Abstract
Rickettsia are obligate intracellular bacteria often associated with ticks and best known for causing human diseases (rickettsiosis), including typhus fever and sporadic cases of serious infection. In this study, we conducted a large survey of ticks in French Guiana to understand the overall diversity of Rickettsia in this remote area largely covered by dense rainforests. Out of 819 individuals (22 tick species in six genera), 252 (30.8%) samples were positive for Rickettsia infection. Multilocus typing and phylogenetic analysis identified 19 Rickettsia genotypes, but none was 100% identical to already known Rickettsia species or strains. Among these 19 genotypes, we identified two validated Rickettsia species, Rickettsia amblyommatis (spotted fever group) and Rickettsia bellii (bellii group), and characterized a novel and divergent Rickettsia phylogenetic group, the guiana group. While some tick hosts of these Rickettsia genotypes are among the most common ticks to bite humans in French Guiana, their potential pathogenicity remains entirely unknown. However, we found a strong association between Rickettsia genotypes and their host tick species, suggesting that most of these Rickettsia genotypes may be nonpathogenic forms maintained through transovarial transmission.
Subject terms: Infectious-disease epidemiology, Bacterial infection
Introduction
Members of the Rickettsia genus are obligate intracellular bacteria of eukaryotes1–3. The best known Rickettsia species are major human pathogens that include the etiological agents of the epidemic typhus, R. prowazekii, the Rocky Mountain spotted fever, R. rickettsii, and the flea-borne spotted fever, R. felis1,4,5. Most of these pathogenic Rickettsia species have a zoonotic life cycle and are transmitted by blood-feeding arthropods such as ticks, mites, lice and fleas, which commonly serve as ecological bridges for transmission from wildlife to humans and domestic animals1,4,5. However, members of the Rickettsia genus are actually more widespread than previously recognized: ecological surveys are uncovering substantial Rickettsia diversity associated with blood-feeding arthropods but also with non-blood-feeding arthropods, protozoa, algae and plants1–3.
There are currently more than 30 recognized Rickettsia species but the advent of multilocus sequence typing (MLST) and molecular phylogenetics has recently led to the description of several new putative species1,2,4,6,7. Historically, Rickettsia were classified into a few major groups based on serological characteristics, but subsequent DNA sequencing led to classification of at least 10 distinct phylogenetic groups1,2,6. Three Rickettsia groups – the spotted fever group, the typhus group and the transitional group – are the subject of intensive study since they all include major pathogenic species and are commonly found in blood-feeding arthropods1. In addition, another Rickettsia group widely found in ticks but also in many other arthropods, the bellii group, is also commonly studied. This group is thought to be basal to the three other major Rickettsia groups and is largely composed of nonpathogenic Rickettsia species and strains1–3,7.
Despite considerable research effort, the diversity of Rickettsia remains largely unknown in most remote geographic regions and in undersampled arthropod taxa. Interestingly, a recent survey reported the presence of 22 tick species in French Guiana8, a vast equatorial land located on the northeast coast of South America and mostly covered by dense rainforests including old-growth forests, which are biodiversity hotspots. French Guiana’s human population (ca. 250,000 inhabitants) is concentrated principally in a handful of towns spread along the coastline and main rivers, while the interior is largely uninhabited. We know little on the presence, diversity and effect of Rickettsia on ticks in this region. Only one Rickettsia species of the spotted fever group, Candidatus Rickettsia wissemanii, has been documented in bat soft ticks Ornithodoros hasei caught in French Guiana9. Some of the tick species of French Guiana are also present in adjacent countries such as Brazil, but the presence of Rickettsia has been investigated there in a few of these species including the Cayenne tick Amblyomma cajennense and the arboreal tick A. longirostre7,10–14.
In this study, we conducted a wide molecular survey of Rickettsia in ticks in French Guiana. This survey included 819 field specimens belonging to 22 tick species of the 33 known from French Guiana8. We further used MLST, including gltA, coxA, atpA, ompB and 16S rRNA gene sequences, and phylogenetics for the description of these Rickettsia infections. Lastly, we examined and discussed their genetic proximity with known Rickettsia species and strains.
Results
Detection of Rickettsia
Using total tick DNA extracts, we applied a high-throughput 16S rDNA sequencing approach to characterize the whole bacterial diversity in each tick specimen and then to detect the presence of Rickettsia. We assayed for the presence of Rickettsia in 819 individual ticks collected in French Guiana and belonging to six genera and 22 species: Amblyomma (16 species, 686 specimens), Rhipicephalus (2 species, 16 specimens), Ixodes (1 species, 6 specimens), Dermacentor (1 species, 97 specimens), Haemaphysalis (1 species, 8 specimens) and Ornithodoros (1 species, 6 specimens) (Fig. 1 and Table 1).
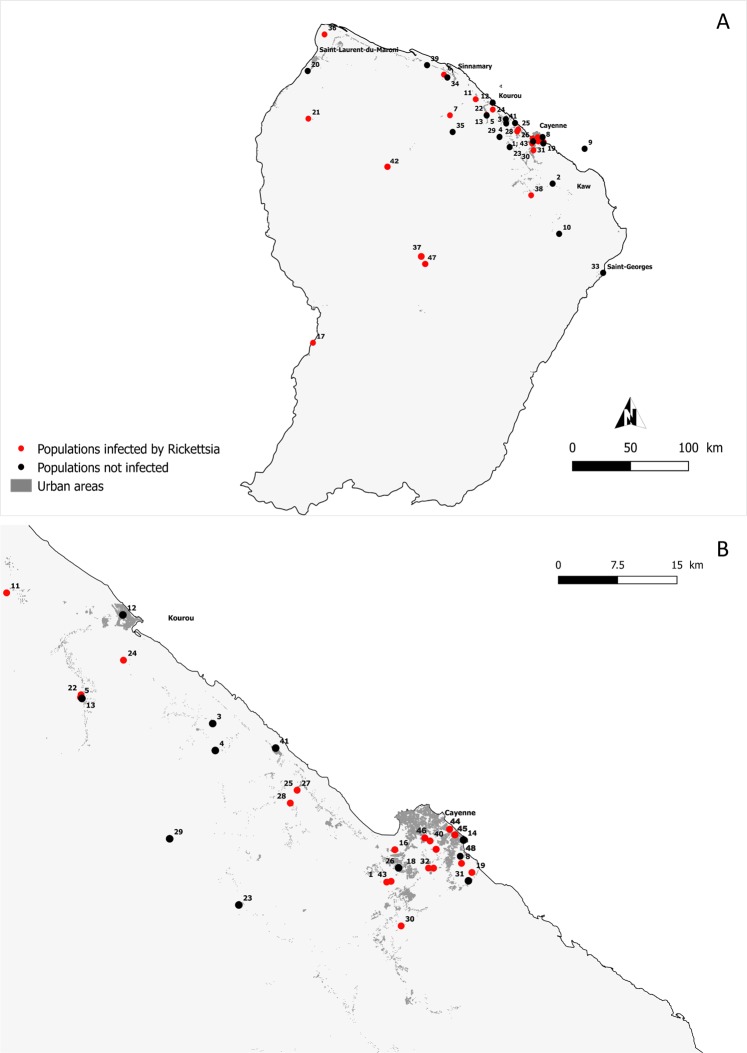
Figure 1.
Location of sampling sites in French Guiana. Localities are represented by dots and numbers correspond to the sampling locality number given in Table S1. (B) is the magnification of the area bounded by the translucid grey rectangle in (A). Red and black dots indicate sampling localities where Rickettsia was detected or not, respectively.
Table 1.
List of tick species and sampling localities included in the analysis, with details on the sample size (n), and the prevalence of Rickettsia.
| Ticks Species | Locality (# on Fig. 1) | Questing/Feeding ticks | n examined | n Rickettsia-positive - % | ||
|---|---|---|---|---|---|---|
| Ixodidae (hard ticks): | ||||||
| 1- | Amblyomma cajennense sensu stricto (Fabricius, 1787) | 7 localities (#1,2,6,7,11,13,43) | Questing | 351 | 88 | 25% |
| 2- | A. calcaratum Neumann, 1899 | 1 locality (#22) | Feeding | 1 | 0 | 0% |
| 3- | A. coelebs Neumann, 1899 | 4 localities (#2,6,7,34) | Questing | 14 | 4 | 29% |
| 4- | A. dissimile Koch, 1884 | 5 localities (#1,17,36,44,45) | Feeding | 24 | 16 | 67% |
| 5- | A. geayi Neumann, 1899 | 5 localities (#8,19,21,32,38) | Feeding | 16 | 10 | 62% |
| 6- | A. goeldii Neumann, 1899 | 1 locality (#38) | Feeding | 5 | 4 | 80% |
| 7- | A. humerale Koch, 1844 | 4 localities (#13,15,42,47) | Feeding | 10 | 5 | 50% |
| 8- | A. latepunctatum Tonelli-Rondelli, 1939 | 2 localities (#7,23,33) | Questing | 4 | 4 | 100% |
| 9- | A. longirostre (Koch, 1844) | 14 localities (#8,19–31) | Feeding | 130 | 106 | 82% |
| 10- | A. naponense (Packard, 1869) | 3 localities (#2,33,42) | Questing and Feeding | 5 | 1 | 20% |
| 11- | A. oblongoguttatum Koch, 1844 | 3 localities (#6,7,11) | Questing and Feeding | 95 | 0 | 0% |
| 12- | A. pacae Aragão, 1911 | 2 localities (#11,31) | Questing and Feeding | 7 | 0 | 0% |
| 13- | A. romitii Tonelli-Rondelli, 1939 | 1 locality (#41) | Feeding | 2 | 0 | 0% |
| 14- | A. rotundatum Koch, 1844 | 2 localities (#5,39) | Questing and Feeding | 6 | 0 | 0% |
| 15- | A. scalpturatum Neumann, 1906 | 5 localities (#5,10,11,35,37) | Questing and Feeding | 8 | 0 | 0% |
| 16- | A. varium Koch, 1844 | 5 localities (#1,16,32,43,44) | Questing and Feeding | 8 | 5 | 63% |
| 17- | Dermacentor nitens Neumann, 1897 | 1 locality (#4) | Feeding | 97 | 0 | 0% |
| 18- | Haemaphysalis juxtakochi Cooley, 1946 | 3 localities (#5,7,11) | Feeding | 8 | 3 | 38% |
| 19- | Ixodes luciae Senevet, 1940 | 3 localities (#18,40,46) | Feeding | 6 | 6 | 100% |
| 20- | Rhipicephalus microplus (Canestrini, 1888) | 1 locality (#3) | Questing and Feeding | 10 | 0 | 0% |
| 21- | R. sanguineus sensu lato (Latreille, 1806) | 2 localities (#12,14) | Feeding | 6 | 0 | 0% |
| Argasidae (soft ticks): | ||||||
| 22- | Ornithodoros capensis sensu stricto Neumann, 1901 | 1 locality (#9) | Feeding | 6 | 0 | 0% |
| Total | 819 | 252 | 31% | |||
Of the 819 specimens, 252 (30.8%) exhibited Rickettsia 16S rDNA reads. The 567 remaining specimens (69.2%) were devoid of any of Rickettsia 16S rDNA reads but had satisfactory DNA template quality, as shown by the positive amplification of other bacterial 16S rDNA reads commonly detected in ticks (including Coxiella- and Francisella-like endosymbionts15–18). Of the 22 tick species examined, 10 species (6/16 Amblyomma species, 1/1 of Dermacentor, 2/2 of Rhipicephalus and 1/1 of Ornithodoros) were not infected by Rickettsia (Table 1 and S1). The 12 other tick species (10/16 Amblyomma species, 1/1 Haemaphysalis and 1/1 Ixodes) were Rickettsia-positive for at least one of the examined specimens (Fig. 1, Tables 1 and S1). The detection rate of Rickettsia did not co-vary with the screening effort, i.e., the number of examined specimens per tick species (Spearman’s rank correlation, n = 22, rs = 0.20, p = 0.37): the tick species observed with Rickettsia infections were not those for which we examined more specimens. This is best exemplified by (1) Rickettsia-positive tick species for which we examined few specimens, such as I luciae (n = 6 examined specimens and all were Rickettsia-positive) and A. goeldii (n = 5 examined specimens and 4 were Rickettsia-positive), and (2) Rickettsia-negative tick species for which we examined a large number of specimens, such as A. oblongoguttatum (n = 95 examined specimens but none positive) and D. nitens (n = 97 examined specimens but none positive) (Fig. 1, Tables 1 and S1).
The prevalence of Rickettsia differed substantially between the 12 infected tick species (Fisher’s exact test, p = 2.10−16): while prevalence is low to moderate in some tick species (e.g., 4 infected specimens of the 14 examined in A. coelebs; 29%), it is significantly higher in other species (e.g., 106 infected specimens of the 130 examined in A. longirostre; 82%) (Tables 1 and S1). Prevalence of Rickettsia varied significantly between sampling localities of two tick species, A. cajennense (7 localities, with prevalence ranging from 0 to 48%; Fisher’s exact test, p = 3.10−5) and A. longirostre (14 localities, with prevalence ranging from 0 to 100%; Fisher’s exact test, p = 4.10−6) (Table S1). However, prevalence of Rickettsia did not vary significantly between sampling localities of the nine other tick species for which distinct geographic populations were examined (Fisher’s exact tests, all p > 0.05) (Table S1). Furthermore, there was no obvious difference in prevalence of Rickettsia between localities from peri-urban, agricultural and urban areas (Kruskal-Wallis test, H = 0.68, df = 3, p = 0.08).
Diversity of Rickettsia
The diversity of Rickettsia in French Guiana ticks was examined using sequences from one to five genetic markers (gltA, 16S rRNA, atpA, ompB and coxA). Overall, the examination of this multilocus data set led to the identification of 19 distinct Rickettsia genotypes (FG019a–FG019s hereafter; Table 2), as detailed below.
Table 2.
Sequence profiles of the five genes in the 19 Rickettsia genotypes (FG019a–FG019s) identified in this study.
| Ticks Species | Rickettsia MLST genotypes | Genes | n | ||||
|---|---|---|---|---|---|---|---|
| gltA | 16S rDNA | atpA | ompB | coxA | |||
| Ixodidae (hard ticks): | |||||||
| Amblyomma cajennense sensu stricto (Fabricius, 1787) | FG019a | a | a | a | a | a | 13 |
| A. coelebs Neumann, 1899 | FG019b | c | b | b | c | b | 3 |
| A. dissimile Koch, 1884 | FG019c | d | c | c | d | c | 2 |
| FG019d | e | c | c | d | _ | 1 | |
| FG019e | f | d | d | e | _ | 1 | |
| FG019f | g | c | c | d | c | 2 | |
| A. geayi Neumann, 1899 | FG019c | d | c | c | d | c | 1 |
| A. goeldii Neumann, 1899 | FG019g | d | f | e | f | d | 1 |
| FG019h | h | e | f | g | e | 1 | |
| A. humerale Koch, 1844 | FG019i | d | c | e | i | d | 2 |
| FG019j | i | g | g | h | _ | 1 | |
| A. latepunctatum Tonelli-Rondelli, 1939 | FG019c | d | c | c | d | c | 1 |
| FG019k | i | g | g | j | f | 1 | |
| FG019l | j | e | a | e | g | 1 | |
| A. longirostre (Koch, 1844) | FG019m | b | a | a | b | a | 4 |
| A. naponense (Packard, 1869) | FG019n | k | h | h | _ | _ | 1 |
| A. varium Koch, 1844 | FG019o | d | k | i | f | d | 1 |
| FG019p | l | i | j | e | h | 2 | |
| Haemaphysalis juxtakochi Cooley, 1946 | FG019q | d | c | e | _ | _ | 1 |
| FG019r | i | c | g | _ | _ | 1 | |
| Ixodes luciae Senevet, 1940 | FG019s | g | k | e | f | d | 3 |
Letters a–k represent the different alleles at each gene locus. Dash indicates an absence of PCR product. n, number of specimens for each Rickettsia genotype (on the basis of multilocus typing of 44 representative tick samples).
First, Rickettsia sequences from the gltA gene were taken from a subsample of 92 infected specimens from the 12 infected species (one to 47 specimens per infected species were examined; Table 2). On the basis of DNA sequencing, 12 distinct gltA genotypes with 84.9–99.8% pairwise nucleotide identity were characterized from the 92 specimens examined. Six tick species of the 12 infected species harbored each only one gltA genotype: A. cajennense, A. coelebs, A. longirostre, A. geayi, A. naponense and I. luciae. In each of the six other tick species, two to four distinct gltA genotypes were found. We characterized four gltA genotypes from the seven sequenced A. dissimile specimens (Table 2).
Second, we amplified four additional bacterial markers (16S rRNA, atpA, ompB and coxA) from 44 representative tick samples infected by the 12 Rickettsia gltA genotypes (Table 2). We then obtained 10 genotypes of 16S rRNA (97.7–99.9% pairwise nucleotide identity), 10 atpA genotypes (85.4–99.8%), 10 ompB genotypes (78.1–99.8%) and eight coxA genotypes (86.1–99.8%). While the 16S rRNA and atpA gene fragments were amplified from the 44 samples, the ompB and coxA were only amplified from 41 and 38 samples, respectively (Table 2). The diversity at the 16S rRNA, atpA, ompB and coxA gene fragments was consistent with the results inferred from the gltA sequences: Rickettsia infections with distinct gltA sequences have distinct sequences at the other gene markers. No 16S rRNA, atpA, ompB and coxA sequence variation was observed within tick species in which only one gltA genotype was detected (i.e., A. cajennense, A. coelebs, A. longirostre, A. geayi, A. naponense and I. luciae). However, the combined use of these five markers allowed the distinction of additional Rickettsia genetic variation not detected with the single gltA gene sequences. Indeed, while one of the Rickettsia infections of A. dissimile and one of A. humerale shared the same gltA sequence (the gltA sequence type #d in Table 2), their atpA, ompB and coxA (but not 16S rRNA) gene sequences were different, showing that they were thus two distinct Rickettsia genotypes. The examination of gltA, 16S rRNA, atpA, ompB and coxA gene sequences thus led to the identification of 19 Rickettsia genotypes (FG019a–FG019s; Table 2). Only one of these Rickettsia genotypes, FG019c, was shared by several tick species (A. dissimile, A. geayi and A. latepunctatum). Each of the 18 other Rickettsia genotypes (FG019a, FG019b and FG019d–FG019s) was found in only one tick species (Table 2).
Phylogeny of Rickettsia
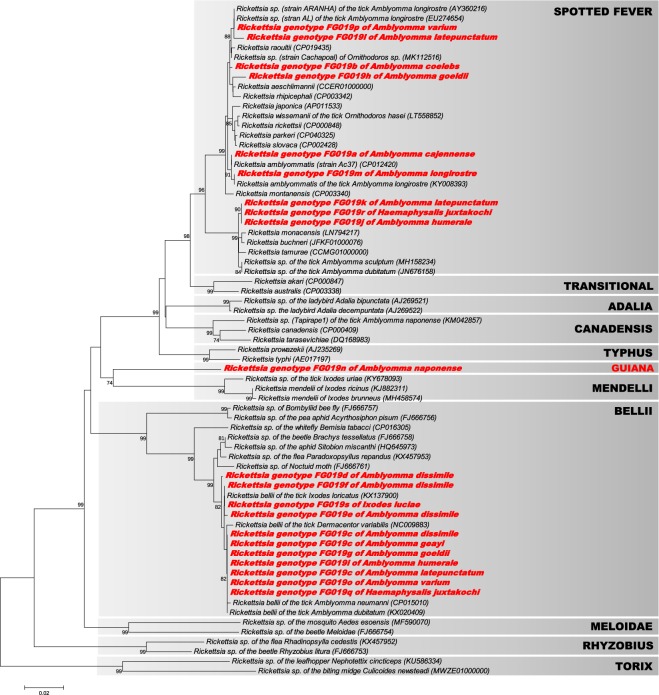
The phylogenetic relationships between the Rickettsia infections were first estimated using the 92 gltA sequences from the 12 infected tick species found in this study, as well as gltA sequences from representative Rickettsia species and strains available in GenBank (Fig. 2). The closest relatives of the Rickettsia found in French Guiana were also included in the analyses. No recombination events were detected for the gltA data set using both the RDP and GENCONV methods (all p > 0.23). The ML phylogenetic analysis based on the gltA sequences showed that the 19 Rickettsia genotypes (FG019a–FG019s) found in this study consisted of three distinct groups (Fig. 2):
Nine Rickettsia genotypes clustered with known members of the spotted fever group, including R. amblyommatis (Rickettsia FG019a and FG019m of A. cajennense and A. longirostre, respectively), R. tamurae, R. buchneri and R. monacensis (FG019j, FG019k and FG019r of A. humerale, A. latepunctatum and H. juxtakochi, respectively), R. aeschlimannii and R. rhipicephali (FG019h of A. goeldii) and Rickettsia sp. strains AL (FG019l and FG019p of A. latepunctatum and A. varium, respectively). The Rickettsia FG019b of A. coelebs also belonged to the spotted fever group within a cluster including R. raoultii, R. aeschlimannii and R. rhipicephali.
Nine other Rickettsia genotypes clustered with known members of the R. bellii group previously found in other tick species. These genotypes included FG019c (of A. dissimile, A. geayi and A. latepunctatum), FG019d (A. dissimile), FG019e (A. dissimile), FG019f (A. dissimile), FG019g (A. goeldii), FG019i (A. humerale), FG019o (A. varium), FG019q (H. juxtakochi) and FG019s (I. luciae).
The last Rickettsia genotype, FG019n of A. naponense, was genetically distant to all other known Rickettsia species and groups. It showed a low level of gltA nucleotide identity (<88%) with all other Rickettsia species and groups. This Rickettsia genotype was the single member of a novel and highly divergent group, here provisionally called the guiana group (Fig. 2). It is noteworthy that this strain is also distantly related to the Rickettsia sp. clone Tapirape1 (canadensis group; Fig. 2), which was previously found in A. naponense from Brazil19.
Figure 2.
Phylogeny of Rickettsia constructed using maximum-likelihood (ML) estimations based on gltA gene sequences (589 unambiguously aligned nucleotide sites; best-fit approximation for the evolutionary model: GTR + G + I). Sequences from Rickettsia characterized in this study are shown in red. Only one gltA sequence per Rickettsia genotype and per tick species is shown. Sequences from representative Rickettsia groups, species and strains available in GenBank were also added to the analysis. The grey boxes delineate the different Rickettsia groups (their names are indicated in upper case), including the novel guiana group described in this study. Bacterial name, host species and GenBank accession numbers are shown on the tree. Branch numbers indicate percentage bootstrap support for major branches (1000 replicates; only bootstrap values >70% are shown). The scale bar is in units of substitution/site.
However, none of the Rickettsia genotypes found in this study is closely related to the single species already reported from French Guiana, Candidatus Rickettsia wissemanii (Fig. 2).
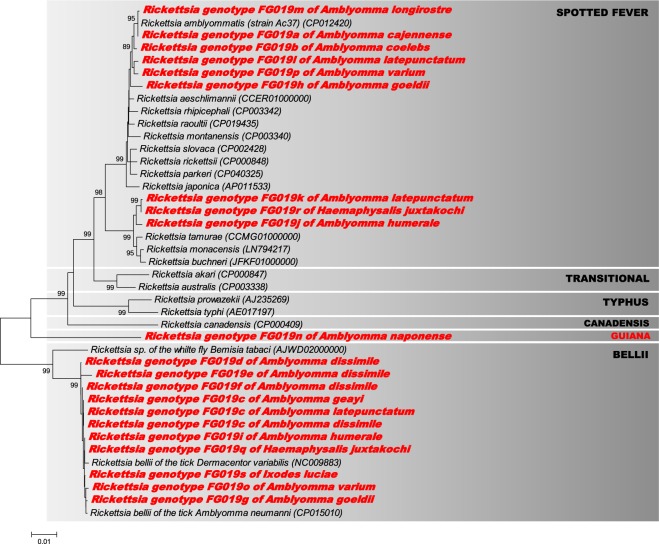
A second analysis was performed to refine the intrageneric phylogeny of Rickettsia. For this, we used the Rickettsia 16S rRNA, atpA, ompB and coxA sequences from the 19 Rickettsia genotypes identified in the present work, as well as sequences of representative Rickettsia species and strains available in GenBank. The analysis of single and concatenated gene sequences did not detect significant recombination events in the data set using both RDP and GENCONV methods (all p > 0.08). When the sequences were examined separately for each gene, we obtained the same phylogenetic pattern as observed with the ML analysis based on gltA gene sequences with the partitioning of the 19 Rickettsia genotypes into the same three different groups (i.e., spotted fever, bellii and guiana) (Figs. S1–S4). Indeed, the examination of the16S rRNA and atpA gene sequences of Rickettsia FG019n genotype of A. naponense (neither ompB nor coxA could be amplified from this Rickettsia strain; see Table 2) corroborated the existence of the guiana group: the 16S rRNA, atpA and gltA single-gene phylogenies (Figs. 2, S1 and S2) and the gltA, 16S rRNA and atpA concatenated phylogeny (Fig. 3) showed that the FG019n genotype is highly divergent from all other known Rickettsia groups, species and strains.
Figure 3.
Phylogeny of Rickettsia constructed using maximum-likelihood (ML) estimations based on concatenated 16S rDNA, gltA and atpA sequences (1886 unambiguously aligned nucleotide sites; best-fit approximation for the evolutionary model: GTR + G + I). Sequences from Rickettsia characterized in this study are shown in red. Only one 16S rDNA, gltA and atpA concatenated sequence per Rickettsia genotype and per tick species is shown. Sequences from representative Rickettsia groups, species and strains available in GenBank were also added to the analysis. The grey boxes delineate the different Rickettsia groups (their names are indicated in upper case), including the novel guiana group described in this study. Bacterial name, host species and GenBank accession numbers are shown on the tree. Branch numbers indicate percentage bootstrap support for major branches (1000 replicates; only bootstrap values >70% are shown). The scale bar is in units of substitution/site.
Analyses of a multilocus data set (based on the 16S rRNA, gltA and atpA genes) further showed that the nine Rickettsia genotypes (FG019c–g, FG019i, FG019o, FG019q and FG019s) belonging to the bellii group always clustered together with the R. bellii strains previously found in other American tick species, such as A. neumanni (Argentina) and D. variabilis (USA) (Figs. 3 and S1–4). These nine Rickettsia genotypes can therefore be considered as members of the R. bellii species. None of these nine Rickettsia genotypes were 100% identical to already known R. bellii members.
The multilocus data set also showed that the nine Rickettsia genotypes (FG019a, FG019b, FG019h, FG019j–m, FG019p and FG019r) belonging the spotted fever group can be split into two subgroups:
The first subgroup included the Rickettsia FG019j (A. humerale), FG019k (A. latepunctatum) and FG019r (H. juxtakochi), which clustered with R. tamurae, R. buchneri and R. monacensis although remaining substantially divergent from them at each gene marker (Figs. 2, 3 and S1–4).
The second subgroup included the Rickettsia FG019a (A. cajennense), FG019m (A. longirostre), FG019b (A. coelebs), FG019h (A. goeldii), FG019l (A. latepunctatum) and FG019p (A. varium), which all clustered with R. amblyommatis on the basis of multilocus analyses (Fig. 3). However, only FG019a and FG019m consistently clustered with R. amblyommatis at each gene marker (Figs. 2 and S1–4), showing that these two genotypes can be considered as members of the R. amblyommatis species. None of these two Rickettsia genotypes were 100% identical to already known R. amblyommatis members, however. The four other Rickettsia genotypes (FG019b, FG019h, FG019l and FG019p) cannot be classified into specific species due to a lack of consensus between the phylogenetic trees (Figs. 2, 3 and S1–4). Indeed, while the 16S rDNA genotype of FG019b is more closely related to R. amblyommatis (Fig. S1), its atpA genotype is more closely related to R. montanensis (Fig. S2). Although these last four Rickettsia genotypes may each represent a novel species, additional gene sequencing is required to determine their precise phylogenetic proximity with other members of the spotted fever group.
Discussion
We found here that Rickettsia infections are common in French Guiana ticks, a pattern also observed among ticks of other South American regions7,10–13,20. The incidence of infection varied between tick species of French Guiana: 12 of the 22 tick species examined, including Amblyomma, Haemaphysalis and Ixodes species, harbored Rickettsia, and when present, prevalence ranged from 10 to 100%, with significant variations between sampling localities of some species. We further found that these Rickettsia infections are remarkably diverse. Multilocus strain typing revealed the presence of 19 Rickettsia genotypes of different phylogenetic origins. Of these 19 genotypes, 18 were found each in only one tick species. Remarkably, more than one Rickettsia genotype was found within half of the infected tick species, meaning that this intraspecific variation of infection is common in tick species of French Guiana. The single Rickettsia species known from this region, Candidatus Rickettsia wissemanii9, was not detected in the present study. Altogether, this means that at least 20 different Rickettsia genotypes are circulating in ticks in French Guiana.
None of the 19 Rickettsia genotypes we identified in French Guiana had been documented before this study. Multilocus typing showed that 11 of these Rickettsia genotypes can be assigned to two validated Rickettsia species, namely R. amblyommatis (two genotypes) and R. bellii (nine genotypes). These two Rickettsia species are widely present among Central and South American ticks, each infecting more than 10 species7,11–13,21–26. Their presence in French Guiana was therefore expected, but the observation of novel genotypes indicates the presence of important geographic variability: R. amblyommatis and R. bellii have probably radiated within their respective regions, including French Guiana, into different genotypes. Overall, this confirms that R. amblyommatis and R. bellii have the widest host range and the broadest geographic distribution among all Rickettsia species reported from South America, as suggested in early studies7,25. Besides the R. amblyommatis and R. bellii genotypes, the eight other Rickettsia genotypes are rarer, since they are apparently endemic to French Guiana and cannot be assigned to formerly validated species. While a few genotypes remain unclassified within the spotted fever group, we described one novel Rickettsia genotype, which belongs to a novel and divergent group, the guiana group. It is noteworthy that the guiana group has an intermediate phylogenetic position between the spotted fever and bellii Rickettsia groups, since it is more related on the basis of its gltA sequence to the rare species R. mendelii, which was found only in Europe27,28.
Most of human pathogenic Rickettsia species are vectored by hard ticks4,29. This leads to the question of the pathogenicity of the 19 Rickettsia genotypes we found in French Guiana and the associated health risk. Since none of the 19 Rickettsia genotypes was previously described before this study, no evidence of their pathogenicity currently exists, even for those belonging to validated Rickettsia species. Indeed, although R. amblyommatis and R. bellii are commonly found in the ticks of French Guiana, and more broadly in American ticks7,11–13,21–26, they have never been found in vertebrate hosts, suggesting that they are nonpathogenicspecies. Interestingly, while the Cayenne tick A. cajennense is one of the most common ticks found in French Guiana, blood-feeding on many different hosts, including humans8,30,31, R. amblyommatis (infecting here 25% of the A. cajennense specimens examined) were never detected in humans or animals: while French Guiana is an outermost region of the European Union, with technical and financial resources that close to European countries, no case was notified to date. Another intriguing point is the apparent specificity of Rickettsia genotypes to tick species: 18 out of 19 Rickettsia genotypes were detected in only a single tick species. Even generalist tick species, such as A. cajennense and A. dissimile, feeding on (and sharing) a variety of vertebrate hosts8,30,31, did not share the same Rickettsia genotypes. These observations may indicate that at least some of the Rickettsia genotypes in French Guiana are present in ticks but not in vertebrate hosts.
The persistence means of the 19 Rickettsia genotypes remain unknown in French Guiana. As pointed out in a recent study32, the current view in rickettsiology has a strong anthropocentric bias and tends to describe all novel Rickettsia species as pathogenic forms. However, most of the novel Rickettsia species or strains discovered in recent years are also found exclusively in arthropods and never in vertebrates1–3,33,34. In ticks, as for many other arthropods, some Rickettsia are maternally inherited endosymbionts with poorly known effects on tick biology. This is the case for R. buchneri in the black-legged tick I. scapularis35, R. peacockii in the American dog tick D. variabilis36, and R. vini in the tree-hole tick I. arboricola16,37. These nonpathogenic Rickettsia may interact with a variety of tick-borne pathogens34, including Anaplasma marginale38, Borrelia burgdorferi39 and also other Rickettsia40,41. Indeed, the endosymbiont R. peacockii may possibly hamper the multiplication of the spotted fever agent, R. rickettsii40, and may also block transovarial transmission colonization of R. rickettsii, R. montana and R. rhipicephali40,41. In French Guiana, further studies are needed to test this hypothesis of endosymbiosis by observing transstadial and transovarial transmission in ticks.
To conclude, this study revealed substantial diversity of Rickettsia, including novel genotypes, specie and group, in ticks in French Guiana. This underlines the need to better document Rickettsia diversity in diverse regions, and more especially in remote regions. A recent meta-analysis suggests that more than 20% of terrestrial arthropods may be infected by Rickettsia, with ticks hosting most of this bacterial diversity29, as observed in this study. In many arthropods other than ticks, Rickettsia are nonpathogenic, undergo exclusive maternal transmission to offspring, and may function as both a mutualist and reproductive manipulator2,3,42,43. Overall, adaptations of Rickettsia to this diversity of hosts encompass an array of parasitic, but also mutualistic, interactions1–3. In French Guiana, the effect of the19 Rickettsia genotypes on human and animal health as well as on tick physiology and reproduction remains to be elucidated.
Materials and Methods
Tick collection
A collection of 819 specimens from 22 tick species, collected in 38 sample sites of French Guiana in 2016 and 2017, was used (Tables 1 and S1). Questing ticks were collected from the vegetation using a drag-flag method over sites covering three types of ecological conditions (periurban, agricultural and natural). Ticks were also directly collected in nests or on hosts (including humans, four domestic animal species and wild animal species; see Table S1). All ticks were stored in 75% ethanol until examination. For each tick specimen, species were formally identified through morphological examination (using dichotomous keys30,44) and DNA sequencing in a previous study8.
Detection of Rickettsia
To avoid external bacterial contaminants, ticks were processed with commercial bleach diluted at 1% for 30 s and then rinsed for 1 min in three successive baths of DNA-free water following a published protocol45. For each tick specimen, total DNA was further extracted from whole body using a genomic DNA extraction kit according to the manufacturer’s instructions (DNeasy Blood & Tissue extraction kit, Qiagen). The presence of Rickettsia within each DNA template was investigated through high-throughput 16S rDNA sequencing. To this aim, a 251-bp portion of the V4 variable region of the bacterial 16S rDNA was amplified from whole-body DNA samples using the universal forward and reverse primers listed in Table S4. Each PCR product from individual samples was tagged with a unique 35-base barcode using the Nextera Index Kit (Illumina, San Diego, CA, USA). PCR amplifications were performed in duplicates for each sample. PCR reactions were conducted using a Multiplex PCR Kit (Qiagen). Amplified bacterial 16S rDNA products were purified and sequenced on an Illumina MiSeq platform (GenSeq, Montpellier University) and 250-bp end sequence reads were obtained. All bioinformatic analyses were conducted using the pipeline Frogs (https://github.com/geraldinepascal/FROGS) as follows46: primers were removed from paired-end sequences with Cutadapt47, and these sequences were merged into contigs with FLASH48 before filtering by length (251 bp ± 10 bp). Chimaeras were removed with VSEARCH49, then sequences were clustered using SWARM50. We obtained an average number of 29,206 bacterial 16S rDNA reads per tick specimen. Sequences with 97% similarity were clustered together and identified as an operational taxonomic unit (OTU). Each representative OTU sequence was aligned and taxonomically assigned using the Silva database (https://www.arb-silva.de/). To eliminate the possibility of contamination, we included four mock DNA extractions under identical conditions using water, buffers and kits utilized for the experimental samples followed by Illumina Miseq analysis of 16S rDNA reads. The negative controls provided only a handful of reads that did not correspond to the bacterial genera found in the tick samples.
Molecular typing of Rickettsia
A random subset of DNA templates for which Rickettsia reads were obtained through high-throughput 16S rDNA sequencing were used for Rickettsia multilocus typing. These Rickettsia infections were genotyped using independent PCR assays based on gltA, coxA, ompB, atpA and 16S rRNA, using semi-nested or nested PCR assays (Table S2). To prevent possible contamination, different parts of this process were physically separated from one another, in entirely separate rooms. All amplicons were also sequenced to control for false-positive amplifications. Gene features, primers and PCR conditions are detailed in Table S2.
Seminested and nested PCR amplifications were performed as follows: the first PCR run with the external primers was performed in a 10-μL volume containing approximately 20 ng of genomic DNA, 3 mM of each dNTP (Thermo Scientific), 8 mM of MgCl2 (Roche Diagnostics), 3 μM of each primer, 1 μL of 10× PCR buffer (Roche Diagnostics) and 0.5 U of Taq DNA polymerase (Roche Diagnostics). A 1-μL aliquot of the PCR product from the first reaction was then used as a template for the second round of amplification. The second PCR was performed in a total volume of 25 μL and contained 8 mM of each dNTP (Thermo Scientific), 10 mM of MgCl2 (ThermoScientific), 7.5 μM of each of the internal primers, 2.5 μL of 10× PCR buffer (Thermo Scientific) and 1.25 U of Taq DNA polymerase (Thermo Scientific). All PCR amplifications were performed under the following conditions: initial denaturation at 93 °C for 3 min, 35 cycles of denaturation (93 °C, 30 s), annealing (Tm = 52–56 °C, depending on primers, 30 s), extension (72 °C, 1 min), and a final extension at 72 °C for 5 min. Known positive and negative individuals were used as controls in each PCR assay. All PCR products were visualized through electrophoresis in a 1.5% agarose gel. Positive PCR products were purified and sequenced in both directions (EUROFINS). The chromatograms were manually inspected and cleaned with CHROMAS LITE (http://www.technelysium.com.au/chromas_lite.html) and sequence alignments were done using CLUSTALW51, both implemented in MEGA7. Genotype naming (ie,) was based on the following rationale: the genotype FG019a means French Guiana 2019 genotype a. Novel nucleotide sequences were deposited in the GenBank nucleotide database (Accession numbers: gltA, MT009163-MT009163; 16S rRNA, MT006105-MT006125; coxA, MT009148-MT009162; ompB, MT009184-MT009201; atpA, MT009127-MT009147).
Molecular phylogenetics
The GBLOCKS52 program with default parameters was used to remove poorly aligned positions and to obtain unambiguous sequence alignments. All sequence alignments were also checked for putative recombinant regions using the RDP3 computer analysis package53. Given a set of aligned nucleotide sequences, RDP3 can rapidly analyze these with a range of powerful nonparametric recombination detection methods, including the GENECON54 and RDP55. Phylogenetic relationships were evaluated between Rickettsia strains using gltA, coxA, ompB, atpA and 16S rRNA gene sequences. The evolutionary models most closely fitting the sequence data were determined using Akaike information criterion with the MEGA7 program56. Phylogenetic analyses were based on maximum likelihood (ML) analyses. A ML heuristic search, using a starting tree obtained by neighbor-joining, was conducted, and clade robustness was further assessed by bootstrap analysis using 1000 replicates in MEGA756.
Ethics approval
The use of the genetic resources was declared to the French Ministry of the Environment under reference TREL19028117S/156 and #150401230100, in compliance with the Access and Benefit Sharing procedure implemented by the Loi pour la Reconquête de la Biodiversité. The capture of ticks in the Grand Connétable protected area was authorized by the Prefecture of French Guiana by prefectoral decree R03-2016-09-23-003. All animals were handled in strict accordance with good animal practices as defined by the French code of practice for the care and use of animals for scientific purposes, established by articles R214-87 to R214-137 of the French rural code.
Supplementary information
Acknowledgements
Financial support was provided by the French government’s Programmes Investissement d’Avenir (Laboratoire d’Excellence CEBA, the MicroBIOMES Strategic project 2016–2018 and the MiTick Annual project 2016). F.B. benefits from a PhD fellowship financed by the CEBA Laboratoire d’Excellence and University of Montpellier. We are grateful to Mathis Petit and Cécile Richard-Hansen from the Office National de la Chasse et de la Faune Sauvage (ONCFS) and Tanguy Deville for their precious help in tick sampling. We wish to thank all members of LabEx CEBA who provided tick specimens, in particular Benoît de Thoisy, Philippe Gaucher, François Catzeflis, Frederic Delsuc and Denis Blanchet. We are grateful to Stéphane Garnier and Rolland Ruffine, to the Groupe d'Étude et de Protection des Oiseaux en Guyane (GEPOG) and to members of Institut Pasteur de Guyane, in particular to Isabelle Dufour, Stanislas Talaga and Agathe Chavy, for support in the field. We also thank Xavier Baudrimont from the Direction de l’Alimentation, de l’Agriculture et de la Forêt de Guyane (DAAF) and to breeders for having allowed us to collect ticks on their cattle and horses. We also acknowledge useful discussions with members of the Tiques et Maladies à Tiques (TMT) French group.
Author contributions
F.B. and O.D. designed the study and wrote the manuscript. F.B., M.B. and O.D. collected the samples. F.B., M.B. and R.B. performed the molecular typing. F.B. and O.D. performed the phylogenetic analyses. All authors agreed to the final version of the manuscript.
Data availability
Nucleotide sequences of Rickettsia were deposited in the GenBank nucleotide database (Accession numbers: 16S rRNA: [MT006105-MT006125]; gltA: [MT009163-MT009183]; ompB: [MT009184-MT009201]; atpA: [MT009127-MT009147]; coxA: [MT009148-MT0091462]).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-59488-0.
References
- 1.Weinert, L. A. The diversity and phylogeny of Rickettsia. Parasite Diversity and Diversification: Evolutionary Ecology Meets Phylogenetics (2015)
- 2.Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM. Evolution and diversity of Rickettsia bacteria. BMC Biol. 2009;7:6. doi: 10.1186/1741-7007-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perlman SJ, Hunter MS, Zchori-Fein E. The emerging diversity of Rickettsia. Proc. Biol. Sci. 2006;273:2097–2106. doi: 10.1098/rspb.2006.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997;10:694–719. doi: 10.1128/CMR.10.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parola P, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin. Microbiol. Rev. 2013;26:657–702. doi: 10.1128/CMR.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labruna MB. Ecology of Rickettsia in South America. Ann. N. Y. Acad. Sci. 2009;1166:156–166. doi: 10.1111/j.1749-6632.2009.04516.x. [DOI] [PubMed] [Google Scholar]
- 8.Binetruy F, Chevillon C, de Thoisy B, Garnier S, Duron O. Survey of ticks in French Guiana. Ticks and Tick-borne. Dis. 2019;10:77–85. doi: 10.1016/j.ttbdis.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Tahir D, et al. New Rickettsia species in soft ticks Ornithodoros hasei collected from bats in French Guiana. Ticks Tick-borne Dis. 2016;7:1089–1096. doi: 10.1016/j.ttbdis.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Guedes E, Leite RC, Pacheco RC, Silveira I, Labruna MB. Rickettsia species infecting Amblyomma ticks from an area endemic for Brazilian spotted fever in Brazil. Rev. Bras. Parasitol. Vet. 2011;20:308–311. doi: 10.1590/S1984-29612011000400009. [DOI] [PubMed] [Google Scholar]
- 11.Amoêdo-Lima M, et al. Ticks and tick-associated spotted fever group Rickettsia from birds in the Southwestern Brazilian Amazon. Rev. Colombiana de. Cienc. Pecuarias. 2018;31:26–35. doi: 10.17533/udea.rccp.v31n1a04. [DOI] [Google Scholar]
- 12.Ogrzewalska M, Literak I, Martins TF, Labruna MB. Rickettsial infections in ticks from wild birds in Paraguay. Ticks Tick. Borne Dis. 2014;5:83–89. doi: 10.1016/j.ttbdis.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 13.McIntosh D, et al. Detection of Rickettsia bellii and Rickettsia amblyommii in Amblyomma longirostre (Acari: Ixodidae) from Bahia state, Northeast Brazil. Braz. J. Microbiol. 2015;46:879–883. doi: 10.1590/S1517-838246320140623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogrzewalska M, Uezu A, Labruna MB. Ticks (Acari: Ixodidae) infesting wild birds in the eastern Amazon, northern Brazil, with notes on rickettsial infection in ticks. Parasitol. Res. 2010;106:809–816. doi: 10.1007/s00436-010-1733-1. [DOI] [PubMed] [Google Scholar]
- 15.Lalzar I, Friedmann Y, Gottlieb Y. Tissue tropism and vertical transmission of Coxiella in Rhipicephalus sanguineus and Rhipicephalus turanicus ticks. Environ. Microbiol. 2014;16:3657–3668. doi: 10.1111/1462-2920.12455. [DOI] [PubMed] [Google Scholar]
- 16.Duron O, et al. Evolutionary changes in symbiont community structure in ticks. Mol. Ecol. 2017;26:2905–2921. doi: 10.1111/mec.14094. [DOI] [PubMed] [Google Scholar]
- 17.Duron O, et al. Tick-bacteria mutualism depends on b vitamin synthesis pathways. Curr. Biol. 2018;28:1896–1902.e5. doi: 10.1016/j.cub.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 18.Clayton KA, Gall CA, Mason KL, Scoles GA, Brayton KA. The characterization and manipulation of the bacterial microbiome of the Rocky Mountain wood tick, Dermacentor andersoni. Parasit. Vectors. 2015;8:632. doi: 10.1186/s13071-015-1245-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soares HS, et al. Ticks and rickettsial infection in the wildlife of two regions of the Brazilian Amazon. Exp. Appl. Acarol. 2015;65:125–140. doi: 10.1007/s10493-014-9851-6. [DOI] [PubMed] [Google Scholar]
- 20.Ogrzewalska M, Literak I, Cardenas-Callirgos JM, Capek M, Labruna MB. Rickettsia bellii in ticks Amblyomma varium Koch, 1844, from birds in Peru. Ticks Tick. Borne Dis. 2012;3:254–256. doi: 10.1016/j.ttbdis.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Bermúdez CSE, Troyo A. A review of the genus Rickettsia in Central America. Res. Rep. Trop. Med. 2018;9:103–112. doi: 10.2147/RRTM.S160951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopes, M. G. et al. Ticks and rickettsiae from wildlife in Belize, Central America. Parasit Vectors9 (2016). [DOI] [PMC free article] [PubMed]
- 23.Polsomboon S, et al. Molecular detection and identification of Rickettsia species in ticks (acari: ixodidae) collected from Belize, Central America. J. Med. Entomol. 2017;54:1718–1726. doi: 10.1093/jme/tjx141. [DOI] [PubMed] [Google Scholar]
- 24.Muñoz-Leal S, Marcili A, Fuentes-Castillo D, Ayala M, Labruna MB. A relapsing fever Borrelia and spotted fever Rickettsia in ticks from an Andean valley, central Chile. Exp. Appl. Acarol. 2019;78:403–420. doi: 10.1007/s10493-019-00389-x. [DOI] [PubMed] [Google Scholar]
- 25.Krawczak FS, Labruna MB, Hecht JA, Paddock CD, Karpathy SE. Genotypic characterization of Rickettsia bellii reveals distinct lineages in the United States and South America. Biomed. Res. Int. 2018;2018:8505483. doi: 10.1155/2018/8505483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogrzewalska M, Pacheco RC, Uezu A, Ferreira F, Labruna MB. Ticks (Acari: Ixodidae) infesting wild birds in an Atlantic forest area in the state of São Paulo, Brazil, with isolation of Rickettsia from the tick Amblyomma longirostre. J. Med. Entomol. 2008;45:770–774. doi: 10.1093/jmedent/45.4.770. [DOI] [PubMed] [Google Scholar]
- 27.Hajduskova E, et al. ‘Candidatus Rickettsia mendelii’, a novel basal group rickettsia detected in Ixodes ricinus ticks in the Czech Republic. Ticks Tick. Borne Dis. 2016;7:482–486. doi: 10.1016/j.ttbdis.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Stańczak J, Biernat B, Racewicz M, Zalewska M, Matyjasek A. Prevalence of different Rickettsia spp. in Ixodes ricinus and Dermacentor reticulatus ticks (Acari: Ixodidae) in north-eastern Poland. Ticks Tick. Borne Dis. 2018;9:427–434. doi: 10.1016/j.ttbdis.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ. The incidence of bacterial endosymbionts in terrestrial arthropods. Proc. Biol. Sci. 2015;282:20150249. doi: 10.1098/rspb.2015.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Floch H, Fauran P. Ixodides de la Guyane et des Antilles Françaises. Publ. Inst. Pasteur Guyane Fr. Inini. 1958;19:1–94. [PubMed] [Google Scholar]
- 31.Nava, S., Venzal, J. M., Acuña, D. G., Martins, T. F. & Guglielmone, A. A. Ticks of the Southern Cone of America: Diagnosis, Distribution, and Hosts with Taxonomy, Ecology and Sanitary Importance. (Academic Press, 2017).
- 32.Labruna MB, Walker DH. Rickettsia felis and changing paradigms about pathogenic rickettsiae. Emerg. Infect. Dis. 2014;20:1768–1769. doi: 10.3201/eid2010.131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darby AC, Cho N-H, Fuxelius H-H, Westberg J, Andersson SGE. Intracellular pathogens go extreme: genome evolution in the rickettsiales. Trends genetics: TIG. 2007;23:511–520. doi: 10.1016/j.tig.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Bonnet, S. I., Binetruy, F., Hernández-Jarguín, A. M. & Duron, O. The tick microbiome: why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front Cell Infect Microbiol7 (2017). [DOI] [PMC free article] [PubMed]
- 35.Kurtti TJ, et al. Rickettsia buchneri sp. nov., a rickettsial endosymbiont of the blacklegged tick Ixodes scapularis. Int. J. Syst. Evol. Microbiol. 2015;65:965–970. doi: 10.1099/ijs.0.000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Felsheim RF, Kurtti TJ, Munderloh UG. Genome sequence of the endosymbiont Rickettsia peacockii and comparison with virulent Rickettsia rickettsii: Identification of virulence factors. PLOS ONE. 2009;4:e8361. doi: 10.1371/journal.pone.0008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novakova, M., Costa, F. B., Krause, F., Literak, I. & Labruna, M. B. Rickettsia vini n. sp. (Rickettsiaceae) infecting the tick Ixodes arboricola (Acari: Ixodidae). Parasit Vectors9 (2016). [DOI] [PMC free article] [PubMed]
- 38.Gall CA, et al. The bacterial microbiome of Dermacentor andersoni ticks influences pathogen susceptibility. ISME J. 2016;10:1846–1855. doi: 10.1038/ismej.2015.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steiner FE, et al. Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. J. Med. Entomol. 2008;45:289–297. doi: 10.1093/jmedent/45.2.289. [DOI] [PubMed] [Google Scholar]
- 40.Burgdorfer, W., Hayes, S. F & Mavros, A. J. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii. Rickettsiae and rickettsial diseases (1980).
- 41.Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- 42.Behar A, McCormick LJ, Perlman SJ. Rickettsia felis infection in a common household insect pest, Liposcelis bostrychophila (Psocoptera: Liposcelidae) Appl. Environ. Microbiol. 2010;76:2280–2285. doi: 10.1128/AEM.00026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gottlieb Y, et al. Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae) Appl. Environ. Microbiol. 2006;72:3646–3652. doi: 10.1128/AEM.72.5.3646-3652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones, E. K., Clifford, C. M., Keirans, J. E. & Kohls, G. M. Ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma in the Western Hemisphere. Brigham Young University Science Bulletin, Biological Series 17 (1972).
- 45.Binetruy F, Dupraz M, Buysse M, Duron O. Surface sterilization methods impact measures of internal microbial diversity in ticks. Parasites Vectors. 2019;12:268. doi: 10.1186/s13071-019-3517-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escudié F, et al. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinforma. 2018;34:1287–1294. doi: 10.1093/bioinformatics/btx791. [DOI] [PubMed] [Google Scholar]
- 47.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 48.Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinforma. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rognes T, Flouri T, Nichols B, Quince C, Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahé F, Rognes T, Quince C, de Vargas C, Dunthorn M. Swarm: robust and fast clustering method for amplicon-based studies. PeerJ. 2014;2:e593. doi: 10.7717/peerj.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson, J. D., Gibson, T. J. & Higgins, D. G. Multiple sequence alignment using ClustalW and ClustalX. Curr Protoc Bioinformatics Chapter 2, Unit 2.3 (2002). [DOI] [PubMed]
- 52.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 53.Martin DP, et al. RDP3: a flexible and fast computer program for analyzing recombination. Bioinforma. 2010;26:2462–2463. doi: 10.1093/bioinformatics/btq467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sawyer S. Statistical tests for detecting gene conversion. Mol. Biol. Evol. 1989;6:526–538. doi: 10.1093/oxfordjournals.molbev.a040567. [DOI] [PubMed] [Google Scholar]
- 55.Martin D, Rybicki E. RDP: detection of recombination amongst aligned sequences. Bioinforma. 2000;16:562–563. doi: 10.1093/bioinformatics/16.6.562. [DOI] [PubMed] [Google Scholar]
- 56.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Nucleotide sequences of Rickettsia were deposited in the GenBank nucleotide database (Accession numbers: 16S rRNA: [MT006105-MT006125]; gltA: [MT009163-MT009183]; ompB: [MT009184-MT009201]; atpA: [MT009127-MT009147]; coxA: [MT009148-MT0091462]).