Abstract
Background
The Ontario Breast Screening Program expanded in July 2011 to screen high-risk women age 30–69 years with annual magnetic resonance imaging (MRI) and digital mammography. This study examined the benefits of screening with mammography and MRI by age and risk criteria.
Methods
This prospective cohort study included 8782 women age 30–69 years referred to the High Risk Ontario Breast Screening Program from July 2011 to June 2015, with final results to December 2016. Cancer detection rates, sensitivity, and specificity of MRI and mammography combined were compared with each modality individually within risk groups stratified by age using generalized estimating equation models. Prognostic features of screen-detected breast cancers were compared by modality using Fisher exact test. All P values are two-sided.
Results
Among 20 053 screening episodes, there were 280 screen-detected breast cancers (cancer detection rate = 14.0 per 1000, 95% confidence interval [CI] = 12.4 to 15.7). The sensitivity of mammography was statistically significantly lower than that of MRI plus mammography (40.8%, 95% CI = 29.3% to 53.5% vs 96.0%, 95% CI = 92.2% to 98.0%, P < .001). In mutation carriers age 30–39 years, sensitivity of the combination was comparable with MRI alone (100.0% vs 96.8%, 95% CI = 79.2% to 100.0%, P = .99) but with statistically significantly decreased specificity (78.0%, 95% CI = 74.7% to 80.9% vs 86.2%, 95% CI = 83.5% to 88.5%, P < .001). In women age 50–69 years, combining MRI and mammography statistically significantly increased sensitivity compared with MRI alone (96.3%, 95% CI = 90.6% to 98.6% vs 90.9%, 95% CI = 83.6% to 95.1%, P = .02), with a small but statistically significant decrease in specificity (84.2%, 95% CI = 83.1% to 85.2% vs 90.0%, 95% CI = 89.2% to 90.9%, P < .001).
Conclusions
Screening high risk women age 30–39 years with annual MRI only may be sufficient for cancer detection and should be evaluated further, particularly for mutation carriers. Among women age 50–69 years, detection is most effective when mammography is included with annual MRI.
Women who have inherited a highly penetrant breast cancer predisposition gene have an elevated lifetime risk of breast cancer compared with the general population. Among BRCA1/2 mutation carriers, the cumulative risk of developing breast cancer by age 70 years is 45% to 87% (1–3), and at an earlier age (4). Women with a strong family history of breast cancer or who have undergone therapeutic chest radiation before age 30 years are also at comparable increased risk (5–9).
Several observational studies have demonstrated that women at high risk for breast cancer based on their family history and/or genetic testing benefit from screening with breast magnetic resonance imaging (MRI) in addition to mammography (10–21). The combined sensitivity of MRI and mammography ranged from 90% to 100%, with breast cancers detected at a much earlier stage than with mammography alone in the same population (11,13,17,19,21).
Expert guidelines recommend that women with a lifetime breast cancer risk of 20% or greater (22–25) or a history of chest radiation therapy (26) begin annual screening with MRI and mammography at age 25–30 years. However, it is unknown whether the performance of MRI screening in previous observational studies or academic centers can also be achieved in a community setting. Uncertainties also exist regarding whether recommendations for high-risk women should be individualized dependent on their age and/or risk criteria. Some groups have reported a higher interval cancer rate in younger women with BRCA1 mutations (18) and have suggested that MRI screening every 6 months might be more appropriate. The National Institute for Health and Care Excellence guidelines recommend annual MRI screening of high-risk women primarily from age 30–49 years (27). The value of adding mammography to MRI for women younger than 40 years has also been questioned given the higher breast density and lower sensitivity of mammography in this age group (17), particularly for BRCA1/2 mutation carriers (28,29) and women with a strong familial breast cancer risk (30). Recent studies among women with prior chest radiation therapy have reported an improvement in screening sensitivity with the addition of mammography to MRI (31–34), with minimal decrease in specificity (32).
Based on recommendations from the Ontario Program in Evidence-Based Care (25) and the Ontario Health Technology Advisory Committee (35), the Ontario Breast Screening Program (OBSP) expanded its services in 2011 to include annual MRI in addition to digital mammography for high-risk women age 30–69 years (36). Follow-up of this large cohort screened at 30 centers across the province provides a unique opportunity to examine the benefits and harms of the combination of mammography and MRI within a population-based screening program. Because all women are screened both with MRI and mammography, performance measures can be compared with each individually within risk groups stratified by age. Prognostic features of screen-detected cancers were also compared by modality.
Methods
Study Cohort
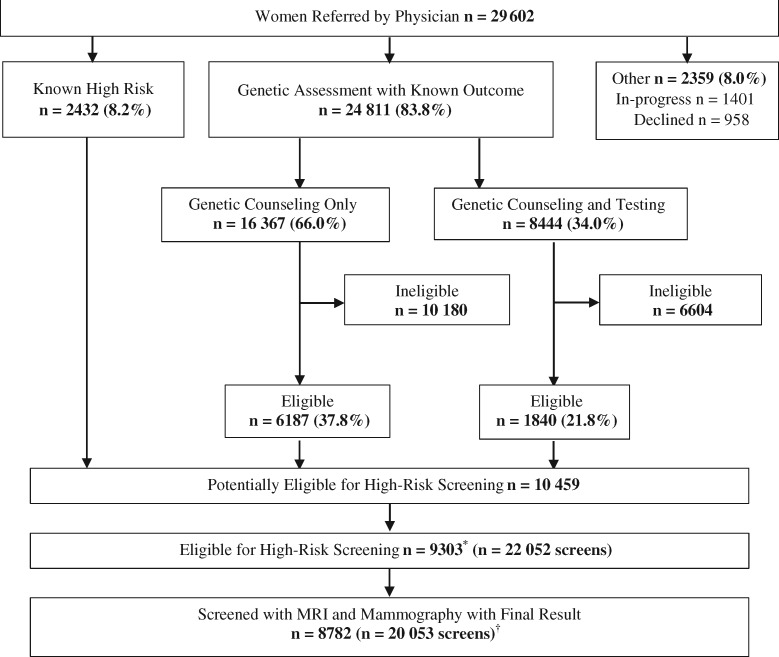
The cohort was identified from 29 602 asymptomatic women age 30–69 years referred by their physician to the High Risk OBSP between July 1, 2011, and June 30, 2015, with a final result to December 31, 2016 (Figure 1). Of these women, 2432 (8.2%) were previously known to meet one of the eligibility criteria and 24 811 (83.8%) completed genetic assessment. Of the 10 459 women eligible for screening, 1156 either declined (n = 403), deferred (n = 282), died (n = 3), had bilateral mastectomy (n = 146), or were not enrolled for reasons unknown (n = 322). Among the 9303 women who underwent screening, screening episodes that included only a mammogram (n = 577) or MRI (or ultrasound) (n = 689) were excluded because the other screening test was unavailable. Screening episodes that included an ultrasound and mammogram (n = 649) or breast cancers found incidentally on prophylactic mastectomy (n = 15) and those without a final result (n = 69) were also excluded. The study was approved by the University of Toronto Research Ethics Board, and informed consent was not required.
Figure 1.
Women age 30–69 years referred to the High Risk Ontario Breast Screening Program between July 1, 2011, and June 30, 2015, with final result to December 31, 2016. *Excludes 1156 women who declined (n = 403), deferred (n = 282), died (n = 3), had bilateral mastectomy (n = 146), or reasons unknown (n = 322). †Excludes 521 women with mammogram-only screens (n = 577), MRI (or ultrasound)-only screens (n = 689), mammogram and ultrasound screens (n = 649), mammogram and MRI screens for breast cancers found incidentally on prophylactic mastectomy (n = 15), or screens without a final result (n = 69). MRI = magnetic resonance imaging.
Clinical Pathway
A clinical pathway was developed for the identification, referral, and genetic assessment of asymptomatic women at potential high risk (37). High-risk criteria included: women with a deleterious mutation in BRCA1/2 or other gene(s) predisposing to a markedly elevated breast cancer risk; untested first-degree relatives of a gene mutation carrier; women with a family history and an estimated personal lifetime breast cancer risk of 25% or higher; and women who had radiation therapy to the chest (before age 30 years and at least 8 years previously). Women who met at least one of the high-risk criteria were eligible even if they had a prior history of breast and/or other cancers or breast implants or had a unilateral mastectomy.
Women were assessed for risk of breast cancer and referred to the program by their physician. If there was prior knowledge that the woman met at least one of the high risk criteria, she was enrolled and eligible for screening. If the woman was a first-degree relative of a mutation carrier and had not previously had genetic assessment or had a personal or family history of breast or ovarian cancer suggestive of a hereditary breast cancer syndrome, she was referred to a genetics clinic for further risk assessment by a genetic counsellor. Women found to be mutation carriers and/or assessed as having a 25% or greater personal lifetime risk of breast cancer based on the International Breast Cancer Intervention Study model (38) or the Breast and Ovarian Analysis of Disease Incidence of Carrier Estimation Algorithm (39) were enrolled. Women with contraindications to MRI were scheduled for breast ultrasound (12,13,19).
At High Risk OBSP centers, quality assurance on the equipment met that specified by the Canadian Association of Radiologists’ Mammography Accreditation Program, and radiologists and technologists were accredited under the Canadian Association of Radiologists’ Mammography Accreditation Program. Centers conducted MRI-guided biopsy on site or had a partnership with a facility that did and were affiliated with a Breast Assessment Site. The minimum MRI standards included a 1.5 Tesla magnet, gadolinium enhancement (0.1–0.2 mmol/kg), and a dedicated breast coil with bilateral axial or sagittal acquisition.
Diagnostic interpretation of lesions detected on mammograms or MRIs was based on assessment categories of the American College of Radiology Breast Imaging Reporting and Data System 5th edition (40). Screening or assessment results reported as “normal/benign” (categories 1 and 2) were considered negative results, whereas results reported as “probably benign” (category 3), “abnormal requiring further imaging” (category 0), or “suspicious for breast cancer” (category 4 or 5) were considered positive results.
Mammograms were usually performed before MRI studies, within 1 month of each other. Although different radiologists may interpret mammogram and MRI studies, they were aware of the mammogram results before interpreting MRI studies as well as all previous imaging and clinical history. If either or both modalities were positive based on referral criteria, the radiologists indicated their recommendations for assessment. Masses were biopsied if either MRI and/or mammography features were suspicious for malignancy and were pathologically confirmed to be benign, ductal carcinoma in situ (DCIS), or invasive cancer.
Data Collection
Data were obtained from routine information collected for all women screened within the High Risk OBSP from Cancer Care Ontario’s Integrated Client Management System database. The requisition for high-risk screening form included data on physician visit date, referral method, high-risk criteria, and medical history. For women referred for genetic assessment, the breast cancer genetic assessment results form collected data on genetic counseling and/or testing dates and results, and screening eligibility. Information on screening dates, type (initial, rescreen), and results were recorded on the screening report, and details about diagnostic imaging or biopsy dates and outcomes on the assessment results form. Women with interval cancers were identified from record linkage using AutoMatch (41) with the Ontario Cancer Registry estimated to be 98% complete for breast cancer (42).
Tumor characteristics were collected from surgical and pathological reports obtained from the Ontario Cancer Registry for any primary DCIS or invasive breast cancer of any histologic type diagnosed after enrollment. Reports were reviewed by a trained abstractor and overseen by a breast pathologist (SJD). Tumor size was defined as the greatest diameter of the largest invasive carcinoma. Lymph node status was defined as positive if at least one sentinel or other axillary lymph node contained at least one cluster of malignant cells larger than 0.2 mm. For breast cancers treated with neoadjuvant therapy, tumor size and nodal status were defined at the time of diagnosis, before treatment initiation. Tumor size for neoadjuvant cases was estimated to be larger than 2 cm unless otherwise specified in the pathology and any referenced imaging report.
Tumor morphology was coded using the International Classification of Diseases for Oncology (43). Stage was coded using the TNM classification scheme (44). Tumors were graded using the Nottingham combined histologic grading system (45). Estrogen, progesterone, and HER2 protein receptors were coded as positive or negative (46–48).
Performance Measures
The performance measure definitions used for this study were primarily those adopted by the Canadian Partnership Against Cancer (49) and other international screening programs (50,51). Abnormal recall rate was defined as the percentage of screens referred for further testing because of an abnormal screening result. Biopsy rate was defined as the percentage of screens with a core or open surgical biopsy. Cancer detection rate was defined as the number of screen-detected invasive or DCIS breast cancers per 1000 screening examinations. For screening episodes with a final result, screen-detected cancers (true positive) were defined as breast cancers diagnosed within 12 months of a positive screening MRI and/or mammogram. Interval cancers (false negative) were defined as cancers diagnosed within 12 months of a negative screening MRI and mammogram. Sensitivity was defined as the percentage of all cancers (true positive + false negative) that were screen-detected (true positive). Specificity was defined as the percentage of women without breast cancer (true negative + false positive) who had a true negative screening MRI and mammogram. To allow for a follow-up period of 12 months from the start of screening, sensitivity and specificity analysis included screening episodes up to December 31, 2015.
Statistical Analysis
The unit for analyses was the screening episode including MRI and mammography with a final result after recommended work-up and/or biopsy; women may have had more than one screening round during the study. Performance measures were examined by screening result based on the following definitions: “mammography” refers to an abnormal mammography result, irrespective of MRI result; “MRI” refers to an abnormal MRI result irrespective of mammography result; “MRI plus mammography” refers to at least one modality having an abnormal result. Cancer detection rate, sensitivity, and specificity were further stratified by risk criteria and age group at index screen. A generalized estimating equation model using a binomial distribution with logit link function and independent working correlation matrix was used to compare performance measures by screening result overall and in stratified analyses with MRI plus mammography as the reference. Multivariable models adjusted for factors related to women and screen type (52). The inversed logits were used to estimate performance measures and approximate 95% confidence intervals (CIs) by screening result based on the least squares means of fixed effects.
Prognostic features of screen-detected primary breast cancers were examined in three mutually exclusive groups. “Mammography only” refers to an abnormal mammography result and a normal MRI result. “MRI only” refers to an abnormal MRI result and a normal mammography result. “MRI and mammography” refers to an abnormal MRI and mammogram result. Fisher exact test was performed to compare prognostic characteristics of screen-detected cancers by screening result.
Analyses were conducted using SAS version 9.4 (53). A two-tailed 5% statistical significance level was used for statistical tests.
Results
The final cohort included 8782 women who underwent 20 053 MRI and mammography screening examinations with a final result (Figure 1). Women excluded (n = 521) were more likely to be age 50–69 years (34.7% vs 26.4%; P < .001) or postmenopausal (41.4% vs 36.2%; P = .03). MRIs (94.0%) were usually within 30 days of the mammogram, and 85.5% of screening episodes were within 15 months (mean = 13.5 months).
The majority of screened women (73.6%) were age 30–49 years at their initial screening examination, had at least two screening rounds (68.3%), were premenopausal (62.2%), and had a family history and estimated personal lifetime breast cancer risk of 25% or higher (72.6%) (Table 1).
Table 1.
Characteristics of women and screens by age at screen (first, index), number of screening episodes, menopausal status, risk criteria, and prior breast cancer
| Characteristic | Women (n = 8782) | Screens (n = 20 053) |
|---|---|---|
| No. (%) | No. (%) | |
| Age at screen (first, index), y | ||
| 30–39 | 3029 (34.5) | 5589 (27.9) |
| 40–49 | 3434 (39.1) | 8051 (40.2) |
| 50–69 | 2319 (26.4) | 6413 (32.0) |
| No. of screening episodes | ||
| 1 | 2790 (31.8) | 8782 (43.8) |
| 2 | 2667 (30.4) | 5992 (29.9) |
| 3 | 1757 (20.0) | 3325 (16.6) |
| 4–5 | 1568 (17.9) | 1954 (9.7) |
| Menopausal status | ||
| Pre- | 5465 (62.2) | 11 762 (58.7) |
| Post- | 3107 (35.4) | 7957 (39.7) |
| Unknown | 210 (2.4) | 334 (1.7) |
| Risk criteria* | ||
| Known mutation carrier | 1885 (21.5) | 4812 (24.0) |
| BRCA1 | 851 (45.2) | 2118 (44.0) |
| BRCA2 | 869 (46.1) | 2217 (46.1) |
| BRCA1 and BRCA2 | 8 (0.4) | 21 (0.4) |
| Other mutation | 157 (8.3) | 456 (9.5) |
| Family history and ≥25% risk | 6376 (72.6) | 13 896 (69.3) |
| Untested, first-degree relative | 197 (2.2) | 466 (2.3) |
| Chest radiation therapy | 324 (3.7) | 879 (4.4) |
| Prior breast cancer | ||
| No | 8512 (96.9) | 19 427 (96.9) |
| Yes | 270 (3.1) | 626 (3.1) |
If a woman met more than one risk criterion, the following hierarchy was selected to classify her: known carrier; family history and ≥25% lifetime risk; untested, first-degree relative; chest radiation therapy.
The biopsy rate was highest for MRI plus mammography (7.1%) (Table 2). There were 280 screen-detected breast cancers (cancer detection rate: 14.0 per 1000, 95% CI = 12.4 to 15.7). The cancer detection rate for MRI plus mammography was higher on the initial screens (17.1 per 1000, 95% CI = 14.6 to 20.0) compared with rescreens (11.5 per 1000, 95% CI = 9.7 to 13.7). By age, the detection rate for MRI plus mammography was greater among women age 50–69 years (19.3 per 1000, 95% CI = 16.2 to 23.0). By risk group, the detection rate for MRI plus mammography was highest among known mutation carriers (26.8 per 1000, 95% CI = 22.6 to 31.8).
Table 2.
Abnormal screens, biopsies, and cancers detected by screen type, age group, and risk criteria*
| Performance measures | MRI plus mammography† |
Mammography† |
MRI† |
|||||
|---|---|---|---|---|---|---|---|---|
| No. | Rate (95% CI) | No. | Rate (95% CI) | P ‡ | No. | Rate (95% CI) | P ‡ | |
| Abnormal screens, %§ | 4472 | 22.3 (21.7 to 22.9) | 2003 | 10.0 (9.6 to 10.4) | <.001 | 3121 | 15.6 (15.0 to 16.1) | <.001 |
| Initial | 2594 | 29.5 (28.6 to 30.5) | 1115 | 12.7 (12.0 to 13.4) | <.001 | 1922 | 21.9 (21.0 to 22.8) | <.001 |
| Rescreen | 1878 | 16.7 (16.0 to 17.4) | 888 | 7.9 (7.4 to 8.4) | <.001 | 1199 | 10.6 (10.1 to 11.2) | <.001 |
| Biopsies, %§ | 1429 | 7.1 (6.8 to 7.5) | 426 | 2.1 (1.9 to 2.3) | <.001 | 1315 | 6.6 (6.2 to 6.9) | <.001 |
| Initial | 845 | 9.6 (9.0 to 10.3) | 265 | 3.0 (2.7 to 3.4) | <.001 | 786 | 9.0 (8.4 to 9.6) | <.001 |
| Rescreen | 584 | 5.2 (4.8 to 5.6) | 161 | 1.4 (1.2 to 1.7) | <.001 | 529 | 4.7 (4.3 to 5.1) | <.001 |
| Cancers detected per 1000 screens‡,‖ | 280 | 14.0 (12.4 to 15.7) | 109 | 5.4 (4.5 to 6.6) | <.001 | 263 | 13.1 (11.6 to 14.8) | .24 |
| Screen type | ||||||||
| Initial | 150 | 17.1 (14.6 to 20.0) | 60 | 6.8 (5.3 to 8.8) | <.001 | 144 | 16.4 (13.9 to 19.3) | .11 |
| Rescreen | 130 | 11.5 (9.7 to 13.7) | 49 | 4.3 (3.3 to 5.7) | <.001 | 119 | 10.6 (8.8 to 12.6) | .25 |
| Age at index screen, y | ||||||||
| 30–39 | 60 | 10.7 (8.3 to 13.9) | 26 | 4.7 (3.2 to 6.8) | <.001 | 57 | 10.2 (7.8 to 13.3) | .62 |
| 40–49 | 96 | 11.9 (9.8 to 14.5) | 35 | 4.3 (3.1 to 6.1) | <.001 | 90 | 11.2 (9.1 to 13.7) | .44 |
| 50–69 | 124 | 19.3 (16.2 to 23.0) | 48 | 7.5 (5.6 to 9.9) | <.001 | 116 | 18.1 (15.1 to 21.7) | .41 |
| Risk criteria¶ | ||||||||
| Known mutation carrier | 129 | 26.8 (22.6 to 31.8) | 52 | 10.8 (8.2 to 14.2) | <.001 | 121 | 25.1 (21.1 to 30.0) | .42 |
| Family history and ≥25% risk# | 131 | 9.4 (7.9 to 11.2) | 48 | 3.5 (2.6 to 4.6) | <.001 | 122 | 8.8 (7.3 to 10.5) | .36 |
| Untested, first-degree relative | 5 | 10.7 (4.5 to 25.6) | 3 | 6.4 (2.1 to 19.9) | .24 | 5 | 10.7 (4.5 to 25.6) | .99 |
| Chest radiation therapy | 15 | 17.1 (10.3 to 28.1) | 6 | 6.8 (3.1 to 15.1) | .01 | 15 | 17.1 (10.3 to 28.1) | .99 |
n = 20 053 screens: initial n = 8782; rescreen n = 11 271. CI = confidence interval; MRI = magnetic resonance imaging.
Mammography refers to an abnormal mammogram result irrespective of MRI result; MRI refers to an abnormal MRI result irrespective of mammogram result; MRI plus mammography refers to an abnormal MRI and/or abnormal mammogram result.
Compared with MRI plus mammography.
Adjusted for repeated measures within women.
n = 280 cancers among n = 278 women.
If a woman met more than one risk criterion, the following hierarchy was selected to classify her: known carrier; family history ≥25% lifetime risk; untested, first-degree relative; chest radiation therapy.
Based on International Breast Cancer Intervention Study and/or Breast and Ovarian Analysis of Disease Incidence of Carrier Estimation Algorithm.
Sensitivity was 96.0% (95% CI = 92.2% to 98.0%) for MRI plus mammography, and as single modalities 40.8% (95% CI = 29.3% to 53.5%) for mammography and 90.8% (95% CI = 84.7% to 94.7%) for MRI (Table 3). Among age groups, a statistically significant increase in sensitivity for the combination compared with MRI alone was observed for women age 40–49 years (92.4%, 95% CI = 85.1% to 96.3% vs 85.9%, 95% CI = 77.3% to 91.6%, P = .01) and age 50–69 years (96.3%, 95% CI = 90.6% to 98.6% vs 90.9%, 95% CI = 83.6% to 95.1%, P = .02). Of the screen-detected cancers in mutation carriers, MRI missed one of 26 cancers in those age 30–39 years and one of 32 cancers in those age 40–49 years. However, in mutation carriers age 50–69 years, the combination found five (9.2%) more breast cancers than MRI alone (92.7%, 95% CI = 82.1% to 97.2% vs 83.5%, 95% CI = 71.0% to 91.3%, P = .02). Of the screen-detected cancers in women with a family history and lifetime risk of 25% or higher, MRI missed two of 23 cancers in those age 30–39 years, five of 45 cancers in those age 40–49 years, and one of 49 cancers in those age 50–69 years.
Table 3.
Adjusted sensitivity (%) and 95% CI stratified by age group and risk criteria (n = 257)
| Characteristics | Total cancers (screen-detected + interval)† | MRI plus mammography* |
Mammography* |
MRI* |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Screen-detected cancers | Sensitivity (95% CI) | Screen-detected cancers | Sensitivity (95% CI) | P ‡ | Screen-detected cancers | Sensitivity (95% CI) | P ‡ | ||
| Overall§ | 257 | 245 | 96.0 (92.2 to 98.0) | 97 | 40.8 (29.3 to 53.5) | <.001 | 230 | 90.8 (84.7 to 94.7) | <.001 |
| Age at index screen, y | |||||||||
| 30–39‖ | 55 | 54 | 98.2 (88.6 to 99.7) | 24 | 43.1 (30.8 to 56.3) | <.001 | 51 | 92.7 (82.3 to 97.2)| | .10 |
| 40–49‖ | 94 | 87 | 92.4 (85.1 to 96.3) | 32 | 32.9 (24.1 to 43.1) | <.001 | 81 | 85.9 (77.3 to 91.6) | .01 |
| 50–69‖ | 108 | 104 | 96.3 (90.6 to 98.6) | 41 | 38.2 (29.4 to 47.9) | <.001 | 98 | 90.9 (83.6 to 95.1) | .02 |
| Known carrier, ¶ | 115 | 109 | 94.8 (89.0 to 97.6) | 45 | 38.7 (30.0 to 48.1) | <.001 | 102 | 88.7 (81.6 to 93.2) | <.001 |
| 30–39# | 26 | 26 | 100.0 | 9 | 33.3 (17.5 to 54.1) | <.001 | 25 | 96.8 (79.2 to 100.0) | .99 |
| 40–49‖ | 34 | 32 | 94.2 (81.0 to 98.4) | 11 | 31.6 (17.7 to 49.8) | <.001 | 31 | 91.3 (77.4 to 97.0) | .31 |
| 50–69‖ | 55 | 51 | 92.7 (82.1 to 97.2) | 25 | 45.2 (32.1 to 58.9) | <.001 | 46 | 83.5 (71.0 to 91.3)| | .02 |
| Family history and ≥25% risk, ¶, ** | 122 | 117 | 95.9 (90.5 to 98.3) | 43 | 35.2 (27.2 to 44.2) | <.001 | 109 | 89.3 (82.4 to 93.7) | <.001 |
| 30–39# | 23 | 23 | 100.0 | 11 | 47.8 (28.8 to 67.5) | <.001 | 21 | 91.3 (71.1 to 100.0) | .99 |
| 40–49‖ | 50 | 45 | 89.6 (77.5 to 95.6) | 18 | 34.7 (22.8 to 48.9) | <.001 | 40 | 79.3 (65.9 to 88.4) | .02 |
| 50–69# | 49 | 49 | 100.0 | 14 | 28.1 (17.2 to 42.4) | <.001 | 48 | 98.1 (87.3 to 100.0) | .99 |
| Untested, first-degree relative¶,#, †† | 5 | 5 | 100.0 | 3 | 63.6 (17.2 to 93.7) | <.001 | 5 | 100.0 | .99 |
| Chest radiation therapy¶,#, †† | 15 | 14 | 93.9 (77.8 to 98.5) | 6 | 39.5 (16.7 to 68.1) | <.001 | 14 | 93.9 (77.8 to 98.5) | .99 |
Mammography refers to an abnormal mammogram result irrespective of MRI result; MRI refers to an abnormal MRI result irrespective of mammogram result; MRI plus mammography refers to an abnormal MRI and/or abnormal mammogram result. CI = confidence interval; MRI = magnetic resonance imaging.
n = 257 cancers among n = 256 women (n = 245 screen-detected cancers among n = 244 women; n = 12 interval cancers among n = 12 women).
Compared with MRI plus mammography.
Adjusted for age at index screen, screen type (initial, rescreen), prior breast cancer, and risk criteria.
Adjusted for age at index screen and screen type (initial, rescreen).
If a woman met more than one risk criterion, the following hierarchy was selected to classify her: known carrier; family history ≥25% lifetime risk; untested, first-degree relative; chest radiation therapy.
Adjusted for age at index screen.
Based on International Breast Cancer Intervention Study and/or Breast and Ovarian Analysis of Disease Incidence of Carrier Estimation Algorithm.
Excluded from stratified analysis by age group because of small numbers.
The combination statistically significantly reduced specificity to 81.0% (95% CI = 78.7% to 83.1%) compared with 91.8% (95% CI = 90.6% to 92.8%) for mammography alone and 87.3% (95% CI = 85.6% to 88.9%) for MRI alone (Table 4). By age, the smallest decrease in specificity from adding mammography to MRI compared with MRI alone was among women age 50–69 years (84.2%, 95% CI = 83.1% to 85.2% vs 90.0%, 95% CI = 89.2% to 90.9%, P < .001). When stratified by risk and age group, the largest decrease in specificity between the combination and MRI was among mutation carriers age 30–39 years (78.0%, 95% CI = 74.7% to 80.9% vs 86.2%, 95% CI = 83.5% to 88.5%, P < .001).
Table 4.
Adjusted specificity (%) and 95% CI stratified by age group and risk criteria (n = 16 915 screens)
| Characteristics | True-negative + false-positive tests | MRI plus mammography* |
Mammography* |
MRI* |
|||
|---|---|---|---|---|---|---|---|
| True- negative tests | Specificity (95% CI) | True- negative tests | Specificity (95% CI) | True- negative tests | Specificity (95% CI) | ||
| Overall† | 16 915 | 13 168 | 81.0 (78.7 to 83.1) | 15 217 | 91.8 (90.6 to 92.8)‡ | 14 354 | 87.3 (85.6 to 88.9)‡ |
| Age at index screen, y | |||||||
| 30–39§ | 4868 | 3527 | 74.1 (72.7 to 75.4) | 4262 | 88.6 (87.6 to 89.5)‡ | 3921 | 81.9 (80.8 to 83.1)‡ |
| 40–49§ | 6770 | 5199 | 77.0 (76.0 to 78.1) | 6091 | 90.2 (89.5 to 90.9)‡ | 5686 | 84.3 (83.4 to 85.2)‡ |
| 50–69§ | 5277 | 4442 | 84.2 (83.1 to 85.2) | 4864 | 92.3 (91.5 to 93.0)‡ | 4747 | 90.0 (89.2 to 90.9)‡ |
| Known carrier, ‖ | 4209 | 3525 | 84.2 (83.0 to 85.4) | 3874 | 92.5 (91.6 to 93.3)‡ | 3775 | 90.2 (89.2 to 91.1)‡ |
| 30–39§ | 844 | 649 | 78.0 (74.7 to 80.9) | 742 | 88.7 (86.3 to 90.7)‡ | 720 | 86.2 (83.5 to 88.5)‡ |
| 40–49§ | 1146 | 916 | 80.0 (77.4 to 82.3) | 1043 | 91.2 (89.3 to 92.7)‡ | 995 | 87.0 (84.8 to 88.9)‡ |
| 50–69§ | 2219 | 1960 | 88.0 (86.5 to 89.4) | 2089 | 94.0 (92.9 to 95.0)‡ | 2060 | 92.7 (91.5 to 93.7)‡ |
| Family history & ≥25% risk, ‖, ¶ | 11 545 | 8 720 | 76.3 (75.5 to 77.1) | 10 279 | 89.6 (89.0 to 90.2)‡ | 9591 | 83.8 (83.1 to 84.5)‡ |
| 30–39§ | 3603 | 2575 | 73.3 (71.7 to 74.9) | 3143 | 88.4 (87.3 to 89.4)‡ | 2874 | 81.3 (80.0 to 82.7)‡ |
| 40–49§ | 5191 | 3933 | 76.1 (74.9 to 77.3) | 4652 | 89.9 (89.0 to 90.7)‡ | 4312 | 83.4 (82.4 to 84.4)‡ |
| 50–69§ | 2751 | 2212 | 80.4 (78.8 to 81.9) | 2484 | 90.4 (89.2 to 91.5)‡ | 2405 | 87.5 (86.2 to 88.7)‡ |
| Untested, first-degree relative§, ‖, # | 402 | 322 | 81.5 (77.0 to 85.3) | 370 | 93.0 (89.9 to 95.2)‡ | 344 | 86.9 (83.2 to 89.9)‡ |
| Chest radiation therapy§, ‖, # | 759 | 601 | 79.4 (75.8 to 82.5) | 694 | 91.7 (89.3 to 93.6)‡ | 644 | 85.1 (82.2 to 87.7)‡ |
Mammography refers to an abnormal mammogram result irrespective of MRI result; MRI refers to an abnormal MRI result irrespective of mammogram result; MRI plus mammography refers to an abnormal MRI and/or abnormal mammogram result. CI = confidence interval; MRI = magnetic resonance imaging.
Adjusted for age at index screen, screen type (initial, rescreen), prior breast cancer, and risk criteria.
Compared with MRI plus mammography (P < .001).
Adjusted for age at index screen and screen type (initial, rescreen).
If a woman met more than one risk criterion, the following hierarchy was selected to classify her: known carrier; family history ≥25% lifetime risk; untested, first-degree relative; chest radiation therapy.
Based on International Breast Cancer Intervention Study and/or Breast and Ovarian Analysis of Disease Incidence of Carrier Estimation Algorithm.
Excluded from stratified analysis by age group because of small numbers.
Of the 57 screen-detected DCIS cases, mammography detected only five (8.8%) missed by MRI, whereas MRI detected 36 (63.1%) missed by mammography (Table 5). Of the 28 low-grade invasive cancers, mammography detected only two (7.1%) missed by MRI compared with 22 (78.6%) detected by MRI. MRI-detected invasive cancers were more likely to be stage I (88.6%) and lymph node negative (96.2%) compared with cancers seen on both modalities. The majority of the 12 interval cancers were invasive (66.7%), and of these, a higher proportion were histologic grade III (75%), stage I (71.4%), and none was diagnosed in mutation carriers age 30 to 39 years (Table 6).
Table 5.
Prognostic characteristics of first primary screen-detected breast cancers by screening result (n = 244)*
| Characteristics | All cancers No. (%) | Screening result† |
||
|---|---|---|---|---|
| MRI and mammography (n = 87) |
Mammography only (n = 12) |
MRI only (n = 145) |
||
| No. (%) | ||||
| Breast cancer type | ||||
| DCIS | 57 (23.4) | 16 (18.4) | 5 (41.7) | 36 (24.8) |
| Invasive | 187 (76.6) | 71 (81.6) | 7 (58.3) | 109 (75.2) |
| Tumor size, cm | ||||
| ≤1.0 | 92 (52.9) | 27 (43.5) | 4 (57.1) | 61 (58.1) |
| 1.1–2.0 | 55 (31.6) | 21 (33.9) | 1 (14.3) | 33 (31.4) |
| >2.0 | 27 (15.5) | 14 (22.6) | 2 (28.6) | 11 (10.5) |
| Missing‡ | 13 | 9 | 0 | 4 |
| Histologic grade | ||||
| 1 | 28 (16.3) | 4 (6.2) | 2 (28.6)§ | 22 (22.0)§ |
| 2 | 83 (48.3) | 31 (47.7) | 4 (57.1) | 48 (48.0) |
| 3 | 61 (35.5) | 30 (46.2) | 1 (14.3) | 30 (30.0) |
| Missing | 15 | 6 | 0 | 9 |
| Lymph nodes | ||||
| Positive | 26 (14.6) | 21 (32.3) | 1 (14.3) | 4 (3.8) |
| Negative | 152 (85.4) | 44 (67.7) | 6 (85.7) | 102 (96.2)§ |
| Missing | 9 | 6 | 0 | 3 |
| Stage at diagnosis | ||||
| I | 139 (80.3) | 41 (67.2) | 5 (71.4) | 93 (88.6)§ |
| II | 31 (17.9) | 17 (27.9) | 2 (28.6) | 12 (11.4) |
| III | 3 (1.7) | 3 (4.9) | 0 (0.0) | 0 (0.0) |
| Missing‡ | 14 | 10 | 0 | 4 |
| Estrogen receptor | ||||
| Positive | 133 (73.9) | 50 (73.5) | 6 (85.7) | 77 (73.3) |
| Negative | 47 (26.1) | 18 (26.5) | 1 (14.3) | 28 (26.7) |
| Missing | 7 | 3 | 0 | 4 |
| Progesterone receptor | ||||
| Positive | 116 (64.4) | 43 (63.2) | 5 (71.4) | 68 (64.8) |
| Negative | 64 (35.6) | 25 (36.8) | 2 (28.6) | 37 (35.2) |
| Missing | 7 | 3 | 0 | 4 |
| HER2 | ||||
| Positive | 18 (10.4) | 10 (15.4) | 0 (0.0) | 8 (7.9) |
| Negative | 155 (89.6) | 55 (84.6) | 7 (100.0) | 93 (92.1) |
| Missing | 14 | 6 | 0 | 8 |
| Triple negative | ||||
| No | 138 (77.1) | 52 (76.5) | 6 (85.7) | 80 (76.9) |
| Yes | 41 (22.9) | 16 (23.5) | 1 (14.3) | 24 (23.1) |
| Missing | 8 | 3 | 0 | 5 |
Excludes women with a prior breast cancer (n = 34): MRI and mammography (n = 5); MRI only (n = 24); mammography only (n = 5). DCIS = ductal carcinoma in situ; MRI = magnetic resonance imaging.
MRI and mammography refers to an abnormal result both on MRI and mammography; MRI only refers to an abnormal result on MRI only; mammography only refers to an abnormal result on mammography only.
Includes cancers treated with neoadjuvant therapy (n = 8 MRI and mammography; n = 1 MRI only).
Compared with MRI and mammography (P < .05).
Table 6.
Prognostic characteristics of interval breast cancers (n = 12)
| No. | Risk criteria | Prior breast cancer | Age at diagnosis, y | Screen type | Breast cancer type | Histologic grade | Invasive tumor size, cm | Lymph nodes | Invasive stage at diagnosis | Receptor status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | BRCA1 carrier | Yes | 48 | Initial | Invasive | 3 | 1.7 | Negative | 1 | ER(+) PR(+)* |
| 2 | Other known carrier | No | 66 | Initial | Invasive | 3 | 0.8 | Positive | 1 | ER(+) PR(+)* |
| 3 | BRCA2 carrier | No | 69 | Rescreen | DCIS† | 3 | —† | —† | —† | —† |
| 4 | BRCA2 carrier | No | 52 | Rescreen | Invasive | 3 | 0.7 | Negative | 1 | ER(−) PR(−) HER2(−) |
| 5 | BRCA1 & BRCA2 carrier | No | 65 | Initial | Invasive | 3 | 0.6 | Negative | 1 | ER(−) PR(−) HER2(−) |
| 6 | BRCA2 carrier | No | 46 | Rescreen | Invasive | 3 | —‡ | Positive | —‡ | ER(−) PR(−) HER2(−) |
| 7 | Family history and ≥25% risk | No | 48 | Rescreen | Invasive | 3 | 0.8 | Negative | 1 | ER(−) PR(−) HER2(+) |
| 8 | Family history and ≥25% Risk | No | 47 | Initial | Invasive | 1 | 2.2 | Negative | 2 | ER(+) PR(+) HER2(−) |
| 9 | Family history and ≥25% risk | No | 46 | Rescreen | DCIS† | 3 | —† | —† | —† | —† |
| 10 | Family history and ≥25% risk | No | 47 | Initial | Invasive | 2 | 3.3 | Negative | 2 | ER(+) PR(+) HER2(−) |
| 11 | Family history and ≥25% risk | No | 48 | Initial | DCIS† | 2 | —† | —† | —† | —† |
| 12 | Chest radiation | No | 32 | Initial | DCIS† | 2 | —† | —† | —† | —† |
Information on Her2 receptor status is missing.
DCIS = ductal carcinoma in situ; ER = estrogen receptor; PR = progesterone receptor; — = information on tumor size, lymph nodes, stage, and receptors not applicable.
This case had neoadjuvant therapy; information on tumor size and stage is not available.
Discussion
To the best of our knowledge, the High Risk OBSP is the first population-based breast screening program for high-risk women. Although many of the screening centers did not have experience with screening MRI before program initiation, the results compare favorably to those reported in meta-analyses of observational studies (10,21) and case series from academic centers (16–19).
Not surprisingly, when stratified by risk, the highest cancer detection rates were among mutation carriers (26.8 per 1000) and when stratified by age, in women age 50–69 years (19.3 per 1000), comparable to previous studies (13,16–19). In our cohort, 23% of screen-detected cancers were DCIS and the majority were detected by MRI only. This is consistent with results from the Toronto MRI study (20) but not those of earlier studies, which reported a lower DCIS rate (11,14,21) likely attributable to inexperience recognizing the characteristic non-mass enhancement on MRI (54).
In the High Risk OBSP, the benefit of adding mammography to MRI was particularly small among mutation carriers age 30–39 years. Mammography detected only one additional cancer compared with 25 detected by MRI, whereas the combination reduced specificity by 8.2%. A recent meta-analysis based on observational studies also found that adding mammography to MRI did not statistically significantly improve sensitivity in BRCA1 mutation carriers age 40 years or younger (29). In addition, no interval cancers were diagnosed in mutation carriers age 30–39 years, suggesting that annual MRI may be sufficient in this subgroup and does not justify the higher cost and reduced specificity of more-frequent MRI screening (18). The use of mammography in addition to MRI when screening high-risk women age 30–39 years requires further consideration, particularly among BRCA1/2 mutation carriers, in whom the risk of radiation-induced cancers may be higher because of a defect in DNA repair (3).
In our cohort, had women age 50–69 years been screened with mammography alone, more than one-half of the screen-detected cancers would have been undetected. Our findings do not support the National Institute for Health and Care Excellence guidelines recommending screening MRI in high-risk women only until age 50 years, except in women with dense breasts (27). Contrary to findings in younger women, the addition of mammography to MRI in older women resulted in high sensitivity with little loss of specificity, particularly among mutation carriers. Similar results have been reported for BRCA1/2 mutation carriers older than 50 years (28, 29). These findings are not unexpected given sensitivity and specificity of mammography is higher in older compared with younger women in the general population (17,55).
The main strength of our study was the inclusion of women screened in an organized screening program, therefore ensuring all radiologists and equipment met minimum quality standards and methods of evaluation and follow-up were similar for all women. Our study had a few limitations. Women excluded were primarily age 50–69 years or postmenopausal; this was, however, unlikely to appreciably alter results because they comprised only 5.6% of the cohort. Information on mammographic density was not collected; it is therefore unknown how this may have affected our results. Our results for women with previous chest radiation therapy are limited by sample size and require further follow-up. Although radiologists are aware of mammogram results before interpreting MRI and may be influenced, referral is independent of mammogram findings. Lastly, owing to the small number of women with mutations in genes other than BRCA1 or BRCA2, we are unable to comment specifically on this subgroup.
Our performance measures demonstrate that a population-based, high-risk breast screening program of annual MRI plus mammography delivers comparable results to those reported in prospective cohort studies and tertiary academic centers. Screening high-risk women age 30–39 years with an annual MRI only may be sufficient and should be evaluated further, particularly for mutation carriers. Among women age 50–69 years, screening is most effective when mammography is included with annual MRI.
Funding
This work was supported by the Canadian Cancer Society (grant number 316078).
Notes
The funder had no involvement in the design, conduct or reporting of the study; the writing of the manuscript; or the decision to publish the manuscript. The authors declare that they have no financial disclosures or conflicts of interest. We thank Cancer Care Ontario for use of its data.
References
- 1. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen S, Parmigiani G.. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Narod SA, Foulkes WD.. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4(9):665–676. [DOI] [PubMed] [Google Scholar]
- 4. van der Kolk DM, de Bock GH, Leegte BK, et al. Penetrance of breast cancer, ovarian cancer and contralateral breast cancer in BRCA1 and BRCA2 families: high cancer incidence at older age. Breast Cancer Res Treat. 2010;124(3):643–651. [DOI] [PubMed] [Google Scholar]
- 5.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58, 209 women with breast cancer and 101, 986 women without the disease. Lancet. 2001;358(9291):1389–1399. [DOI] [PubMed] [Google Scholar]
- 6. Pharoah PD, Day NE, Duffy S, et al. Family history and the risk of breast cancer: a systematic review and meta-analysis. Int J Cancer. 1997;71(5):800–809. [DOI] [PubMed] [Google Scholar]
- 7. Bevier M, Sundquist K, Hemminki K.. Risk of breast cancer in families of multiple affected women and men. Breast Cancer Res Treat. 2012;132(2):723–728. [DOI] [PubMed] [Google Scholar]
- 8. Henderson TO, Amsterdam A, Bhatia S, et al. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152(7):444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290(4):465–475. [DOI] [PubMed] [Google Scholar]
- 10. Granader EJ, Dwamena B, Carlos RC.. MRI and mammography surveillance of women at increased risk for breast cancer: recommendations using an evidence-based approach. Acad Radiol. 2008;15(12):1590–1595. [DOI] [PubMed] [Google Scholar]
- 11. Kriege M, Brekelmans CT, Peterse H, et al. Tumor characteristics and detection method in the MRISC screening program for the early detection of hereditary breast cancer. Breast Cancer Res Treat. 2007;102(3):357–363. [DOI] [PubMed] [Google Scholar]
- 12. Kuhl C, Weigel S, Schrading S, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol. 2010;28(9):1450–1457. [DOI] [PubMed] [Google Scholar]
- 13. Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476. [DOI] [PubMed] [Google Scholar]
- 14. Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet. 2005;365(9473):1769–1778. [DOI] [PubMed] [Google Scholar]
- 15. Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244(2):381–388. [DOI] [PubMed] [Google Scholar]
- 16. Passaperuma K, Warner E, Causer PA, et al. Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer. 2012;107(1):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riedl CC, Luft N, Bernhart C, et al. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J Clin Oncol. 2015;33(10):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rijnsburger AJ, Obdeijn IM, Kaas R, et al. BRCA1-associated breast cancers present differently from BRCA2-associated and familial cases: long-term follow-up of the Dutch MRISC Screening Study. J Clin Oncol. 2010;28(36):5265–5273. [DOI] [PubMed] [Google Scholar]
- 19. Sardanelli F, Podo F, Santoro F, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging (the High Breast Cancer Risk Italian 1 Study): final results. Invest Radiol. 2011;46(2):94–105. [DOI] [PubMed] [Google Scholar]
- 20. Warner E, Hill K, Causer P, et al. Prospective study of breast cancer incidence in women with a BRCA1 or BRCA2 mutation under surveillance with and without magnetic resonance imaging. J Clin Oncol. 2011;29(13):1664–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warner E, Messersmith H, Causer P, et al. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148(9):671–679. [DOI] [PubMed] [Google Scholar]
- 22. Bevers TB, Anderson BO, Bonaccio E, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw. 2009;7(10):1060–1096. [DOI] [PubMed] [Google Scholar]
- 23. Cardoso F, Loibl S, Pagani O, et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012;48(18):3355–3377. [DOI] [PubMed] [Google Scholar]
- 24. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 25.Warner E, Messersmith H, Causer P, et al. Magnetic resonance imaging screening of women at high risk for breast cancer. Program in Evidence-based Care Evidence-based Guideline No.: 15-11 Version 2. Toronto (ON): Cancer Care Ontario; 2012.
- 26. Mulder RL, Kremer LC, Hudson MM, et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013;14(13):e621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Collaborating Centre for Cancer. Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer Cardiff, UK: National Institute for Health and Care Excellence; 2013. [PubMed]
- 28. Phi XA, Houssami N, Obdeijn IM, et al. Magnetic resonance imaging improves breast screening sensitivity in BRCA mutation carriers age ≥50 years: evidence from an individual patient data meta-analysis. J Clin Oncol. 2015;33(4):349–356. [DOI] [PubMed] [Google Scholar]
- 29. Phi XA, Saadatmand S, De Bock GH, et al. Contribution of mammography to MRI screening in BRCA mutation carriers by BRCA status and age: individual patient data meta-analysis. Br J Cancer. 2016;114(6):631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phi XA, Houssami N, Hooning MJ, et al. Accuracy of screening women at familial risk of breast cancer without a known gene mutation: individual patient data meta-analysis. Eur J Cancer. 2017;85:31–38. [DOI] [PubMed] [Google Scholar]
- 31. Freitas V, Scaranelo A, Menezes R, et al. Added cancer yield of breast magnetic resonance imaging screening in women with a prior history of chest radiation therapy. Cancer. 2013;119(3):495–503. [DOI] [PubMed] [Google Scholar]
- 32. Ng AK, Garber JE, Diller LR, et al. Prospective study of the efficacy of breast magnetic resonance imaging and mammographic screening in survivors of Hodgkin lymphoma. J Clin Oncol. 2013;31(18):2282–2288. [DOI] [PubMed] [Google Scholar]
- 33. Sung JS, Lee CH, Morris EA, et al. Screening breast MR imaging in women with a history of chest irradiation. Radiology. 2011;259(1):65–71. [DOI] [PubMed] [Google Scholar]
- 34. Tieu MT, Cigsar C, Ahmed S, et al. Breast cancer detection among young survivors of pediatric Hodgkin lymphoma with screening magnetic resonance imaging. Cancer. 2014;120(16):2507–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Health Quality Ontario. Cancer screening with digital mammography for women at average risk for breast cancer, magnetic resonance imaging (MRI) for women at high risk: an evidence-based analysis. Ont Health Technol Assess Ser. 2010;10(3):1–55. [PMC free article] [PubMed] [Google Scholar]
- 36. Chiarelli AM, Prummel MV, Muradali D, et al. Effectiveness of screening with annual magnetic resonance imaging and mammography: results of the initial screen from the Ontario High Risk Breast Screening Program. J Clin Oncol. 2014;32(21):2224–2230. [DOI] [PubMed] [Google Scholar]
- 37. Eisen A, Blackmore KM, Meschino WS, et al. Genetic assessment wait time indicators in the High Risk Ontario Breast Screening Program. Mol Genet Genomic Med. 2018;6(2):213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tyrer J, Duffy SW, Cuzick J.. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. [DOI] [PubMed] [Google Scholar]
- 39. Antoniou AC, Pharoah PP, Smith P, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91(8):1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American College of Radiology. ACR BI-RADS Atlas: Breast Imaging Reporting and Data System Reston, VA: American College of Radiology; 2013.
- 41. Jaro MA. Probabilistic linkage of large public health data files. Stat Med. 1995;14(5–7):491–498. [DOI] [PubMed] [Google Scholar]
- 42. Holowaty EJ, Marrett LD, Fehringer G, Methods Cancer Incidence in Ontario: Trends and Regional Variations in the 1980s. Toronto, Ontario: Publications Ontario; 1995. [Google Scholar]
- 43.World Health Organization. International Classification of Diseases for Oncology: ICD-O. Geneva: WHO; 2000. [Google Scholar]
- 44.American Joint Committee on Cancer. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. New York: Springer; 2010. [Google Scholar]
- 45. Elston CW, Ellis IO.. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. [DOI] [PubMed] [Google Scholar]
- 46. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134(7):e48–e72. [DOI] [PubMed] [Google Scholar]
- 47. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. [DOI] [PubMed] [Google Scholar]
- 48. Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. [DOI] [PubMed] [Google Scholar]
- 49.Canadian Partnership Against Cancer. Breast Cancer Screening in Canada: Monitoring and Evaluation of Quality Indicators – Results Report, January 2011 to December 2012 Toronto, Canada: Canadian Partnership Against Cancer; 2016.
- 50.National Health Service Breast Screening Program. Consolidated Guidance on Standards for the NHS Breast Screening Programme. Sheffield, UK: NHS Cancer Screening Programmes; 2005. [Google Scholar]
- 51.BreastScreen Australia. BreastScreen Australia National Accreditation Handbook – March 2015. Canberra: Commonwealth of Australia; 2015.
- 52. Pepe MS. The Statistical Evaluation of Medical Tests for Classification and Prediction. Oxford: Oxford University Press; 2003:35–64. [Google Scholar]
- 53.SAS Institute Inc. Statistical Analysis Software 9.4 ed. Cary, NC: SAS Institute; 2013.
- 54. Facius M, Renz DM, Neubauer H, et al. Characteristics of ductal carcinoma in situ in magnetic resonance imaging. Clini Imaging. 2007;31(6):394–400. [DOI] [PubMed] [Google Scholar]
- 55. Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92(13):1081–1087. [DOI] [PubMed] [Google Scholar]