acad. Sci. USA. 1990;87:5568–72. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/S0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 28.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn. Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Schafer JH, et al. Homocysteine and Cognitive Function in a Population-Based Study of Older Adults. J. Am. Geriatr. Soc. 2005;53:381–388. doi: 10.1111/j.1532-5415.2005.53153.x. [DOI] [PubMed] [Google Scholar]
- 30.Kruman II, et al. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J. Neurosci. 2000;20:6920–6. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleck SJ. Cardiovascular adaptations to resistance training. Medicine and science in sports and exercise. 1988;20:S146–S151. doi: 10.1249/00005768-198810001-00010. [DOI] [PubMed] [Google Scholar]
- 32.Colcombe SJ, et al. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A. Biol. Sci. Med. Sci. 2006;61:1166–70. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 33.Greenwood PM. Functional plasticity in cognitive aging: Review and hypothesis. Neuropsychology. 2007;21:657–673. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- 34.Niemann C, Godde B, Staudinger UM, Voelcker-Rehage C. Exercise-induced changes in basal ganglia volume and cognition in older adults. Neuroscience. 2014;281:147–163. doi: 10.1016/j.neuroscience.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Eckardt, N. & Rosenblatt, N. J. Instability resistance training decreases motor noise during challenging walking tasks in older adults: a 10-week double-blinded RCT. Front. Aging Neurosci. (2019). [DOI] [PMC free article] [PubMed]
- 36.Koziol LF, et al. Consensus paper: The cerebellum’s role in movement and cognition. Cerebellum. 2014;13:151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borreani S, et al. Exercise intensity progression for exercises performed on unstable and stable platforms based on ankle muscle activation. Gait Posture. 2014;39:404–409. doi: 10.1016/j.gaitpost.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Anderson K, Behm DG. Trunk muscle activity increases with unstable squat movements. Can. J. Appl. Physiol. 2005;30:33–45. doi: 10.1139/h05-103. [DOI] [PubMed] [Google Scholar]
- 39.Calatayud J, et al. Core muscle activity in a series of balance exercises with different stability conditions. Gait Posture. 2015;42:186–192. doi: 10.1016/j.gaitpost.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Kempermann G. The neurogenic reserve hypothesis: what is adult hippocampal neurogenesis good for? Trends Neurosci. 2008;31:163–169. doi: 10.1016/j.tins.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Hötting K, Röder B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013;37:2243–2257. doi: 10.1016/j.neubiorev.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Moreau D, Conway ARA. Cognitive enhancement: a comparative review of computerized and athletic training programs. Int. Rev. Sport Exerc. Psychol. 2013;6:155–183. doi: 10.1080/1750984X.2012.758763. [DOI] [Google Scholar]
- 43.Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin. Geriatr. Med. 2011;27:101–10. doi: 10.1016/j.cger.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nair AK, et al. Clock drawing test ratings by dementia specialists: interrater reliability and diagnostic accuracy. J. Neuropsychiatry Clin. Neurosci. 2010;22:85–92. doi: 10.1176/jnp.2010.22.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez MN, Charter RA, Mostafavi B, Nibut LP, Smith WE. Psychometric properties of the Folstein Mini-Mental State Examination. Assessment. 2005;12:137–44. doi: 10.1177/1073191105275412. [DOI] [PubMed] [Google Scholar]
- 46.Dias N, et al. Die Deutsche version der Falls Efficacy Scale-International Version (FES-I) Z. Gerontol. Geriatr. 2006;39:297–300. doi: 10.1007/s00391-006-0400-8. [DOI] [PubMed] [Google Scholar]
- 47.Kempen GIJM, et al. Cross-cultural validation of the Falls Efficacy Scale International (FES-I) in older people: results from Germany, the Netherlands and the UK were satisfactory. Disabil. Rehabil. 2007;29:155–62. doi: 10.1080/09638280600747637. [DOI] [PubMed] [Google Scholar]
- 48.Parmelee PA, Katz IR. Geriatric depression scale. J. Am. Geriatr. Soc. 1990;38:1379. doi: 10.1111/j.1532-5415.1990.tb03461.x. [DOI] [PubMed] [Google Scholar]
- 49.Frey I, Berg A. Physical activity counseling: Assessment of physical activity by questionnaire. Eur. J. Sport Sci. 2002;2:1–6. doi: 10.1080/17461390200072406. [DOI] [Google Scholar]
- 50.Benke T, Karner E, Delazer M. FAB-D: German version of the Frontal Assessment Battery. J. Neurol. 2013;260:2066–2072. doi: 10.1007/s00415-013-6929-8. [DOI] [PubMed] [Google Scholar]
- 51.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/WNL.55.11.1621. [DOI] [PubMed] [Google Scholar]
- 52.Hsu CL, Nagamatsu LS, Davis JC, Liu-Ambrose T. Examining the relationship between specific cognitive processes and falls risk in older adults: a systematic review. Osteoporos. Int. 2012;23:2409–24. doi: 10.1007/s00198-012-1992-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waters GS, Caplan D. The reliability and stability of verbal working memory measures. Behav. Res. Methods. Instrum. Comput. 2003;35:550–64. doi: 10.3758/BF03195534. [DOI] [PubMed] [Google Scholar]
- 54.Turner, M. & Ridsdale, J. The Digit Memory Test. Dyslexia Action (2004).
- 55.Wechsler, D. Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, TX NCS Pearson (2008).
- 56.Eileen De Monte V, Malke Geffen G, Kwapil K. Test-retest Reliability and Practice Effects of a Rapid Screen of Mild Traumatic Brain Injury. J. Clin. Exp. Neuropsychol. 2005;27:624–632. doi: 10.1080/13803390490918589. [DOI] [PubMed] [Google Scholar]
- 57.Troyer AK, Leach L, Strauss E. Aging and Response Inhibition: Normative Data for the Victoria Stroop Test. Aging, Neuropsychol. Cogn. 2006;13:20–35. doi: 10.1080/138255890968187. [DOI] [PubMed] [Google Scholar]
- 58.Penner IK, et al. The stroop task: Comparison between the original paradigm and computerized versions in children and adults. Clin. Neuropsychol. 2012;26:1142–1153. doi: 10.1080/13854046.2012.713513. [DOI] [PubMed] [Google Scholar]
- 59.Strauss GP, Allen DN, Jorgensen ML, Cramer SL. Test-retest reliability of standard and emotional stroop tasks: an investigation of color-word and picture-word versions. Assessment. 2005;12:330–7. doi: 10.1177/1073191105276375. [DOI] [PubMed] [Google Scholar]
- 60.Strauss, E., Sherman, E. M. S., Spreen, O. & Spreen, O. A compendium of neuropsychological tests: administration, norms, and commentary. (Oxford University Press, 2006).
- 61.Gaudino EA, Geisler MW, Squires NK. Construct validity in the trail making test: What makes part B harder? J. Clin. Exp. Neuropsychol. 1995;17:529–535. doi: 10.1080/01688639508405143. [DOI] [PubMed] [Google Scholar]
- 62.Wagner S, Helmreich I, Dahmen N, Lieb K, Tadi A. Reliability of three alternate forms of the trail making tests A and B. Arch. Clin. Neuropsychol. 2011;26:314–321. doi: 10.1093/arclin/acr024. [DOI] [PubMed] [Google Scholar]
- 63.Nafisi Moghadam R, Shajari A, Afkhami-Ardekani M. Influence of physiological factors on thyroid size determined by ultrasound. Acta Med. Iran. 2011;49:302–304. [PubMed] [Google Scholar]
- 64.Atkinson G. Analysis of repeated measurements in physical therapy research: multiple comparisons amongst level means and multi-factorial designs. Phys. Ther. Sport. 2002;3:191–203. doi: 10.1054/ptsp.2002.0123. [DOI] [Google Scholar]
- 65.Cumming, G. Understanding the new statistics: effect sizes, confidence intervals, and meta-analysis. (Routledge, 2012).
- 66.Cohen, J. Statistical Power Analysis for the Behavioral Sciences. (L. Erlbaum Associates, 1988).
- 67.Wetzels R, et al. Statistical Evidence in Experimental Psychology. Perspect. Psychol. Sci. 2011;6:291–298. doi: 10.1177/1745691611406923. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analysed during this study are included in this published article (and its Supplementary Information files).