Abstract
Landmark investigation two decades ago demonstrated sex-based disparities among participants in cancer cooperative group trials. Although federal efforts have aimed to improve representation of female patients in government-sponsored research, less is known about sex disparities in the broader landscape of modern oncologic randomized controlled trials. Using ClinicalTrials.gov, we identified randomized controlled trials related to colorectal or lung cancer (the two most common non-sex-specific disease sites). Among the 147 included trials, the proportion of female patients enrolled on trial was on average 6.8% (95% confidence interval = −8.8% to –4.9%) less than the proportion of female patients in the population by disease site (P < .001). Whereas no statistically significant underrepresentation of women was noted within the 26 cooperative group trials, sex disparities were markedly heightened for the 121 noncooperative-group-sponsored trials. Furthermore, underrepresentation of women did not improve with time. Future efforts should therefore focus on addressing these pervasive sex-based enrollment disparities beyond cooperative group trials alone.
Two decades ago, pivotal evidence demonstrated marked underrepresentation of women enrolled in cancer clinical trials funded by the National Cancer Institute (NCI) (1). Concurrently, federal initiatives were implemented to boost enrollment of female patients in National Institutes of Health sponsored research (2). Since that time, sex disparities among certain site-specific subgroups of NCI-sponsored trials appear to have improved (3,4). The growth of trials with tumor molecular subtype enrollment criteria, particularly in lung cancer, may select for a greater proportion of women (3,4). With limited data on sex-based enrollment disparities among the broader landscape of oncologic trials, we hypothesized that sex disparities exist for cancer clinical trial patients, particularly for those enrolled on non-NCI-supported trials. Therefore, we examined sex disparities among modern oncologic randomized controlled trials (RCTs) for colorectal and lung cancer (the two most common non-sex-specific disease sites), identifying trial-specific factors associated with underrepresentation of women.
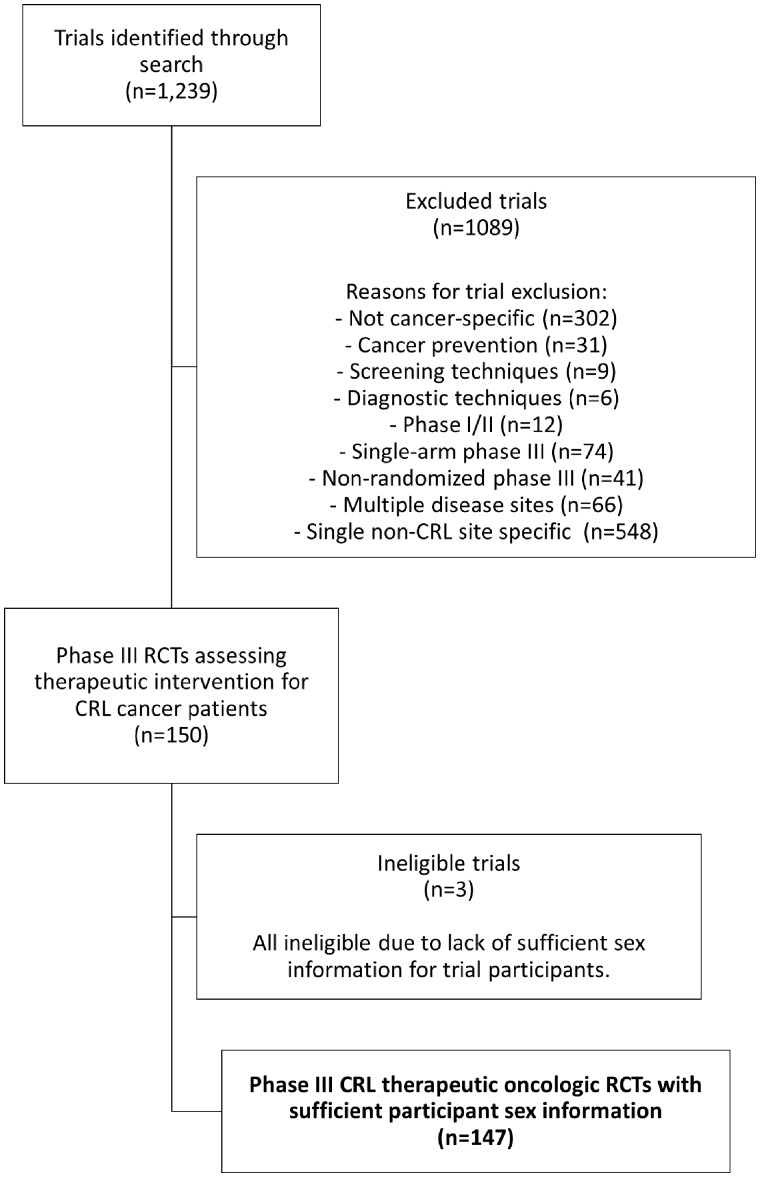
ClinicalTrials.gov was queried to identify colorectal and lung cancer RCTs using the following search parameters: terms: “cancer”; study type: “all studies”; status: excluded “not yet recruiting”; phase: phase III; and study results: “with results.” This yielded 1239 trials, which were then screened for cancer-specific phase III RCTs addressing a therapeutic intervention; only trials addressing single disease sites of colorectal or lung cancer were included (Figure 1). Trials that did not provide the proportion of female patients enrolled in the study were ineligible. For each trial, the proportion of female patients was compared to the proportion of female patients for the relevant disease site based on the NCI Surveillance, Epidemiology, and End Results (SEER) database (5). The SEER-based population proportion of female patients by disease site was also matched to the time period of trial enrollment. Statistical analyses included Wilcoxon signed rank, Mann-Whitney U, and Kruskal-Wallis tests, as well as linear regression modeling and Pearson correlation analysis; analyses were performed using SPSS (Version 22.0) (6). All tests were two-sided, and a P value of less than .05 was considered statistically significant.
Figure 1.
Flowchart of clinical trial screening, eligibility, and inclusion. CRL = colorectal/lung cancer; RCT = randomized controlled trial.
One hundred and forty-seven trials met inclusion criteria (Figure 1); these trials collectively enrolled a total of 100 907 patients, with years of enrollment initiation from 1996 to 2014. For each trial, the difference in proportion of female patients (DPF) was calculated, representing the trial proportion of female patients minus the population proportion of female patients by disease site. For all trials, the mean DPF was −6.8% (95% confidence interval [CI] = −8.8% to –4.9%; P < .001; Table 1). Sex disparities were less pronounced among cooperative group trials, with an mean DPF of −1.1% for cooperative-group-sponsored trials vs −8.0% for noncooperative-group-sponsored trials (P= .001; Table 1). For cooperative group studies only (n = 26), there was no difference between the proportion of female patients enrolled on trial compared with the population (average DPF = −1.1%; 95% CI = −4.9% to +2.5%; P= .51); however, for noncooperative-group-sponsored trials (n = 121), there was a statistically significant difference between the trial proportion of female patients and the population (mean DPF = −8.0%; 95% CI = −10.2% to −5.9%; P < .001). We then identified 16 trials (10.9%) restricted to patients with a molecular subtype associated with a larger proportion of female patients than that of the disease site more generally (such as ALK-rearranged or EGFR-mutant nonsmall-cell lung cancers). These molecular-subtype-restricted trials were associated with a greater proportion of female patients than unrestricted trials (mean DPF = +2.6% vs −8.0%; P= .006; Table 1). Notably, none of these 16 molecular-subtype-restricted studies were sponsored by a cooperative group. Therefore, upon exclusion of the subtype-restricted trials, the sex disparities between cooperative group and noncooperative group trials widened (mean DPF = −1.1% vs −9.7%, respectively; P< .001). Finally, examining all trials (n = 147), the DPF was analyzed by year of enrollment initiation; linear regression modeling revealed no statistically significant changes in sex disparities over time (estimated annual change of +0.1% in trial DPF; 95% CI = −0.5% to +0.7%; P = .68).
Table 1.
Trial factors associated with sex disparities
| Trial factor | No. | Mean DPF, % (95% CI) | P * |
|---|---|---|---|
| All included trials | 147 | −6.8 (−8.8 to −4.9) | <.001 |
| Cooperative group trial | |||
| Yes | 26 | −1.1 (−4.6 to +2.4) | .001 |
| No | 121 | −8.0 (−10.2 to −5.9) | — |
| Industry funding of trial | |||
| Yes | 131 | −7.1 (−9.2 to −5.0) | .18 |
| No | 16 | −4.4 (−8.5 to −0.3) | — |
| Molecular profile restriction criterion† | |||
| Yes | 16 | +2.6 (−3.4 to +8.6) | .006 |
| No | 131 | −8.0 (−9.9 to −6.0) | — |
| Disease site | |||
| Colorectal | 39 | −4.1 (−5.8 to −2.4) | .005 |
| Lung | 108 | −7.8 (−10.3 to −5.3) | — |
| Modality | |||
| Systemic therapy | 132 | −7.2 (−9.2 to −5.2) | .75 |
| Radiotherapy | 4 | −5.6 (−14.5 to +3.4) | — |
| Surgery | 1 | −4.8 (NA) | — |
| Supportive care | 10 | −3.2 (−13.3 to +6.9) | — |
| Systemic therapy subgroup‡ | |||
| Cytotoxic chemotherapy | 32 | −8.0 (−11.8 to −4.2) | .69 |
| Targeted therapy | 100 | −6.9 (−9.2 to −4.6) | — |
| Trial success (primary endpoint met) | |||
| Yes | 60 | −5.2 (−8.6 to −1.9) | .15 |
| No | 61 | −8.5 (−11.2 to −5.9) | — |
For all included trials (n = 147), the P value provided represents the results of a one-sample Wilcoxon signed rank test comparing the median DPF for all trials against a hypothetical population median DPF of 0%. All other P values provided reflect Mann-Whitney U tests or Kruskal-Wallis tests for each trial factor listed. CI = confidence interval; DPF = difference in proportion of female patients (trial minus population).
Molecular profile restriction trials were those that included an enrollment criterion that selected for female patients based on the molecular profile of patients’ tumors; this included trials specifically for patients with ALK-rearranged or EGFR-mutant nonsmall-cell lung cancer (both associated with higher proportions of female patients).
Systemic therapy trials were divided into cytotoxic chemotherapy and targeted therapy; the latter included monoclonal antibodies, small molecule inhibitors, and similar.
These data demonstrate substantial sex-based enrollment disparities among cancer clinical trial participants, primarily within noncooperative-group-supported trials. As cooperative group studies account for only a minority of RCTs in the modern era (17.8% of trials in this analysis), sex disparities among trial participants appear to be far more pervasive than previously reported (1,3,7–9). Although federal interventions focused on National Institutes of Health sponsored research may have improved representation of women among cooperative group trials, underrepresentation of women on the remainder of trials requires further investigation to better understand the basis for these disparities (2,10). This may involve assessment of eligibility criteria that disproportionately affect women, patient and physician perspectives, socioeconomic barriers to participation, equity in trial access, referral patterns for trial consideration, and more (10).
It is noteworthy that the population median age at diagnosis for both colorectal and lung cancers is slightly older for females than males (11). Data from our group recently demonstrated statistically significant age disparities among cancer clinical trial participants, with younger patients treated in colorectal and lung cancer trials than in the population by disease site (12). We assessed the effect of such an interaction between sex and age disparities by comparing trial DPF to the trial difference in median age (DMA); the DMA was calculated as the trial median age minus the SEER population median age by disease site (12). No correlation was found between DPF and DMA (Pearson correlation r = .11; P = .18). We further sought to confirm no effect of age disparities on DPF through a multiple regression model. This model confirmed independent effects of cooperative-group sponsorship (P = .001), use of molecular-subtype-restricted enrollment criteria (P < .001), and disease site (P = .02) on sex disparities; age disparities (DMA) were not associated with DPF in this model (P= .31).
Another potential limitation to the study is the inclusion of only two disease sites; the combination of colorectal and lung cancer trials may not be representative of the broader clinical trials landscape. Examining all non-sex-specific single-disease-site trials, the total enrollment of colorectal and lung trials accounts for 40.7% of trial enrollees (100 907 of 247 931 enrolled patients). Future studies are needed to examine sex-based disparities across other disease sites to assess generalizability of these data.
Sex disparities in participation in cancer RCTs are pervasive, predominantly among trials not sponsored by a cooperative group. Furthermore, underrepresentation of women does not appear to be improving with time. It is imperative that efforts be directed not only at understanding the basis for sex-based disparities among oncologic RCTs but also at identifying and implementing programs to promote enrollment of female patients on noncooperative group trials.
Funding
The authors report no funding for this work.
Notes
The authors report no financial disclosures or conflicts of interests related to this work.
References
- 1. Murthy VH, Krumholz HM, Gross CP.. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. NIH Revitalization Act. Subtitle B: §131-133; 1993.
- 3. Pang HH, Wang X, Stinchcombe TE, et al. Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. J Clin Oncol. 2016;34(33):3992–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fouad MN, Lee JY, Catalano PJ, et al. Enrollment of patients with lung and colorectal cancers onto clinical trials. J Oncol Pract. 2013;9(2):e40–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institutes of Health, National Cancer Institute, Surveillance Epidemiology and End Results Cancer Statistics. SEER Cancer Statistics Review 1975–2015: Table 1.1, Estimated New Cancer Cases and Deaths for 2018, All Races, By Sex. https://seer.cancer.gov/archive/csr/1975_2015/browse_csr.php? sectionSEL=1&pageSEL=sect_01_table.01. Accessed May 16, 2019.
- 6.IBM Corp. IBM SPSS Statistics for Windows, Version 22.0, 2013. Armonk, NY: IBM Corp. [Google Scholar]
- 7. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067. [DOI] [PubMed] [Google Scholar]
- 8. Booth CM, Cescon DW, Wang L, Tannock IF, Krzyzanowska MK.. Evolution of the randomized controlled trial in oncology over three decades. J Clin Oncol. 2008;26(33):5458–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun GH, Steinberg JD, Jagsi R.. The calculus of national medical research policy—the United States versus Asia. N Engl J Med. 2012;367(8):687–690. [DOI] [PubMed] [Google Scholar]
- 10. Jagsi R, Motomura AR, Amarnath S, Jankovic A, Sheets N, Ubel PA.. Under-representation of women in high-impact published clinical cancer research. Cancer. 2009;115(14):3293–3301. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health, National Cancer Institute, Surveillance Epidemiology, and End Results Cancer Statistics. SEER Cancer Statistics Review 1975-2015: Table 1.12, Median Age of Cancer Patients at Diagnosis, 2011–2015, By Primary Cancer Site, Race and Sex. https://seer.cancer.gov/csr/1975_2015/browse_csr.php? sectionSEL=1&pageSEL=sect_01_table.12. Accessed May 16, 2019.
- 12. Ludmir EB, Mainwaring W, Lin TA, et al. Factors associated with age disparities among cancer clinical trial participants [published online ahead of print June 3, 2019]. JAMA Oncol. 2019; doi:10.1001/jamaoncol.2019.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]