Abstract
A paradigm shift is occurring in cancer therapy, where instead of targeting tumor cells, immunotherapy agents (IA) target the immune system to overcome cancer tolerance and to stimulate an antitumor immune response. IA using immune checkpoint inhibitors (CPI) or chimeric antigen receptor T-cells have emerged as the most encouraging approaches to treat cancer patients. CPI are reported to induce moderate-to-severe neurologic immune-related adverse events in less than 1% of patients, whereas chimeric antigen receptor T-cell therapy is associated with frequent neurological toxicities that can be severe or even fatal. Cognitive difficulties have been described following chemotherapy and targeted therapy, but not specifically explored in patients receiving IA. The aim of this review is to establish a picture of the first published studies suggesting some biological and physiopathological effects of IA on cognitive functions among cancer patients. The first results originate from a preclinical study evaluating the role of CPI associated with peripheral radiation on cognitive dysfunction and the recent discovery of the central nervous lymphatic system allowing leukocytes to penetrate the central nervous system. Evaluating possible side effects of IA on cognitive function will be an important challenge for future clinical trials and for better understanding the underlying mechanisms through preclinical animal models.
Increasing survival for cancer patients has transformed long-term patient management, even in patients with metastatic cancers. Current cancer treatments have been associated with negative effects on cognitive functions, with perceived cognitive impairment experienced during and after treatment. These effects may be subjectively assessed by the patient or objectively assessed by neuropsychological tests and/or neuroimaging techniques, including structural and functional magnetic resonance imaging (1,2). Of the survivors, 16% to 60% (according to the studies and principally among female breast cancer patients) had objective cognitive decline (3). The rate of cognitive complaints is higher and reported to be up to 75% in a recent literature review (4). The cognitive impairment induced by chemotherapy, known as “chemofog,” affects learning, memory, attention, executive function, and processing speed (5,6). The mechanisms by which chemotherapy induces brain neurotoxicity and cognitive disorders are hypothesized to involve the release of proinflammatory mediators such as cytokines and chemokines, reactive oxygen species production, microglia activation, astrogliosis, myelin degradation, and likely potential alteration of the blood–brain barrier (7). Some targeted therapies such as antiangiogenic agents have also been associated with cognitive decline (8).
Immunotherapy agents (IA), encompassing monoclonal antibodies directed against immune checkpoints that inhibit T-cell activation, have emerged as a new cancer therapy (9). Checkpoint inhibitors (CPI) are used as monotherapy or in combination. Combination treatment has been found to be more efficacious, as demonstrated in patients with brain metastases from melanoma (10) and in the GL261 syngeneic mouse model of glioblastoma multiforme (11). However, CPI can cause inflammation, which may contribute to changes in neurobiology (7). The association with chemotherapy (12) or with radiotherapy (13) has produced promising results in various cancers but could potentiate neurological toxicities (Figure 1), with a negative effect on cognitive functions. Anticancer immunotherapy encompasses other approaches, such as chimeric antigen receptor (CAR) T-cell therapy, developed to target cancer-related cell-surface molecules (14). The use of CAR T-cells is approved for certain hematological cancers and is currently under investigation in other cancers. The main side effects are linked with cytokine release syndrome (CRS) with some potential frequent severe neurological toxicities (15). These side effects are common and can affect cognitive function.
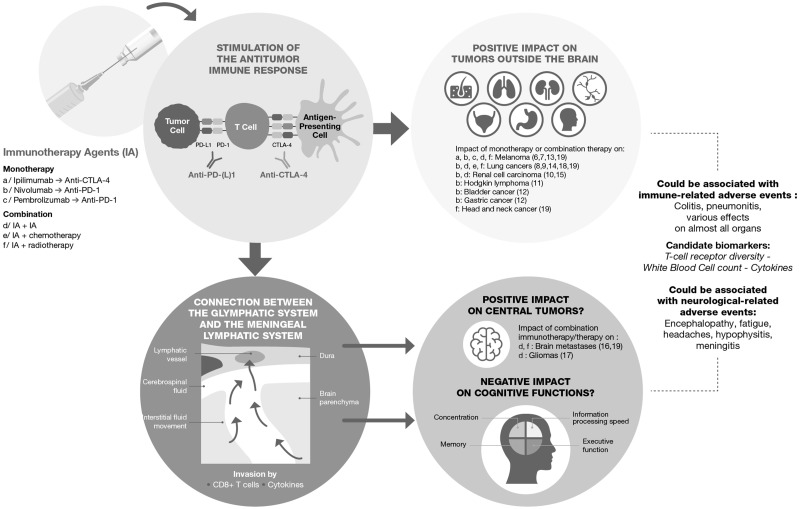
Figure 1.
The potential effect of immunotherapy agents on cognitive functions. Immunotherapy agents, anti-CTLA-4 and anti-PD-(L)1 antibodies, are used in monotherapy or in combined therapy, and they have shown efficacy in numerous tumors outside the brain. The connection between the glymphatic system and the meningeal lymphatic system allows the crossing of T-cells and cytokines and could explain the effect of immunotherapy on central tumors. Louveau and colleagues (38) have put forth a model in which the glymphatic system drains to the meningeal lymphatic vessels via the cerebrospinal fluid. This model in which T-cells may play a role in the central nervous system, could explain the effect of immunotherapy on brain metastases and gliomas. Immunotherapy could be associated with immune-related adverse events likely to affect almost all organs. Candidate biomarkers have been preliminary studied to predict which patients will experience the most clinically significant immune-related adverse events. Neurological-related adverse events are also described, and cognitive difficulties could be one of them.
Nevertheless, in the pivotal studies evaluating CPI, cognitive impairment has not been specifically evaluated, and this potential side effect has probably been underestimated. In view of the effect on cognition of chemotherapy and other cancer treatments, it is essential to characterize any potential cognitive impairment induced by immunotherapy, including the physio-pathological mechanisms and preventive strategies.
Neurological Immune-Related Adverse Events Associated With Immunotherapy
CPI
Immune-related adverse events resulting from potentiation of immune response in checkpoint inhibitors have been described to affect nearly all organs (16). The incidence of immune-related neurological adverse events is probably underestimated, particularly when these events are transient or mild, and do not require a neurological consultation (17). The overall incidence of reported neurological adverse events of any grade was from 3.8% to 6.1% with one agent and 12.0% with combination therapy. Most of these neurological adverse events were grade 1–2 in severity; the incidence of high-grade toxicity (7,8) was less than 1% for all types of treatment (18).
The clinical spectrum of neurological disorders is heterogeneous. Headaches, encephalopathy, and meningitis are the most commonly reported (21%, 19%, and 15%, respectively). Hypophysitis, previously unfamiliar to most oncologists, has also been reported. This refers to a chronic inflammation of the pituitary gland, inducing endocrine disorders (19); it occurs in up to 18% of metastatic melanoma patients treated with ipilimumab, with 5% of patients having grade 3 toxicity or worse (20). This suggests that immunotherapy via hypophysis dysregulation and major global hormonal changes could affect attention and induce fatigue and cognitive complaints without directly causing damage to the brain. In that context, an absence of cortisol response in cancer patients has been associated with an impaired delayed recall performance (21), suggesting also an indirect neuroendocrine regulation of cognitive functions.
Fatigue is one of the more frequent side effects reported by patients receiving immunotherapy, with an incidence greater than 20% for PD-1 and CTLA-4 inhibitors (22). Cognitive complaints have been associated with mood and fatigue (23), but this relationship is less clear for objective cognitive decline. Moreover, fatigue is a well-recognized clinical problem in autoimmune diseases such as systemic lupus erythematosus. Therefore, it is not surprising that fatigue is a potential immune-related neurological adverse event, possibly as a result of induced autoimmunity.
There has been no analysis dedicated to the potential effect of CPI on cognition in the pivotal studies, even though neurologic defects have been reported. A unique clinical pilot study has been conducted in 15 adult cancer patients treated with ipilimumab, nivolumab, or pembrolizumab to assess cognitive impairment (24). Two validated cognitive screening tests were used, the Montreal Cognitive Assessment and the Nine Images test of the Seine-Saint-Denis district, before the start of treatment and 3 months later. The authors concluded that cognitive deterioration in this small series of patients was associated with previous cytotoxic chemotherapy and not immunotherapy per se. However, this study has several major limitations. The small sample size was not sufficient to draw definite conclusions, and the effect of immunotherapy should have been evaluated in two groups: chemo-naive and chemo-exposed patients. The methodology of future studies should be optimized with the use of a battery of neuropsychological cognitive tests, the inclusion of a control group, and adequate consideration of practice effect.
CAR T-Cells
The question of neurological immune-related adverse events is even more relevant because treatment with CAR T-cells is associated with CRS, which can affect any organ, including the nervous system, and with CAR T-cell–related encephalopathy syndrome (CRES) caused by the activation of T-cells and subsequent release of cytokines, as well as the recruitment and activation of other immune cells (15).
CRS corresponds to a massive release of cytokines and granulocyte colony-stimulating factor by the T-cells' neighboring immune cells, producing what is sometimes called a cytokine storm (25). All CAR T-cell therapies elicit some degree of CRS; 27% have severe CRS requiring intensive care support (26). Even when well managed, CRS can range in severity from low-grade constitutional symptoms to a high-grade syndrome associated with life-threatening multiorgan dysfunction (27).
CAR T-cell–related encephalopathy syndrome is the second most common adverse event, and it corresponds to a central nervous system toxicity mediated by CAR T-cells: It represents a major cause of morbidity and mortality potentially hindering the expansion of CAR T-cell treatments. Such neurological adverse events are not rare, with up to 40% of patients developing severe and sometimes even fatal neurological symptoms (26,27). Although CRES can occur without CRS, patients usually present both simultaneously (91%) (25,28). These problems occur frequently and require specific neurologic monitoring, especially of cognitive functions.
Potential Physiopathological Mechanisms and Candidate Biomarkers of Immune-Related Cognitive Adverse Events
CPI
A preclinical study has explored the effect of CPI, alone or in combination with noncranial radiotherapy, on behavioral and cognitive performance both in tumor-free and tumor-bearing mice. The authors observed that the combined treatment achieved the best tumor control but was accompanied by behavioral, cognitive, and neuroinflammatory changes. The cognitive impairments affecting executive functions were accompanied by increased microglial activation in mice receiving immunotherapy alone or in combination. Finally, proinflammatory cytokines and growth factors were statistically significantly higher in tumor-bearing mice receiving immunotherapy or combined treatment compared with mice receiving no treatment (6). It should be noted that an abscopal effect has been described, by which radiotherapy at one site may lead to regression of nonirradiated metastatic cancer at distant sites (29), suggesting activation of immune actors mediating anticancer functions. Some studies have shown that combining radiotherapy with immunotherapy can effectively boost abscopal response rates (30). The association of immunotherapy and noncranial radiotherapy may exacerbate the detrimental effect of immunotherapy on cognitive functioning via the abscopal effect. In parallel, radiation-induced necrosis following the association of immunotherapy and brain radiotherapy appears as an issue. The overall rate of radiation necrosis following immunotherapy in combination or sequence with stereotactic cerebral radiosurgery has been reported to be 27% (31). Thus, the association of immunotherapy and cerebral radiotherapy may impair cognitive functioning via radiation necrosis. Reliable clinical or molecular biomarkers predicting which patients will experience the most considerable immune-related adverse events have not been identified (32). Nevertheless, candidate biomarkers have been studied in a preliminary manner even though no evidence of direct impact on cognitive functions has been provided so far (Figure 1). A potential biomarker could be the increase in T-cell–receptor diversity (33). White blood cell count could be a biomarker because increased eosinophil levels in the blood of anti-CTLA4–treated melanoma patients correlated with immune-related adverse events (34). Cytokines play a role in the response to immunotherapy and could constitute biomarkers of potential cognitive difficulties because their dysregulated levels in chemotherapy-treated patients can be associated with cognitive impairment (35). Studies converged to establish that some cytokines could have causal roles by crossing the blood–brain barrier, leading to systemic communication between peripheral cytokines and the brain (36). Recently, a toxicity score has been defined, and it consists of the expression of 11 cytokines statistically significantly upregulated in melanoma patients with severe immune-related toxicities at baseline and early in therapy with PD-1 inhibitors alone or in combination with anti-CTLA4 (37).
CAR T-Cells
CAR T-cell–treated cancer patients with grade greater than or equal to 4 CRS exhibited higher concentrations of interferonγ, interleukin (IL)6, IL8, IL10, IL15, monocyte chemoattractant protein 1, tumor necrosis factor receptor p55, and macrophage inflammatory protein 1β. Similarly, in B-ALL patients treated with CD19 CAR T-cells, elevated levels of IL1α, IL2, IL3, IL5, IL6, IL10, IL15, IP10, interferonγ, granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, and monocyte chemoattractant protein 1 were described in those with severe neurotoxicity (22). Brain examination in patients who died from neurotoxicity followed by CD19 CAR T-cells showed vascular lesions and necrosis with perivascular CD8+ T-cell infiltration (28), suggesting that neurological deficits and toxicity are mediated through direct cerebral invasion by CAR T-cells and in situ cytokine release.
The central nervous system, in large part because of the protective nature of the blood–brain barrier, was traditionally believed to be an immune-privileged organ incapable of surveillance by peripheral immunity. However, the recent discovery of lymphatic conduits residing within the meninges associated with the glymphatic system—a unique system of perivascular tunnels involving astroglial cells draining interstitial fluids through and out of brain parenchyma for degradation and removal into the circulatory system—suggests that the brain may be surveyed by the peripheral immune system (38). These discoveries strongly support the notion that the peripheral effect of immunotherapy on the immune system could reach the brain and induce cognitive decline (Figure 1).
Neurological disorders are important issues in patients treated with CPI and CAR T-cell therapies. Following CPI treatment, neurological disorders including cognitive decline are relatively rare but may be severe and can appear at the time of the treatment or later. There are emerging data on cognitive dysfunction in patients treated with CPI. Nonetheless, targeted and prospective studies are recommended. CAR T-cells have been associated with frequent and severe neurological disorders, which usually appear soon after the infusion and can include adverse cognitive events.
The cognitive follow-up of patients treated with CPI or CAR T-cells should be encouraged during and after treatment and a cognitive evaluation for future clinical trials should be promoted. An understanding of the involved mechanisms could be accomplished, as suggested by the International Cognition and Cancer Task Force guidelines, with functional imaging, development of studies with animal models, and objective and subjective cognitive evaluation of the treated patients.
Funding
None to list.
Notes
FJ discloses that she has received grants from Bristol-Myers Squibb; all other authors declare no other potential conflict of interest. The Cancer and Cognition Platform is supported by the Ligue Nationale contre le Cancer. The authors thank Laura Besnier, Medideo, for professional medical writing and editorial assistance.
References
- 1. McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ.. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123(3):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ.. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30(20):2500–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C.. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012;38(7):926–934. [DOI] [PubMed] [Google Scholar]
- 4. Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR.. An update on cancer‐ and chemotherapy‐related cognitive dysfunction: current status. Semin Oncol. 2011;38(3):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Joly F, Giffard B, Rigal O, et al. Impact of cancer and its treatments on cognitive function: advances in research from the Paris International Cognition and Cancer Task Force Symposium and update since 2012. J Pain Symptom Manage. 2015;50(6):830–841. [DOI] [PubMed] [Google Scholar]
- 6. McGinnis GJ, Friedman D, Young KH, et al. Neuroinflammatory and cognitive consequences of combined radiation and immunotherapy in a novel preclinical model. Oncotarget. 2017;8(6):9155–9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santos JC, Pyter LM.. Neuroimmunology of behavioral comorbidities associated with cancer and cancer treatments. Front Immunol. 2018;9:1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joly F, Heutte N, Duclos B, et al. Prospective evaluation of the impact of antiangiogenic treatment on cognitive functions in metastatic renal cancer. Eur Urol Focus. 2016;2(6):642–649. [DOI] [PubMed] [Google Scholar]
- 9. Dempke WCM, Fenchel K, Uciechowski P, Dale SP.. Second- and third-generation drugs for immuno-oncology treatment—the more the better? Eur J Cancer. 2017;74:55–72. [DOI] [PubMed] [Google Scholar]
- 10. Caponnetto S, Draghi A, Borch TH, et al. Cancer immunotherapy in patients with brain metastases. Cancer Immunol Immunother. 2018;67(5):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reardon DA, Gokhale PC, Klein SR, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016;4(2):124–135. [DOI] [PubMed] [Google Scholar]
- 12. Lazzari C, Karachaliou N, Bulotta A, et al. Combination of immunotherapy with chemotherapy and radiotherapy in lung cancer: is this the beginning of the end for cancer? Ther Adv Med Oncol. 2018;10:1758835918762094.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Deng W, Li N, et al. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol. 2018;9:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davila ML, Sadelain M.. Biology and clinical application of CAR T cells for B cell malignancies. Int J Hematol. 2016;104(1):6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559–574. [DOI] [PubMed] [Google Scholar]
- 17. Wick W, Hertenstein A, Platten M.. Neurological sequelae of cancer immunotherapies and targeted therapies. Lancet Oncol. 2016;17(12):e529–e541. [DOI] [PubMed] [Google Scholar]
- 18. Cuzzubbo S, Javeri F, Tissier M, et al. Neurological adverse events associated with immune checkpoint inhibitors: review of the literature. Eur J Cancer. 2017;73:1–8. [DOI] [PubMed] [Google Scholar]
- 19. Weber JS, Kähler KC, Hauschild A.. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. [DOI] [PubMed] [Google Scholar]
- 20. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. [DOI] [PubMed] [Google Scholar]
- 21. Andreano JM, Waisman J, Donley L, Cahill L.. Effects of breast cancer treatment on the hormonal and cognitive consequences of acute stress. Psychooncology. 2012;21(10):1091–1098. [DOI] [PubMed] [Google Scholar]
- 22. Touat M, Talmasov D, Ricard D, Psimaras D.. Neurological toxicities associated with immune-checkpoint inhibitors. Curr Opin Neurol. 2017;30(6):659–668. [DOI] [PubMed] [Google Scholar]
- 23. Bray VJ, Dhillon HM, Vardy JL.. Systematic review of self-reported cognitive function in cancer patients following chemotherapy treatment. J Cancer Surviv. 2018;12(4):537–559. [DOI] [PubMed] [Google Scholar]
- 24. Cuzzubbo S, Belin C, Chouahnia K, et al. Assessing cognitive function in patients treated with immune checkpoint inhibitors: a feasibility study. Psychooncology. 2018;27(7):1861–1864. [DOI] [PubMed] [Google Scholar]
- 25. Santomasso BD, Park JH, Salloum D, et al. Clinical and biologic correlates of neurotoxicity associated with CAR T cell therapy in patients with B-cell Acute lymphoblastic leukemia (B-ALL). Cancer Discov. 2018;8(8):958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weber JS, Yang JC, Atkins MB, Disis ML.. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33(18):2092–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC.. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18(5):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J.. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol. 2018;11(1):104.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fang P, Jiang W, Allen P, et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J Neurooncol. 2017;133(3):595–602. [DOI] [PubMed] [Google Scholar]
- 32. Johnson DB, Balko JM.. Biomarkers for immunotherapy toxicity: are cytokines the answer? Clin Cancer Res. 2019;25(5):1452–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fong L, Oh DY, Cham J, et al. T cell repertoire diversification is associated with immune related toxicities following CTLA-4 blockade in cancer patients. Cancer Res. 2016;77(6):1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schindler K, Harmankaya K, Kuk D, et al. Correlation of absolute and relative eosinophil counts with immune-related adverse events in melanoma patients treated with ipilimumab; ASCO Annual Meeting Proceedings. J Clin Oncol. 2014;32(suppl 15):9096. [Google Scholar]
- 35. Castel H, Denouel A, Lange M, Tonon MC, Dubois M, Joly F.. Biomarkers associated with cognitive impairment in treated cancer patients: potential predisposition and risk factors. Front Pharmacol. 2017;8:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheung YT, Ng T, Shwe M, et al. Association of proinflammatory cytokines and chemotherapy associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol. 2015;26(7):1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lim SY, Lee JH, Gide TN, et al. Circulating cytokines predict immune-related toxicity in melanoma patients receiving anti-PD-1-based immunotherapy. Clin Cancer Res. 2019;25(5):1557–1563. [DOI] [PubMed] [Google Scholar]
- 38. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]