Abstract
Background
Epidemiologic data are inconsistent regarding the vitamin E-lung cancer association, and no study to our knowledge has examined serologic changes in vitamin E status in relation to subsequent risk.
Methods
In a cohort of 22 781 male smokers in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study, we ascertained 3184 lung cancer cases during up to 28 years of observation. Cox proportional hazards models examined whether higher serum alpha-tocopherol concentrations at baseline, 3 years, or the interval change were associated with lower lung cancer risk. All statistical tests were two-sided.
Results
After adjustment for age, body mass index, smoking intensity and duration, serum total cholesterol, and trial intervention group, we found lower lung cancer risk in men with high baseline alpha-tocopherol (fifth quintile [Q5] vs Q1, hazard ratio [HR] = 0.76, 95% confidence interval [CI] = 0.66 to 0.87, Ptrend < .001). A similar reduction in risk was seen for serum alpha-tocopherol at 3 years (Q5 vs Q1, HR = 0.78, 95% CI = 0.67 to 0.91, Ptrend = .004). The inverse risk association appeared stronger for younger men and those who had smoked fewer years but was similar across trial intervention groups. We also found reduced risk among men not supplemented with vitamin E who had a lower serum alpha-tocopherol at baseline and greater increases in concentrations at 3 years (third tertile vs first tertile of serum alpha-tocopherol change, HR = 0.74, 95% CI = 0.59 to 0.91, P = .005).
Conclusions
Higher vitamin E status, as measured by serum alpha-tocopherol concentration, as well as repletion of a low vitamin E state, was related to decreased lung cancer risk during a 28-year period. Our findings provide evidence supporting the importance of adequate physiological vitamin E status for lung cancer risk reduction.
Lung cancer is the leading malignancy worldwide in terms of both incidence and mortality, accounting for 11.6% of all cancer diagnoses and 18.4% of cancer-related deaths globally (1). Tobacco smoking is the primary modifiable risk factor for the disease, with other factors including genetic susceptibility, diet, vitamin supplements, physical activity, and occupational and environmental exposures also contributing to its development (2).
The role of vitamin E in lung carcinogenesis remains unclear, however. Vitamin E is a hydrophobic fat-soluble micronutrient encompassing tocopherol and tocotrienol compounds (3). Alpha-tocopherol is considered the most biologically active form in humans, and it exhibits several biological properties that inhibit the development or progression of cancer, including its antioxidant function (4–7). Experimental data along with some observational studies led to controlled trials that tested the cancer-preventive effects of alpha-tocopherol supplementation with inconsistent results, including for lung cancer (8–10). Altogether, epidemiologic evidence regarding the association between serum vitamin E (ie, alpha-tocopherol) status and lung cancer risk is inconsistent, with most studies having limited sample sizes and lacking data for histological subtypes. For example, several case-control and cohort studies (but not all [11]) suggest a weak inverse association between circulating alpha-tocopherol and lung cancer risk (12–16) overall or restricted to specific subgroups (17).
Our previous prospective analysis of this hypothesis included 1144 cases of lung cancer with 10 years of follow-up in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study cohort and provided evidence to support a role for higher prediagnostic serum alpha-tocopherol being related to reduced lung cancer risk in male smokers (12), with the association appearing stronger among younger men and lighter smokers. The present investigation extended that analysis and included 28 years of lung cancer incidence (n = 3184 cases) following measurement of serum alpha-tocopherol both at baseline and 3 years.
Methods
Study Population
The study population included 29 133 Finnish male smokers ages 50–69 years who were enrolled in the ATBC Study (ClinicalTrials.gov Identifier: NCT00342992) that was conducted in southwestern Finland between 1985 and 1988. Using a 2 × 2 factorial, randomized, double-blinded, placebo-controlled prevention trial design, participating men were assigned to one of four trial intervention groups: alpha-tocopherol (dl-alpha-tocopheryl-acetate, 50 mg/d), beta-carotene (20 mg/d), both supplements, or placebo for 5–8 years (median: 6.1 years). At baseline (presupplementation) and at 3 years, overnight fasting blood samples were collected and protected from light, processed to serum, aliquoted, and stored at −70°C until analysis. At enrollment, height and weight were measured, and questionnaires were used to collect behavioral and lifestyle information, including smoking, alcohol consumption, medical history, diet, and supplement use. All participants provided written informed consent at enrollment. The ATBC Study has been approved by institutional review boards at the US National Cancer Institute and the Finnish National Institute for Health and Welfare.
Serum alpha-tocopherol, beta-carotene, and retinol were assayed using high-performance liquid chromatography in one laboratory (18). Total and high-density lipoprotein cholesterol concentrations were measured using an enzymatic assay (19). For serum alpha-tocopherol, the interbatch coefficients of variation were 2.2%. Participants with missing serum alpha-tocopherol at baseline or 3 years were excluded from the present analysis. In addition, to minimize the potential influence of selection bias, the first 3 years (1096 days) of follow-up for all the participants were excluded. This resulted in a final cohort size of 22 781 participants, with the rates of missing baseline and 3-year serum alpha-tocopherol measurements being, respectively, 0.1% (only 31 of 29 133 participants) and 21.5% (6278 of 29 133 participants). Men included in the final analysis were followed from 1096 days (3 years) until the date of primary lung cancer diagnosis, death, or the end of follow-up (December 31, 2015), whichever occurred first.
Identification of Primary Lung Cancer Case Patients
Primary cancer of the lung or bronchus (International Classification of Disease-9 = 162), as the main outcome (n = 3184), was ascertained through the Finnish Cancer Registry, which represents approximately 100% case coverage nationwide (20). Medical records of all cancer cases diagnosed before April 1994 were collected from hospital and pathology laboratories and reviewed for cancer confirmation by one or two pathologists and/or pulmonary cytologists. After April 1994, medical records of 25% of the lung cancer cases were obtained and reviewed by one study physician. Therefore, the histologic subtype information for the lung cancer cases was either based on central review of histopathologic and cytologic specimens or obtained solely from the Finnish Cancer Registry. In the present study, 30.0% of the lung cancers were squamous cell carcinoma, 14.7% were small cell carcinoma, and 13.4% were adenocarcinoma, with the remaining cases being of less common histological types (eg, large cell or neuroendocrine tumors) or unknown histology.
Statistical Analysis
Multivariable-adjusted Cox proportional hazards regression, with attained follow-up time as the time metric, was used to examine hazard ratios (HRs) and confidence intervals (CIs) for associations between serum alpha-tocopherol (quintiles) and lung cancer risk. We selected potential confounders, a priori, as previous studies suggested (12,21), for adjustment in multivariable regressions including age at baseline (continuous), body mass index (BMI, continuous), years of cigarette smoking (continuous), number of cigarettes smoked daily (continuous), serum total cholesterol concentration (continuous), and trial intervention group (four supplement categories). BMI status and serum total cholesterol measures were missing for less than 0.1% of the participants in this analysis at baseline. We further adjusted for family history of lung cancer and education status. Tests for linear trend across quintiles of serum alpha-tocopherol were conducted by assigning cohort members the median value of serum alpha-tocopherol for each quintile and entering this new variable into the above-mentioned Cox proportional hazards regression model. Kaplan-Meier plots and log-rank tests compared differences in cumulative lung cancer incidence according to baseline serum alpha-tocopherol quintiles. To account for a possible nonlinear association between baseline serum alpha-tocopherol and lung cancer risk, we used cubic restricted splines with 4-knots and treated serum alpha-tocopherol as a continuous variable. Knots were selected at fifth, 25th, 75th, and 95th percentiles of serum alpha-tocopherol concentrations.
Stratified analyses were conducted based on age at baseline (<55, 55–59, or ≥60 years), years of cigarette smoking (<32, 32–40, or ≥40 years), number of cigarettes smoked daily (<20 or ≥20), BMI (<24.5, 24.5–27.6, or ≥27.6 kg/m2), trial intervention group assignment, and years of follow-up (<10 or ≥10 years; given that we had excluded the first 3 years of follow-up [ie, 1096 days], the cohort observation period for men with ≥10 years of follow-up started 13 years [3 + 10 years] after original study enrollment). We also performed stratified analyses by trial alpha-tocopherol supplementation intervention arm (yes or no) according to age at baseline (<60 or ≥60 years), years of cigarette smoking (<40 or ≥40 years), and BMI (<27.6 or ≥27.6 years). P values for interaction were assessed through likelihood ratio tests by comparing regression models with and without the cross-product terms for each assessed factor (categorized into two or three levels) and baseline serum alpha-tocopherol concentration (quintiles). The serum alpha-tocopherol-lung cancer association was also examined within each of the three major histological subtypes (ie, squamous cell carcinoma, small cell carcinoma, and adenocarcinoma).
Using a similar Cox regression approach, we performed analyses to examine changes in serum alpha-tocopherol from baseline to the 3-year follow-up (as tertiles) in relation to lung cancer risk according to alpha-tocopherol supplementation intervention arm (yes or no). Sensitivity analyses were also performed to test whether the associations between serum alpha-tocopherol change and lung cancer risk were modified by baseline serum alpha-tocopherol concentration (low: <10.5; high: ≥10.5 mg/L).
Using a likelihood ratio test, the proportional hazard assumption was tested for the Cox proportional hazards regression models by assigning the cross-product term of follow-up time and the exposures of interest, and no evidence of violation was observed. All the analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, NC). All reported P values were two-sided. A P value of less than .05 was considered statistically significant.
Results
Among the 22 781 men in this analytical cohort, we identified 3184 lung cancer cases during up to 28 years of follow-up (median = 16.2 years). The median and mean baseline serum alpha-tocopherol concentrations in the cohort were 15.9 mg/L and 17.0 mg/L, respectively, and the median concentration in the fifth quintile was nearly 2 times higher than that in the first quintile (Table 1). Compared with participants in the lowest quintile, men with higher serum alpha-tocopherol smoked less and had higher BMI. Alpha-tocopherol concentrations were positively associated with serum retinol, beta-carotene, and total cholesterol; fruit, vegetable, red meat, and coffee consumption; and the dietary antioxidants vitamins A, C, and E (Table 1).
Table 1.
Baseline characteristics of Finnish male smokers by quintile of baseline serum alpha-tocopherol concentration in the ATBC Study
| Characteristics | Baseline serum alpha-tocopherol quintile |
P | ||||
|---|---|---|---|---|---|---|
| Q1 (n = 4623) | Q2 (n = 4571) | Q3 (n = 4413) | Q4 (n = 4590) | Q5 (n = 4584) | ||
| Age at blood collection, y | 57 (53–62) | 56 (53–61) | 56 (53–60) | 56 (53–60) | 56 (53–60) | <.001† |
| BMI, kg/m2 | 25.3 (22.8–28.0) | 25.7 (23.5–28.2) | 25.9 (23.7–28.3) | 26.1 (24.0–28.5) | 26.8 (24.7–29.2) | <.001† |
| Cigarettes per day | 20 (15–25) | 20 (15–25) | 20 (15–25) | 20 (15–25) | 20 (15–25) | <.001† |
| Years of cigarette smoking | 37 (32–42) | 36 (31–42) | 35 (30–40) | 36 (30–40) | 36 (30–40) | <.001† |
| History of diabetes, % | 4.0 | 3.4 | 3.0 | 3.5 | 6.3 | <.001‡ |
| Serum nutrients at baseline | ||||||
| Serum alpha-tocopherol, mg/L | 8.4 (7.6–9.0) | 10.3 (9.9–10.6) | 11.6 (11.3–11.9) | 13.1 (12.7–13.6) | 15.9 (14.9–17.7) | <.001† |
| Serum retinol, µg/L | 545 (470–626) | 563 (492–643) | 578 (506–657) | 591 (518–672) | 621 (544–709) | <.001† |
| Serum beta-carotene, µg/L | 134 (85–201) | 168 (113–250) | 188 (127–280) | 203 (134–299) | 202 (131–315) | <.001† |
| Serum total cholesterol, mmol/L | 5.3 (4.7–5.8) | 5.8 (5.3–6.3) | 6.2 (5.7–6.8) | 6.6 (6.0–7.3) | 7.2 (6.5–7.9) | <.001† |
| Dietary intake per day | ||||||
| Total energy, kcal | 2626 (2174–3165) | 2652 (2199–3150) | 2640 (2199–3135) | 2588 (2151–3101) | 2549 (2127–3060) | <.001† |
| Fruit, g | 97 (49–161) | 104 (56–167) | 115 (61–178) | 117 (66–186) | 120 (67–191) | <.001† |
| Vegetables, g | 86 (54–132) | 95 (61–143) | 103 (67–152) | 107 (69–159) | 115 (77–167) | <.001† |
| Red meat, g | 64 (47–86) | 66 (49–88) | 66 (50–88) | 66 (49–88) | 65 (49–87) | .02† |
| Coffee intake, g | 550 (330–770) | 600 (440–770) | 600 (440–770) | 600 (440–770) | 550 (420–770) | .003† |
| Alcohol or ethanol, g | 12.0 (2.6–27.4) | 10.7 (2.3–25.2) | 10.6 (2.5–24.2) | 10.0 (2.4–23.7) | 10.7 (2.7–24.3) | <.001† |
| Total vitamin A, µg | 1579 (1094–2272) | 1656 (1145–2405) | 1649 (1145–2411) | 1667 (1127–2421) | 1646 (1109–2382) | <.001† |
| Vitamin C, mg | 87 (62–117) | 91 (67–121) | 95 (71–126) | 97 (72–130) | 99 (74–132) | <.001† |
| Total vitamin E, mg | 9.4 (7.3–12.1) | 10.4 (8.0–13.5) | 10.9 (8.4–14.3) | 11.4 (8.7–15.6) | 12.4 (9.3–17.1) | <.001† |
| Alpha-tocopherol, mg | 8.1 (6.3–10.3) | 8.8 (6.9–11.6) | 9.4 (7.3–12.2) | 9.8 (7.4–13.4) | 10.7 (8.0–14.8) | <.001† |
| Gamma-tocopherol, mg | 4.0 (2.2–7.2) | 5.1 (2.8–9.4) | 6.0 (3.3–10.7) | 6.8 (3.6–12.8) | 8.4 (4.4–14.9) | <.001† |
Median (interquartile range) unless otherwise indicated. ANOVA = analysis of variance; ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention; BMI = body mass index; Q = quintile.
P value based on ANOVA test (continuous variable).
P value based on χ2 test (categorical variable).
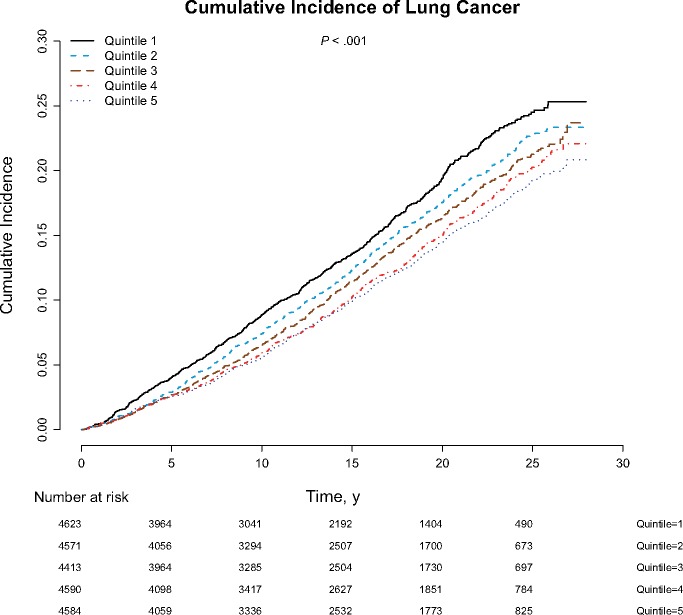
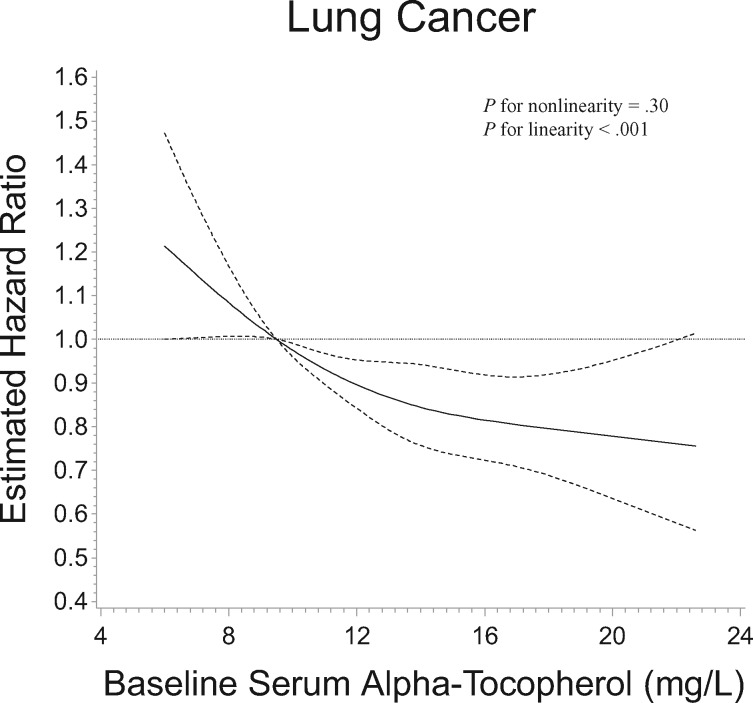
After multivariable adjustment, higher serum alpha-tocopherol concentrations by quintiles at baseline and 3 years, as well as average and maximum concentrations of two measurements, were statistically significantly associated with 21%–24% lower lung cancer risk compared with those in the lowest quintiles, with clear dose-response trends (Ptrend < .001, Ptrend = .004, Ptrend < .001, and Ptrend = .008, respectively; Table 2). Further adjustment for family history of lung cancer and education did not materially alter the associations (Supplementary Table 1, available online). The Kaplan-Meier plot demonstrated that men in the lowest quintile of serum alpha-tocopherol had statistically significantly increased cumulative lung cancer incidence compared with those in the higher quintiles (log-rank test P < .001; Figure 1). To examine the baseline serum alpha-tocopherol-lung cancer risk dose-response association, the restricted 4-knot cubic spline regression showed a statistically significant inverse linear dose-response association between alpha-tocopherol and lung cancer relative risk, with higher risk below the reference value of 9.5 mg/L (corresponding to the first quintile cutoff value) and progressively lower risk for men with higher serum alpha-tocopherol concentrations (Pfor linearity < .001; Pfor nonlinearity = .30; Figure 2).
Table 2.
Multivariable-adjusted hazard ratios and 95% confidence intervals of lung cancer by quintile of serum alpha-tocopherol concentration at baseline and at 3 years among Finnish male smokers in the ATBC Study*
| Serum measure | Event/total person-years of follow-up | Lung cancer incidence rate† | HR (95% CI) |
|---|---|---|---|
| Baseline alpha-tocopherol, range, mg/L | |||
| Q1: <9.5 | 695/66 962 | 10.38 | 1.00 (Ref) |
| Q2: ≥9.5 to <10.9 | 681/72 593 | 9.38 | 0.92 (0.82 to 1.02) |
| Q3: ≥10.9 to <12.3 | 629/71 879 | 8.75 | 0.87 (0.78 to 0.98) |
| Q4: ≥12.3 to <14.2 | 613/75 329 | 8.14 | 0.80 (0.71 to 0.90) |
| Q5: ≥14.2 | 566/73 821 | 7.67 | 0.76 (0.66 to 0.87) |
| Ptrend‡ | <.001 | ||
| 3-Year alpha-tocopherol, range, mg/L | |||
| Q1: <11.4 | 698/67 294 | 10.37 | 1.00 (Ref) |
| Q2: ≥11.4 to <13.6 | 646/74 157 | 8.71 | 0.85 (0.76 to 0.95) |
| Q3: ≥13.6 to <15.9 | 632/72 951 | 8.66 | 0.82 (0.73 to 0.93) |
| Q4: ≥15.9 to <19.0 | 609/74 088 | 8.22 | 0.77 (0.67 to 0.88) |
| Q5: ≥19.0 | 599/72 095 | 8.31 | 0.78 (0.67 to 0.91) |
| Ptrend‡ | .004 | ||
| Mean alpha-tocopherol at baseline and 3 y, range, mg/L | |||
| Q1: <10.8 | 701/67 336 | 10.41 | 1.00 (Ref) |
| Q2: ≥10.8 to <12.5 | 663/73 594 | 9.01 | 0.86 (0.77 to 0.96) |
| Q3: ≥12.5 to <14.1 | 630/72 655 | 8.67 | 0.83 (0.74 to 0.94) |
| Q4: ≥14.1 to <16.3 | 608/74 059 | 8.21 | 0.79 (0.70 to 0.90) |
| Q5: ≥16.3 | 582/72 941 | 7.98 | 0.76 (0.66 to 0.87) |
| Ptrend‡ | <.001 | ||
| Maximum alpha-tocopherol at baseline or 3 y, range, mg/L | |||
| Q1: <11.7 | 708/67 948 | 10.42 | 1.00 (Ref) |
| Q2: ≥11.7 to <13.9 | 635/73 945 | 8.59 | 0.84 (0.75 to 0.94) |
| Q3: ≥13.9 to <16.1 | 626/72 576 | 8.63 | 0.82 (0.73 to 0.93) |
| Q4: ≥16.1 to <19.2 | 622/75 028 | 8.29 | 0.79 (0.69 to 0.90) |
| Q5: ≥19.2 | 593/71 086 | 8.34 | 0.79 (0.68 to 0.92) |
| Ptrend‡ | .008 | ||
Cox proportional hazards regression using follow-up time as the time metric to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of associations between measures of serum alpha-tocopherol (quintiles) and incidence of lung cancer, and adjusted for age at baseline, body mass index, years of cigarette smoking, number of cigarettes smoked daily, serum total cholesterol concentration, and trial intervention group. ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention; Q = quintile; Ref = referent.
Crude lung cancer incidence rate per 1000 person-years.
Two-sided Ptrend is based on the statistical significance of the coefficient of the quintile variable (median value within each quintile).
Figure 1.
Kaplan-Meier plot comparing cumulative incidence of lung cancer according to baseline serum alpha-tocopherol concentration quintile in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. The two-sided log-rank test was used to test the differences across serum alpha-tocopherol strata.
Figure 2.
Cubic restricted spline regression for estimated relative risk of lung cancer according to baseline serum alpha-tocopherol concentration in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. The reference value (9.5 mg/L; relative risk = 1.00) corresponds to the cutoff value of the first quintile of serum alpha-tocopherol concentration. The two-sided P values were derived from the likelihood ratio tests for nonlinearity and the linear relation.
We assessed effect modification of the serum alpha-tocopherol-lung cancer association by age, years of smoking, smoking intensity, BMI, and trial intervention assignment. The inverse risk association appeared stronger for younger men and those having smoked fewer years (the latter Pinteraction = .04). Although the interaction with follow-up time was formally statistically significant, the risk estimates for the 0–10 and 10 or more year periods were nearly identical (quintile 5 [Q5 vs Q1], HR = 0.77, 95% CI = 0.63 to 0.95 and 0.76, 95% CI = 0.63 to 0.91, Ptrend = .006 and .002, respectively) (Table 3). Men in the lower tertile of BMI also appeared to have experienced a stronger inverse alpha-tocopherol-lung cancer association (first and second tertile of BMI, Q5 vs Q1: HR = 0.78, 95% CI = 0.61 to 0.99; and HR = 0.60, 95% CI = 0.47 to 0.75, Ptrend = .02 and <.001, respectively), whereas the estimated associations differed little across the trial supplementation groups (Table 3). The trial alpha-tocopherol supplement did not modify the serum-risk interactions with age, years of smoking, or BMI (Supplementary Table 2, available online).
Table 3.
Multivariable-adjusted hazard ratios and 95% confidence intervals of lung cancer by quintile of baseline serum alpha-tocopherol concentration, stratified by age at baseline, smoking, BMI, and intervention assignment, among Finnish male smokers in the ATBC Study*
| Stratified characteristics | No. of events | Baseline serum alpha-tocopherol quintiles, range |
P trend † | P interaction ‡ | ||||
|---|---|---|---|---|---|---|---|---|
| Q1 <9.5 mg/L | Q2 9.5–10.9 mg/L | Q3 10.9–12.3 mg/L | Q4 12.3–14.2 mg/L | Q5 ≥14.2 mg/L | ||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Age at baseline, y | ||||||||
| <55 | 947 | 1.00 (Ref) | 0.78 (0.64 to 0.95) | 0.76 (0.62 to 0.93) | 0.62 (0.49 to 0.78) | 0.59 (0.46 to 0.75) | <.001 | .32 |
| 55–59 | 1045 | 1.00 (Ref) | 0.93 (0.77 to 1.13) | 0.88 (0.72 to 1.07) | 0.90 (0.73 to 1.11) | 0.76 (0.59 to 0.96) | .03 | |
| ≥60 | 1192 | 1.00 (Ref) | 1.02 (0.86 to 1.21) | 0.96 (0.79 to 1.15) | 0.86 (0.71 to 1.05) | 0.94 (0.76 to 1.17) | .33 | |
| Years of smoking | ||||||||
| <32 | 482 | 1.00 (Ref) | 0.77 (0.59 to 1.02) | 0.76 (0.57 to 1.004) | 0.57 (0.42 to 0.78) | 0.45 (0.31 to 0.66) | <.001 | .04 |
| 32–40 | 1069 | 1.00 (Ref) | 0.86 (0.71 to 1.04) | 0.78 (0.64 to 0.95) | 0.72 (0.59 to 0.89) | 0.65 (0.51 to 0.82) | <.001 | |
| ≥40 | 1633 | 1.00 (Ref) | 1.01 (0.87 to 1.17) | 0.96 (0.82 to 1.13) | 0.93 (0.79 to 1.11) | 0.97 (0.80 to 1.17) | .59 | |
| No. of cigarettes smoked daily | ||||||||
| <20 | 903 | 1.00 (Ref) | 0.87 (0.71 to 1.07) | 0.86 (0.70 to 1.07) | 0.80 (0.63 to 0.999) | 0.69 (0.53 to 0.90) | .006 | .17 |
| ≥20 | 2281 | 1.00 (Ref) | 0.94 (0.83 to 1.06) | 0.87 (0.76 to 1.00) | 0.80 (0.69 to 0.93) | 0.80 (0.68 to 0.94) | .002 | |
| BMI (range), kg/m2 | ||||||||
| <24.5 | 1215 | 1.00 (Ref) | 1.00 (0.85 to 1.18) | 0.99 (0.83 to 1.18) | 0.85 (0.70 to 1.04) | 0.78 (0.61 to 0.99) | .02 | .30 |
| 24.5–27.6 | 1084 | 1.00 (Ref) | 0.79 (0.65 to 0.95) | 0.74 (0.61 to 0.90) | 0.68 (0.55 to 0.83) | 0.60 (0.47 to 0.75) | <.001 | |
| ≥27.6 | 881 | 1.00 (Ref) | 0.97 (0.78 to 1.20) | 0.88 (0.70 to 1.10) | 0.89 (0.71 to 1.13) | 0.95 (0.74 to 1.21) | .70 | |
| Alpha-tocopherol supplementation | ||||||||
| Yes | 1590 | 1.00 (Ref) | 0.94 (0.80 to 1.09) | 0.88 (0.75 to 1.04) | 0.82 (0.69 to 0.98) | 0.77 (0.63 to 0.93) | .0043 | |
| No | 1594 | 1.00 (Ref) | 0.89 (0.77 to 1.04) | 0.86 (0.73 to 1.01) | 0.77 (0.65 to 0.92) | 0.75 (0.62 to 0.91) | .002 | .98 |
| β-Carotene supplementation | ||||||||
| Yes | 1612 | 1.00 (Ref) | 0.88 (0.76 to 1.03) | 0.84 (0.71 to 0.98) | 0.81 (0.68 to 0.96) | 0.77 (0.64 to 0.94) | .008 | .79 |
| No | 1572 | 1.00 (Ref) | 0.95 (0.82 to 1.11) | 0.90 (0.77 to 1.06) | 0.79 (0.66 to 0.94) | 0.75 (0.62 to 0.91) | <.001 | |
| Years of follow-up | ||||||||
| <10 | 1391 | 1.00 (Ref) | 0.91 (0.78 to 1.07) | 0.84 (0.71 to 1.00) | 0.78 (0.65 to 0.94) | 0.77 (0.63 to 0.95) | .006 | .01 |
| ≥10 | 1793 | 1.00 (Ref) | 0.94 (0.81 to 1.09) | 0.91 (0.78 to 1.06) | 0.82 (0.69 to 0.97) | 0.76 (0.63 to 0.91) | .002 | |
Cox proportional hazards regression using follow-up time as the time metric to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of associations between baseline serum alpha-tocopherol (quintiles) and incidence of lung cancer, adjusted for age at baseline, body mass index (BMI), years of cigarette smoking, number of cigarettes smoked daily, serum total cholesterol concentration, and trial intervention group. ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention; Q = quintile; Ref = referent.
Two-sided Ptrend is based on the statistical significance of the coefficient of the quintile variable (median value within each quintile).
Two-sided Pinteraction is based on the statistical significance of the cross-product term added to multivariable models.
The association between serum alpha-tocopherol and lung cancer appeared to be somewhat stronger for squamous cell carcinoma (Ptrend = .03) than for small cell carcinoma and adenocarcinoma (Table 4). We observed substantially reduced risk among men with fewer years of smoking for all three histologic types, however (Q5 vs Q1, HR = 0.60, 95% CI = 0.42 to 0.85; HR = 0.51, 95% CI = 0.31 to 0.85; and HR = 0.67, 95% CI = 0.40 to 1.13; Ptrend = .002, .02, and .09, respectively; Table 4).
Table 4.
Multivariable-adjusted hazard ratios and 95% confidence intervals for associations between quintile of baseline serum alpha-tocopherol concentration and lung cancer according to tumor histology and smoking years in the ATBC Study*
| Histological type | Quintiles of baseline serum alpha-tocopherol, range |
P trend † | ||||
|---|---|---|---|---|---|---|
| Q1 <9.5 mg/L | Q2 9.5–10.9 mg/L | Q3 10.9–12.3 mg/L | Q4 12.3–14.2 mg/L | Q5 ≥14.2 mg/L | ||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Squamous cell carcinoma (n = 959) | 1.00 (Ref) | 0.92 (0.76 to 1.12) | 0.82 (0.67 to 1.02) | 0.70 (0.56 to 0.87) | 0.80 (0.63 to 1.02) | .03 |
| <40 y of smoking | 1.00 (Ref) | 0.76 (0.57 to 1.01) | 0.79 (0.60 to 1.06) | 0.51 (0.37 to 0.71) | 0.60 (0.42 to 0.85) | .002 |
| ≥40 y of smoking | 1.00 (Ref) | 1.08 (0.83 to 1.41) | 0.82 (0.60 to 1.11) | 0.91 (0.67 to 1.24) | 1.03 (0.73 to 1.43) | .91 |
| Small cell carcinoma (n = 469) | 1.00 (Ref) | 1.09 (0.82 to 1.45) | 0.92 (0.68 to 1.26) | 0.85 (0.62 to 1.18) | 0.83 (0.58 to 1.18) | .14 |
| <40 y of smoking | 1.00 (Ref) | 0.74 (0.50 to 1.12) | 0.66 (0.43 to 1.01) | 0.63 (0.40 to 0.97) | 0.51 (0.31 to 0.85) | .02 |
| ≥40 y of smoking | 1.00 (Ref) | 1.58 (1.05 to 2.39) | 1.30 (0.83 to 2.05) | 1.15 (0.71 to 1.85) | 1.30 (0.78 to 2.17) | .76 |
| Adenocarcinoma (n = 427) | 1.00 (Ref) | 1.08 (0.80 to 1.45) | 0.83 (0.59 to 1.15) | 1.04 (0.75 to 1.45) | 0.81 (0.55 to 1.19) | .27 |
| <40 y of smoking | 1.00 (Ref) | 1.10 (0.73 to 1.66) | 0.72 (0.46 to 1.13) | 0.91 (0.58 to 1.43) | 0.67 (0.40 to 1.13) | .09 |
| ≥40 y of smoking | 1.00 (Ref) | 1.06 (0.68 to 1.64) | 1.00 (0.61 to 1.62) | 1.25 (0.77 to 2.03) | 1.03 (0.59 to 1.80) | .77 |
Cox proportional hazard regression using follow-up time as the time metric to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of associations between baseline serum alpha-tocopherol (quintiles) and incidence of lung cancer by tumor histology and years of smoking and adjusted for age at baseline, body mass index (BMI), years of cigarette smoking, number of cigarettes smoked daily, serum total cholesterol concentration, and trial intervention group. ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention; Q = quintile; Ref = referent.
Two-sided Ptrend is based on the statistical significance of the coefficient of the quintile variable (median value within each quintile).
The median difference in serum alpha-tocopherol from baseline to 3 years for men who did or did not receive the trial alpha-tocopherol supplement, respectively, was 5.7 mg/L (interquartile range = 3.7 to 7.9 mg/L) and 0.79 mg/L (interquartile range = −0.34 to 1.93 mg/L). Overall, the difference in serum alpha-tocopherol from baseline to 3 years was not associated with lung cancer risk, with the exception of lower risk among men below the median of baseline serum alpha-tocopherol (<10.5 mg/L) who did not receive the trial alpha-tocopherol supplement but who had greater increases in serum alpha-tocopherol (tertiles 1–3 of change of serum alpha-tocopherol had HR = 1.00 (referent), 0.86, 95% CI = 0.69 to 1.06; and HR = 0.74, 95% CI = 0.59 to 0.91, Ptrend = .004) (Table 5).
Table 5.
Multivariable-adjusted hazard ratios and 95% confidence intervals for associations between changes in serum alpha-tocopherol concentration from baseline to 3 years and lung cancer by trial alpha-tocopherol supplementation and baseline serum alpha-tocopherol concentration in the ATBC Study*
| Baseline and change in serum alpha-tocopherol | No alpha-tocopherol supplementation |
Alpha-tocopherol supplementation |
||
|---|---|---|---|---|
| No. of events | HR (95% CI) | No. of events | HR (95% CI) | |
| Lower and higher baseline serum alpha-tocopherol combined† | ||||
| Lowest tertile of change (≥ –56.3 to <0.09 mg/L) | 506 | 1.00 (Referent) | 536 | 1.00 (Referent) |
| 2nd tertile of change (≥0.09 to <1.51 mg/L) | 564 | 1.03 (0.91 to 1.17) | 540 | 0.98 (0.86 to 1.10) |
| 3rd tertile of change (≥1.51 mg/L) | 524 | 0.97 (0.86 to 1.10) | 514 | 0.96 (0.85 to 1.09) |
| Ptrend‡ | 0.60 | 0.53 | ||
| Lower baseline serum alpha-tocopherol (<10.5 mg/L) | ||||
| Lowest tertile of change (≥ –6.7 to <0.09 mg/L) | 136 | 1.00 (Referent) | 162 | 1.00 (Referent) |
| 2nd tertile of change (≥0.09 to <1.51 mg/L) | 235 | 0.86 (0.69 to 1.06) | 220 | 1.08 (0.88 to 1.32) |
| 3rd tertile of change (≥1.51 mg/L) | 211 | 0.74 (0.59 to 0.91) | 189 | 0.99 (0.80 to 1.23) |
| Higher baseline serum alpha-tocopherol (≥10.5 mg/L) | ||||
| Lowest tertile of change (≥ –56.3 to <0.09 mg/L) | 370 | 0.74 (0.56 to 0.99) | 374 | 0.99 (0.75 to 1.31) |
| 2nd tertile of change (≥0.09 to <1.51 mg/L) | 329 | 0.82 (0.62 to 1.08) | 320 | 0.91 (0.69 to 1.20) |
| 3rd tertile of change (≥1.51 mg/L) | 313 | 0.82 (0.62 to 1.09) | 325 | 0.94 (0.71 to 1.24) |
Cox proportional hazards regression examined hazard ratios (HRs) and 95% confidence intervals (CIs) of associations between serum alpha-tocopherol changes from baseline to 3-year follow-up (tertiles) and lung cancer risk by trial alpha-tocopherol supplementation status, and adjusted for age at baseline, body mass index, years of cigarette smoking, number of cigarettes smoked daily, serum total cholesterol concentration, and trial intervention group. “Change of alpha-tocopherol levels” = “3-year alpha-tocopherol levels minus alpha-tocopherol concentration at baseline.” A change in alpha-tocopherol that is greater than zero indicates that concentrations increased compared with baseline, and changes less than zero indicate lower levels at 3 years. ATBC = Alpha-Tocopherol, Beta-Carotene Cancer Prevention.
Multivariable-adjusted Cox models further adjusted for baseline serum alpha-tocopherol (quintiles).
Two-sided Ptrend is based on the statistical significance of the coefficient of the tertile variable (median value within each quintile).
Discussion
In this large trial-based cohort study of male smokers, we observed a dose-dependent inverse association between prospectively measured serum alpha-tocopherol concentrations and lung cancer risk based on nearly 3200 cases, two separate serum measurements, and adjustment for several potential confounding factors. Men in the highest serum quintile had 24% reduced risk of lung cancer compared with men in the lowest quintile. There were stronger inverse associations among men younger than 60 years, men with BMI of less than 27.6 kg/m2, and men with fewer than 40 years of smoking. Interestingly, the association was not modified by the trial vitamin E supplementation, and it persisted during the entire 28-year observation period. Of note, we found statistically significantly lower lung cancer risk among men who had lower serum vitamin E concentrations at baseline that increased by the third year through nonsupplementation means (ie, likely through diet).
Alpha-tocopherol is considered the dominant form of vitamin E for humans and is preferentially maintained in blood and tissues. Because the hepatic alpha-tocopherol transfer protein preferentially binds alpha-tocopherol, this protein can prevent its degradation (22). It has been widely accepted that alpha-tocopherol and other vitamin E compounds can act as efficient antioxidants in vivo, protecting against cellular oxidative damage by scavenging free radicals and inhibiting lipid peroxidation (7). In addition, alpha-tocopherol has other cancer preventive properties, including inhibiting endogenous nitrosamine or nitrosoamide formation, suppressing cell proliferation, inhibiting tumor angiogenesis, inducing apoptosis, and enhancing immunity (5,6,23).
Several epidemiologic studies have reported that vitamin E status (blood levels or intake) is inversely associated with specific cancers, including oropharynx and larynx, esophagus and stomach, lung, pancreas, and bladder (12,24–28). The present study of lung cancer shows a stronger inverse association primarily in younger men and those with fewer cumulative years of smoking, possibly representing the need for higher vitamin E exposure earlier in the carcinogenic process; that is, any potential protective association of alpha-tocopherol may be overshadowed by longer exposure to, and accumulation of, tobacco-induced carcinogens. The stronger inverse association in younger men could also result from the age-related decline in lipoprotein lipase activity, which could reduce alpha-tocopherol release from the chylomicrons and alter alpha-tocopherol transport and metabolism (29–31). A nested case-control study of tin miners in China also showed an inverse serum alpha-tocopherol-lung cancer association in men younger than 60 years (17).
Alpha-tocopherol cannot be synthesized physiologically in humans, and the dominant dietary sources are vegetable oils, whole grains, nuts (eg, almonds), seeds (eg, sunflower), and some vegetables and fruit (32,33). There are other bioactive nutrients in vitamin E-rich foods, including fiber, polyphenols, phytosterols, additional tocopherols, minerals (such as iron, magnesium, and zinc), other vitamins (such as B vitamins and folacins), and other antioxidants (such as alpha- and beta-carotene) (34–36). These bioactive nutrients have multiple biological functions such as reducing oxidative stress and inflammation (37–39), and they coexist with alpha-tocopherol in vitamin E-rich foods so we cannot preclude the possibility that they contribute to some of the observed lung cancer association with serum alpha-tocopherol. Adjusting for vitamin E-rich foods (including vegetable oils, fish, rye, wheat, and eggs) in the main analytical models did not, however, statistically significantly alter the inverse serum alpha-tocopherol association (baseline Q5 vs Q1, HR = 0.80, 95% CI = 0.70 to 0.92, Ptrend = .0008). This supports a role for higher serum alpha-tocopherol that is not accounted for by the other bioactive constituents in vitamin E-rich foods.
Serum alpha-tocopherol concentrations are at least partially determined by the above-mentioned dietary factors and can be increased through vitamin E supplementation. In the unsupplemented group, we showed that men with relatively low baseline concentrations that increased within 3 years experienced a one-fourth reduction in lung cancer risk. This is consistent with a beneficial role for dietary modification in raising vitamin E status. By contrast, the trial alpha-tocopherol supplementation-related increase in serum concentrations was not associated with risk, findings substantiated by previously reported trial results from the ATBC Study and the Selenium and Vitamin E Cancer Prevention Trial, with both studies having shown no overall alpha-tocopherol supplementation effects on lung cancer risk, either during the intervention [ATBC relative risk = 0.99; Selenium and Vitamin E Cancer Prevention Trial HR = 1.00) (8,9), or post-trial (ATBC relative risk = 1.01) (10). In addition, findings from the Shanghai Women’s Health Study of Chinese female nonsmokers showed source-specific vitamin E-lung cancer associations, with total dietary tocopherol intake being inversely associated with risk vs greater lung cancer risk for vitamin E supplement use (40). Taken together, these findings argue against a beneficial role for vitamin E supplementation in lung cancer prevention, whereas improved vitamin E status through dietary means, particularly among those with low serum concentrations, may be beneficial.
To the best of our knowledge, this is the largest prospective investigation of vitamin E status and lung cancer to date. The primary strengths of the study include validity of the exposure assessment (high-performance liquid chromatography platform assays) and outcome ascertainment from the population-based registers. The serum alpha-tocopherol measurements provide integrated assessments of exogenous and endogenous exposures and metabolism, and therefore represent more accurate assessments of vitamin E biological status than estimates from dietary questionnaires. Compared with the vast majority of studies that included a single blood sample measurement, we measured alpha-tocopherol concentrations both at baseline and 3 years, which improved classification of exposure status and yielded consistent findings.
Study limitations include the possibility that unmeasured confounders affected the observed association even though we adjusted for a large number of relevant covariates available. Collinearity of higher serum alpha-tocopherol status with other beneficial dietary or lifestyle factors, or specific genotypes, is also possible. The ATBC Study itself constitutes a relatively homogeneous population of Finnish male smokers, which may limit generalizability of our findings to other populations, including former smokers, nonsmokers, women, and other races and ethnicities. Further studies are warranted to investigate such associations in broader, more diverse populations.
In summary, we found higher vitamin E status, as measured by serum alpha-tocopherol concentrations from two blood collections taken 3 years apart, were related in a dose-dependent manner to decreased lung cancer incidence during a 28-year period. The inverse association was observed primarily among younger men and those with fewer cumulative years of cigarette smoking. Our findings provide evidence supporting the importance of adequate physiological vitamin E status for reduction in risk of lung cancer.
Funding
The ATBC Study is supported by the Intramural Research Program of the US National Cancer Institute, National Institutes of Health, and by US Public Health Service contract HHSN261201500005C from the National Cancer Institute, Department of Health and Human Services.
Notes
The authors declare no conflicts of interest.
The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Supplementary Material
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Malhotra J, Malvezzi M, Negri E, et al. Risk factors for lung cancer worldwide. Eur Respir J. 2016;48(3):889–902. [DOI] [PubMed] [Google Scholar]
- 3. Das Gupta S, Suh N.. Tocopherols in cancer: an update. Mol Nutr Food Res. 2016;60(6):1354–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ju J, Picinich SC, Yang Z, et al. Cancer-preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2010;31(4):533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shklar G, Schwartz JL.. Vitamin E inhibits experimental carcinogenesis and tumour angiogenesis. Eur J Cancer B Oral. 1996;32B(2):114–119. [DOI] [PubMed] [Google Scholar]
- 6. Stone WL, Papas AM.. Tocopherols and the etiology of colon cancer. J Natl Cancer Inst. 1997;89(14):1006–1014. [DOI] [PubMed] [Google Scholar]
- 7. Traber MG, Atkinson J.. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43(1):4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Albanes D, Heinonen OP, Taylor PR, et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88(21):1560–1570. [DOI] [PubMed] [Google Scholar]
- 9. Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Virtamo J, Taylor PR, Kontto J, et al. Effects of alpha-tocopherol and beta-carotene supplementation on cancer incidence and mortality: 18-year postintervention follow-up of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Int J Cancer. 2014;135(1):178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Epplein M, Franke AA, Cooney RV, et al. Association of plasma micronutrient levels and urinary isoprostane with risk of lung cancer: the Multiethnic Cohort Study. Cancer Epidemiol Biomarkers Prev. 2009;18(7):1962–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woodson K, Tangrea JA, Barrett MJ, et al. Serum alpha-tocopherol and subsequent risk of lung cancer among male smokers. J Natl Cancer Inst. 1999;91(20):1738–1743. [DOI] [PubMed] [Google Scholar]
- 13. Menkes MS, Comstock GW, Vuilleumier JP, et al. Serum beta-carotene, vitamins A and E, selenium, and the risk of lung cancer. N Engl J Med. 1986;315(20):1250–1254. [DOI] [PubMed] [Google Scholar]
- 14. Stahelin HB, Gey KF, Eichholzer M, et al. Plasma antioxidant vitamins and subsequent cancer mortality in the 12-year follow-up of the prospective Basel Study. Am J Epidemiol. 1991;133(8):766–775. [DOI] [PubMed] [Google Scholar]
- 15. Goodman GE, Schaffer S, Omenn GS, et al. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from Beta-Carotene and Retinol Efficacy Trial. Cancer Epidemiol Biomarkers Prev. 2003;12(6):518–526. [PubMed] [Google Scholar]
- 16. Comstock GW, Alberg AJ, Huang HY, et al. The risk of developing lung cancer associated with antioxidants in the blood: ascorbic acids, carotenoids, alpha-tocopherol, selenium, and total peroxyl radical absorbing capacity. Am J Epidemiol. 2008;168(7):831–840. [DOI] [PubMed] [Google Scholar]
- 17. Ratnasinghe D, Tangrea JA, Forman MR, et al. Serum tocopherols, selenium and lung cancer risk among tin miners in China. Cancer Causes Control. 2000;11(2):129–135. [DOI] [PubMed] [Google Scholar]
- 18. Milne DB, Botnen J.. Retinol, alpha-tocopherol, lycopene, and alpha- and beta-carotene simultaneously determined in plasma by isocratic liquid chromatography. Clin Chem. 1986;32(5):874–876. [PubMed] [Google Scholar]
- 19. Kattermann R, Jaworek D, Moller G, et al. Multicentre study of a new enzymatic method of cholesterol determination. J Clin Chem Clin Biochem. 1984;22(3):245–251. [DOI] [PubMed] [Google Scholar]
- 20. Korhonen P, Malila N, Pukkala E, et al. The Finnish Cancer Registry as follow-up source of a large trial cohort--accuracy and delay. Acta Oncol. 2002;41(4):381–388. [DOI] [PubMed] [Google Scholar]
- 21.The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- 22. Peh HY, Tan WS, Liao W, et al. Vitamin E therapy beyond cancer: tocopherol vs tocotrienol. Pharmacol Ther. 2016;162(1):152–169. [DOI] [PubMed] [Google Scholar]
- 23. Sigounas G, Anagnostou A, Steiner M.. dl-alpha-tocopherol induces apoptosis in erythroleukemia, prostate, and breast cancer cells. Nutr Cancer. 1997;28(1):30–35. [DOI] [PubMed] [Google Scholar]
- 24. Edefonti V, Hashibe M, Parpinel M, et al. Vitamin E intake from natural sources and head and neck cancer risk: a pooled analysis in the International Head and Neck Cancer Epidemiology consortium. Br J Cancer. 2015;113(1):182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor PR, Qiao YL, Abnet CC, et al. Prospective study of serum vitamin E levels and esophageal and gastric cancers. J Natl Cancer Inst. 2003;95(18):1414–1416. [DOI] [PubMed] [Google Scholar]
- 26. Jeurnink SM, Ros MM, Leenders M, et al. Plasma carotenoids, vitamin C, retinol and tocopherols levels and pancreatic cancer risk within the European Prospective Investigation into Cancer and Nutrition: a nested case-control study: plasma micronutrients and pancreatic cancer risk. Int J Cancer. 2015;136(6):E665–E676. [DOI] [PubMed] [Google Scholar]
- 27. Stolzenberg-Solomon RZ, Sheffler-Collins S, Weinstein S, et al. Vitamin E intake, alpha-tocopherol status, and pancreatic cancer in a cohort of male smokers. Am J Clin Nutr. 2009;89(2):584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen F, Li Q, Yu Y, et al. Association of vitamin C, vitamin D, vitamin E and risk of bladder cancer: a dose-response meta-analysis. Sci Rep. 2015;5:9599.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohn JS, McNamara JR, Cohn SD, et al. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res. 1988;29(4):469–479. [PubMed] [Google Scholar]
- 30. Krasinski SD, Cohn JS, Schaefer EJ, et al. Postprandial plasma retinyl ester response is greater in older subjects compared with younger subjects. Evidence for delayed plasma clearance of intestinal lipoproteins. J Clin Invest. 1990;85(3):883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Borel P, Mekki N, Boirie Y, et al. Postprandial chylomicron and plasma vitamin E responses in healthy older subjects compared with younger ones. Eur J Clin Invest. 1997;27(10):812–821. [DOI] [PubMed] [Google Scholar]
- 32. Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347–362. [DOI] [PubMed] [Google Scholar]
- 33. Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med. 2014;72:76–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slavin JL, Jacobs D, Marquart L, et al. The role of whole grains in disease prevention. J Am Diet Assoc. 2001;101(7):780–785. [DOI] [PubMed] [Google Scholar]
- 35. Kris-Etherton PM, Hu FB, Ros E, et al. The role of tree nuts and peanuts in the prevention of coronary heart disease: multiple potential mechanisms. J Nutr. 2008;138(9):1746S–1751S. [DOI] [PubMed] [Google Scholar]
- 36. Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr. 1999;70(3):475S–490S. [DOI] [PubMed] [Google Scholar]
- 37. Jenkins DJ, Kendall CW, Josse AR, et al. Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. J Nutr. 2006;136(12):2987–2992. [DOI] [PubMed] [Google Scholar]
- 38. Torabian S, Haddad E, Rajaram S, et al. Acute effect of nut consumption on plasma total polyphenols, antioxidant capacity and lipid peroxidation. J Hum Nutr Diet. 2009;22(1):64–71. [DOI] [PubMed] [Google Scholar]
- 39. Jiang R, Jacobs DR Jr, Mayer-Davis E, et al. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163(3):222–231. [DOI] [PubMed] [Google Scholar]
- 40. Wu QJ, Xiang YB, Yang G, et al. Vitamin E intake and the lung cancer risk among female nonsmokers: a report from the Shanghai Women's Health Study. Int J Cancer. 2015;136(3):610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.