We thank the editors for the opportunity to respond to a recent letter by Habel and Buist regarding our study on cancer outcomes in patients who did not undergo locoregional treatment after diagnosis with ductal carcinoma in situ (DCIS).
To substantiate their claim of “artificially low” ipsilateral invasive breast cancer (iIBC) estimates, Habel and Buist compare our findings against estimates from the NSABP-17 trial, which compared lumpectomy against lumpectomy with radiation (1). We find this comparison problematic. First, NSABP-17 (1985–1990) predated the reporting period of our study (1992–2014) and recurrence rates have decreased substantially over this time span (2). Breast cancer screening as well as the diagnosis and treatment of DCIS have changed markedly in the last three decades, so that DCIS patients who are diagnosed in most contemporary clinics are likely at much lower risk than those diagnosed in the 1980s.
Moreover, a direct comparison of crude iIBC risks between two studies is meaningful only if the competing mortality risks are similar. Indeed, the cumulative incidence of iIBC directly depends on the magnitude of the competing risk of all-cause mortality, and because the latter was much higher in our study (24% after 10 years) compared with NSABP-17 (9% after 10 years), a direct comparison of crude iIBC risks is not appropriate. In view of these issues, we believe that it is more meaningful to compare our findings against more contemporary cohorts and to use net risk (Kaplan-Meier) estimates instead of competing risk estimates. For example, a large, prospective, single-institution cohort estimated a 10-year net risk of iIBC among women ages 40 years and older who underwent guideline-concordant care to be 6.5% (no confidence interval given) (3). This is roughly one-half the 10-year net iIBC risk we found in our SEER-based no-surgery cohort of 12.1% (95% confidence interval = 10.0% to 14.7%).
Another major concern raised was that of systematic underreporting of iIBC events in SEER prior to 2007, at which time new multiple primary coding rules were introduced (4). To assess their claim, we stratified the net risk estimates of iIBC by years of diagnosis of 1992–1999, 2000–2007, and 2008–2014 (Figure 1). We found no difference in net risk between the three groups (P = .74, log-rank test), supporting that there was no systematic underreporting before 2007.
Figure 1.
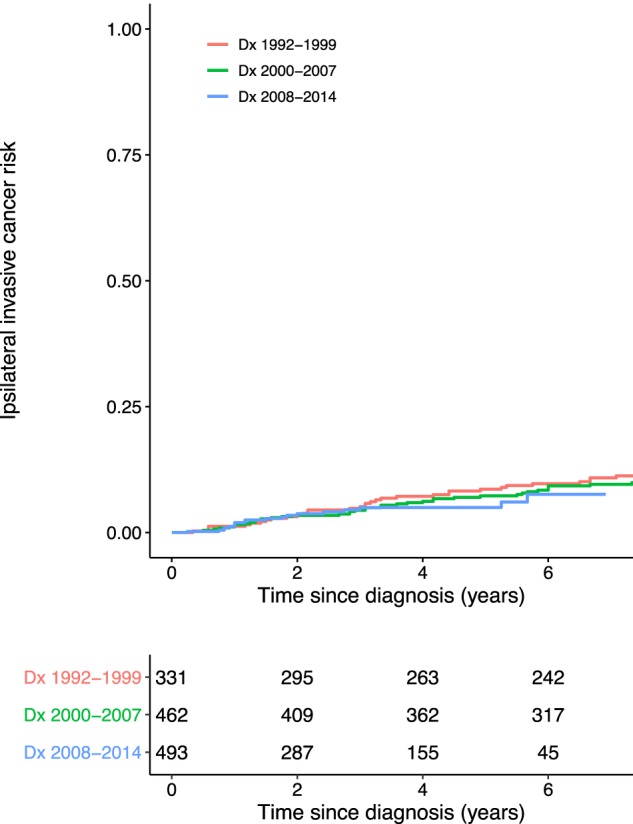
Net risk of ipsilateral invasive breast cancer (iIBC) in patients without locoregional treatment, based on US National Cancer Institute’s Surveillance, Epidemiology, and End Results program (1992–2014). The cumulative net risk of iIBC is shown for patients diagnosed between 1992 and 1999, 2000 and, and 2008 and 2014, respectively. The number of patients at risk is shown beneath the figure. Dx = date of diagnosis.
In summary, we do not find the proposed studies to be comparable with our study cohort, nor did we find evidence of systematic underreporting of iIBC before 2007. However, we entirely agree with Habel and Buist that these estimates require corroboration by additional data sources. To this end, we have collected detailed patient-level follow-up data from primary source documentation on a subset of more than 20 000 DCIS patients from the American College of Surgeon’s National Cancer Database. Preliminary analyses of these data support the range of iIBC risk seen in our SEER-based study. Finally, we note that the most definitive estimates will be provided by ongoing prospective randomized studies such as the Comparison of Operative to Monitoring and Endocrine Therapy, Low Risk DCIS, and LOw Risk DCIS trials, which compare oncologic outcomes between active surveillance against surgical care in patients with low-risk DCIS (5).
Notes
The authors do not have any conflicts of interest to disclose.
References
- 1. Wapnir IL, Dignam JJ, Fisher B, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103(6):478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Subhedar P, Olcese C, Patil S, Morrow M, Van Zee KJ.. Decreasing recurrence rates for ductal carcinoma in situ: analysis of 2996 women treated with breast-conserving surgery over 30 years. Ann Surg Oncol. 2015;22(10):3273–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cronin PA, Olcese C, Patil S, Morrow M, Van Zee KJ.. Impact of age on risk of recurrence of ductal carcinoma in situ: outcomes of 2996 women treated with breast-conserving surgery over 30 years. Ann Surg Oncol. 2016;23(9):2816–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson C, Peace S, Adamo P, Fritz A, Percy-Laurry A, Edwards B.. The 2007 multiple primary and histology coding rules. National Cancer Institute, Surveillance, Epidemiology and End Results Program. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 5. Hwang ES, Hyslop T, Lynch T, et al. The COMET (Comparison of Operative versus Monitoring and Endocrine Therapy) trial: a phase III randomised controlled clinical trial for low-risk ductal carcinoma in situ (DCIS). BMJ Open. 2019;9(3):e026797.. [DOI] [PMC free article] [PubMed] [Google Scholar]


