Abstract
High‐affinity antibodies are produced during multiple processes in germinal centres (GCs), where follicular helper T (Tfh) cells interact closely with B cells to support B‐cell survival, differentiation and proliferation. Recent studies have revealed that a specialised subset of regulatory T cells, follicular regulatory T (Tfr) cells, especially fine‐tune Tfh cells and GC B cells, ultimately regulating GC reactions. Alterations in frequencies or function of Tfr cells may result in multiple autoantibody‐mediated or autoantibody‐associated diseases. This review discusses recent insights into the physiology and pathology of Tfr cells, with a special emphasis on their potential roles in human diseases. Discrepancies are common among studies, reflecting the limited understanding of Tfr cells. Further exploration of the mechanisms of Tfr cells in these diseases and thus targeting Tfr cells may help reinstate immune homeostasis and provide novel immunotherapy.
Keywords: follicular helper T cells, follicular regulatory T cells, germinal centre responses, human diseases, humoral immunity
This review discusses the physiology and pathology of follicular regulatory T (Tfr) cells, which remains largely unknown. Numerical and functional alterations of Tfr cells are observed in a multitude of diseases, predominantly in autoimmune diseases. Better methods to obtain bona fide Tfr cells in humans and more precise designation for Tfr cells are required to assess their underlying mechanisms, ultimately developing novel immunotherapy.
Introduction
Germinal centres (GCs) are secondary lymphoid organs in which somatic hypermutation, affinity maturation and class switch recombination occur, thus producing high‐affinity antibodies in humoral immunity.1 During the multistep process of GC reactions, follicular helper T (Tfh) cells interact closely with B cells to support B‐cell survival, differentiation and proliferation via direct contact and soluble signal.2 It was not until 10 years ago that Tfh cells were broadly acknowledged among immunologists with B‐cell leukaemia/lymphoma 6 (Bcl‐6) discovered as a lineage‐defining transcription factor (TF) of Tfh cells.2 Although various mechanisms have been defined, the understanding of GC reactions is still elusive.
A subset of regulatory T (Treg) cells expressing C‐X‐C chemokine receptor type 5 (CXCR5) was first established in human tonsils.3, 4 Then, in 2011, three separate articles described their physiology in mice.5, 6, 7 CXCR5+Foxp3+ cells present different transcriptional signatures compared with traditional Treg cells, making them a distinct subset of Treg cells, termed follicular regulatory T (Tfr) cells. High expression of CXCR5 directs Tfr cells to the B‐cell follicle by gradients of C‐X‐C motif chemokine ligand 13 (CXCL13), and then, they fine‐tune the GC responses. They express, simultaneously, markers of Treg cells including Foxp3, IL‐10, GITR and CTLA‐4 and markers of Tfh cells including Bcl‐6, PD‐1 and ICOS, thus possessing dual characteristics of Tfh cells and traditional Treg cells. Considering the potential expression of autoreactive T‐cell receptors (TCRs), Tfr cells are more similar to Treg cells than Tfh cells.8 Nonetheless, Tfr cells together with Tfh cells harbour less diverse repertoires relative to Treg cells.9 Recent studies have investigated this population in a wide range of diseases and have gained some progress.
This review discusses the physiology, pathology, discrepancies and challenges of Tfr cells, especially their alterations and potential roles in human diseases.
Distribution, differentiation and development of Tfr cells
Follicular regulatory T cells have been found in the spleen, lymph nodes (LNs), lymph or other lymphoid tissues such as Peyer's patches, and also in blood. Few Tfr cells are located within the GC, whereas most of them with low levels of PD‐1 are located surrounding the GC.10 Unlike Tfh cells, which are derived from naïve CD4+T cells,11 Tfr cells are mainly derived from thymic Treg cells.5, 6, 7 In addition, Tfr cells are also derived from Foxp3− precursors in a PD‐L1–dependent manner using certain adjuvants.12 The full differentiation of Tfr cells undergoes multistage and multifactorial processes (Figure 1). A model proposed by Fonseca et al. 13 explains that after interaction with dendritic cells (DCs), CXCR5−Foxp3+ thymic Treg cells differentiate into early‐stage Tfr (eTfr) cells and then either enter the circulation or migrate to the T‐B border. After interacting with cognate B cells at the T‐B border, eTfr cells become intermediate Tfr (iTfr) cells. With loss of CD25 expression in GCs, signatures of iTfr cells are further strengthened into matured Tfr (mTfr) cells, which can potently suppress Tfh cells and GC B cells. There are two perspectives on the origination of circulating Tfr (cTfr) cells: one advocates eTfr cells,14 and the other supports lymphoid‐resident mTfr cells.15 cTfr cells may remigrate to follicles and GCs after reactivation.15, 16
Figure 1.
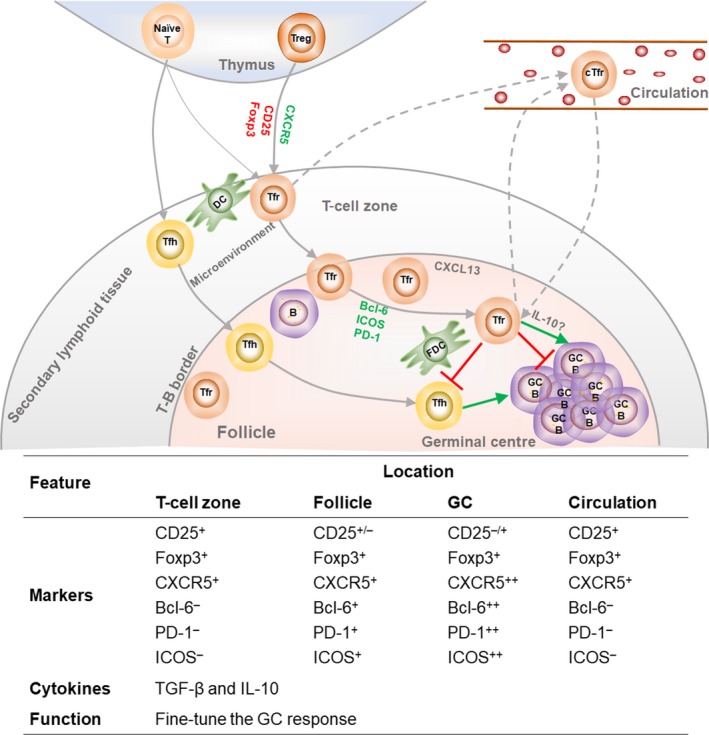
Differentiation and features of follicular regulatory T (Tfr) cells. Thymic Treg cells and naïve CD4+T are primed by dendritic cells (DCs) with antigen presentation and signals from the microenvironment at the edge of the T‐cell zone; CD4+CD25+Foxp3+ thymic Treg cells and Foxp3– precursors are then differentiated into early‐stage Tfr cells with lower levels of CD25 and Foxp3. Full differentiation of Tfr and Tfh cells requires stimulation from cognate B cells at the T‐B border. Mature Tfr cells especially fine‐tune Tfh cells and GC B cells, ultimately regulating germinal centre reactions. Markers that are upregulated in the process of Tfr‐cell differentiation are represented in green font, and markers that are downregulated are in red font.
Signals that facilitate Tfr‐cell development
T‐cell receptor and costimulatory signals through CD28 and ICOS are indispensable for Tfr‐cell development.5, 17, 18 Attenuated Tfr cells appear in CD28‐deficient cells,5 CD28‐deficient mice17, 18 and ICOS‐deficient mice.18
Bcl‐6 is a significant TF of Tfr and Tfh cells that prompts the expression of CXCR5. Deletion of Bcl‐6 in cells or in mice results in the absence of Tfr production.5, 7
Foxp3 and chromatin‐modifying enzyme Ezh2 prompt suppressive capacity and the transcriptional programme of Tfr cells.19 Once they lose Foxp3 expression, they turn into ex‐Tfr cells with an aberrant transcriptional programme and impaired suppressive capacity. Ezh2‐deficient Tfr cells also exhibit reduced suppressive function by altering the Tfr‐cell transcriptional programme distinct from Foxp3.
The development of Treg cells requires Ca2+ influx through Ca2+ release–activated Ca2+ channels (formed by STIM and ORAI), which mediates sustained and potent Ca2+ influx and is referred to as store‐operated Ca2+ entry or SOCE.20 Stim1/2‐deficient Treg cells eliminate Ca2+ signalling and cannot differentiate into Tfr cells, which may be because of impaired TFs such as B lymphocyte–induced maturation protein 1 (Blimp‐1) and signalling pathways such as CXCR5.20 SOCE also regulates Tfr‐ and Tfh‐cell differentiation via NFAT2‐dependent molecules including PD‐1, ICOS and CXCR5 and NFAT2‐mediated TFs including IRF4, BATF and Bcl‐6 expression.21 In addition to upregulating CXCR5, Tfr cells also require NFAT2 for migration.22
The role of mammalian target of rapamycin (mTOR) signalling in T‐cell development and function is intricate. It is reported that mTORC1 is a negative regulator of conventional Treg‐cell differentiation, while it also plays a critical role in Treg‐cell homeostasis and suppressive capacity.23 Nevertheless, both mTORC1 and mTORC2 are indispensable for Tfh‐cell development.24 In contrast, Roquin suppresses the PI3K‐mTOR signalling, thereby inhibiting conversion of Treg to Tfr cells.25 In particular, mTORC1 signalling prompts early differentiation and suppressive function of Tfr cells via p‐STAT3‐TCF‐1‐Bcl‐6 axis.26
It is found that TRAF3, TCF1/LEF1, Id2/Id3 and ICOS/P85α‐osteopontin are essential for the development of Tfr cells. The ablation of TRAF3 reduces the generation of Tfr cells by inhibiting ICOS expression.27 In TCF1/LEF1‐conditional knockout mice, Tfr‐cell development is abolished with impaired Bcl‐6 expression, and single knockout results in partial reduction of Tfr cells.28 Members of the helix–loop–helix family Id2 and Id3 regulate specific transcription signatures of Tfr cells by modulating CXCR5 and Foxp3 expression.29 The activation of ICOS promotes interaction of p85α (the regulatory subunit of PI3K) with osteopontin and thus maintains Bcl‐6 expression.30
miR‐10a may potentially promote Tfr‐cell differentiation by targeting Bcl‐6 and corepressor Ncor2 simultaneously, thereby diminishing the conversion of Treg cells to Tfh cells.31
Signals that inhibit Tfr‐cell development
The balance between Blimp‐1 and Bcl‐6 is subtle for the development of Tfr cells. Tfr cells express Blimp‐1 and Bcl‐6 simultaneously, whereas Tfh cells express only Bcl‐6.5 Blimp‐1 is critical for the differentiation and suppressive function of Treg cells, while Bcl‐6 is suggested to be important to maintain a Tfh‐like phenotype of Tfr cells.5 Significantly increased Tfr cells are observed in mice with Blimp‐1 specifically deleted.5, 32 However, Blimp‐1–deficient Tfr cells attenuate the suppression of antibody generation by B cells,32 suggesting that Blimp‐1 regulates Tfr function.
An increased frequency of Tfr cells is observed in the LNs from PD‐1−/− mice, and the transfer of PD‐1−/−CD4+CXCR5−Foxp3+ cells into recipient mice results in augmented differentiation and suppressive ability of Tfr cells, which is mediated by PD‐L1.18 Therefore, the PD‐1–PD‐L1 pathway can inhibit differentiation and function of Tfr cell. However, PD‐1−/− Tfr cells are able to home to GCs.
The addition of soluble OX40L results in increased immunoglobulin generation in a coculture system, indicating that the OX40L/OX40 axis impairs Tfr‐cell function.33
IL‐21 plays multifaceted roles in impairing the number and function of Tfr cells. The percentage of Tfr cells is significantly increased in BXD2‐Il21−/− mice, and the addition of IL‐21 also results in defective Tfr cell–mediated suppression of GC reactions.34 Altogether, high concentrations of IL‐21 inhibit Tfr commitment and impair their suppressive capacity while enhancing Tfh differentiation, which is mediated by downregulating p‐AKT while upregulating p‐Stat3.34 Furthermore, IL‐21 can enhance B‐cell metabolism and function, thus augmenting insensitivity of B cells to Tfr cell–mediated suppression. By enhancing Glut1 levels on Tfr cells, IL‐21 may also alter Tfr‐cell metabolism.35
It is conjectured that miR‐15b/16 may inhibit Tfr‐cell development, as they repress the expression of Rictor and mTOR, which are essential for early differentiation and effector function of Tfr cells.26, 36
The roles of miR‐17–92, miR‐155, IL‐2, STAT‐3 and AKT remain elusive. The miR‐17–92 cluster is found to promote the differentiation of Tfr cells by targeting Pten and promoting PI3K/AKT/mTOR signalling using genetic overexpression cells.25 In addition, miR‐17–92 is validated to promote Tfh‐cell differentiation, and the inhibition of Pten is implicated in their early differentiation.37 While an increased ratio of Tfr/Tfh cells is also found in chronic GVHD mice conditionally deficient for miR‐17–92, whether the underlying mechanism is attributed to selective inhibition of Tfr cells or increased apoptosis in Tfh cells deserves more investigation.38 miR‐155 overexpression results in the lack of Tfr cells by inhibiting the expression of CTLA‐4.39 Conversely, it is speculated that miR‐155 might promote Tfr‐cell differentiation by inhibiting SOCS1.40 High IL‐2 levels preclude Tfr‐cell development by promoting Blimp‐1,41 while dnTGF‐βRII Il2ra−/− mice have impaired Tfr‐cell development, which may be mediated by regulating Bcl‐6 and Nrp‐1 expression.42 The activation of p‐STAT3 by IL‐21 counteracts Tfr cell–mediated inhibition of Tfh cells.34 However, the deletion of STAT3 in Treg cells also results in loss of Tfr cells with enhanced generation of antigen‐specific IgG.43 Likewise, mTORC1 signalling prompts Tfr‐cell development by activating STAT3.26 AKT is required for regulating the proliferation and survival of B cells.44 The transfer of Tfr cells into experimental autoimmune myasthenia gravis (EAMG) mice downregulates p‐AKT and thus inactivates AKT in B cells.32 Paradoxically, inhibition of p‐AKT by IL‐21 downregulates Foxp3 expression and therefore impairs Tfr‐cell commitment.34
Mechanisms of Tfr‐cell effector function
Bcl6fl/flFoxp3cre mice (Tfr cell–specific depletion) exhibit lower levels of IgG, increased levels of IgA and decreased avidity to human immunodeficiency virus (HIV)‐1 antigen.45 In addition, higher levels of IFN‐γ, IL‐10 and IL‐21 are produced in Tfh cells from Bcl6−/− mice. The alteration in the cytokine milieu may influence the selection of B cells, ultimately resulting in abnormal GC reactions.
CTLA‐4 is intended to serve as a vital mediator for Tfr cells to fully exert suppressive function.46, 47, 48 Deletion of CTLA‐4 results in compromised effector function of Tfr cell with accumulating Tfr cells.46, 47 As a coinhibiting molecule, CTLA‐4 may downregulate costimulatory ligands B7‐1 and B7‐2 on antigen‐presenting cells49 and directly control Tfh‐cell differentiation by regulating CD28 engagement.50
Follicular regulatory T cells inhibit the expression of specific effector genes and central metabolic (i.e. Myc and mTOR) and anabolic (i.e. serine biosynthesis and one‐carbon metabolism, and purine metabolism) pathways in GC B and Tfh cells.35 Interestingly, such suppression is durable and lasts even in the absence of Tfr cells. The sustained inhibition is associated with epigenetic changes in B cells and can be overcome by IL‐21.
Follicular regulatory T cells express both the IL‐1 decoy receptor IL‐1R2 and the IL‐1 antagonist receptor IL‐1Ra, while Tfh cells express only the IL‐1R1 agonist receptor.51 IL‐1β prompts Tfh cells to secret IL‐4 and IL‐21; however, Tfr cells suppress the cytokine secretion to a similar extent as recombinant IL‐1Ra (Anakinra). Therefore, it has been proposed that the suppressive function is mediated by IL‐1R2 or IL‐1Ra on Tfr cells.
Using a new TFR–DTR mouse (Cxcr5 IRES‐LoxP‐STOP‐LoxP‐DTRFoxp3IRES‐CreYFP) strain that can selectively perturb Tfr cells, it has been found that Tfr cells potently regulate early, rather than late, GC responses and control IgE production induced by Tfh13 cells in house dust mite models.52 In addition, Tfr cells regulate memory B‐cell responses by preventing GC formation and facilitate antibody affinity during memory.
RNA sequencing has demonstrated that Tfr cells inhibit the development of a cytotoxic‐like Tfh‐cell subset that highly expresses Eomes proteins and granzyme B in Tfr cell–deficient (Bcl6‐flox/Foxp3‐cre) and Tfr cell–amplified (Blimp1‐flox/Foxp3‐cre) mouse strains.53 Since abnormal cytotoxic Tfh cells are associated with an attenuated GC and antibody responses, Tfr cells are expected to have the potential to help the GC responses.
Considering the substantial similarities between Tfr cells and Treg cells, it is plausible to extrapolate the suppressive function of Tfr cells from Treg cells such as granzyme B and suppressive cytokines. Tfr cells may induce cell death by secreting granzyme B, as Tfr cells also express granzyme B although at a lower level than Treg cells.5 TGF‐β is speculated to be a suppressive cytokine of Tfr cells, because TGF‐β signalling in T cells inhibits Tfh‐cell accumulation, activates self‐reactive B cell and therefore controls autoantibody production.54 IL‐10, however, has aroused controversies in Tfr‐cell effector function. Tfh cells also secrete IL‐10–like Tfr cells, and IL‐10+ Tfh cells can help GC responses in mice, as specific deletion of IL‐10 in Tfh cells leads to impaired humoral immunity during chronic viral infection.55 In addition, Tfr cells suppress IL‐10 production by Tfh cells and in the coculture supernatants.16 Consistent with its suppressive function, IL‐10 inhibition impedes GC responses and humoral immunity.56 Nonetheless, another study found that Tfr cell–derived IL‐10 promotes B‐cell differentiation and GC responses by inducing nuclear FOXO1 translocation in activated B cells, contributing to the dark zone phenotype and affinity maturation during acute viral infection.57
Tfr cells in human diseases and animal models
Little is known about the physiological role and mechanisms of Tfr cells because of their relatively recent studies. Nevertheless, Tfr cells have already been associated with a variety of human diseases, predominantly autoimmune diseases (AIDs; Figure 2). Considering the relative difficulty in obtaining samples from human lymphoid tissues, circulating Tfr (cTfr) cells and circulating Tfh (cTfh) cells have been regarded as the surrogate populations to investigate GC responses in most studies. Although these studies have suggested critical roles that Tfr cells play in various disease settings, many of them emphasise the correlation between numerical alterations of Tfr cells and disease manifestations, devoid of in‐depth mechanism research.
Figure 2.
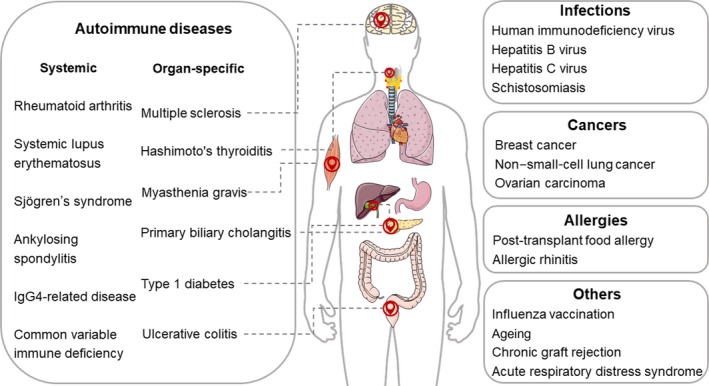
Human diseases and vaccine responses involving follicular regulatory T (Tfr) cells. Tfr cells are involved in influenza vaccination and pathological contexts including autoimmune diseases, infections, cancers, allergies, chronic graft rejection and acute respiratory distress syndrome.
Tfr cells in autoimmune diseases
Altered frequencies and/or the suppressive capacity of cTfr cells have been elucidated in a multitude of AIDs, including systemic AIDs and organ‐specific AIDs (shown in Table 1). Systemic AIDs involve rheumatoid arthritis (RA),58, 59, 60, 61, 62 systemic lupus erythematosus (SLE),33, 63, 64, 65 Sjögren's syndrome (SS),14, 66, 67 ankylosing spondylitis (AS),68 IgG4‐related disease (IgG4‐RD)69 and common variable immune deficiency (CVID).70 Organ‐specific AIDs involve multiple sclerosis (MS),15, 71, 72 myasthenia gravis (MG),73, 74, 75 Hashimoto's thyroiditis (HT),76 primary biliary cholangitis (PBC),77 type 1 diabetes (T1D)78 and ulcerative colitis (UC).79 It is also demonstrated that when the regulatory capacity of Tfr cells is impaired, the expansion of Tfr cells is accompanied by the development of autoimmunity in mice.13, 46, 47
Table 1.
Studies of Tfr cells in patients with AIDs
| Disease | Authors | Molecular phenotype of Tfr cells | Main findings |
|---|---|---|---|
| RA | Pandya et al. 62 |
CD4+CD25+CXCR5+CD127low |
cTfr‐, cTfh‐ |
| Liu et al. 58 |
CD4+CXCR5+Foxp3+ |
cTfr↑, cTfh↑, cTfr/cTfh↑, activated cTfr↑, function of cTfr↑, cTfr is negatively correlated with IgG, ACPA, RF and DAS28 | |
| Romão et al. 59 |
CD4+CXCR5+Foxp3+ |
cTfr↓, cTfh↑, cTfr/cTfh↓, cTfr does not correlate with DAS28, RF and ACPA, cTfr/cTfh is negatively correlated with DAS28 | |
| Niu et al. 60 |
CD4+CXCR5+Foxp3+ |
cTfr↓, cTfh↑, cTfr/cTfh↓, cTfr is negatively correlated with DAS28 | |
| Wang et al. 61 |
CD4+CXCR5+CD127low |
cTfr↑, cTfh↑, cTfr/cTfh↓, cTfr/cTfh is negatively correlated with CRP, ESR, RF, ACPA, IgG and DAS28 | |
| SLE | Ma et al. 65 |
CD4+CXCR5+Foxp3+ |
cTfr↓, cTfr of seronegative anti‐dsDNA exceeds cTfr of seropositive anti‐dsDNA |
| Xu et al. 64 |
CD4+CD25+CD127low–intermediateCXCR5+ |
cTfr↓, PD‐1highICOShigh Tfr↑, Ki‐67+Tfr↑, cTfr is negatively correlated with serum anti‐dsDNA antibody level | |
| Liu et al. 63 |
CD4+CXCR5+Foxp3+ |
cTfr↑, the suppressive function is not altered, cTfr is positively correlated with autoantibodies and SLEDAI scores | |
| Jacquemin et al. 33 | CD4+CD45RA–CXCR5+Foxp3+ | cTfr‐, cTfh↑, cTfh is positively correlated with SLEDAI and plasmablasts | |
| SS | Fonseca et al. 14 |
CXCR5+Foxp3+CD4+/CXCR5+CD25+CD127−CD4+ |
cTfr↑, cTfh‐, cTfr/cTfh↑ and is associated with antibody levels |
| Fonseca et al. 67 |
CXCR5+CD25+Foxp3+CD4+ |
cTfr↑, cTfr/cTfh↑ and is correlated with anti–SSA/Ro 60, anti–SSA/Ro 52 and pathologic lymphocytic infiltration in minor salivary glands | |
| Ivanchenko et al. 66 |
CXCR5+Foxp3+CD4+ |
cTfr↑, cTfr/cTfh↑, the expression of PD‐1 on Tfr↑ | |
| AS | Shan et al. 68 |
Foxp3+CXCR5+CD4+ |
cTfr↑, cTfh↑, cTfr/cTfh↑, and Tfr is negatively correlated with cTfh cells and the level of serum IL‐21 after treatment |
| IgG4‐RD | Ito et al. 69 |
CD3+CD4+CXCR5+PD‐1+CD25+CD127− |
IL‐10+–producing cTfr↑, cTfr is positively correlated with serum IgG4, ratio of IgG4/IgG, number of organs involved and soluble IL‐2 receptor |
| CVID | Cunill et al. 70 |
CD4+CXCR5+CD25highCD127low |
cTfr↓ in smB− patients, cTfh↑ |
| MS | Dhaeze et al. 15 |
CD4+CD25+CD4+CD25hiCD127loCXCR5+PD‐1+ |
cTfr↓, cTfh↑, cTfr/cTfh↓, the suppressive function of cTfr↓ |
| Jones et al. 72 |
CD4+CXCR5+Foxp3+ |
cTfr‐, cTfh‐, CD45RA+Foxp3lo resting Tfr↓, CD45RA−Foxp3lo non‐cTfr↑, Helios↓ | |
| Puthenparampil et al. 71 |
CD3+CD4+CXCR5+CD25+CD127dim |
Tfr↓, cTfr/cTfh↓, cTfr/cTfh is associated with abnormal IgG production in blood and cerebrospinal fluid | |
| MG | Wen et al. 74 | CD4+CXCR5+Foxp3+ | cTfr↓, cTfh↑, cTfr/cTfh is negatively correlated with the disease activity |
| Cui et al. 75 |
CD4+Foxp3+CXCR5+ICOS+ |
cTfr↓, cTfh↑ | |
| Zhao et al. 73 |
CD4+CXCR5+Foxp3+ |
cTfr↓, cTfh↑, cTfr/cTfh is the lowest in GMG patients | |
| HT | Zhao et al. 76 |
CD4+CXCR5+CD25intermediate–highCD127low |
cTfr↑, cTfr/cTfh↑, expression of ICOS, PD‐1 on Tfr↑, CTLA‐4↓ |
| PBC | Zheng et al. 77 | CD4+CXCR5+CD127loCD25hi | cTfr↓, cTfh↑, ICOS+cTfr↑, cTfr/cTfh is inversely correlated with disease progression, drug response and level of serum IgM |
| T1D | Xu et al. 78 |
CD4+CD19–Foxp3+CXCR5+ ICOS+/CD4+CD19– Foxp3+CXCR5+PD‐1+ |
cTfr↓, cTfh↑, CXCR5+ PD‐1+ cTfr is positively correlated with fasting serum C‐peptide levels in T1D patients |
| UC | Wang et al. 79 | Foxp3+CXCR5+CD4+ | cTfr↓, IL‐10+cTfr↓, cTfh↑, IL‐21+cTfh↑, cTfr and cTfr/cTfh are negatively correlated with disease activity |
Unless stated in the corresponding text, frequencies of Tfr cells and Tfh cells and the suppressive function of Tfr cells are compared between patients and healthy subjects; ↓, lower level compared with healthy subjects; ↑, higher level compared with healthy subjects; ‐, no statistically significant difference between patients and healthy subjects.
ACPA, anticitrullinated protein antibodies; AIDs, autoimmune diseases; AS, ankylosing spondylitis; CRP, C‐reactive protein; cTfh, circulating follicular helper T; cTfr, circulating follicular regulatory T; CVID, common variable immune deficiency; DAS28, disease activity score in 28 joint; dsDNA, double‐stranded DNA; ESR, erythrocyte sedimentation rate; GMG, generalised myasthenia gravis; HT, Hashimoto's thyroiditis; IgG, immunoglobulin G; IgG4‐RD, IgG4‐related disease; MG, myasthenia gravis; MS, multiple sclerosis; NA, not available; PA‐IgG, platelet antibody IgG; PBC, primary biliary cholangitis; PLT, platelet counts; RA, rheumatoid arthritis; RF, rheumatoid factor; SLE, systemic lupus erythematosus; SLEDAI, systemic lupus erythematosus disease activity index; smB, switched memory phenotype B cells; SS, Sjögren's syndrome; T1D, type 1 diabetes; Tfr, follicular regulatory T; UC, ulcerative colitis.
Increased58, 61 and decreased59, 60 frequencies of cTfr cells are found in RA patients, while no significant difference is found in early‐stage RA.62 The frequency of cTfr cells has negative58, 60 or no correlation59 with disease activity, and the ratio of cTfr/cTfh is correlated with disease activity.59, 61 Moreover, increased cTfr cells with enhanced suppressive function and activated CD45RA−Foxp3high cTfr subset have been found in patients with RA in stable remission compared with patients with active RA and healthy controls.58 Percentages of cTfr and cTfh cells are decreased when prescribed with methotrexate.61
Intriguingly, divergences are also observed in patients with SLE. Lower frequencies of cTfr cells were observed in two studies,64, 65 while they were higher in another study.63 Despite the total reduction in one study, active subpopulations PD‐1highICOShigh Tfr cells and Ki‐67+Tfr cells are increased in SLE patients.64 The frequency of cTfr cells is negatively correlated with serum anti‐dsDNA antibody levels and with disease activity64 and is significantly lower in seropositive anti‐dsDNA patients than in seronegative anti‐dsDNA patients.65 In contrast, another study has found a positive correlation with clinical severity and autoantibodies IgG and IgA.63 The suppressive function of cTfr cells is not altered in newly diagnosed SLE patients.63 In addition, the imbalance of cTfr cells can be improved after effective therapy.64, 65 Reduced frequency and potentially impaired inhibitory capacity of CD4+PD‐1+CXCR5+Foxp3+ Tfr cells are found in spleens from BXD2 autoimmune lupus mice.80 In the presence of NFAT2‐deficient Tfr cells, the expression of CXCR5 is downregulated, and lupus‐like disease is exacerbated in chromatin‐immunised mice.22
Elevated cTfr levels and cTfr/cTfh ratios in SS patients have reached a consensus,14, 66, 67 especially in patients with high autoantibody levels.14 The expression of PD‐1 on cTfr cells is increased.66 Activated PD‐1+ICOS+cTfh cells are closely associated with SS disease activity compared with patients with non‐SS/sicca syndrome, and the increased ratio of cTfr/cTfh indicates the formation of ectopic lymphoid structures within minor salivary glands, typically in patients with focal sialadenitis.67 Furthermore, cTfr cells do not preferentially inhibit humoral responses because of the lack of full B cell–suppressive capacity, limiting class switch recombination, and they show a naïve‐like phenotype. These cells are not emerged from the thymus but are produced in peripheral lymphoid organs during the GC reaction, leaving the tissue and then entering the blood.14 Thus, the increased frequency of the cTfr cells indicates ongoing humoral activity,14 and the cTfr/cTfh ratio is suggested to be considered as a strong predictor of SS diagnosis and focal sialadenitis in patients suffering sicca symptoms.67 Impaired cTfr cells contribute to decreased saliva flow rates and enhanced salivary gland–specific antibodies, tissue destruction and IgG deposition in a mouse experimental SS model, indicative of the development of disease.81
Percentages of cTfr cells and cTfh cells are significantly higher in patients with AS.68 In addition, the frequency of cTfr cells is negatively associated with serum IgA in AS patients before treatment and is negatively associated with cTfh cells and the level of serum IL‐21 after 1 month of standard treatment in drug responders.68
IL‐10+–producing cTfr cells are increased in patients with IgG4‐RD.69 In addition, the frequency of cTfr cells is positively correlated with serum IgG4, ratio of IgG4/IgG, number of organs involved and soluble IL‐2 receptor in IgG4‐RD patients. The percentages of blood and tonsillar Tfr cells are increased with ageing, especially at the ages of IgG4‐RD high prevalence.
The frequency of cTfr cells is decreased only in CVID patients with ≤2% of IgD−CD27+ (switched memory phenotype) B cells (smB−), while the frequency of cTfh cells is increased in both smB− and smB+ patients.70 Moreover, CD4+CXCR5+CD25highCD127lowcTfr cells exert inhibitory capacity as nonfollicular CD4+CXCR5−CD25highCD127low cells.
In MS patients, significantly lower frequencies of cTfr cells were found in two studies,15, 71 as are Tfr cells in cerebrospinal fluid.71 In addition, a lower cTfr/cTfh ratio is related to higher IgG production and circulating B‐cell percentage.71 Notwithstanding, in patients with early clinical phase clinically isolated syndrome, a neurological disturbance often occurs before the development of MS, and the proportions of cTfr cells and cTfh cells are not significantly different from healthy controls.72 Specifically, proportions of proinflammatory Th17‐like cTfr cells15 and cytokine‐producing CD45RA−Foxp3lo non‐cTfr cells72 are increased, while the proportion of suppressive fraction CD45RA+Foxp3lo resting cTfr cells72 is reduced in MS patients, which may explain the impaired suppressive function of cTfr cells. This impairment may be because of a defect in CTLA‐4 signalling and that the most potent Tfr cells home to the lymph organs to inhibit the ongoing GC response.15
Decreased cTfr cells are found in MG patients compared with healthy controls.73, 74, 75 The ratio of cTfr/cTfh is positively correlated with the expression of the autoimmune regulator gene in peripheral blood73 but negatively correlated with the disease severity of MG.73, 74 In addition, a significantly decreased frequency of cTfr cells and an increased frequency of cTfh cells are observed in generalised MG (GMG) patients compared with untreated ocular MG (OMG) patients,74 and the cTfr/cTfh ratio is the lowest in the GMG patients, as compared to the OMG patients, and higher in healthy controls.73 After glucocorticoid treatment, cTfr cells and cTfh cells in MG patients restore immune homeostasis.74
A significantly increased percentage of cTfr cells and the ratio of cTfr/cTfh are observed in patients with HT, and Th2‐like cTfr cells are significantly upregulated, while CTLA‐4 is downregulated on Tfr cells, which may contribute to the impaired humoral immune function of cTfr cells in HT.76
Decreased cTfr cells and increased cTfh cells are found in patients with PBC.77 Meanwhile, both ICOS+cTfr cells and CTLA‐4+cTfh cells are increased, and the ratio of cTfr/cTfh is inversely correlated with disease progression, drug response and the level of serum IgM. The effector memory phenotype (CCR7loPD‐1hi) in cTfr cells and cTfh cells is significantly increased, while the central memory phenotype (CCR7hiPD‐1lo) is decreased in PBC patients, and the frequency of effector memory cTfr cells is positively correlated with the level of serum alkaline phosphatase. Based on the expression of CXCR3 and CCR6, CD4+CXCR5+T cells are further classified into three types: Tfh1 type (CXCR3+CCR6–), Tfh2 type (CXCR3–CCR6–) and Tfh17 type (CXCR3–CCR6+). The ratio of (Tfr2+Tfr17)/Tfr1 is decreased, but the ratio of (Tfh2+Tfh17)/Tfh1 is increased in PBC patients, which may imply the ongoing humoral immune response.
Significantly decreased CD4+CD19−Foxp3+CXCR5+ICOS+ and CD4+CD19−Foxp3+CXCR5+PD‐1+cTfr cells and the PD‐1+cTfr/cTfh ratio are found in patients with both T1D and type 2 diabetes (T2D).78 Between these two different markers, only the frequency of CXCR5+PD‐1+ cTfr cells is positively correlated with fasting serum C‐peptide levels in T1D patients, which is contrary to that of T2D patients. However, in only T1D patients, the frequency of Tfr cells is correlated with levels of positive autoantibodies. In addition, cTfr cells decrease significantly after 1‐year follow‐up with the progress of T1D. Impaired suppressive function of cTfr cells is also observed in T1D patients. Decreased cTfr cells are further validated in nonobese diabetic mice and are associated with ongoing diabetes. Notably, an adoptive transfer of Tfr cells effectively prevents diabetes onset.
Decreased frequencies of cTfr cells are observed in UC patients.79 And the subtype IL‐10+Foxp3+CXCR5+ cells are decreased, but cTfh cells and the subtype IL‐21+Foxp3−CXCR5+ cells are expanded in UC.
Although the emphasis of Tfr‐cell studies is mainly on AIDs, vaccine responses14, 15 and other diseases including infections,82, 83, 84, 85, 86, 87, 88 cancers,89, 90, 91 allergies,92, 93 chronic graft rejection/graft‐versus‐host disease (GVHD)94 and acute respiratory distress syndrome (ARDS)95 have also been under investigation (shown in Table 2).
Table 2.
Studies of Tfr cells in patients with non‐AIDs and vaccine responses
| Disease | Authors | Molecular phenotype of Tfr cells | Main findings |
|---|---|---|---|
| HIV | Colineau et al. 87 |
CD3+CD4+CD45RA–CCR7−CXCR5+Foxp3+ |
GC Tfr↑ |
| Miles et al. 85 |
CD4+Foxp3+CD20+IgD+ |
GC Tfr↑, proliferation↑, apoptosis↓, regulatory function↑ | |
| Miller et al. 84 |
CXCR5+CD25+ CD127−(Tfr) and CXCR5+PD1+CD25+CD127−(GC Tfr) |
Tfr↑, GC Tfr↑, Tfh↑, GC Tfh↑, and Tfr is highly permissive to R5‐tropic HIV‐1 | |
| CHB | Wu et al. 82 |
CD4+CXCR5+Foxp3+ |
cTfr↑, cTfr/cTfh↑ and are positively correlated with FIB‐4 and APRI |
| HCV | Cobb et al. 83 |
CD4+CXCR5+ PD‐1+CD25+Foxp3+ |
Tfr↑, expression of CTLA‐4 on Tfr↑, suppression capacity↑ |
|
CHB CHC |
Wang et al. 88 |
CD4+CXCR5+Foxp3+ |
cTfr↑, cTfh↓, cTfr is positively correlated with serum HBV DNA, HBsAg and ALT in CHB patients, and also HCV RNA and ALT in CHC patients |
| Schistosomiasis | Chen et al. 86 |
CD3+CD4+CXCR5+Foxp3+ |
cTfr↑, CD45RA− cTfr↑ |
| Breast cancer | Faghih Z et al. 91 |
CD4+Bcl6+CXCR5int/hi |
Tfr↑, Tfh↑ |
| NSCLC | Guo et al. 90 | CD4+CXCR5+ ICOS+Foxp3+ | cTfr↑, cTfh↑ |
| Ovarian carcinoma | Li et al. 89 |
CD4+CD25+CD127−CXCR5+/CD4+CD25+CD127− CXCR5+Foxp3+ |
cTfr↑, the expression of TGFB1 and IL10↑, suppression capacity↑ |
| LTFA | Bruyne et al. 93 |
CD3+CD4+CD45RO+CXCR5+CD25+ Foxp3+ |
cTfr↓ |
| AR | Yao et al. 92 |
CD3+CD4+CD45RAlowCXCR5high/CD3+CD4+CD45RAlowCXCR5+CD25highCD127lowFoxp3+ |
Tfr↓ and is negatively correlated with antigen‐specific IgE production and disease activity, the suppression capacity↓ |
| CGR | Chen et al. 94 |
CD4+CXCR5+ICOS+Foxp3+CD127− |
Tfr↓, IL‐21–Tfh↑ in AMR patients |
| ARDS | Li et al. 95 |
CD4+Foxp3+CXCR5+ |
cTfr↑, expression of CTLA‐4 and IL‐10 on Tfr↓, the suppression capacity↓ |
| Influenza vaccination | Dhaeze et al. 15 |
CD4+CD25+CD127−CXCR5+ PD‐1+ |
cTfr↑ |
| Fonseca et al. 14 |
CXCR5+Foxp3+CD4+/CXCR5+CD25+CD127−CD4+ |
cTfr↑ |
AIDs, autoimmune diseases; ALT, serum alanine transaminase; AMR, antibody‐mediated rejection; APRI, aspartate transaminase‐to‐platelet ratio index; AR, allergic rhinitis; ARDS, acute respiratory distress syndrome; CGR, chronic graft rejection; CHB, chronic hepatitis B; CHC, chronic hepatitis C; FIB‐4, fibrosis index based on four factors; GC, germinal centre; HCV, hepatitis C virus; HIV, human immunodeficiency virus; LTFA, post‐transplant food allergy; NSCLC, non–small‐cell lung cancer; Tfr, follicular regulatory T.
Tfr cells in infections
A greater proportion of Tfr cells is found in chronically HIV+ spleens,87 lymphoid tissues84, 85 and tonsils.84, 87 The expansion of Tfr cells is mediated by HIV viral replication, IDO and TGF‐β signalling, enhanced proliferation and weakened apoptosis, and regulatory DC.85 Moreover, tonsil Tfr cells exhibit increased regulatory function and dysregulated activity of Tfh cells during HIV infection.85 Tfr cells are highly permissive to R5‐tropic HIV‐1, probably because of the elevated expression of CCR5 and an enhanced proliferative state, and the heightened permissivity leads to persistent HIV‐1 replication in vivo.84 Paradoxically, increased CD4+Foxp3+CD20+IgD+ and Foxp3+CD25+CXCR5+CCR7–CD4+ Tfr cells,85, 96 unchanged CD4+CD25+Foxp3+CXCR5+PD1hiBcl‐6+ Tfr cells97 and decreased CXCR5+CCR7−Foxp3+CD25+CD4+ Tfr cells98 are discovered in lymphoid tissues from rhesus macaques during chronic Simian immunodeficiency virus (SIV) infection, and the discrepancies are presumably attributed to distinct gating strategies. In contrast to chronic infection, Foxp3+CD25+CXCR5+CCR7−CD4+ Tfr cells are reduced during acute SIV infection, and the decreased ratio of Tfr/Tfh, rather than the frequency of Tfr cells, is correlated with anti‐dsDNA antibody and antiphospholipid antibody levels.96 In addition, the frequency of Tfr cells is negatively correlated with the avidity of antibodies recognising SIV‐gp120.98 The percentage of Tfr cells is also increased during chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection,82, 83, 88 which is more pronounced in patients with liver cirrhosis82 and HBeAg+ chronic hepatitis B (CHB).88 The percentage of IL‐10–producing tonsillar Tfr cells is increased, and the expression of CTLA‐4 on Tfr cells is upregulated.83 In addition, the frequency of cTfr cells and the ratio of cTfr/cTfh are positively correlated with fibrosis index based on four factors and the aspartate transaminase‐to‐platelet ratio index.82 Conversely, one study found that an increased number of cTfr cells are accompanied by higher levels of serum HBV DNA, HBsAg and serum alanine transaminase (ALT) in CHB patients and HCV RNA and ALT in chronic hepatitis C (CHC) patients,88 while another study indicated no correlations between cTfr cells and HBV DNA and ALT.82 The suppressive capacity of Tfr cells against Tfh cells is enhanced after exposure to HCV.83 The percentage of cTfr cells is also increased in patients with schistosomiasis, and most are CD45RA− cTfr cells, reflecting memory‐like properties.86 It seems that the increase in Tfr cells in infections has gained consensus in humans. However, divergences exist in mouse models. Both cTfr cells and cTfh cells were observed to be expanded in mice infected with influenza.16, 99 Botta et al. 41 found that Tfr cells in the lung‐draining mediastinal LNs fail to accumulate at the peak of influenza infection, but after the immune response subsides, a fraction of Treg cells downregulates CD25 and then differentiates into Tfr cells. Tfr cell–deficient mice exhibit enhanced immunity to influenza viruses, indicating that Tfr cells may be targets in infectious disease.81 Using mice infected with orthopox and influenza A viruses, it was observed that Tfh cells expand more prominently than Tfr cells and that both lymphoid and circulating Tfh/Tfr ratios can be regarded as early predictors of long‐lived protective humoral immune responses.100
Tfr cells in cancers
Increased frequencies of Tfr cells89, 90, 91 and Tfh cells90, 91 are found in tumor‐draining LNs91 and peripheral blood89, 90 from patients with breast cancer,91 non–small‐cell lung cancer (NSCLC)90 and ovarian carcinoma.89 Tumor‐infiltrating Tfr cells exhibit a higher proportion than cTfr cells.89 In addition, Tfr cells from ovarian cancer patients exhibit higher TGF‐β (TGFB1) and IL‐10 (IL10) gene transcription levels, particularly the tumor‐infiltrating Tfr cells.89 The frequency of Tfr cells is not correlated with metastasis or the progression of breast cancer,91 whereas the frequency of cTfh cells is correlated with clinical stage and histological subtypes of NSCLC patients.90 Notably, when cocultured with CD8+T cells in ovarian cancer patients, Tfr cells inhibit the activation of CD8+T cells in an IL‐10–dependent manner, indicative of enhanced suppressive function.89
Tfr cells in allergies
Frequencies of Tfr cells are found to be lower in peripheral blood from post‐transplant food allergy patients93 and in tonsils from allergic rhinitis (AR) patients.92 The phenotype and number of cTfr cells are correlated with tonsillar Tfr cells.92 Moreover, cTfr cells in AR patients show impaired function specifically in suppressing IgE expression rather than other immunoglobulin types. The number and function of cTfr cells are negatively correlated with antigen‐specific IgE production and disease activity in AR patients. Once patients get relief after allergen immunotherapy, the frequency and function of cTfr cells are recovered.92 Tfr cells have also been investigated in a food allergy mouse model to measure peanut‐specific antibody responses.53
Tfr cells in other disease settings
In renal transplant patients with chronic renal allograft dysfunction, frequencies of Tfr cells in peripheral blood and renal grafts from those with antibody‐mediated rejection (AMR) are significantly decreased, while the frequency of IL‐21–producing Tfh cells is increased, relative to those non‐AMR patients.94 However, Tfr cells exhibit equivalent suppressive function between AMR and non‐AMR patients. An increase in the Tfr‐cell ratio inhibits the expression of IL‐21 in Tfh cells, the proliferation and differentiation of B cells, and IgG and IgA secretion of plasma cells.94 The miR‐17–92 cluster is proved to facilitate Tfh‐cell differentiation and impair Tfr/Tfh balance, thus accelerating the development of chronic GVHD in mice.38 In addition, Tfr cells prompt lymphangiogenesis and Breg proliferation, exerting an atheroprotective effect in Apoe−/− mice transferred with Tfr cells.101
Follicular regulatory T cells are significantly elevated in peripheral blood mononuclear cells (PBMCs) and in mini‐bronchoalveolar lavage (BAL) during the onset of ARDS. Notably, Tfr cells account for a small proportion of Treg cells in PBMCs and a major proportion in mini‐BAL. Compared with non‐Tfr Treg cells, Tfr cells exhibit lower levels of CTLA‐4 and IL‐10 and weaker suppression capacity towards autologous CD4+CD25−T cells but enhanced capacity to induce IL‐10+Breg cells.95
Tfr cells in vaccine responses
The number of cTfr cells is increased after influenza vaccination in healthy subjects14, 15 and is positively correlated with anti‐influenza antibodies. Among cTfr cells, memory Tfr cells (CD45RO+CD45RA− Tfr) are significantly increased, whereas naïve Tfr cells (CD45RO−CD45RA+) are decreased significantly after vaccination.15
Tfr cells in ageing
The expansion of Tfr cells in ageing is corroborated in patients with NSCLC90 and IgG4‐RD.69 The percentages of cTfr cells and cTfh cells are higher in patients older than 60 years, but the cTfr/cTfh ratio is decreased with age.90 Nevertheless, the suppressive capacity of Tfr cells decreases with ageing.69 Both Tfr cells and Tfh cells expand with age in mice,99, 102 and Tfr cells are more pronounced.102 However, male mice exhibit a higher percentage of Tfr cells than their female counterparts regardless of age.99 Aged and young Tfr cells exhibit identical suppressive capacity but a distinct phenotype with enhanced PD‐1 and decreased ICOS expression.102
Challenges and unsolved questions of Tfr cells
Despite the fact that great progress has been made in the physiology of Tfr cells in recent years, much is unknown, and significant phenotypic and functional heterogeneity of Tfr cells still exists among various disease settings. The uniform methodology to identify and purify Tfr cells is a technical challenge. Actually, the defining markers such as Foxp3, CD25103 and CXCR5104 are also upregulated during effector T (Teff)‐cell activation. Unlike Treg cells, recent studies have shown that bona fide Tfr cells do not express IL‐2Ra (CD25),41, 45, 51, 105 which is inconsistent with previous description of the CD25+Tfr cell. Thus, most previous studies investigating the physiology of Tfr cells may be mixtures of Tfr cells and Treg cells. Notably, both CD25+ and CD25– Tfr cells exist in follicles and GCs.105 Therefore, it is imperative to designate corresponding markers in different anatomic locations. Tfr cells were analysed initially by total Treg depletion,5, 7, 46 adoptive transfer along with other T cells,5, 7, 18, 26 and then mice with deletion of Roquin25 and NFAT2,22 but these studies may manifest nonspecific effects or nonphysiological function of Tfr cells.106 Recently, a novel model using Bcl6fl/flFoxp3cre mice has been under investigation, and the findings about Tfr‐cell function are sharply distinct from previous studies.41, 45, 57 Nevertheless, Bcl6fl/flFoxp3cre mouse model has its limits, and a more recent study has used a Cxcr5IRES‐LoxP‐STOP‐LoxP‐DTRFoxp3IRES‐CreYFP mouse strain.52 Therefore, the establishment of uniform markers both phenotypically and functionally to unambiguously define bona fide Tfr cells in corresponding anatomic locations and of models to analyse Tfr cells specifically and physiologically will greatly deepen our understanding of Tfr cells.
Restricted by the samples, cTfr cells are often chosen as surrogate indicators, but the origin, phenotype and function between cTfr and GC Tfr cells have not yet reached a final conclusion (shown in Table 3). However, the kinetics of LN Tfr cells are found to be similar to those of cTfr cells.18 It is acknowledged that cTfr cells are derived from lymphoid tissues and are at least activated by DC.14, 15, 16 Two studies conducted in patients with X‐linked agammaglobulinaemia14 and anti‐CD20 antibody rituximab treatment,107 both B cell–deficient, found that cTfr‐ and cTfh‐cell populations are not influenced. In addition, cTfr cells comprise a phenotypically distinct population compared with GC Tfr cells, displaying similar or lower levels of CXCR5 and lower or even no ICOS, PD‐1 or Bcl‐6.14, 15, 16 Resembling,15 attenuated16 and incomplete14 effector function of cTfr cells is described by sorting cTfr cells with distinct strategies. RNA‐seq has also validated that cTfr cells separate from GC Tfr cells, and even LN Tfr cells only partially overlap with splenic Tfr cells in mice immunised with NP‐OVA.19 More specifically, LN Tfr cells exhibit higher levels of ICOS and CTLA‐4, similar levels of Ki‐67, but lower levels of PD‐1 relative to splenic Tfr cells. When comparing their suppressive ability, LN Tfr cells are more potent for inhibiting class switching than splenic Tfr cells.19 Thus, whether cTfr cells can be regarded as an alternative to investigate bona fide Tfr cells needs more validation. cTfr cells might have the capacity to home to secondary lymphoid organs after reactivation15, 16 and recirculate quickly (about a few hours) through the blood,16 whereas their putative remigration and subsequent reactions have not yet studied.
Table 3.
Studies comparing cTfr and GC Tfr cells
| Authors | Gating | Samples | Origin | Phenotype | Function |
|---|---|---|---|---|---|
| Sage et al. 16 |
CD4+ICOS+CXCR5+Foxp3+/GITR+CD19– |
Mice immunised with NP‐OVA or NP‐HEL in CFA | Primed by DC, do not require B cells | Memory‐like, persist in vivo for a long time, express similar levels of CXCR5 but lower ICOS, similar proportions in cell cycle | Similar to LN Tfr cells but with a much lower capacity |
| Dhaeze et al. 15 |
CD4+CD25+CD127−CXCR5+PD‐1+ |
Non‐AIDs adult patients with routine tonsillectomies | Lymphoid‐resident Tfr cells after a GC response | Express lower levels of follicular markers (CXCR5, PD‐1, Bcl‐6 and ICOS) but similar levels of regulatory markers (Foxp3 and Helios) with comparable Foxp3 methylation status and higher levels of CD31, CCR7 and CD62L, display a memory phenotype and higher percentage of Th1‐like phenotype | Comparable suppressive function with tonsil‐derived Tfr cells |
| Fonseca et al. 14 |
CXCR5+Foxp3+CD4+/CXCR5+CD25+CD127−CD4+ |
Healthy children with routine tonsillectomies | Peripheral lymphoid tissues before T‐B interaction | Naïve‐like phenotype (high levels of CD45RA, CCR7, CD62L and CD27 and low levels of HLA‐DR), CD45RO−Foxp3lo resting cells are the majority, do not express ICOS, PD‐1 or Bcl‐6 | Able to suppress activation of B cells and proliferation of Tfh cells, do not inhibit class switch recombination |
AIDs, autoimmune diseases; CFA, complete Freund's adjuvant; cTfr, circulating follicular regulatory T; DC, dendritic cell; GC, germinal centre; HLA‐DR, human leucocyte antigen–DR; LN, lymph node; NP‐HEL, 4‐hydroxy‐3‐nitrophenylacetyl hapten–conjugated hen egg lysozyme; NP‐OVA, 4‐hydroxy‐3‐nitrophenylacetyl hapten–conjugated ovalbumin.
Animal models are generally used to reflect putative human physiology and pathology. Studies by two groups have revealed differences in cTfr function between humans and mice. Sage et al. 16, 18, 35, 46 showed that cTfr cells in mice can inhibit antibody generation, while Fonseca et al. 14 reported that cTfr cells in humans are not fully suppressed. Partly because of environmental exposure, murine immune system and immune responses are actually different from those of humans.108 From this viewpoint, applications of murine knowledge directly to humans are circumscribed. Furthermore, the origin and TCR repertoire of Tfr cells have been studied only in mice.
Generally speaking, Tfr cells are deemed as repressors of GC reactions, so it is speculated that decreased Tfr/Tfh ratios are associated with enhanced autoantibody generation. Nevertheless, the alterations of Tfr cells in homogeneous diseases are inconsistent and even opposite. Study participants with different stages of the disease and therapeutic regimens may account for part of the reason because methotrexate, a first‐line medication for RA, has reduced the frequencies of both Tfr and Tfh cells.61 In addition, distinct markers to define Tfr cells in the same anatomic locations and the same markers to define Tfr cells in distinct anatomic locations have brought about great confusion. A possible interpretation for increased Tfr cells in autoantibody‐mediated diseases is that it is a compensative response as a result of increased frequency of Tfh cells, attempting to restore immune homeostasis. Another is that suppressive function of Tfr cells is impaired in disease settings; thus, the overall effect of controlling GC responses is still attenuated. In addition, it has also been proposed that the microenvironment while thymic Treg cells differentiate into eTfr cells has altered so that eTfr cells have difficulty in getting access to GCs, leaving them in the peripheral blood.
Also unknown is whether Tfr cells convert from suppressive to stimulatory function in GC responses. Although most studies have demonstrated that Tfr cells function as inhibitors of Tfh and GC B cells, it has been proven that Tfr cells do not influence Tfh or GC B‐cell gross population in a Bcl6fl/flFoxp3cre mouse model.45 Furthermore, Tfr cells help maintain high levels of high‐affinity antigen‐specific antibodies5, 45 and regulate the isotype switch of antibodies.45 They also promote the GC response through the secretion of IL‐10 and the suppression of cytotoxic Tfh cells.53, 57 Thus, Tfr cells might play a complicated role in the fine‐tuning of GC response and act as ‘helper cells’ in certain scenarios.
Recently, Treg cells have been proven to be not terminally differentiated populations and have some degree of instability and plasticity under inflammatory conditions, suggesting that Treg cells may lose Foxp3 expression or even acquire the properties of Teff cells. In addition, Teff cells are resistant to suppression by Treg cells in some AIDs.109 Foxp3 instability in Tfr cells has recently been demonstrated, which results in attenuated suppressive capacity of Tfr cells.19 The so‐called ex‐Tfr cells lose their previous transcriptional programme, rendering them more similar to Tfh cells.19 Considering the shared similarities between Tfr and Treg cells, whether plasticity exists in Tfr cells and whether Tfh and B cells are resistant to Tfr‐cell suppression under pathological conditions remain unknown.
Since Tfr cells play such a significant role in regulating antibody production, it is attractive to target Tfr cells to restore immune homeostasis. Altered Tfr/Tfh might be implicated in causes or effects of the above‐mentioned diseases. Intraperitoneally injected all‐trans retinoic acid in EAMG rats and caspase‐1 inhibitor in mice have ameliorated disease severity concomitant with an increased frequency of Tfr cells and decreased frequency of Tfh cells.110, 111 Baicalin treatment has also been found to ameliorate lupus nephritis in MRL/lpr lupus‐prone mice by enhancing the expansion of Tfr cells and suppressing the differentiation of Tfh cells.112 Methods to enhance Treg cells numerically and functionally have been under clinical trials in AIDs,109 so we wonder whether Tfr cells can be applied for therapeutic interventions in AIDs, infections, cancers, allergies and other disorders. According to their role in inhibiting antibody generation, regulation of Tfr cells may strengthen the efficacy of vaccines.
Perspectives
The discovery of Tfr cells has provided novel insights into the regulation of humoral immunology, although it is still in the nascent stage. Future emphasis should be put on intricate factors that influence the differentiation and function of Tfr cells. Further elucidation of Tfr cell–suppressive mechanisms is essential for Tfr cell–related therapeutics. Tfr cells have been studied in a wide range of diseases, predominantly AIDs, and most of the samples derive from peripheral blood. A major challenge in human research is to obtain lymphoid tissues to assess bona fide Tfr cells. LN fine‐needle aspirates (FNAs), a minimally invasive method primarily used for cancer pathology surveillance, may provide access to GCs in humans.113 LN FNAs have already been applied in a pioneering longitudinal study for human GC investigation.114 Undoubtedly, the establishment of a precise definition of Tfr cells in different anatomic locations and relevant animal models is of equal importance. Substantial discrepancies and problems remain to be solved, and these answers will undoubtedly improve immunotherapy.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
This article was supported by the National Natural Science Foundation of China (No. 81573802, 81503426, 81874383).
References
- 1. Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol 2012; 30: 429–457. [DOI] [PubMed] [Google Scholar]
- 2. Crotty S. T Follicular helper cell biology: a decade of discovery and diseases. Immunity 2019; 50: 1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim HW, Hillsamer P, Banham AH et al Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol 2005; 175: 4180–4183. [DOI] [PubMed] [Google Scholar]
- 4. Lim HW, Hillsamer P, Kim CH. Regulatory T cells can migrate to follicles upon T cell activation and suppress GC‐Th cells and GC‐Th cell‐driven B cell responses. J Clin Invest 2004; 114: 1640–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Linterman MA, Pierson W, Lee SK et al Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med 2011; 17: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wollenberg I, Agua‐Doce A, Hernandez A et al Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol 2011; 187: 4553–4560. [DOI] [PubMed] [Google Scholar]
- 7. Chung Y, Tanaka S, Chu F et al Follicular regulatory T cells expressing Foxp3 and Bcl‐6 suppress germinal center reactions. Nat Med 2011; 17: 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maceiras AR, Almeida S, Mariotti‐Ferrandiz E et al T follicular helper and T follicular regulatory cells have different TCR specificity. Nat Commun 2017; 8: 15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ritvo PG, Saadawi A, Barennes P et al High‐resolution repertoire analysis reveals a major bystander activation of Tfh and Tfr cells. Proc Natl Acad Sci USA 2018; 115: 9604–9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sayin I, Radtke AJ, Vella LA et al Spatial distribution and function of T follicular regulatory cells in human lymph nodes. J Exp Med 2018; 215: 1531–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011; 29: 621–663. [DOI] [PubMed] [Google Scholar]
- 12. Aloulou M, Carr EJ, Gador M et al Follicular regulatory T cells can be specific for the immunizing antigen and derive from naive T cells. Nat Commun 2016; 7: 10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fonseca VR, Ribeiro F, Graca L. T follicular regulatory (Tfr) cells: dissecting the complexity of Tfr‐cell compartments. Immunol Rev 2019; 288: 112–127. [DOI] [PubMed] [Google Scholar]
- 14. Fonseca VR, Agua‐Doce A, Maceiras AR et al Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci Immunol 2017; 2: eaan1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dhaeze T, Peelen E, Hombrouck A et al Circulating follicular regulatory T cells are defective in multiple sclerosis. J Immunol 2015; 195: 832–840. [DOI] [PubMed] [Google Scholar]
- 16. Sage PT, Alvarez D, Godec J et al Circulating T follicular regulatory and helper cells have memory‐like properties. J Clin Invest 2014; 124: 5191–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Linterman MA, Denton AE, Divekar DP et al CD28 expression is required after T cell priming for helper T cell responses and protective immunity to infection. elife 2014; 3:e03180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sage PT, Francisco LM, Carman CV et al The receptor PD‐1 controls follicular regulatory T cells in the lymph nodes and blood. Nat Immunol 2013; 14: 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hou S, Clement RL, Diallo A et al FoxP3 and Ezh2 regulate Tfr cell suppressive function and transcriptional program. J Exp Med 2019; 216: 605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vaeth M, Wang YH, Eckstein M et al Tissue resident and follicular Treg cell differentiation is regulated by CRAC channels. Nat Commun 2019; 10: 1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaeth M, Eckstein M, Shaw PJ et al Store‐operated Ca2+ entry in follicular T cells controls humoral immune responses and autoimmunity. Immunity 2016; 44: 1350–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaeth M, Muller G, Stauss D et al Follicular regulatory T cells control humoral autoimmunity via NFAT2‐regulated CXCR5 expression. J Exp Med 2014; 211: 545–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng H, Yang K, Cloer C et al mTORC1 couples immune signals and metabolic programming to establish T(reg)‐cell function. Nature 2013; 499: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng H, Cohen S, Guy C et al mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity 2016; 45: 540–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Essig K, Hu D, Guimaraes JC et al Roquin suppresses the PI3K‐mTOR signaling pathway to inhibit T helper cell differentiation and conversion of Treg to Tfr cells. Immunity 2017; 47: 1067–1082. [DOI] [PubMed] [Google Scholar]
- 26. Xu L, Huang Q, Wang H et al The kinase mTORC1 promotes the generation and suppressive function of follicular regulatory T cells. Immunity 2017; 47: 538–551. [DOI] [PubMed] [Google Scholar]
- 27. Chang JH, Hu H, Jin J et al TRAF3 regulates the effector function of regulatory T cells and humoral immune responses. J Exp Med 2014; 211: 137–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang BH, Wang K, Wan S et al TCF1 and LEF1 control Treg competitive survival and Tfr development to prevent autoimmune diseases. Cell Rep 2019; 27: 3629–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyazaki M, Miyazaki K, Chen S et al Id2 and Id3 maintain the regulatory T cell pool to suppress inflammatory disease. Nat Immunol 2014; 15: 767–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leavenworth JW, Verbinnen B, Yin J et al A p85α‐osteopontin axis couples the receptor ICOS to sustained Bcl‐6 expression by follicular helper and regulatory T cells. Nat Immunol 2015; 16: 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takahashi H, Kanno T, Nakayamada S et al TGF‐β and retinoic acid induce the microRNA miR‐10a, which targets Bcl‐6 and constrains the plasticity of helper T cells. Nat Immunol 2012; 13: 587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang G, Yang X, Zhang J et al Transcriptional repressor Blimp1 regulates follicular regulatory T‐cell homeostasis and function. Immunology 2018; 153: 105–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jacquemin C, Augusto JF, Scherlinger M et al OX40L/OX40 axis impairs follicular and natural Treg function in human SLE. JCI Insight 2018; 3:e122167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ding Y, Li J, Yang P et al Interleukin‐21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol 2014; 66: 2601–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sage PT, Ron‐Harel N, Juneja VR et al Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol 2016; 17: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh Y, Garden OA, Lang F et al MicroRNA‐15b/16 enhances the induction of regulatory T cells by regulating the expression of Rictor and mTOR. J Immunol 2015; 195: 5667–5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Baumjohann D, Kageyama R, Clingan JM et al The microRNA cluster miR‐17 approximately 92 promotes TFH cell differentiation and represses subset‐inappropriate gene expression. Nat Immunol 2013; 14: 840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu Y, Schutt S, Paz K et al MicroRNA‐17‐92 is required for T‐cell and B‐cell pathogenicity in chronic graft‐versus‐host disease in mice. Blood 2018; 131: 1974–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chao G, Li X, Ji Y et al MiR‐155 controls follicular Treg cell‐mediated humoral autoimmune intestinal injury by inhibiting CTLA‐4 expression. Int Immunopharmacol 2019; 71: 267–276. [DOI] [PubMed] [Google Scholar]
- 40. Maul J, Alterauge D, Baumjohann D. MicroRNA‐mediated regulation of T follicular helper and T follicular regulatory cell identity. Immunol Rev 2019; 288: 97–111. [DOI] [PubMed] [Google Scholar]
- 41. Botta D, Fuller MJ, Marquez‐Lago TT et al Dynamic regulation of T follicular regulatory cell responses by interleukin 2 during influenza infection. Nat Immunol 2017; 18: 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li L, Yang SH, Yao Y et al Block of both TGF‐β and IL‐2 signaling impedes Neurophilin‐1+ regulatory T cell and follicular regulatory T cell development. Cell Death Dis 2016; 7: e2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu H, Xie MM, Liu H et al Stat3 is important for follicular regulatory T cell differentiation. PLoS One 2016; 11: e155040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Limon JJ, Fruman DA. Akt and mTOR in B cell activation and differentiation. Front Immunol 2012; 3: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wu H, Chen Y, Liu H et al Follicular regulatory T cells repress cytokine production by follicular helper T cells and optimize IgG responses in mice. Eur J Immunol 2016; 46: 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sage PT, Paterson AM, Lovitch SB et al The coinhibitory receptor CTLA‐4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 2014; 41: 1026–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wing JB, Ise W, Kurosaki T et al Regulatory T cells control antigen‐specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA‐4. Immunity 2014; 41: 1013–1025. [DOI] [PubMed] [Google Scholar]
- 48. Chao G, Li X, Ji Y et al CTLA‐4 regulates T follicular regulatory cell differentiation and participates in intestinal damage caused by spontaneous autoimmunity. Biochem Biophys Res Commun 2018; 505: 865–871. [DOI] [PubMed] [Google Scholar]
- 49. Walker LS, Sansom DM. Confusing signals: recent progress in CTLA‐4 biology. Trends Immunol 2015; 36: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang CJ, Heuts F, Ovcinnikovs V et al CTLA‐4 controls follicular helper T‐cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci USA 2015; 112: 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ritvo PG, Churlaud G, Quiniou V et al Tfr cells lack IL‐2Rα but express decoy IL‐1R2 and IL‐1Ra and suppress the IL‐1‐dependent activation of Tfh cells. Sci Immunol 2017; 2: eaan0368. [DOI] [PubMed] [Google Scholar]
- 52. Clement RL, Daccache J, Mohammed MT et al Follicular regulatory T cells control humoral and allergic immunity by restraining early B cell responses. Nat Immunol 2019; 20: 1360–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xie MM, Fang S, Chen Q et al Follicular regulatory T cells inhibit the development of granzyme B‐expressing follicular helper T cells. JCI Insight 2019; 4:e128076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCarron MJ, Marie JC. TGF‐β prevents T follicular helper cell accumulation and B cell autoreactivity. J Clin Invest 2014; 124: 4375–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xin G, Zander R, Schauder DM et al Single‐cell RNA sequencing unveils an IL‐10‐producing helper subset that sustains humoral immunity during persistent infection. Nat Commun 2018; 9: 5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guthmiller JJ, Graham AC, Zander RA et al Cutting edge: IL‐10 is essential for the generation of germinal center B cell responses and anti‐plasmodium humoral immunity. J Immunol 2017; 198: 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Laidlaw BJ, Lu Y, Amezquita RA et al Interleukin‐10 from CD4+ follicular regulatory T cells promotes the germinal center response. Sci Immunol 2017; 2: pii: eaan4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu C, Wang D, Lu S et al Increased circulating follicular Treg cells are associated with lower levels of autoantibodies in patients with rheumatoid arthritis in stable remission. Arthritis Rheumatol 2018; 70: 711–721. [DOI] [PubMed] [Google Scholar]
- 59. Romão VC, Fonseca JE, Agua‐Doce A et al T follicular regulatory cells are decreased in patients with established treated rheumatoid arthritis with active disease: comment on the article by Liu et al. Arthritis Rheumatol 2018; 70: 1893–1895. [DOI] [PubMed] [Google Scholar]
- 60. Niu Q, Huang ZC, Wu XJ et al Enhanced IL‐6/phosphorylated STAT3 signaling is related to the imbalance of circulating T follicular helper/T follicular regulatory cells in patients with rheumatoid arthritis. Arthritis Res Ther 2018; 20: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang X, Yang C, Xu F et al Imbalance of circulating Tfr/Tfh ratio in patients with rheumatoid arthritis. Clin Exp Med 2019; 19: 55–64. [DOI] [PubMed] [Google Scholar]
- 62. Pandya JM, Lundell AC, Hallstrom M et al Circulating T helper and T regulatory subsets in untreated early rheumatoid arthritis and healthy control subjects. J Leukoc Biol 2016; 100: 823–833. [DOI] [PubMed] [Google Scholar]
- 63. Liu C, Wang D, Song Y et al Increased circulating CD4+CXCR5+FoxP3+ follicular regulatory T cells correlated with severity of systemic lupus erythematosus patients. Int Immunopharmacol 2018; 56: 261–268. [DOI] [PubMed] [Google Scholar]
- 64. Xu B, Wang S, Zhou M et al The ratio of circulating follicular T helper cell to follicular T regulatory cell is correlated with disease activity in systemic lupus erythematosus. Clin Immunol 2017; 183: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ma L, Zhao P, Jiang Z et al Imbalance of different types of CD4+ forkhead box protein 3 (FoxP3)+ T cells in patients with new‐onset systemic lupus erythematosus. Clin Exp Immunol 2013; 174: 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ivanchenko M, Aqrawi LA, Bjork A et al FoxP3+ CXCR5+ CD4+ T cell frequencies are increased in peripheral blood of patients with primary Sjogren's syndrome. Clin Exp Immunol 2019; 195: 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fonseca VR, Romão VC, Agua‐Doce A et al The ratio of blood T follicular regulatory cells to T follicular helper cells marks ectopic lymphoid structure formation while activated follicular helper T cells indicate disease activity in primary Sjögren's syndrome. Arthritis Rheumatol 2018; 70: 774–784. [DOI] [PubMed] [Google Scholar]
- 68. Shan Y, Qi C, Zhao J et al Higher frequency of peripheral blood follicular regulatory T cells in patients with new onset ankylosing spondylitis. Clin Exp Pharmacol Physiol 2015; 42: 154–161. [DOI] [PubMed] [Google Scholar]
- 69. Ito F, Kamekura R, Yamamoto M et al IL‐10+ T follicular regulatory cells are associated with the pathogenesis of IgG4‐related disease. Immunol Lett 2019; 207: 56–63. [DOI] [PubMed] [Google Scholar]
- 70. Cunill V, Clemente A, Lanio N et al Follicular T cells from smB‐ common variable immunodeficiency patients are skewed toward a Th1 phenotype. Front Immunol 2017; 8: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Puthenparampil M, Zito A, Pantano G et al Peripheral imbalanced TFH/TFR ratio correlates with intrathecal IgG synthesis in multiple sclerosis at clinical onset. Mult Scler 2019; 25: 918–926. [DOI] [PubMed] [Google Scholar]
- 72. Jones AP, Trend S, Byrne SN et al Altered regulatory T‐cell fractions and Helios expression in clinically isolated syndrome: clues to the development of multiple sclerosis. Clin Transl Immunology 2017; 6: e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhao S, Ding J, Wang S et al Decreased expression of circulating Aire and increased Tfh/Tfr cells in myasthenia gravis patients. Biosci Rep 2018; 38: pii: BSR20180096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wen Y, Yang B, Lu J et al Imbalance of circulating CD4+CXCR5+FOXP3+ Tfr‐like cells and CD4+CXCR5+FOXP3‐ Tfh‐like cells in myasthenia gravis. Neurosci Lett 2016; 630: 176–182. [DOI] [PubMed] [Google Scholar]
- 75. Cui Y, Chen F, Guo Y et al Changes of circulating Tfr and Tfh cells in children with myasthenia gravis. Chin J Microbiol Immunol 2016; 36: 746–752. [Google Scholar]
- 76. Zhao J, Chen Y, Xu Z et al Increased circulating follicular regulatory T cells in Hashimoto's thyroiditis. Autoimmunity 2018; 51: 345–351. [DOI] [PubMed] [Google Scholar]
- 77. Zheng J, Wang T, Zhang L et al Dysregulation of circulating Tfr/Tfh ratio in primary biliary cholangitis. Scand J Immunol 2017; 86: 452–461. [DOI] [PubMed] [Google Scholar]
- 78. Xu X, Shen M, Zhao R et al Follicular regulatory T cells are associated with β‐cell autoimmunity and the development of type 1 diabetes. J Clin Endocrinol Metab 2019; 104: 4199–4213. [DOI] [PubMed] [Google Scholar]
- 79. Wang X, Zhu Y, Zhang M et al The shifted balance between circulating follicular regulatory T cells and follicular helper T cells in patients with ulcerative colitis. Clin Sci (Lond) 2017; 131: 2933–2945. [DOI] [PubMed] [Google Scholar]
- 80. Kim YU, Lim H, Jung HE et al Regulation of autoimmune germinal center reactions in lupus‐prone BXD2 mice by follicular helper T cells. PLoS One 2015; 10: e120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fu W, Liu X, Lin X et al Deficiency in T follicular regulatory cells promotes autoimmunity. J Exp Med 2018; 215: 815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wu X, Su Z, Cai B et al Increased circulating follicular regulatory T‐like cells may play a critical role in chronic hepatitis B virus infection and disease progression. Viral Immunol 2018; 31: 379–388. [DOI] [PubMed] [Google Scholar]
- 83. Cobb DA, Kim OK, Golden‐Mason L et al Hepatocyte‐derived exosomes promote T follicular regulatory cell expansion during hepatitis C virus infection. Hepatology 2018; 67: 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller SM, Miles B, Guo K et al Follicular regulatory T cells are highly permissive to R5‐Tropic HIV‐1. J Virol 2017; 91: pii: e00430-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Miles B, Miller SM, Folkvord JM et al Follicular regulatory T cells impair follicular T helper cells in HIV and SIV infection. Nat Commun 2015; 6: 8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen X, Li W, Zhang Y et al Distribution of peripheral memory T follicular helper cells in patients with Schistosomiasis japonica . PLoS Negl Trop Dis 2015; 9: e4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Colineau L, Rouers A, Yamamoto T et al HIV‐infected spleens present altered follicular helper T cell (Tfh) subsets and skewed B cell maturation. PLoS One 2015; 10: e140978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wang L, Qiu J, Yu L et al Increased numbers of CD5+CD19+CD1dhighIL‐10+ Bregs, CD4+Foxp3+ Tregs, CD4+CXCR5+Foxp3+ follicular regulatory T (TFR) cells in CHB or CHC patients. J Transl Med 2014; 12: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li L, Ma Y, Xu Y. Follicular regulatory T cells infiltrated the ovarian carcinoma and resulted in CD8 T cell dysfunction dependent on IL‐10 pathway. Int Immunopharmacol 2019; 68: 81–87. [DOI] [PubMed] [Google Scholar]
- 90. Guo Z, Liang H, Xu Y et al The role of circulating T follicular helper cells and regulatory cells in non‐small cell lung cancer patients. Scand J Immunol 2017; 86: 107–112. [DOI] [PubMed] [Google Scholar]
- 91. Faghih Z, Erfani N, Haghshenas MR et al Immune profiles of CD4+ lymphocyte subsets in breast cancer tumor draining lymph nodes. Immunol Lett 2014; 158: 57–65. [DOI] [PubMed] [Google Scholar]
- 92. Yao Y, Wang ZC, Wang N et al Allergen immunotherapy improves defective follicular regulatory T cells in patients with allergic rhinitis. J Allergy Clin Immunol 2019; 144: 118–128. [DOI] [PubMed] [Google Scholar]
- 93. De Bruyne R, Gevaert P, Van Winckel M et al Raised immunoglobulin A and circulating T follicular helper cells are linked to the development of food allergy in paediatric liver transplant patients. Clin Exp Allergy 2015; 45: 1060–1070. [DOI] [PubMed] [Google Scholar]
- 94. Chen W, Bai J, Huang H et al Low proportion of follicular regulatory T cell in renal transplant patients with chronic antibody‐mediated rejection. Sci Rep 2017; 7: 1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li H, Zhou R, Wang C et al T follicular regulatory cells infiltrate the human airways during the onset of acute respiratory distress syndrome and regulate the development of B regulatory cells. Immunol Res 2018; 66: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Fan W, Demers AJ, Wan Y et al Altered ratio of T follicular helper cells to T follicular regulatory cells correlates with autoreactive antibody response in simian immunodeficiency virus‐infected rhesus macaques. J Immunol 2018; 200: 3180–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chowdhury A, Del REP, Tharp GK et al Decreased T follicular regulatory cell/T follicular helper cell (TFH) in simian immunodeficiency virus‐infected rhesus macaques may contribute to accumulation of TFH in chronic infection. J Immunol 2015; 195: 3237–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Blackburn MJ, Zhong‐Min M, Caccuri F et al Regulatory and helper follicular T cells and antibody avidity to simian immunodeficiency virus glycoprotein 120. J Immunol 2015; 195: 3227–3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Arsenovic‐Ranin N, Petrovic R, Zivkovic I et al Influence of aging on germinal centre reaction and antibody response to inactivated influenza virus antigens in mice: sex‐based differences. Biogerontology 2019; 20: 475–496. [DOI] [PubMed] [Google Scholar]
- 100. Eldi P, Chaudhri G, Nutt SL et al Viral replicative capacity, antigen availability via hematogenous spread, and high TFH:TFR ratios drive induction of potent neutralizing antibody responses. J Virol 2019; 93: pii: e01795-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Baptista D, Mach F, Brandt KJ. Follicular regulatory T cell in atherosclerosis. J Leukoc Biol 2018; 104: 925–930. [DOI] [PubMed] [Google Scholar]
- 102. Sage PT, Tan CL, Freeman GJ et al Defective TFH cell function and increased TFR cells contribute to defective antibody production in aging. Cell Rep 2015; 12: 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Grant CR, Liberal R, Mieli‐Vergani G et al Regulatory T‐cells in autoimmune diseases: challenges, controversies and–yet–unanswered questions. Autoimmun Rev 2015; 14: 105–116. [DOI] [PubMed] [Google Scholar]
- 104. Schaerli P, Loetscher P, Moser B. Cutting edge: induction of follicular homing precedes effector Th cell development. J Immunol 2001; 167: 6082–6086. [DOI] [PubMed] [Google Scholar]
- 105. Wing JB, Kitagawa Y, Locci M et al A distinct subpopulation of CD25− T‐follicular regulatory cells localizes in the germinal centers. Proc Natl Acad Sci USA 2017; 114: E6400–E6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Xie MM, Dent AL. Unexpected help: follicular regulatory T cells in the germinal center. Front Immunol 2018; 9: 1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wallin EF, Jolly EC, Suchanek O et al Human T‐follicular helper and T‐follicular regulatory cell maintenance is independent of germinal centers. Blood 2014; 124: 2666–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tao L, Reese TA. Making mouse models that reflect human immune responses. Trends Immunol 2017; 38: 181–193. [DOI] [PubMed] [Google Scholar]
- 109. Sharabi A, Tsokos MG, Ding Y et al Regulatory T cells in the treatment of disease. Nat Rev Drug Discov 2018; 17: 823. [DOI] [PubMed] [Google Scholar]
- 110. Wang CC, Zhang M, Li H et al Caspase‐1 inhibitor regulates humoral responses in experimental autoimmune myasthenia gravis via IL‐6‐dependent inhibiton of STAT3. Neurosci Lett 2017; 656: 169–176. [DOI] [PubMed] [Google Scholar]
- 111. Xie X, Mu L, Yao X et al ATRA alters humoral responses associated with amelioration of EAMG symptoms by balancing Tfh/Tfr helper cell profiles. Clin Immunol 2013; 148: 162–176. [DOI] [PubMed] [Google Scholar]
- 112. Yang J, Yang X, Yang J et al Baicalin ameliorates lupus autoimmunity by inhibiting differentiation of Tfh cells and inducing expansion of Tfr cells. Cell Death Dis 2019; 10: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Havenar‐Daughton C, Carnathan DG, Torrents DLPA et al Direct probing of germinal center responses reveals immunological features and bottlenecks for neutralizing antibody responses to HIV Env trimer. Cell Rep 2016; 17: 2195–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Cirelli KM, Carnathan DG, Nogal B et al Slow delivery immunization enhances HIV neutralizing antibody and germinal center responses via modulation of immunodominance. Cell 2019; 177: 1153–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]


