Highlights
-
•
Occurrence of pesticide residues in organic samples is approximately 5 times lower than in conventional.
-
•
The impact of analytical methods with low LOQs (<10 µg kg−1) has shown not to be relevant in the majority of the cases.
-
•
A wide scope in the analytical methods presented a high influence for organic production evaluation.
-
•
Very polar compounds and metabolites represented 64% of the total detections.
Keywords: Authorized pesticides, Ionic polar compounds, Organic food, GC–MS/MS, LC–MS/MS, IC-Q-Orbitrap-MS/MS
Abstract
In the last decade, the consumption trend of organic food has increased dramatically worldwide. Since only a few pesticides are authorized in organic crops, concentrations are expected to range at zero or ultra-trace levels. In this context, the aim of the present study was to investigate the need for an improvement in the residue controls at very low concentrations (<0.010 mg kg−1) and to assess the impact of the scope of the analytical methods for this type of crops. For that purpose, a monitoring study for fruit and vegetable samples covering a wide range of pesticides (3 2 8) at low LOQs (0.002–0.005 mg kg−1) was developed. The results showed that the impact of applying analytical methods with low LOQs was not very relevant in the majority of the cases. However, a wide scope presented a high influence on this evaluation, especially regarding the inclusion of very polar compounds and metabolites.
1. Introduction
Pesticides are widely applied in agriculture to increase production yield and to ensure crop quality (Bauer, Luetjohann, Rohn, Jantzen, & Kuballa, 2018). From the European Union (EU), there are many efforts to achieve a sustainable use of these compounds to avoid the increase of pesticide levels in the environment and food. Consumption of pesticide-contaminated food via one’s daily diet is a major source of exposure to pesticides, which may pose adverse effects to humans leading to acute, chronic, or sub chronic problems (Ferrer Amate, Unterluggauer, Fischer, Fernández-Alba, & Masselter, 2010). Fruit and vegetables are recognized as the group of food containing higher pesticide residue levels compared to others, since most of them are eaten raw (Stachniuk, Szmagara, Czeczko, & Fornal, 2017). Hence, food control is necessary to monitor pesticide residues in food commodities prior to their introduction into the market and it is regulated by official directions, established by authorities such as the European Food Safety Authority (EFSA).
Recently, the high concern about the presence of pesticide residue is one of the key drivers that promotes the increase of consume of organic fruit and vegetables, indicated by the doubling of organic horticulture from 2003 to 2013 worldwide (Dorais & Alsanius, 2015). This galloping rate of organic produce is reflected on the existence of policy support for organic farming as well as government and industry funding for research.
In the EU, organic farming is supported by Regulations (EC) No 2018/848 (Council of the European Union & European Parliament, 2018) and 889/2008/EC (European Commission, 2008), with detailed rules on production, labelling, certain authorized compounds (such as pyrethroids, spinosad, etc.) and control via an organic action plan (European Commission, 2014). The EU organic food market is the second largest in the world behind the US (Martínez Bueno, Díaz-Galiano, Rajski, Cutillas, & Fernández-Alba, 2018).
The presence of pesticides residues in organic fruit and vegetables is insufficiently reported in the scientific literature (EFSA, 2017). This fact is also reflected in the last EFSA report, where organic food comprised the 6.5% of the total samples (Food & Authority, 2018). It is noteworthy that no specific MRLs are established for organic food produced in accordance with Regulation (EC) No 2018/848 (Council of the European Union & European Parliament, 2018) and hence the MRLs set in Regulation (EC) No 396/2005 (European Commission, 2005) apply equally to organic and to conventional food.
This lack of data, also recognized by European Commission (2011), entails insufficient control and inspection for fraud detection in organic products, which no matter being organic-labelled, they may have been produced conventionally.
Multiresidue gas chromatography coupled to tandem mass spectrometry (GC–MS/MS) and liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) methods are widely recognized as ideal, highly specific and sensitive for testing food products. The high selectivity provided by GC and LC-MS/MS allows the determination multi-class pesticides in one run. High resolution mass spectrometry (HRMS) enables the acquisition of a theoretically unlimited number of pesticides and metabolites in a single run, by means of accurate mass measurements (<5 ppm mass error) combined with high resolving power (>20 k FWHM), limiting the risk of false identifications in such complex matrices, where endogenous matrix components of similar mass may be co-eluted (Nannou, Boti, & Albanis, 2018). Combined with ion chromatography (IC) allows the multiresidue analysis of the most polar pesticides (Rajski, Diaz Galiano, Cutillas, & Fernández-Alba, 2017).
To this context, the aim of this work was to investigate the effect of residue controls at low concentrations (<10 mg kg−1) and the impact of the pesticide scope in the analytical results. For this purpose, it was conducted a monitoring study of 136 commercial organic samples originated from 16 different countries and 4 different commodity groups. For the analysis of the samples there were applied analytical methods based on GC–MS/MS, LC-MS/MS and IC-Q-Orbitrap-MS/MS techniques covering 328 pesticides and transformation products, belonging to different chemical classes.
2. Material and methods
2.1. Reagents and materials
A set of 328 multiclass pesticides and transformation products used in fruit and vegetables were selected for the study (see supplementary material Tables S1–S3). The pesticides were chosen as representatives of the different pesticide classes (herbicides, insecticides and fungicides). High-purity pesticide standards were supplied by Dr Ehrenstorfer (LCG Standards, Middlesex, UK) and Sigma-Aldrich (Steinheim, Germany) and were stored at −20 °C. Individual standard stock solutions (1000–2000 mg L-1) were prepared in acetonitrile (LC and GC pesticides) and stored at −20 °C. Ionic polar compounds stock solutions (1000–2000 mg L-1) were prepared in water and methanol and were stored in plastic vials in the dark at −20 °C.
HPLC-grade acetonitrile and formic acid (purity 98%) were supplied by Sigma-Aldrich (Steinheim, Germany). LC-MS grade water was obtained from Fisher Scientific (Geel, Belgium). Pierce LTQ Velos ESI Negative Ion Calibration Solution was provided by Thermo Fisher Scientific (Waltham, MA). Primary–secondary amine (PSA) Bond-Elut was obtained from Supelco (Bellefonte, PA, USA). Sodium chloride was purchased from J.T.Baker (Deventer, The Netherlands). Disodium hydrogencitrate sesquihydrate and calcium chloride was obtained from Sigma- Aldrich (Steinheim, Germany). Anhydrous magnesium sulphate was supplied by Panreac (Barcelona, Spain). C18, Bond-Elut Enhanced Matrix Removal d-SPE and Bond-Elut Final Polish was purchased from Agilent Technologies (Santa Clara, CA, USA).
2.2. Sample preparation
For the monitoring study, 136 samples with the organic certification coming from 16 different countries were purchased in local markets of Spain, Portugal and Uruguay. These samples were representative of 4 commodity groups according to the EU/SANTE/2017/11813 (SANTE/11813/2017, 2018) (high water content, high acid and water content, high oil content and intermediate water content and difficult and unique commodities). The organic matrices analyzed in the study are included in Table 1.
Table 1.
Number of organic matrices analyzed in the present study according to their commodity group.
| High water content | “Difficult/unique commodities | ||||
|---|---|---|---|---|---|
| Apple | 6 | Potato | 3 | Anise | 1 |
| Artichoke | 1 | Pumpkin | 2 | Black tea | 2 |
| Aubergine | 2 | Spinach | 1 | Chamomile | 2 |
| Banana | 3 | Sweet potato | 1 | Cayenne | 1 |
| Beans | 2 | Tomato | 6 | Cumin | 1 |
| Beet root | 1 | Zucchini | 3 | Curcuma | 1 |
| Broccoli | 3 | High acid and water content | Green tea | 7 | |
| Carrot | 4 | Cherry | 1 | Linden | 1 |
| Celery | 2 | Balckberry | 1 | Oolong tea | 1 |
| Cherimoya | 1 | Blueberry | 1 | Oregano | 1 |
| Cauliflower | 2 | Grape fruit | 1 | Parsley | 1 |
| Cucumber | 3 | Lemon | 3 | Pepper (spice) | 2 |
| Garlic | 5 | Mandarin | 2 | Red tea | 2 |
| Ginger | 1 | Orange | 3 | Roiboos | 1 |
| Fennel | 1 | Pineapple | 2 | Rosemary | 2 |
| Kiwi | 3 | Raspberry | 2 | Thyme | 3 |
| Leek | 2 | Strawberry | 1 | White tea | 2 |
| Lettuce | 2 | High oil content & intermediate water content | Yerba Mate | 1 | |
| Mango | 2 | Avocado | 2 | ||
| Mushroom | 2 | Olive oil | 7 | ||
| Onion | 4 | Soya oil | 1 | ||
| Pear | 3 | Sunflower oil | 2 | ||
| Pepper | 4 | ||||
Extracts for LC-QqQ-MS/MS and GC-QqQ-MS/MS analysis, were prepared using citrate QuEChERS sample preparation method (Anastassiades, Lehotay, Štajnbaher, & Schenck, 2003). For a group of matrices (dry herbs, high oil content and spices), citrate QuEChERS method with some modifications according to the matrix type was applied. Extraction method details and validation data of the modified methods are included in previous publications of this group (Dias, Cutillas, Lozano, Pizzutti, & Fernández-Alba, 2016; E. Hakme et al., 2018, Lozano et al., 2012, Rajski et al., 2013, Rajski et al., 2013, Vázquez et al., 2016). A scheme of each one extraction method used in this work has been included in supplementary material (Figs. S1 and S2).
Briefly, the methods involve citrate buffered extraction of pesticide residues with acetonitrile, followed by salting out with magnesium sulfate and sodium chloride. The clean-up step of dry herbs was carried out employing calcium chloride instead magnesium sulfate (Lozano et al., 2012). Oils and spices were extracted using QuEChERS protocol with EMR-Lipid d-SPE clean-up (Dias et al., 2016, Hakme et al., 2018, Vázquez et al., 2016).
For the analysis of ionic polar pesticides by IC-Q-Orbitrap-MS/MS, all matrices were extracted according to a previously published validated method (Rajski et al., 2017) consisting in a modification of the QuPPe method (European Commission, 2017).
2.3. LC-QqQ-MS/MS analysis
An Agilent UPLC 1290 Series (Agilent Technologies, Palo Alto, CA, USA) coupled to an Agilent Technologies 6490 TripleQuad LC/MS was used for this study. The UPLC was equipped with a reversed-phase C8 analytical column of 2.1 mm × 100 mm and 1.8 mm particle size (Agilent Technologies, Santa Clara, CA). Gradient LC elution was performed with 0.1% formic acid, 5 mM ammonium formate and 2% ultrapure water in methanol as mobile phase A and 0.1% formic acid, 5 mM ammonium formate and 2% methanol in ultrapure water as mobile phase B. The mobile phase composition is as follows: 20% A (2 min), 13 min linear gradient to 100% A (3 min), 2.5 min post-run time back to the initial conditions. The flow rate was 0.3 mL min−1 and the injection volume 5 µL. Analytical parameters of the mass spectrometer are published in a previous work (Gil García, Martínez Galera, Uclés, Lozano, & Fernández-Alba, 2018). Table S1 shows the acquisition parameters of the analyzed pesticides by LC-QqQ-MS/MS (see supplementary material).
2.4. GC-QqQ-MS/MS analysis
Analyses of GC compounds were performed on an Agilent Intuvo 9000 GC system equipped with an Agilent 7693 autosampler and an Agilent 7010 GC–MS/MS triple quadrupole. The samples were injected using a multimode injector inlet in splitless mode, through an Agilent ultra-inert inlet liner with a glass wool frit. The injection volume was 1 µL. Analytical conditions are published in a previous work (Elena Hakme, Lozano, Uclés, & Fernández-Alba, 2017). Table S2 shows the list of compounds and their corresponding acquisition parameters (see supplementary material).
2.5. IC-Q-Orbitrap-MS/MS analysis
For the IC separation, a Dionex Integrion IC system (Thermo Scientific, San Jose, CA) was used. The IC system was coupled to a Q Exactive™Focus mass spectrometer (Thermo Scientific, Bremen, Germany) equipped with a heated electrospray ionization source (HESI-II; Thermo Scientific). Analytical conditions are published in a previous work (Rajski et al., 2017). The list of analyzed compounds, masses of ions, and collision energies are shown in Table S3 (see supplementary material).
2.6. Method validation
Extraction methods were validated in previous works (Dias et al., 2016; E. Hakme et al., 2018, Lozano et al., 2012, Rajski et al., 2017, Rajski et al., 2013, Rajski et al., 2013, Vázquez et al., 2016) and extended for lower LOQs. The validation of the extraction methods were performed according to the EU quality control procedures (SANTE/11813/2017, 2018). The analytical parameters evaluated were selectivity, sensitivity, linearity, recovery, repeatability and matrix effect. The analytical methods involved sample preparation procedures for the analysis by LC-QqQ-MS/MS and GC-QqQ-MS/MS in matrices of high-water content, high-acid content and high-water content, dry herbs, spices and oily fruits and products. Moreover, the analytical methods involve one sample preparation method for compounds analyzed by IC-Q-Orbitrap-MS/MS.
2.7. Quality control
In order to check the correct performance of the analytical procedure, several internal standards were added in different steps of the process. The whole procedure, from the extraction step was controlled by using the procedural internal standards (PIS) carbendazim-d3, malathion d-10 and dichlorvos-d6 for LC. Malathion-d10, dichlorvos-d6 and triphenyl phosphate were used as PIS in GC and glyphosate 13C for IC. The procedure was considered as properly performed when the recovery for the PIS was in the range 60–140%. If those values were outside this range, the extraction was repeated. The injection was also controlled using an injection internal standard (IS), by checking the areas of dimethoate-d6 and lindane-d6 for LC an GC, respectively. The sample extract was considered correctly injected if the area was not deviating more than ±30% from the average area of the calibration standards.
3. Results and discussion
3.1. Limits of quantification
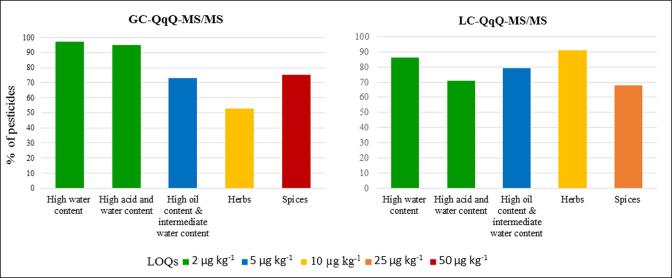
The sensitivity of the method was calculated in terms of limit of quantitation’s (LOQs), which was estimated as the lowest concentration of the analyte validated with the acceptable accuracy in which the quantifier and qualifier selected reaction monitoring transitions (SRM1 and SRM2) had a signal-to noise-ratio ≥3. Fig. 1 shows the LOQs obtained for each method developed by GC and LC analysis, expressed as percentage of the total of pesticides analyzed (3 2 8). The obtained LOQs ranged from 2 to 5 µg kg−1 for more than 70% of the analytes in three of the studied commodity groups (matrices with high water content, with high acid and water content and matrices with high oil content and intermediate water content). Concerning to difficult matrix samples, between 50% (by GC) and 90% (by LC) of the studied pesticides shown LOQs of 10 µg kg−1 in herb matrices, while around 70% presented LOQs values lower than 50 µg kg−1 in spices matrices. All the ionic pesticides analyzed by IC-Q-Orbitrap-MS/MS shown LOQs of 10 µg kg−1 in the four commodity groups studied. In all cases, the LOQs values achieved were far below the maximum residue levels established for conventional products.
Fig. 1.
LOQs obtained for each matrix type, analyzed using GC-QqQ-MS/MS and LC-QqQ-MS/MS.
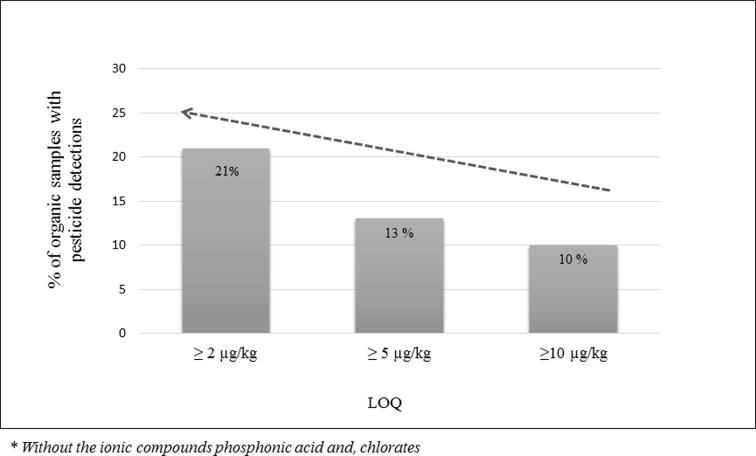
Fig. 2 shows the percentage of organic samples with positive pesticide detections above the LOQs, considering different reference levels. As can be seen, only 10% of all organic samples analyzed exceed the suggested reference level (10 µg kg−1). The results obtained are in line with a recent report by EFSA (EFSA, 2018). It is informed that around 16% of organic vegetables analyzed contained pesticide residue concentrations at measurable concentrations above 10 µg kg−1. Nonetheless, the number of samples with positive pesticide detections increase if the maximum suggested reference values decrease below to 10 µg kg−1. Thus, 13% of the total organic samples showed quantified residues exceeding 5 µg kg−1, while if the reference level applied was 2 µg kg−1, 21% of the samples analyzed presented pesticide residues. These results show that the use of analytical methods with low LOQs are not very relevant for the evaluation.
Fig. 2.
Percentage of organic samples with positive pesticide detections considering different limit of quantitation (LOQs) levels.
3.2. Pesticide residues occurrence in organic samples
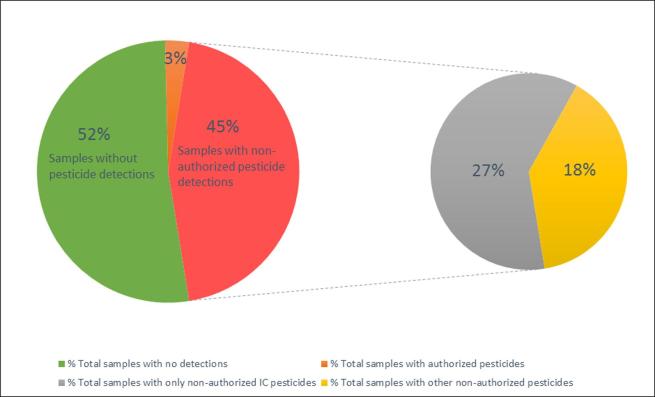
In total, 136 commercial samples labelled with organic certification, coming from 13 different countries and representative of 4 commodity groups according to the EU/SANTE/2017/11813 (SANTE/11813/2017, 2018) were included in the study. Fig. 3 shows the pesticide residues occurrence in the organic samples tested. As can be seen, no pesticide residues were detected above the LOQs achieved for the developed methods in 52% of the organic samples tested (71 samples). Authorized pesticide residues by organic production regulation were detected in 3% of the cases (4 samples), while 45% (61 samples) contained one or more non-authorized pesticides, most of them only ionic polar pesticides (27%, phosphonic acid and chlorates). These results are in agreement with a recent work carried out in Ireland. The authors found pesticide residues in 15 of the 27 organic samples tested (55%) (Tobin, Walsh, Garvey, & Larkin, 2014). The results obtained in this study about pesticide residues occurrence in organic samples were slightly higher than those published in a recent report by EFSA. It informed that 83% of the organic samples analyzed in 2016 were free of quantifiable residues and the percentage of organic samples containing residues in concentrations within the legal limits was only 16% (EFSA, 2018).
Fig. 3.
Pesticide residues occurrence in the organic samples tested.
Among the seven ionic polar compounds studied (glyphosate, chlorate, phosphonic acid, perchlorate), including metabolites (n-acetyl glyphosate, AMPA, n-acetyl-AMPA), only three substances were detected in the analyzed organic fruit, vegetable and vegetal oil samples (chlorate, phosphonic acid, perchlorate). Glyphosate and its main metabolites (n-acetyl glyphosate, AMPA and n-acetyl-AMPA) were not detected in any sample.
Regarding to the four commodity groups evaluated, the percentage of samples with quantifiable pesticide residues is presented in Table 2. The results shown that out of 136 organic samples tested, pesticide residues were detected in 55% of the samples with high water content, 35% in samples with high acid and water content, 8% in samples with high oil content and intermediate water content and 56% in difficult samples. Therefore, attending to the commodity groups studied, samples with high water content (such as spinach, sweet potato, potato, red pepper or tomato) and difficult samples (i.e. thyme, oregano) exhibited similar occurrence percentages, followed by the samples with high acid and water content (i.e. mandarin or lemon).
Table 2.
Percentage of analyzed samples with pesticides per commodity group.
| Commodity group* | N° of analyzed samples per commodity group | % Samples with pesticides | % Samples with pesticides (without ionic pesticides) |
|---|---|---|---|
| High water content | 75 | 55 | 28 |
| High acid and water content | 17 | 35 | 24 |
| High oil content & intermediate water content | 12 | 8 | 8 |
| Difficult/unique commodities | 32 | 56 | 25 |
Commodity groups according to the EU Document SANTE/2017/11813
On the other hand, of the 136 commercial samples labelled with organic certification, coming from 13 different countries, 32 samples were grown in non-EU countries, while 104 were EU samples. Overall, similar pesticide residues percentages were found in samples coming from non-EU and EU countries, 53% and 47%, respectively.
The results of the analysis of 328 pesticide residues using GC–MS/MS, LC-MS/MS and IC-Q-Orbitrap-MS/MS were summarized in Table 3. A total of 24 different pesticides were detected, of which 10 were fungicides, 6 herbicides and 7 insecticides. Most samples with pesticide residues detections contained a single pesticide residue (34%), while two or more were found in 17% of samples, at concentration levels between 2 and 1433 µg kg−1. Three pesticides (prometon, MCPA and chlorates) not included in the multiannual control programme of pesticide residues of the Union (Commission, 2020) were found. These compounds were detected at concentrations above the legal limits stablished by the European legislation for each combination pesticide/commodity (20.4 µg kg−1 of prometon in garlic, 59.75 µg kg−1 of MCPA in thyme and between 10.15 and 16.83 µg kg−1 of chlorates in red pepper, sweet potato, tomato, onion and lettuce). Thus, the MRLs were exceeded in a total for 7 organic samples (5%), of which only one sample (0.7%) was non-compliant taking into account the measurement uncertainty. Only the herbicide prometon exceed the legal limit (10 µg kg−1) established by default for the European legislation on MRLs (European Commission, 2005). The same exceedance rate was published in a recent report by EFSA. It informed that 0.7% of organic vegetables analyzed in 2016 contained residue concentrations exceeding the MRLs established for conventional products (EFSA, 2018).
Table 3.
Summary of pesticide residues quantified in the organic samples.
| Pesticide | Category | Number of quantifications | Concentration Range (μg kg−1) | Matrices |
|---|---|---|---|---|
| Phosphonic acid** | Fungicide | 43 | 10.1–1433.0 | Allspice, anise, apple, avocado, banana, blackberry, broccoli, cayenne, celery, cucumber,garlic, green pepper, kiwi,lettuce, linden, mandarin, mate tea, mushroom, onion, oregano, pear, potato,rosemary ,spinach, sweet potato, tea, thyme,zucchini |
| Chlorpropham | Herbicide | 6 | 2.2–109.5 | Leek, onion, pear, potato, raspberry, red pepper |
| Chlorate (**) | Herbicide, biocide | 5 | 10.2–16.8 | Lettuce, onion, red pepper, sweet potato,tomato |
| Azoxystrobin | Fungicide | 4 | 2.1–34.9 | Garlic |
| Chlorpyrifos | Acaricide, insecticide | 3 | 9.8–101.3 | Cayenne, olive oil, sweet potato |
| Spinosad (*) | Insecticide | 3 | 4.2–68.3 | Pear, spinach |
| Tebuconazole | Fungicide | 3 | 3.3–25.0 | Cayenne, garlic, pear |
| 3-Chloroaniline** | Metabolite | 2 | 53.0–84.0 | Chamomile, cayenne |
| Fludioxinil | Fungicide | 2 | 6.1–7.3 | Pineapple |
| Imidacloprid | Insecticide | 2 | 7.1–65.5 | Red tea, thyme |
| Prometon (**) | Herbicide | 2 | 2.2–20.4 | Garlic |
| Boscalid | Fungicide | 1 | 2.1 | Garlic |
| Carbendazim | Fungicide | 1 | 22.9 | Garlic |
| Cypermethrin | Acaricide, insecticide | 1 | 96.0 | Chamomile |
| Fenhexamid | Fungicide | 1 | 2.3 | Artichoke |
| Fenvalerate | Acaricide, insecticide | 1 | 2.1 | Lemon |
| Fluazifop | Herbicide | 1 | 30.1 | Oregano |
| Iprodione | Fungicide, nematicide | 1 | 5.4 | Leek |
| Lambda-cyhalothrin* | Insecticide | 1 | 3.4 | Mandarin |
| Mandipropamid | Fungicide | 1 | 28.0 | Parsley |
| MCPA (**) | Herbicide | 1 | 59.8 | Thyme |
| Phosmet | Insecticide | 1 | 15.2 | Olive oil |
| Propyzamide | Herbicide | 1 | 4.3 | Lemon |
| Quinoxyfen | Fungicide | 1 | 30.6 | Dry parsley |
Pesticides authorized by organic production.
Pesticides not included in the multiannual control pogramme of the European Union (Commission, 2020).
Two authorized pesticides by organic production were detected: spinosad and λ-cyhalothrin (Table 3). Both pesticides, are listed in Annex II of Reg. 889/2008/EC as substances authorized for organic farming. Spinosad was the most frequent authorized pesticide detected (3 times), at concentrations ranged from 4.2 to 68.3 µg kg−1 in spinach and pear, respectively. The other authorized insecticide, λ -cyhalothrin was only detected in one sample (mandarin) at 3.4 µg kg−1. In both cases, the residue levels found were lower than their MRLs (Spinosad: 15000 µg kg−1 in spinach and 300 µg kg−1 in pear; λ -cyhalothrin: 200 µg kg−1 in mandarin).
On the other hand, regarding non-authorized pesticide the fungicide phosphonic acid was the substance detected more frequently (43 detections), followed by the herbicides chlorpropham (6 times) and chlorates (5 times) and the fungicide azoxystrobin (4 times). Table 3 presents the non-authorized pesticide residues concentrations found in the organic samples tested. Considering these results, the improved of analytic methodologies for the detection of pesticide residues (including ionic polar pesticides) is a powerful tool to expand the monitoring of organic food commodities as well as for a better evaluation and detection of possible fraud in organic crops.
3.2.1. Fungicides
As can be seen in Table 3, phosphonic acid was the most frequently detected fungicide with 43 positive samples. Detection frequency of phosphonic acid in the organic samples tested in the present study is highly remarkable, since it represents the 31.6% of the total analyzed samples. The concentration levels ranged from 10.1 to 1433 µg kg−1. The highest concentration of phosphonic acid was observed in a garlic sample (1433 μg kg−1). Considerable levels of phosphonic acid (>100 μg kg−1) were also found in kiwi (1074 μg kg−1), banana (319.5 μg kg−1), pear (277.6 μg kg−1) and cucumber (238.6 μg kg−1). The residue definition of fosetyl aluminum according to Regulation (EC) No. 396/2005 (European Commission, 2005) is specified as the “sum of fosetyl, phosphonic acid and their salts, expressed as fosetyl”. The inclusion of phosphonic acid in the residue definition of fosetyl-Al initiated the analysis of phosphonic acid as a synthetic pesticide. Residues of phosphonic acid in crops can occur as a result of applying phosphonic acid itself as a fertilizer plant protection product containing or applying fosetyl aluminum, which degrades to phosphonic acid. Foliar phosphorus fertilizers and some preparations approved for organic farming can be also a source of phosphonic acid (Thao & Yamakawa, 2009). Furthermore, residues in crops can occur as well resulting from the application of plant protection products containing phosphonic acid as metabolite (Malusà and Tosi, 2005, Wieland et al., 2014). Phosphonic acid can accumulate and be stored for years and remobilized by the plant, suggesting a long-term persistence and non-predictable residual effects in fruit and vegetables (Bauer, Luetjohann, Rohn, Kuballa, & Jantzen, 2018). Nevertheless, phosphonic acid is not listed in Annex II of Reg. 889/2008/EC (European Commission, 2008) as a product authorized for organic farming, hence its application in organic crops is not allowed and its residues should not appear in the next future (Wieland et al., 2014).
Azoxystrobin, tebuconazole and fludioxonil were also found in four, three and two samples, respectively. Azoxystrobin was quantified in garlic samples at concentration ranges from 2.1 to 34.9 µg kg−1. A similar level of this fungicide was found in a study carried out in organic New Zealand tomatoes (Cressey, Vannoort, & Malcolmb, 2009). The authors detected azoxystrobin in 1 of the 11 organic samples analyzed at 60 µg kg−1. Tebuconazole was present at levels between 3.3 and 25 µg kg−1 in cayenne, garlic and pear, being detected the highest concentration in a cayenne sample. Fludioxonil was found at trace levels (from 6.1 to 7.3 µg kg−1) in pineapple organic samples. Other fungicides (boscalid, carbendazim, fenhexamid, iprodione, mandipropamid and quinoxyfen) were also detected only once (Table 3). In all cases, the fungicide levels found in this study were below the MRLs established for conventional products.
3.2.2. Herbicides
The herbicide chlorpropham exhibited the highest incident rate, being found in 6 samples, representing 4.4% of the total analyzed samples. Furthermore, it was the substance quantified at higher concentration levels ranged from 2.2 to 109.5 µg kg−1 (see Table 3). Chlorpropham concentrations were below to 10 μg kg−1 in fruiting and leafy matrices, while root and tuber matrices presented values between 10 and 100 μg kg−1 (onion and potato). Its main metabolite (3-chloroaniline) was detected in 2 samples of the 136 organic samples analyzed. It was only found in samples classified within difficult commodity group. The highest concentrations were observed in a cayenne sample (84 µg kg−1) followed by a chamomile sample (53 µg kg−1).
Chlorate was the following herbicide/biocide with the highest incident rate, being found in 5 samples at concentrations ranged from 10.2 to 16.8 µg kg−1. MRLs are not applied for chlorate residues in food of plant origin. However, according to the Regulation (EU) No 396/2005, a reference level of 10 μg kg−1 is applied in such matrices (European Commission, 2005). The highest determined concentration was 16.8 μg kg−1 (in red pepper), which is above the MRL of 10 μg kg−1. The same holds for tomato, onion, lettuce and sweet potato samples, showing concentrations of 15.1 μg kg−1, 14.6 μg kg−1, 12.7 μg kg−1 and 10.2 μg kg−1, respectively. Nonetheless, if an uncertainty of 50% is applied to all the above-mentioned concentrations, the findings do not exceed the suggested reference level. The use of chlorate as a pesticide and of sodium chlorate as a component of biocides are no longer authorized in the EU since 2008 (Kaufmann-horlacher et al., 2016). The potential pathways through which food can be contaminated with chlorate are not yet finally elucidated. However, it is reported that chlorate residues arise in many cases by using chlorinated water either for irrigation in the field or post-harvest for various food processing (i.e. washing of crops and disinfection of surfaces in food production premises, or production /processing of food hydro-cooling procedures) (EFSA, 2015a). In the last EFSA report, MRL exceedances in organic products are reported mainly for chlorate (Food & Authority, 2018). Chlorate residues are not necessarily linked to a use as a pesticide and consequently many experts have expressed their concerns of the feasibility of setting MRLs for chlorate (>10 μg kg−1), mainly in processed food.
The herbicide prometon was found at concentrations of 2.2 and 20.4 µg kg−1 in two garlic samples. Prometon is used for the control of most annual and many perennial broad-leaved weeds, grasses and brush weeds on non-crop areas. Persistence in soil can be as much as one year, depending on soil type, moisture and the application rate used (Tomlin, 2009). Prometon is not included in the Regulation (EC) No. 396/2005 (European Commission, 2005). Thereby, a default MRL of 10 μg kg−1 is applied, according to the European legislation on MRLs. In this study, prometon levels were exceed in one of the samples analyzed, taking into account the measurement uncertainty.
Other three herbicides were detected only once. The MCPA was quantified at levels above the MRL established (50 µg kg−1) in a thyme sample at 59.8 µg kg−1. Fluazifop and propyzamide were found at concentrations of 30.1 µg kg−1 in oregano and 4.3 µg kg−1 in lemon, respectively.
Overall, in terms of food safety, three herbicides (prometon, chlorates and MCPA) were found in this study above the MRLs established by Regulation (EU) No 396/2005 for conventional products. However, only prometon presented levels over legal limits taking into account the measurement uncertainty.
3.2.3. Insecticides
As can be seen in Table 3, chlorpyrifos was the most frequently detected insecticide (3 times) at concentrations between 9.8 and 101.3 µg kg−1. By matrix types, chlorpyrifos residue concentrations were 9.8 μg kg−1 (olive oil), 10 μg kg−1 (sweet potato) and 101.3 μg kg−1 (cayenne). The residue levels observed in this study were in the same concentration range to other study carried out in 2014 (Tobin et al., 2014). Here, the authors found chlorpyrifos residues in one organic wine sample at 15.96 μg kg−1.
Imidacloprid was the second insecticide more frequently detected, being found in 2 samples of the total analyzed samples. It was only found in difficult matrices, with residue levels of 7.1 µg kg−1 in red tea and 65.5 µg kg−1 in thyme.
Other insecticides found in the organic samples analyzed were: cypermethrin, fenvalerate and phosmet. They were detected at concentrations of 96 µg kg−1 in chamomile, 2.1 µg kg−1 in lemon and 15.2 µg kg−1 in olive oil, respectively.
None of the insecticides quantified in this study were above the MRLs established by the current EU Regulation (European Commission, 2005) for conventional products taking into account the measurement uncertainty.
3.2.4. Other contaminants detected
Perchlorate was detected in 13% of the total samples, with concentrations varying from 10.3 μg kg−1 to 4390 μg kg−1. Among all the studied commodities, the highest concentrations of perchlorate were observed in spices. Specifically, perchlorate concentration was 4390 μg kg−1 in thyme, followed by 310 μg kg−1 in parsley and 140 μg kg−1 in oregano. Considerable concentrations were also found in spinach, leek, cucumber, avocado and rosemary. Although MRLs for perchlorate are not established yet by European Commission, the level of 100 μg kg−1 as reference value is applied for the majority of fruit and vegetables, with some exceptions such as spices (500 μg kg−1) (EFSA, 2015b). Hence, in the present study only one sample (thyme) was over the suggested reference value. Perchlorate is a chemical ubiquitously detected in water and food. Although it is not a pesticide, it may be considered as environmental contaminant. Water and soil are potential sources of perchlorate contamination in food, since it occurs naturally in the environment, in deposits of nitrate and potash, and can be formed in the atmosphere and precipitate into soil and groundwater. The stability of perchlorate in water and the high solubility of its salts in it may lead to its presence in water supplies (Krynitsky, Niemann, & Nortrup, 2004). Fairly recently, it has been discovered perchlorates are also formed naturally by the action of lightning on chlorine species in the atmosphere (William S. Eck, 2015). Anthropogenic sources of perchlorate include manufacture and recharge of munitions and solid rocket motors, fireworks, safety flares, blasting explosives, electrochemically-prepared chlorine products, and nitrate fertilizers (Eck, 2015). Perchlorate can also be formed during the degradation of sodium hypochlorite used to disinfect water and can contaminate the water supply. These activities have resulted in perchlorate contamination of water bodies, affecting the crops via soil or irrigation. The evaluation of perchlorate presence is of crucial importance since although it is shown to have thyroid-disrupting effects in experimental animals, it remains controversial as to whether environmentally occurring levels of perchlorate have any effects on humans (Krynitsky et al., 2004).
In summary, this monitoring study, reveals that the occurrence of organic samples with pesticide residues is approximately 5 times lower than in conventional ones. In conventional products the 48% of samples present quantifiable pesticide residues (EFSA, 2018) against the 10% of organic samples with pesticide residues above 10 µg kg−1 detected in our studies (Fig. 2). Therefore, although the use of synthetic pesticides is not allowed in organic farming, organic food samples still contain pesticide residues. As it has been reported by others authors, it can be consequence of environmental conditions (soil, rain, or ground water), nearly conventional farms or cross-contamination during storage (Martínez Bueno et al., 2018, Tobin et al., 2014).
4. Conclusion
Since only a few pesticides are authorized in organic crops, concentrations of most of the studied compounds are expected to range at zero or ultra-trace levels. This might demand sensitive, low-level analyses, meeting the EU criteria with the aim to achieve a trustworthy evaluation of potential fraud in organic production. The results presented in this study show that the occurrence of organic samples with pesticide residues is approximately 5 times lower than in conventional ones. Within the scope of the 328 pesticides analyzed in the multi-residue method, 24 different pesticides, five of them not included in the multiannual control programme of the European Union (MCP), were typically detected (75% of total detections) at concentration levels between 2 and 50 µg kg−1, and only in 11% of the cases concentrations higher than 100 µg kg−1. Of the 136 commercial samples labelled with organic certification tested, 4 samples (3%) contained authorized pesticides by organic production, while 61 samples (45%) exhibited the presence of non-authorized pesticide residues, from them 8% were below 10 µg kg−1. Residues of more than one pesticide (multiple residues) were found in 17% of the samples.
The polar fungicide phosphonic acid was the substance detected more frequently (43 detections), followed by the herbicides chlorpropham and chlorate (6 and 5 detections respectively). Three herbicides not included in the MCP (prometon, chlorate and MCPA) were found at concentrations that exceeded the MRLs established by European legislation for conventional products.
These results show that, even though the use of synthetic pesticides is not allowed in organic farming, organic samples can still contain pesticide residues and need to be evaluated to ensure the lowest levels of pesticides against conventional production. In general, low levels of 1–2 pesticide residues may be a matter of environmental contamination or cross-contamination processes. By contrast, high residue concentrations, or a high number of residues can be indicative of illegal or misuse of pesticides, as in the case of a cayenne sample in which four pesticides were detected at concentration levels between 25 and 101 µg kg−1. In this study, the impact of analytical methods with low LOQs has shown not to be relevant in the majority of the cases. However, a wide scope presented a high influence on this evaluation, especially with the inclusion of very polar compounds and metabolites, representing 64% of the total detections. Consequently, organic control bodies inspections should be carried out in laboratories covering a broad scope of pesticides.
CRediT authorship contribution statement
María del Mar Gómez-Ramos: Investigation, Formal analysis, Writing - original draft, Writing - review & editing. Christina Nannou: Formal analysis. María Jesús Martínez Bueno: Investigation, Writing - review & editing. Ana Goday: Formal analysis. María Murcia-Morales: Formal analysis. Carmen Ferrer: Visualization. Amadeo R. Fernández-Alba: Conceptualization, Supervision, Project administration, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are very grateful to the Regional Government of Andalusia (Junta de Andalucía) for the financial support given to the Project “GOP2I-AL-16-0002”.M. J. Martínez Bueno thanks the Marie Skłodowska-Curie Actions (MSCA) for the individual fellowships (H2020-MSCA-IF-2015_#707816_ORGANIC QUAL RACERS).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2020.100079.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Anastassiades M., Lehotay S.J., Štajnbaher D., Schenck F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. Journal of AOAC International. 2003;86(2):412–431. Retrieved from https://www.scopus.com/results/results.uri?numberOfFields=0&src=s&clickedLink=&edit=&editSaveSearch=&origin=searchbasic&authorTab=&affiliationTab=&advancedTab=&scint=1&menu=search&tablin=&searchterm1=Fast+and+easy+multiresidue+method+employing+acetonitrile+extraction%2Fpartitioning+&field1=TITLE&dateType=Publication_Date_Type&yearFrom=Before+1960&yearTo=Present&loadDate=7&documenttype=All&accessTypes=All&resetFormLink=&st1=Fast+and+easy+multiresidue+method+employing+acetonitrile+extraction%2Fpartitioning+&st2=&sot=b&sdt=b&sl=88&s=TITLE%28Fast+and+easy+multiresidue+method+employing+acetonitrile+extraction%2Fpartitioning+%29&sid=ea174485670701321d5a14be5301d517&searchId=ea174485670701321d5a14be5301d517&txGid=b5ab9888822f76fcb932172ff5190a0e&sort=plf-f&originationType=b&rr= [PubMed] [Google Scholar]
- Bauer A., Luetjohann J., Rohn S., Jantzen E., Kuballa J. Development of a suspect screening strategy for pesticide metabolites in fruit and vegetables by UPLC-Q-Tof-MS. Food Analytical Methods (Lc) 2018:1–17. [Google Scholar]
- Bauer A., Luetjohann J., Rohn S., Kuballa J., Jantzen E. Determination of fosetyl and phosphonic acid at 0.010 mg/kg level by ion chromatography tandem mass spectrometry. Journal of Agricultural and Food Chemistry. 2018;66(1):346–350. doi: 10.1021/acs.jafc.7b03464. [DOI] [PubMed] [Google Scholar]
- Commission, T. H. E. E. (2020). (Text with EEA relevance), 13(396), 6–18.
- Council of the European Union, & European Parliament. (2018). REGULATION (EU) 2018/848 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 30 May 2018. Official Journal of the European Union, 2018(1151), 1–92. Retrieved from https://eur-lex.europa.eu/legalcontent/EN/TXT/?uri=uriserv:OJ.L_.2018.150.01.0001.01.ENG&toc=OJ:L:2018:150:TOC.
- Cressey P., Vannoort R., Malcolmb C. Pesticide residues in conventionally grown and organic New Zealand produce. Food Additives and Contaminants: Part B Surveillance. 2009;2(1):21–26. doi: 10.1080/02652030802684096. [DOI] [PubMed] [Google Scholar]
- Dias J.V., Cutillas V., Lozano A., Pizzutti I.R., Fernández-Alba A.R. Determination of pesticides in edible oils by liquid chromatography-tandem mass spectrometry employing new generation materials for dispersive solid phase extraction clean-up. Journal of Chromatography A. 2016;1462:8–18. doi: 10.1016/j.chroma.2016.07.072. [DOI] [PubMed] [Google Scholar]
- Dorais M., Alsanius B. Advances and Trends in Organic Fruit and Vegetable Farming Research. Horticultural Reviews. 2015;Vol. 43:185–267. [Google Scholar]
- EFSA. (2015a). Chorate risk assessment. https://doi.org/10.1002/ps.865.
- EFSA Statement as regards the presence of perchlorate in food endorsed by the Standing Committee on Plants, Animals Food and Feed. EFSA Journal. 2015;13(2):4002. [Google Scholar]
- EFSA The 2015 European Union report on pesticide residues in food. EFSA Journal. 2017;15(4) doi: 10.2903/j.efsa.2017.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Monitoring data on pesticide residues in food: Results on organic versus conventionally produced food, Technical Report. EFSA Supporting Publications. 2018;15(4) [Google Scholar]
- European Commission Directive 2005/396/EC of the European Parliament and the Council of 223 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Official Journal of the European Communities. 2005 [Google Scholar]
- European Commission Commission regulation (EC) No 889/2008 of 5 September 2008 laying down detailed rules for the implementation of council regulation (EC) no 834/2007 on organic production and labelling of organic products with regard to organic production, labelling and co. Official Journal of the European Union, L. 2008;250(834):1–84. [Google Scholar]
- European Commission. (2011). Working document of the Commission services on official controls in the organic sector, (July).
- European Commission . European Union; 2014. Action Plan for the future of Organic Production in the. [Google Scholar]
- European Commission (2017). Quick Method for the Analysis of Residues of numerous Highly Polar Pesticides in Foods of Plant Origin involving Simulta- neous Extraction with Methanol and LC-MS / MS Determination. Retrieved from http://www.eurl-pesticides.eu/userfiles/file/EurlSRM/meth_QuPPe-PO_EurlSRM.pdf.
- Ferrer Amate C., Unterluggauer H., Fischer R.J., Fernández-Alba A.R., Masselter S. Development and validation of a LC-MS/MS method for the simultaneous determination of aflatoxins, dyes and pesticides in spices. Analytical and Bioanalytical Chemistry. 2010;397(1):93–107. doi: 10.1007/s00216-010-3526-x. [DOI] [PubMed] [Google Scholar]
- Food, E., & Authority, S. (2018). The 2016 European Union report on pesticide residues in food, 16(396). https://doi.org/10.2903/j.efsa.2018.5348. [DOI] [PMC free article] [PubMed]
- Gil García M.D., Martínez Galera M., Uclés S., Lozano A., Fernández-Alba A.R. Ultrasound-assisted extraction based on QuEChERS of pesticide residues in honeybees and determination by LC-MS/MS and GC-MS/MS. Analytical and Bioanalytical Chemistry. 2018;410(21):5195–5210. doi: 10.1007/s00216-018-1167-7. [DOI] [PubMed] [Google Scholar]
- Hakme E., Lozano A., Uclés S., Gómez-Ramos M.M., Fernández-Alba A.R. High-throughput gas chromatography-mass spectrometry analysis of pesticide residues in spices by using the enhanced matrix removal-lipid and the sample dilution approach. Journal of Chromatography A. 2018;1573:28–41. doi: 10.1016/j.chroma.2018.08.046. [DOI] [PubMed] [Google Scholar]
- Hakme Elena, Lozano A., Uclés S., Fernández-Alba A.R. Further improvements in pesticide residue analysis in food by applying gas chromatography triple quadrupole mass spectrometry (GC-QqQ-MS/MS) technologies. Analytical and Bioanalytical Chemistry. 2017;1–16 doi: 10.1007/s00216-017-0723-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann-horlacher I., Benkenstein A., Eichhorn E., Wildgrube C., Scherbaum E., Anastassiades M. European Pesticide Residue Workshop (EPRW) Limassol, Cyprus. 2016. Chlorate and perchlorate residues in food of plant origin chlorate perchlorate. [Google Scholar]
- Krynitsky A.J., Niemann R.A., Nortrup D.A. Determination of perchlorate anion in foods by ion chromatography-tandem mass spectrometry. Analytical Chemistry. 2004;76(18):5518–5522. doi: 10.1021/ac049281+. [DOI] [PubMed] [Google Scholar]
- Lozano A., Rajski Ł., Belmonte-Valles N., Uclés A., Uclés S., Mezcua M., Fernández-Alba A.R. Pesticide analysis in teas and chamomile by liquid chromatography and gas chromatography tandem mass spectrometry using a modified QuEChERS method: Validation and pilot survey in real samples. Journal of Chromatography A. 2012;1268:109–122. doi: 10.1016/j.chroma.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Malusà E., Tosi L. Phosphorous acid residues in apples after foliar fertilization: Results of field trials. Food Additives & Contaminants. 2005;22(6):541–548. doi: 10.1080/02652030500135284. [DOI] [PubMed] [Google Scholar]
- Martínez Bueno M.J., Díaz-Galiano F.J., Rajski Ł., Cutillas V., Fernández-Alba A.R. A non-targeted metabolomic approach to identify food markers to support discrimination between organic and conventional tomato crops. Journal of Chromatography A. 2018;1546:66–76. doi: 10.1016/j.chroma.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Nannou C.I., Boti V.I., Albanis T.A. Trace analysis of pesticide residues in sediments using liquid chromatography–high-resolution Orbitrap mass spectrometry. Analytical and Bioanalytical Chemistry. 2018;410(7):1977–1989. doi: 10.1007/s00216-018-0864-6. [DOI] [PubMed] [Google Scholar]
- Rajski Ł., Diaz Galiano F.J., Cutillas V., Fernández-Alba A.R. Coupling Ion Chromatography to High Resolution Accurate Mass Spectrometry for the Fast and Robust Analysis of Anionic Pesticides in Fruits and. Vegetables. 2017;14:1–8. doi: 10.5740/jaoacint.17-0410. [DOI] [PubMed] [Google Scholar]
- Rajski Ł., Lozano A., Belmonte-Valles N., Uclés A., Uclés S., Mezcua M., Fernandez-Alba A.R. Comparison of three multiresidue methods to analyse pesticides in green tea with liquid and gas chromatography/tandem mass spectrometry. The Analyst. 2013;138(3):921–931. doi: 10.1039/c2an35995b. [DOI] [PubMed] [Google Scholar]
- Rajski Ł., Lozano A., Uclés A., Ferrer C., Fernández-Alba A.R. Determination of pesticide residues in high oil vegetal commodities by using various multi-residue methods and clean-ups followed by liquid chromatography tandem mass spectrometry. Journal of Chromatography A. 2013;1304:109–120. doi: 10.1016/j.chroma.2013.06.070. [DOI] [PubMed] [Google Scholar]
- SANTE/11813/2017. (2018). Guidance document on analytical quality control and validation procedures for pesticide residues analysis in food and feed. European Commission, Health & Consumer Protection Directorate-General, 2–44. https://doi.org/10.13140/RG.2.2.33021.77283.
- Stachniuk A., Szmagara A., Czeczko R., Fornal E. LC-MS/MS determination of pesticide residues in fruits and vegetables. Journal of Environmental Science and Health, Part B. 2017;52(7):446–457. doi: 10.1080/03601234.2017.1301755. [DOI] [PubMed] [Google Scholar]
- Thao H.T.B., Yamakawa T. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Science and Plant Nutrition. 2009;55(2):228–234. [Google Scholar]
- Tobin R., Walsh T., Garvey J., Larkin T. Detection of Pesticide Residues in Organic and Conventional Fruits and Vegetables Available in Ireland Using Gas Chromotography / Tandem Mass Spectrometry (GC-MS/MS) and Liquid Chromotography / Tandem Mass Spectrometry (LC-MS/MS) Detection. Nutrition Health Food Sci. 2014;2(1):1–7. Retrieved from http://symbiosisonlinepublishing.com/nutritionalhealth-foodscience/nutritionalhealth-foodscience13.pdf. [Google Scholar]
- Tomlin, C. (2009). The Pesticide Manual Fifteenth Edition.
- Vázquez P.P., Hakme E., Uclés S., Cutillas V., Galera M.M., Mughari A.R. Large multiresidue analysis of pesticides in edible vegetable oils by using efficient solid-phase extraction sorbents based on quick, easy, cheap, effective, rugged and safe methodology followed by gas chromatography – tandem mass spectrometry. Journal of Chromatography A. 2016;1463:20–31. doi: 10.1016/j.chroma.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Wieland M., Bauer N., Ines D., Kolberg S., Wildgrube C., Anastassiades M., Scherbaum E. European Pesticide Residue Workshop (EPRW) Dublin, Ireland. 2014, EPRWA. 2014. Phosphonic Acid: Pesticide or “Foliar Fertilizier”? residues in organic and conventional samples from the German market; p. 2014. [Google Scholar]
- Eck William S. Wildlife Toxicity Assessments for Chemicals of Military Concern. Elsevier; 2015. Wildlife toxicity assessments for chemicals of military concern; pp. 669–692. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.