Abstract
Introduction:
We determine the level of adherence to the revised Kenya early infant diagnosis (EID) algorithm during implementation of a point-of-care (POC) EID project.
Methods:
Data before (August 2016 to July 2017) and after (August 2017 to July 2018) introduction of POC EID were collected retrospectively from the national EID database and registers for 33 health facilities. We assessed the number of HIV-infected infants who underwent confirmatory testing and received baseline viral load test and proportion of infants with an initial negative result who had a subsequent test.
Results and Discussion:
Significantly higher number of infants accessed confirmatory testing (94.2% versus 38.6%; P < .0001) with POC EID. Baseline viral load test and follow-up testing at 6 months, although higher with POC EID, were not significantly different from the pre-POC EID intervention period.
Conclusion:
The POC EID implementation has the potential to increase proportion of infants who receive confirmatory testing, thus reducing the risk of false-positive results.
Keywords: HIV, point of care, early infant diagnosis, adherence, algorithm
What Do We Already Know About this Topic?
It is now known that point of care (POC) reduces the turnaround time for diagnosis of infants compared to conventional early infant diagnosis (EID), and this allows timely initiation on treatment for HIV-infected infants.
How Does Your Research Contribute to the Field?
Our study demonstrates that POC EID improves retesting of infants with a positive initial test result, thereby reducing the likelihood of false-positive results.
What Are Your Research’s Implications toward Theory, Practice, or Policy?
Our findings demonstrate additional benefit of POC EID and the need to integrate POC EID within the laboratory network, especially in hard-to-reach areas and those with high HIV prevalence.
Introduction
Considerable progress has been made in prevention efforts to reduce new HIV infections among children worldwide. The number of newly infected children has declined by 35% since 2010,1 suggesting that ongoing prevention efforts are making strides toward eliminating new HIV infections among infants. Despite this progress, universal testing of HIV-exposed infants (HEI) born to HIV-positive mothers has not yet been achieved nor has initiation of antiretroviral treatment (ART) for all HIV-infected infants.1 Early infant diagnosis (EID) is essential for ensuring timely ART initiation, which is particularly important given the documented high morbidity and mortality rates among HIV-infected children who do not receive treatment.2 However, according to the latest statistics, only 52% of all HIV-infected children are initiated on treatment.1 Moreover, only an estimated 51% of HEI are tested within the first 2 months of life.1
With the success of prevention of mother-to-child transmission (PMTCT) programs, however, a reduction in HIV transmissions to infants has been realized. With a lower pretest probability of HIV infection, there is an increased likelihood of false-positive results.3 The World Health Organization (WHO) recently recommended the use of confirmatory virologic testing for all infants with an initial positive virologic test.4 The organization also supports either conventional or point-of-care (POC) EID technologies to be used as initial and confirmatory testing. In addition, due to changing transmission dynamics and recent evidence that rapid serologic testing has a high rate of false negatives, the WHO now recommends that the 9-month rapid serologic test may be replaced with a virologic test.4-6
There are a number of times when loss to follow-up (LTFU) occurs throughout the EID cascade, from the time the patient presents for sample collection to the caregiver’s receipt of results and the clinical decision-making. Emerging evidence has shown that POC EID significantly reduces turnaround time (TAT), increases the percentage of results returned to caregivers, and increases the percentage of HIV-infected infants initiated on ART.7-9 However, the impact of POC EID on ensuring confirmatory testing and adhering to additional EID testing time points has not yet been documented.
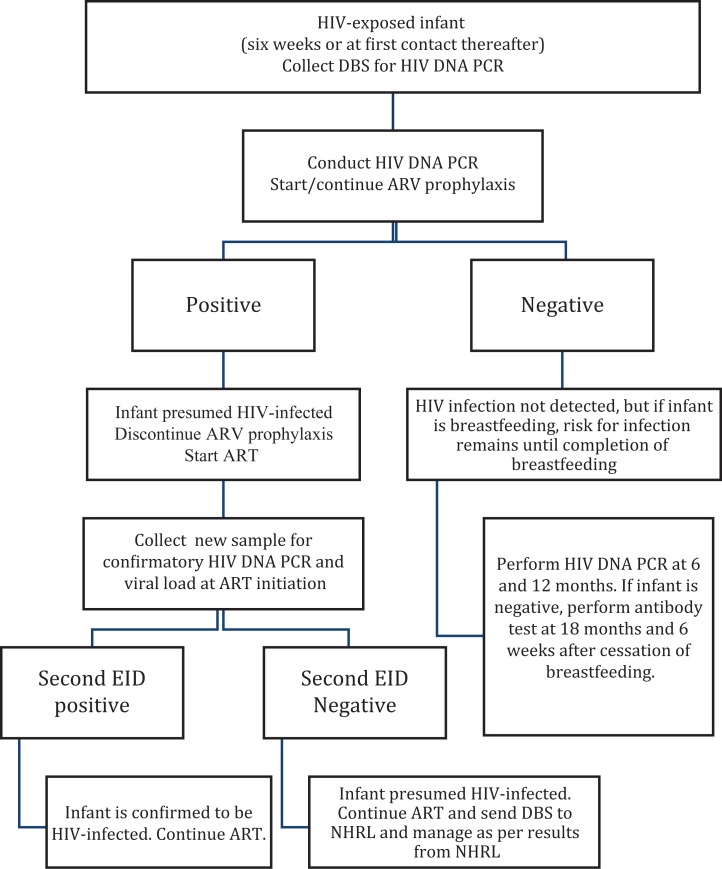
In 2016, Kenya revised its EID algorithm to replace 9-month, serology-based EID testing with virologic EID testing at 6 and 12 months of age (Figure 1). Additionally, the guidelines require that all HEIs with an initial HIV DNA polymerase chain reaction (PCR)-positive result should be initiated on ART as soon as they receive their results, and a new sample collected for a confirmatory PCR and a baseline viral load test. For POC EID, the same algorithm applies where the POC test result functions as a stand-alone result with a confirmatory PCR conducted on the POC device with a newly collected specimen.
Figure 1.
Kenya national early infant diagnosis (EID) algorithm.
However, because viral load testing is yet to be rolled out on POC, the infant baseline viral load will still be transported to the conventional laboratory for testing on a Roche Cobas Ampliprep/Cobas TaqMan HIV-1 test (Roche Molecular Systems, Branchburg, New Jersey). In this article, we evaluate the rate of confirmatory testing and adherence to follow-up testing under Pre-POC EID intervention and post-POC EID intervention.
Methods
The UNITAID/Elizabeth Glaser Pediatric AIDS Foundation (EGPAF) POC EID project (2015-2019) is a multicountry project in 9 African countries. It aims to reduce HIV-related mortality by increasing the number of HIV-positive infants whose HIV status is known, facilitating early return of results and ART initiation through introduction and scale-up of POC EID. Two WHO prequalified POC EID assays, the m-PIMA HIV-1/2 Detect (Abbott Laboratories, Lake Forest, Illinois) and Xpert HIV-1 Qual (Cepheid, Sunnyvale, California) were considered for implementation based on throughput. Implementation of POC EID relies on a hub-and-spoke model, where a hub is a facility with a POC EID platform and a spoke is a facility that refers samples for testing at the hub facility. In Kenya, EGPAF and the Ministry of Health implemented POC EID in 42 hubs and over 700 spoke sites in 12 counties. Implementation began in August 2017 in 3 pilot hub and 36 spoke facilities in 2 counties, namely, Homa Bay and Turkana. The former is the region with the one of the highest HIV prevalence in Kenya, while the latter is a remote region in northern Kenya.
Design
This was a non-randomized pre- and post-POC EID intervention observational cohort evaluation. POC EID was implemented in a stepwise manner. The pre-intervention phase of the study ran from August 2016 to July 2017, while the post-intervention phase was from August 2017 to July 2018.
Setting
The study was conducted in the Homa Bay and Turkana counties, which had a cohort of POC EID clients who completed at least 6 months of follow-up. Homa Bay is located in Western Kenya, along the shores of Lake Victoria and largely inhabited by the Luo community—a fishing community. The HIV prevalence in Homa Bay is the second highest in the country at 20.7%, compared to the national average of 4.9% per the 2018 Kenya HIV Estimates report.10 Turkana County, located on the North Western part of the country, is an arid and semiarid region largely occupied by nomadic pastoralists and with a HIV prevalence of 3.2%.10 Data from a total of 33 health facilities were included in this analysis, representing all facilities that implemented POC EID during the entire post-intervention period. These sites switched EID testing completely from conventional to POC testing. In Homa Bay County, 23 health facilities were selected consisting of 11 hubs and 12 high-volume spokes. The m-Pima platform was implemented in 10 of the 11 hubs with the Xpert platform in the remaining hub. For Turkana County, 1 hub and 9 spokes were included—given that there were lower volumes compared to Homa Bay County. The m-Pima was the platform of choice for Turkana County.
Data Collection and Analysis
Data on HEI were extracted by project staff into a Microsoft Excel file. Patient identification and testing information were extracted from the HEI register and the ministry of health EID database. Treatment information was extracted from the ART registers. In cases where there were gaps in the data, the patient’s HEI card complemented these registers. The EID tracking logs at the facilities were also reviewed to fill in any missing information or in cases where there were inconsistencies. Data for patient identification and testing information for POC EID testing were also extracted from an EID Test Request form used in the POC EID project. The extracted data were then cleaned, anonymized, and imported into STATA software for test of proportions.
For the analysis on adherence to confirmatory testing, as outlined in the EID algorithm, all HEI with an initial positive result pre- and post-intervention were included. We determined whether each sample had a second sample drawn for a confirmatory test and baseline viral load, as outlined in the EID algorithm.
For analysis of adherence to testing at the 6- and 12-month time points, as outlined in the EID algorithm, a cohort of infants with an initial 6-week test were included, pre- and post-POC EID intervention. In the pre-intervention phase, all infants received 6-week testing on conventional central laboratory platforms, and in the post-intervention phase, only infants who received 6-week testing on a POC EID platform were included. We determined the proportion of HEIs who had a follow-up test at the 6- and at 12-month time points if they were found negative in the preceding test. We used the interrupted time series analysis and segmented regression analysis using the Newey-West method to test whether the proportion of infants tested increased after POC EID intervention.
Ethical Approval and Informed Consent
Ethical approval for this analysis was obtained from the Kenyatta National Hospital—University of Nairobi Ethics and Research Committee (KNH-UON ERC; protocol no. P345/04/2016) and Advarra Institutional Review Board (formerly Chesapeake IRB; protocol no. Pro00021804). A waiver for informed consent was granted for retrospective patient-level data collection and analysis.
Results and Discussion
Pre-POC EID intervention data yielded 83 infants with an initial HIV-positive result. Only 32 (38.6%; 95% confidence interval [CI]: 28.8-49.3) had a confirmatory test done and 21 (25.3%; 95% CI: 17.2-35.6) had a baseline viral load test done (Table 1). Post-POC EID intervention data collection yielded 69 infants with an initial HIV-positive result. Confirmatory EID tests were done in 65 (94.2%; 95% CI: 68.0-97.7) infants, and 58 (84.1%; 95% CI: 73.7-90.9) had a baseline viral load test (Table 1). There was significant increase in the proportion of infants with a confirmatory test at the start of the intervention (47.7%; 95% CI: 35.0-60.5; P < .05) and a non-significant increase (28.5%; 95% CI: −7.0 to 63.9) for the baseline viral load test.
Table 1.
Proportion of HIV-Infected Infants with Complete EID Algorithm Pre- and Post-POC EID Intervention.
| Indicators | Pre-POC EID Intervention | Post-POC EID Intervention | Newey-West Coefficient (95% CI) | Test of Significance |
|---|---|---|---|---|
| Number with an initial positive HIV result | 83 | 69 | ||
| Number with a confirmatory EID test done | 32 | 65 | 47.7% (35.0 to 60.5) | <.0001 |
| % Adherence to guideline for confirmatory EID test (95% CI) | 38.6% (28.8% to 49.3%) | 94.2% (68.0% to 97.7%) | ||
| Number with baseline VL test done | 21 | 58 | 28.5% (−7.0 to 63.9) | .105 |
| % Adherence to guideline for baseline VL | 25.3% (17.2% to35.6%) | 84.1% (73.7% to 90.9%) |
Abbreviations: CI, confidence interval; EID, early infant diagnosis; POC, point of care; VL, viral load.
On adherence to testing schedules, a total of 1565 infants who received conventional EID had a negative result at the 6-week/initial test. Of this cohort, 1089 (69.6%; 95% CI: 67.3-71.8) had a follow-up test at 6 months and 1108 (70.8%; 95% CI: 68.5-73.0) had a follow-up test at 12 months, in line with the EID algorithm (Table 2). On the other hand, among the 1240 infants who received POC EID and had a negative result at week 6, 1128 (90.8%; 95% CI: 89.2-92.4) had a follow-up test at month 6, and of the 551 who were eligible for a 12-month follow-up test at the time of data collection, 509 (92.4%; 95% CI: 89.9-94.3) had a follow-up test at month 12. There was an increase in the proportion of infants with follow-up tests at 6- and 12-month time points for HEI with negative results in the post-POC EID intervention phase (Table 2). However, the increase was not significantly different from the pre-POC EID intervention period (−4.0%; 95% CI: −21 to 13.0; P = .62).
Table 2.
Proportion of HIV-Exposed Infants with Complete 12-Month Follow-Up Pre- and Post-POC EID Intervention.a
| Indicators | Pre-POC EID Intervention | Post-POC EID Intervention | Newey-West Coefficient (95% CI) | Test of Significance |
|---|---|---|---|---|
| Number with an initial negative HIV result | 1565 | 1242 | ||
| Number with a 6-month HIV PCR test done | 1089 | 1128 | −4.0% (−21 to 13.0) | 0.62 |
| % Adherence to guideline for 6-month HIV PCR test (95% CI) | 69.6% (67.3% to 71.8%) | 90.8% (89.2% to 92.4%) | ||
| Number with a 12-month HIV PCR test done | 1108 | 509b | Not Done | |
| % Adherence to guideline for of 12-month HIV PCR test | 70.9% (68.5% to 73.0%) | 92.4% (89.9% to 94.3%) |
Abbreviations: CI, confidence interval; EID, early infant diagnosis; PCR, polymerase chain reaction; POC, point of care; VL, viral load.
a Not done: Missing values result in unbalanced data; hence interrupted time series analysis cannot be performed.
b Calculated against 551 infants who were eligible for a 12-month test within a 3-month window from the due date.
The main reasons for attrition were death, LTFU (90 days from due date of the test), and caregivers who moved out of the study area. There were 21 (1.3%) deaths among all HEIs in the pre-POC EID intervention phase, compared to 9 (0.7%) in the post-POC EID intervention phase. Most deaths, 85.7% (n = 18), in the pre-POC EID intervention phase occurred before the 6-month time point. There were 138 (8.8%) cases of LTFU in the pre-POC EID intervention phase and 82 (6.6%) cases in the post-POC EID intervention phase. Additionally, there were 141 (8.9%) transfers out in the pre-POC EID intervention phase, against 70 (5.6%) in the post-POC EID intervention phase.
In the analysis, POC EID implementation was associated with increased proportion of infants with confirmatory testing after an initial HIV-positive EID. On the contrary, there was no significant increase in the proportion of infants with baseline viral load results. Although confirmatory testing for an initial positive EID result has been in the guidelines since 2013, significant improvement did not occur until the introduction of POC EID. As such, this difference between conventional and POC testing may be attributed to access to quick results in the latter, due to reduced TAT where majority of caregivers (over 90%) received results within 7 days.9 Traditionally, laboratory personnel at the facilities collect, package, and ship dry blood spots to conventional laboratories for testing. However, they are not responsible for follow-up of results from the conventional laboratories and adherence to the algorithm, which is usually a responsibility of the facility clinician. With POC, the staffs conducting the test have a responsibility to act on the results, including enhancing adherence to the algorithm, especially for positive results and communication to the clinician.
The main documented reasons for non-adherence were LTFU and patients’ transfer to other facilities beyond the study area. These were higher in the pre-POC EID intervention phase and may result from delayed return of results, especially for the infected infants given that some of the facilities in this analysis are within mobile fishing and nomadic communities. Caregivers may have moved to a different location by the time the infants were due for a confirmatory and baseline viral load test. Other factors may include health workers and caregiver attitude, ineffective patient follow-up mechanism at facility level, and lack of transport for caregivers, among others.11 Finding patients LTFU is difficult and costly.12 Point-of-care testing, as demonstrated in our analysis and in other studies,7,9 clearly reduces LTFU of infants in need of confirmatory EID. This is achieved by enabling faster return of results to caregivers and immediate initiation on treatment. There were more infant deaths in the pre-POC EID intervention phase compared to the post-POC EID intervention phase, possibly due to delays in ART initiation. Most deaths occurred before infants got their 6-month test, consistent with data from other studies that indicate an infant mortality peak between 8 and 12 weeks of age in untreated infants.13
Our analysis had a number of limitations. For example, although training on the introduction of the new algorithms was introduced during the pre-intervention phase, additional training was provided as part of the POC EID introduction. This training focused on the use of POC EID specifically but also may have served as a refresher on EID generally. It is important to note that during the pre-intervention phase, there were a number of active initiatives which may have also biased toward the pre-intervention period. For example, during the pre-intervention phase, the “bring back the mother baby pair campaign” was carried out which followed up on all women and infants who were LTFU from the EID cascade or were inactive. As this analysis is a non-randomized pre/post-intervention observational study, we may have introduced potential biases, including secular trends. For example, the coverage of 6-week EID testing differed between the pre- and post-intervention phases (55% and 67%, respectively). However, as this evaluation looks at the impact of POC EID on follow-up testing (confirmatory or 6-month test) for infants with an initial 6-week EID test, we believe that bias introduced by differences in coverage of 6-week testing between the eras will be low. In addition, over the study period in the assessed sites, there were no additional guideline changes, specific interventions on confirmatory testing, or significant staff changes that would have obviously contributed to this improvement in adherence to the EID algorithm. There may also be outcome ascertainment bias, as the forms used to collect data differed between the pre- and post-intervention period, and the quality of data that were collected retrospectively may differ from that which was collected prospectively. It is not clear if either type of form used would favor one period over the other, but this may have played a role and was not systematically evaluated by the authors. Since early 2016, however, there has been a concerted effort to improve data quality, using routine quality audits and monthly summary reports on key EID indicators. Additionally, there was rapid rollout of electronic medical records at PMTCT clinics to improve the timeliness and quality of data captured that occurred before pre-POC EID intervention data were collected.
Conclusions
Strict adherence to the EID algorithm, including confirmatory EID testing after an initial positive test, improves accuracy of diagnosis and reduces the risk of conveying false-positive results to caregivers. This is to ensure that we do not have infants wrongly diagnosed on lifelong ART, which may be stigmatizing, expose infants to toxicity, and burden the health-care system with unnecessary costs. Adherence to the algorithm also aids in identifying infants who may have been infected during the breastfeeding period and ensuring that they are initiated on treatment to reduce HIV-associated morbidity and mortality. As both 6- and 12-month retesting guidelines are fairly new, there may be a gap in knowledge on the revised guidelines, and this may have contributed to non-adherence to the EID algorithm for both conventional and POC settings, although adherence was lower at the beginning of the pre-intervention period. However, it was surprising that confirmatory testing for infants with presumptive HIV diagnosis was not being implemented, despite this guideline being part of the previous testing guidelines.
Nevertheless, enhanced training regarding the importance of confirmatory testing and adherence to the EID algorithm should be implemented. POC EID may play an important role in ensuring fidelity in adherence to confirmatory and follow-up testing in the EID cascade.
Acknowledgments
The authors are grateful to the study participants, the EGPAF/Unitaid project team, and the Kenya Ministry of Health whose participation made this study possible.
Authors’ Note: C.O.O. and J.C. conceptualized the study and had primary responsibility for interpretation of the data. C.O.O., B.O., and J.O. designed the study. M.W., B.O., and J.O. analyzed the data. C.O.O. and J.C. wrote the paper with assistance from E.M., L.M., R.M., N.B., L.K., and G.G. All authors have read and approved the final article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: UNITAID provided funding for the overall project.
References
- 1. Joint United Nations Programme on HIV/AIDS. Global HIV & AIDS Statistics—2018 Fact Sheet. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2018. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed July 26, 2019. [Google Scholar]
- 2. Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. New Eng J Med. 2008;359(21):2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feucht U, Forsyth B, Kruger M. False-positive HIV DNA PCR testing of infants: implications in a changing epidemic. S Afr Med J. 2012;102(3):149–152. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. HIV Diagnosis and ARV Use in HIV-Exposed Infants: A Programmatic Update. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 5. Urick B, Fong Y, Okiira C, et al. Rapid Serological tests ineffectively screen for HIV exposure in HIV-positive infants. J Acquir Immune Defic Syndr. 2018;77(3):331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wagner AD, Njuguna IN, Andere R, et al. Infant/child rapid serology tests fail to reliably assess HIV exposure among sick hospitalized infants. AIDS (London, England). 2017;31(11):F1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jani IV, Meggi B, Loquiha O, et al. Effect of point-of-care early infant diagnosis on antiretroviral therapy initiation and retention of patients. AIDS. 2018;32(11):1453–63. [DOI] [PubMed] [Google Scholar]
- 8. Mwenda R, Fong Y, Magombo T, et al. Significant patient impact observed upon implementation of point-of-care early infant diagnosis technologies in an observational study in Malawi. Clin Infect Dis. 2018;67(5):701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bianchi F, Cohn J, Sacks E, et al. Evaluation of a routine point-of-care intervention for early infant diagnosis of HIV: an observational study in eight African countries. Lancet HIV. 2019;6(6):e373–e381. [DOI] [PubMed] [Google Scholar]
- 10. Kenya Ministry of Health. National AIDS and STI Control Program (NAS-COP). Nairobi, Kenya: Kenya HIV Estimates Report 2018; 2016. https://nacc.or.ke/wp-content/uploads/2018/11/HIV-estimates-report-Kenya-20182.pdf. Accessed May 26, 2018. [Google Scholar]
- 11. Kiyaga C, Lee HH, Allain JP. Adherence to early infant diagnosis testing algorithm, a challenge to early infant diagnosis program in resource limited settings of Uganda. J HIV Clin Scientific Res. 2015;2(2):030–039. [Google Scholar]
- 12. Forster M, Bailey C, Brinkhof MW, et al. Electronic medical record systems, data quality and loss to follow-up: survey of antiretroviral therapy programmes in resource-limited settings. Bull World Health Organ. 2008;86(12):939–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lilian RR, Kalk E, Bhowan K, et al. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6 weeks of age. J Clin Microbiol. 2012;50(7):2373–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]