Abstract
Background:
This study was to assess the risk of venous thromboembolism (VTE) in patients with peritoneal carcinomatosis (PC) and to evaluate the safety and feasibility of physiotherapy program to prevent VTE during cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC).
Methods:
For VTE prevention, we developed a systematic physiotherapy program consisting of active exercises of both arms and legs, and intermittent pneumatic compression device to massage both legs. This physiotherapy was applied to all patients, and the VTE-related events were recorded and analyzed.
Results:
Cytoreductive surgery + HIPEC was performed on 466 patients with PC. All patients had highest VTE risk, with the median Caprini risk factor score being 11. During the 3-month observation period, 8 patients had 9 (1.9%) clinically symptomatic VTE events, including 8 (1.7%) deep vein thrombosis and 1 (0.2%) pulmonary embolism. Among those, 5 patients received pharmacological treatments with low-molecular-weight heparin, and the other 3 received physical exercises only. All these patients recovered well, and there was no mortality about VTE perioperatively.
Conclusions:
Patients with PC treated by CRS + HIPEC are at highest risk for VTE. The systematic physiotherapy program is safe and feasible to prevent VTE post CRS + HIPEC.
Keywords: venous thromboembolism, peritoneal carcinomatosis, cytoreductive surgery, hyperthermic intraperitoneal chemotherapy, physiotherapy
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE), collectively known as venous thromboembolism (VTE), are common complications of severe diseases and associated with increased morbidity and mortality.1 Compared with cancer-free members of the general population, the incidence of VTE among patients with cancer is much higher (1.8% vs 0.8%), and the relative risk increases with advancing stage which can be up to 27.7-fold for stage IV cancer. Moreover, it is estimated that the risk of VTE among patients having cancer treated with chemotherapy is 16.2 times compared with general population, while 4.1 times for those undergone surgery.2
Peritoneal carcinomatosis (PC), which was once regarded as the terminal stage of cancer, now is treated by cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) in selected cases, because mounting clinical evidence from phase III clinical trials has demonstrated the superior efficacy and acceptable safety of this integrated strategy for PC from gastric cancer,3 colorectal cancer,4 and epithelial ovarian cancer.5 However, it remains a procedure with significant morbidity and mortality, including VTE and PE. One study reported 6 (10%) of 60 patients experiencing VTE,6 and another reported 25 (4.4%) of 562 patients were diagnosed with acute PE.7 Up to now, there has been no reports solely devoted to preventing VTE in patients with PC underwent CRS + HIPEC.
As a long-standing national PC treatment center, we have established specialized CRS + HIPEC protocols for patients with PC from gastrointestinal and pelvic cancers, and a series of experimental researches8 and clinical studies3,9–11 demonstrated the treatment advantages of this strategy. During the development, we have also paid particularly attention to preventing and treating major adverse events, including VTE. Over the past 3 years, a set of combined measures have been formulated to prevent DVT and PE in all patients having PC treated with CRS + HIPEC at our center. This study is to summarize our experience in developing this physiotherapy-based program.
Material and Methods
Design
Our institution is a university-based tertiary-referral cancer center and the only one education and training center on CRS + HIPEC to treat PC, authorized by Beijing Municipal Commission of Health. We have conducted a series of basic,12 translational,13 and clinical14 studies on the diagnosis and treatment of PC. The current study was a retrospectively observational study; and the patient records were prospectively constructed into a comprehensive database. All patients and their authorized caretakers were well educated about the CRS + HIPEC procedures, and standardized informed consent forms were signed from the patients or their authorized caretakers. The study protocol was approved by the institutional review board and the ethics committee of Beijing Shijitan Hospital, Capital Medical University.
Patient Selection
From May 1, 2015, to March 1, 2018, 466 patients having PC treated with CRS + HIPEC at our hospital were enrolled into this retrospective study. All patients were pathologically diagnosed with PC and scheduled to undergo CRS + HIPEC procedure after detailed evaluation including general status, laboratory examinations, computed tomography scan, and dynamic imaging. Major inclusion criteria include: (1) Karnofsky performance status >50; (2) normal peripheral blood white blood cells count ≥3500/mm3 and platelet count ≥80 000/mm3; (3) acceptable liver function, with bilirubin ≤2 × the upper limit of normal (ULN) and aspartic aminotransferase and alanine aminotransferase ≤2 × ULN; (4) acceptable renal function, with serum creatinine ≤1.5 mg/dL; and (5) cardiovascular pulmonary and other major organ functions can stand major operation; Major exclusion criteria include: (1) any lung metastasis, liver metastasis, or prominent retroperitoneal lymph node metastasis during preoperative workup and (2) imaging examination indicating mesenteric contracture.
Perioperative VTE Risk Evaluation
A major consideration in our preoperative management of patients with PC undergoing CRS + HIPEC is to assess the VTE risk. Two evaluation tools were used for this purpose.
The first is the Caprini risk factor score.15 This is a widely used system to evaluate the VTE risk, mainly based on clinical characteristics of the patients. For patients with PC, major risk factors are older age, cancer diagnosis, central venous access, chemotherapy, major surgery lasting over 3 hours, blood transfusion, and others.
The second is Doppler ultrasonography, which was performed on all patients before CRS + HIPEC. If a patient was preoperatively diagnosed as having DVT by Doppler ultrasonography, the intermittent pneumatic compression (IPC) device would not be applied intraoperatively, but the other physiotherapy methods were still used.
During postoperative period, patients with symptoms such as chest pain, dyspnea at rest, and calf swelling or pain were suspected VTE. And only for those suspected patients, computed tomography or Doppler ultrasonography was performed again. If a clot was seen obstructing a pulmonary vessel or lower extremities, PE or DVT was diagnosed.
Cytoreductive Surgery Plus HIPEC Procedure
All CRS + HIPEC procedures were conducted as described before16 by a designated team focusing on PC treatment. Briefly, maximal CRS was performed, including the curative or palliative resection of the primary tumor with acceptable margins, any involved adjacent structures, lymphadenectomy, peritonectomies where peritoneal surfaces were involved by tumor, according to the peritonectomy procedure developed by Sugarbaker,17 and after which the HIPEC was implemented by the open Coliseum technique with each drug dissolved into 3 L of heated saline with temperature 43°C ± 0.5°C, the duration of HIPEC for each drug was 30 minutes with a flow rate of 400 mL/min. The PC index (PCI) and completeness of cytoreduction (CC) were calculated according to the Sugarbaker criteria.18
Postoperative Physiotherapy for VTE Prevention and Treatment
A VTE prevention program is developed based on the characteristics of the procedure and guidelines,19,20 including physiotherapy and pharmacological treatments.
For patients without clinically significant VTE symptoms preoperatively, physiotherapy program is considered as the key prophylactic measures, as summarized in Table 1.
Table 1.
Details of the Systematic Physiotherapy Program.
| Time | Protocol | Frequency |
|---|---|---|
| Intraoperation | IPC | For every operation |
| POD 1-2 | IPC | >20 minutes, twice a day |
| Dorsiflexion–plantarflexion exercises of the feet | >20 times, every 8 hours | |
| Upper body exercisea | >20 times, every 8 hours | |
| POD 3-7 | All measures above | |
| Standing up and slow walking | >3 minutes, every 8 hours | |
| POD 7-10 | All measures above | |
| Walking | >30 minutes, twice a day |
Abbreviations: IPC. intermittent pneumatic compression; POD, postoperation day.
a Including chest-expanding exercise, arm adduction–abduction exercise, washing face, combing hair, and so on
For the prevention of DVT, the dorsiflexion–plantarflexion exercise of the feet with the maximal force can activate both the leg muscles and accelerate the returning blood circulation in the deep veins of both legs. Such exercise is repeated at least 20 times every 8 hours. In addition, we also use IPC device to prevent DVT, from day 1 to 10 after surgery for 20 minutes twice a day.
For the prevention of PE, the chest-expanding exercise could significantly activate the thoracic pump to accelerate the returning blood circulation in both the superior vena cava and inferior vena cava and also accelerate pulmonary circulation. This exercise contains 2 parts. First is the arm up-down exercise, in which the patient extends straight both arms and move arms up overhead as high as possible and then slowly let down. Second is the arm adduction–abduction exercise, in which the patient extends straight both arms and makes the arms adduction and abduction movements. Such exercise is repeated at least 20 times every 8 hours.
For patients with clinically significant VTE, based on the international guidelines,20 pharmacological treatments are implemented, with 4100 AXaIU low-molecular-weight heparin (LMWH) subcutaneously injected twice a day to reduce platelet aggregation for 7 to 10 days.
Screening of the Symptomatic VTE Events
Attentions are especially paid to symptoms such as chest pain, dyspnea at rest, and calf swelling or pain, highly indicating VTE. If calf swelling or pain occurs, the circumference of both legs 10 cm under the patella is measured. Other parameters are reexamined, including vital signs, ultrasound, blood oxygen saturation, chest computed tomography scan, and blood coagulation function including d-dimer. When distinguished from acute coronary syndrome, pneumonia, artery dissection, and pleural effusion, and image examination finding thrombogenesis in the pulmonary artery or lower limb venous, the patient is eventually diagnosed with symptomatic VTE.
Follow-Up
All patients received regular follow-up every month for 3 months. Several different follow-up ways were used: routine outpatient clinical visit, telephone contact, e-mail contact, and WeChat instant communication. Among them, WeChat instant communication was the most efficient and most widespread in China, which could reach everyone with a cellphone. A WeChat follow-up software was designed and used to update our patients’ information. At the clinic, the follow-up package includes physical examination, coagulation function, and Doppler ultrasonography or chest computed tomography scan if necessary. The overall follow-up rate is 100%.
End Point of the Study
The primary end point of the study was the symptomatic VTE. The secondary end point of the study was the safety and feasibility of this systematic physiotherapy program.
Statistical Analysis
Data including clinical information, laboratory results, surgical reports, medical imaging reports, and pathology reports were all collected and analyzed using Microsoft Office 2016 and Statistical Package for Social Science (SPSS, IBM Corporation, SPSS, Armonk, New York). Continuous data were expressed as median and range, analyzed by t test; and category data were presented as number and percentage, analyzed by χ2 test.
Results
The Clinic-Demographic Characteristics
The CRS + HIPEC procedures have been performed on 466 patients with PC, including 25 (5.4%) patients from gastric cancer, 100 (21.5%) from colorectal cancer, 82 (17.6%) from gynecological malignancies, 121 (26.0%) from pseudomyxoma peritonei, 35 (7.5%) from malignant mesothelioma, 16 (3.4%) from primary peritoneum, 55 (11.8%) from retroperitoneal sarcoma, and 32 (6.9%) from miscellaneous malignancies (Table 2). There were 178 (38.2%) male and 288 (61.8%) female patients; 39 (8.4%) patients had prior history of VTE, of which 16 (3.4%) had chemoprophylaxis, and 33 (7.3%) patients were diagnosed as asymptomatic DVT before surgery. The median age was 55 years.
Table 2.
Demographic Characteristics.a
| Items | Value |
|---|---|
| Gender (male/female), % | |
| Male | 178 (38.2) |
| Female | 288 (61.8) |
| Age (years) | 55 (10-81) |
| Karnofsky performance score | 90 (50-100) |
| Prior history of VTE, % | 39 (8.4) |
| Prior history of VTE chemoprophylaxis, % | 16 (3.4) |
| VTE of ultrasound preoperatively, % | 33 (7.2) |
| Caprini risk factor score | 11 (9-17) |
| Primary tumor, % | |
| Carcinoma of the stomach | 25 (5.4) |
| Carcinoma of the colorectum | 100 (21.5) |
| Pseudomyxoma peritonei | 121 (26.0) |
| Malignant peritoneal mesothelioma | 35 (7.5) |
| Carcinoma of ovary | 73 (15.7) |
| Carcinoma of other gynecology | 9 (1.9) |
| Primary peritoneum | 16 (3.4) |
| Retroperitoneal sarcoma | 55 (11.8) |
| Othersb | 32 (6.9) |
| Vascular invasion, % | 108 (23.9) |
Abbreviation: VTE, venous thromboembolism.
a Data are presented as n (%) or median (range).
b Including 2 hepatocellular carcinoma, 1 pancreatic carcinoma, 4 carcinoma from small intestine, 4 gastrointestinal stromal tumor, 1 carcinoma from biliary duct, 2 carcinoma of gallbladder, 2 breast carcinoma, 1 carcinoma from lung, 1 carcinoma from bladder, 1 hemangiopericytoma, 1 inflammatory myofibroblastic tumor, 1 carcinosarcoma, 2 neurofibroma, 1 schwannoma, 3 neuroectodermal, 1 aggressive angiomyxoma, 1 carcinoma from urachus, 1 angiomyolipoma, 1 Ewing sarcoma.
Major CRS + HIPEC Information
The duration of CRS + HIPEC was 10.0 hours (range: 1.6-19.5). The median PCI was 23 (range: 0-39); 257 (60.1%) patients achieved complete or nearly complete cytoreduction, defined as CC score of 0 or 1. The HIPEC regimens used were cisplatin 120 mg and mitomycin C 30 mg in 123 (26.5%) patients; docetaxel 120 mg and mitomycin C 30 mg in 27 (5.8%) patients; or cisplatin 120 mg and docetaxel 120 mg in 259 (55.8%) patients. Of the 466 patients in this study, 429 (92.1%) required intraoperative blood infusion, including 48 (10.3%) patients receiving autologous transfusion.
Evaluation of VTE by Caprini Risk Factor Score
All patients received VTE assessment according to the Caprini’s criteria, which defined total score ≥5 as the highest risk.15 According to this evaluation criteria, the most common risk factors in these patients were major surgery lasting over 3 hours (99.1%), present cancer or chemotherapy (100.0%), central venous access (100.0%), and blood transfusion (92.1%). Other less common risk factors such as history of major surgery and history of VTE are listed in Table 3.
Table 3.
Major Risk Factors in this Study.
| Factors | Score | n (%) |
|---|---|---|
| Age, years | ||
| <40 | 0 | 59 (12.7) |
| 40-59 | 1 | 241 (51.7) |
| 60-74 | 2 | 152 (32.6) |
| ≥75 | 3 | 14 (3.0) |
| Cancer | 3 | 466 (100.0) |
| Central venous access | 1 | 466 (100.0) |
| Present cancer or chemotherapy | 3 | 466 (100.0) |
| Major surgery lasting over 3 hours | 5 | 462 (99.1) |
| Blood transfusion | 1 | 429 (92.1) |
| History of venous thromboembolism | 3 | 39 (8.4) |
| History of major surgery | 1 | 14 (3.0) |
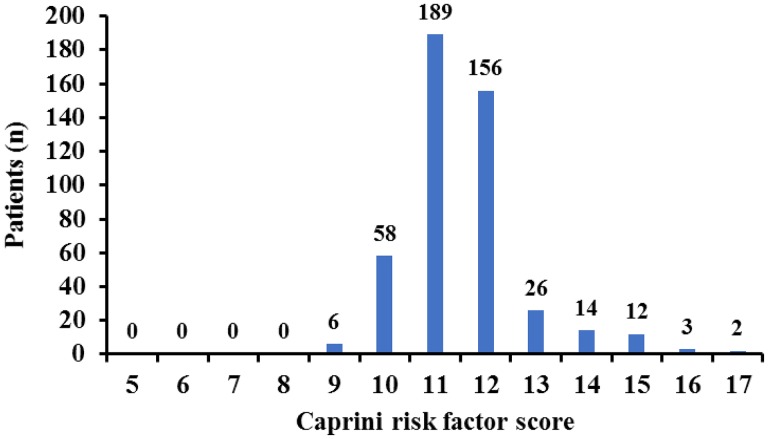
The distribution of total Caprini risk factor score was from 9 to 17 (Figure 1). There were 409 (87.8%) patients in the risk score 9 to 12 group, and 57 (12.2%) patients in the risk score 13 to 17 group.
Figure 1.
The Caprini risk factor score of the patients in this study.
Venous Thromboembolism-Related Clinical Outcomes and Risk Factor Analysis
There were 9 (1.9%) clinically symptomatic VTE events occurred in 8 patients during the 3-month perioperative period. The 9 clinically symptomatic VTE events included 8 (1.7%) DVT and 1 (0.2%) PE. Among the 8 patients with clinically symptomatic VTE, 5 patients received pharmacological treatments with LWMH. For the other 3 patients with Doppler ultrasonography confirmed small DVT, we just encourage the patients to perform standardized physical exercises only, and these patients also recovered well. For the only 1 patient with PE, she was in sound condition during the extended follow-up for 6 months. There was no mortality about VTE perioperatively.
We also conducted comparison study on major risk factors between the patients with VTE and without VTE (Table 4). The occurrence of VTE had a correlation with history of VTE (P = .013) and history of major surgery (P = .019), but no correlation with age, major surgery lasting over 3 hours, and blood transfusion.
Table 4.
Comparison of Patients Having VTE With Non-VTE Patients on Major Risk Factors.
| Factors | VTE (n = 22) | No-VTE (n = 444) | P value |
|---|---|---|---|
| Age, years | .929 | ||
| <40 | 2 | 57 | |
| 40-59 | 12 | 229 | |
| 60-74 | 7 | 145 | |
| ≥75 | 1 | 13 | |
| Cancer | 22 | 444 | – |
| Central venous access | 22 | 444 | – |
| Present cancer or chemotherapy | 22 | 444 | – |
| Major surgery lasting over 3 hours | 22 | 440 | .655 |
| Blood transfusion | 22 | 407 | .158 |
| History of venous thromboembolism | 5 | 34 | .013 |
| History of major surgery | 3 | 11 | .019 |
Abbreviation: VTE, venous thromboembolism.
Note: The aim of boldface is to show number highlight, becuause the P value have statistical significance.
Discussion
Venous thromboembolism is a common complication of severe diseases associated with increased morbidity and mortality and the second leading cause of cancer death.21 The incidence of VTE was 1.6% among patients with common cancer22 and with an estimated annual percentage increase of 4.0% (95% confident interval, 2.9-5.1).23 Due to advanced stage, extensive surgery, chemotherapy, blood transfusion, and central venous access, patients with PC who underwent CRS + HIPEC are more prone to developing VTE. In studies focusing on CRS + HIPEC to treat peritoneal metastases, the incidence of symptomatic DVT ranged from 0.9% to 11.1%, and of PE from 0.5% to 12.8% (Table 5). Moreover, the mortality owing to PE could be as high as 3.3%.24 Huang et al25 even found that the incidence of DVT and PE was from 0.9% to 9.3%. Therefore, it is very important to enhance vigilance on VTE complications in patients with PC and adopt active perioperative prophylaxis.
Table 5.
The Literature-Reported VTE Data on Patients Having PC Treated With CRS + HIPEC.
| Reference | Year | Patient, n | Age, years | Operation Duration, hour | Blood Loss, mL | PCI | CC 0-1, n (%) | VTE, n (%) | DVT, n (%) | PE, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Witkamp et al26 | 2001 | 46 | 56 (34-76) | 10 (5.5-18) | 13,000 (1600-55 000) | NA | NA | NA | NA | 3 (6.5) |
| Verwaal et al4 | 2003 | 48 | 53 (28-69) | 8.1 (5.3-12.8) | 3900 (500-30 000) | NA | 39 (81.3) | NA | NA | 2 (4.2)a |
| Ahmad et al27 | 2004 | 33 | 49 (26-72) | 8.3 (4.1-14.8) | 650 (100-3300) | NA | NA | NA | 1 (3.0) | NA |
| Glehen et al28 | 2004 | 506 | 51 (16-81) | NA | NA | NA | 377 (74.5) | NA | NA | 2 (0.4)b |
| Kusamura et al29 | 2006 | 205 | 52 (22-76) | 8.9 (4.0-22.0) | NA | NA | 182 (88.8) | NA | NA | 1 (0.5) |
| Moran et al30 | 2006 | 100 | 52 (32-74) | NA (3-18) | NA | NA | 65 (65.0) | NA | 5 (5.0) | 4 (4.0) |
| Smeenk et al31 | 2006 | 103 | 57 (30-77) | 9.0 (4.5-18.0) | 8000 (300-55 000) | NA | 87 (84.5) | 10 (9.7)b | NA | NA |
| Hagendoorn et al32 | 2009 | 49 | 55 (40-76) | 7.7 (5.0-11.5) | 1420 (150-5500) | NA | NA | NA | 1 (2.0)c | NA |
| Kerscher et al33 | 2010 | 109 | 54.1d | 6.63 (4-12) | NA | 21.2d | 53 (48.6) | NA | NA | 2 (1.8)b |
| Canda et al34 | 2013 | 115 | 53.4 (20-82) | 6.0 (2.0-12.0) | NA | 14.7 (3-28) | NA | NA | 1 (0.9) | 1 (0.9) |
| Votanopoulos et al35 | 2013 | 925 | NA (11-82) | NA | NA | NA | NA | 26 (2.8)e | 17 (1.8) | 9 (1.0) |
| Wagner et al36 | 2013 | 282 | NA | NA (6.5-12.5) | NA (400-2500) | NA (8-18) | 230 (81.6) | 4 (1.4)b | NA | NA |
| Jimenez et al37 | 2014 | 202 | 53 (25-80) | NA | NA | NA | 170 (84.2) | NA | NA | 5 (2.5)b |
| Konigsrainer et al38 | 2014 | 90 | 55 (18-76) | 7.6 (0.6-17.9) | NA | 20 (3-39) | 62 (68.9) | NA | NA | 7 (7.7) |
| Shen et al39 | 2014 | 27 | 52.2d | NA | NA | NA | NA | 2 (7.4)a | NA | NA |
| Spiliotis et al40 | 2014 | 100 | NA | NA | NA | NA (3-39) | 86 (86.0) | NA | NA | 6 (6.0) |
| Beckert et al41 | 2015 | 381 | 55 (14-77) | 7.6 (0.6-17.9) | NA | 20 (1-39) | 263 (69.0) | 27 (7.1) | 10 (2.6) | 17 (4.5) |
| Coccolini et al42 | 2015 | 54 | 54.5d | 8.85d | NA | 10.1 (1-28) | 54 (100.0) | NA | 1 (1.9)b,e | 1 (1.9)b, e |
| Huang et al25 | 2015 | 800 | 53d | NA | NA | NA (2-31) | 765 (95.6) | 43 (5.4) | NA | NA |
| Lord et al43 | 2015 | 512 | 56 (24-82) | NA | NA | NA | 512 (100.0) | NA | 57 (11.1) | 9 (1.8) |
| Randle et al44 | 2015 | 935 | NA (11-87) | NA (4-25.6) | NA (50-6500) | NA (0-39) | NA | 28 (3.0) | NA | NA |
| Spark et al24 | 2015 | 30 | 53.3d (25-71) | 10.1d | NA | NA | 21 (70.0) | NA | 3 (10.0)b | 2 (6.7) |
| Baumgartner et al45 | 2016 | 247 | 53 (20-86) | 6.7 (3.3-13.3) | 250 (10-4000) | 14 (0-29) | 235 (95.2) | NA | NA | 8 (3.2)f |
| Dagbert et al46 | 2016 | 39 | 58.2d | 5.1d | NA | 15.1d | 38 (97.4) | NA | NA | 5 (12.8)g |
| Martin et al47 | 2016 | 203 | NA | 5.1 (5.1-8.3) h | 300 (200-600)h | NA (6-18) | 173 (85.2) | 14 (6.9) | NA | NA |
| Sargant et al48 | 2016 | 201 | 55 | NA | NA | NA | NA | NA | 10 (5.0)i | 4 (2.0) |
| This study | 2018 | 466 | 55 (10-81) | 10.0 (1.6-19.5) | 600 (0-6100) | 23 (0-39) | 257 (60.1) | 23 (4.9) | 22 (4.7) | 1 (0.2) |
Abbreviations: CC, completeness of cytoreduction; CRS, cytoreductive surgery; DVT, deep vein thrombosis; HIPEC, hyperthermic intraperitoneal chemotherapy; NA, not available; PC, peritoneal carcinomatosis; PCI, peritoneal carcinomatosis index; PE, pulmonary embolism; VTE, venous thromboembolism.
a Within 3 months after surgery.
b Grades 3-5 according to the National Cancer Institute’s Common Toxicity Criteria.
c Grades 2-5.
d Average.
e Within 1 month after surgery.
f Within 2 months after surgery.
g Only for those underwent splenectomy.
h interquartile range.
i Including 2 atrial thromboses.
According to the Chinese guidelines, IPC or grated compression stocking is recommend for all hospitalized patients with cancer, alone with unfractionated heparin or LWMH or factor Xa inhibitor for those with Carprini risk factor score ≥3.19 However, in the international guideline, mechanical methods are not recommended as monotherapy (Grade IIC), while pharmacological prophylaxis of LMWH once a day postoperatively and continued at least 7 to 10 days is recommended (Grade 1A).20 However, the practical importance of physical exercise has not been paid enough attention among clinicians.
In this study of 466 patients, the incidence of VTE (1.9%) is much lower than that of 10.0% compared with another similar retrospective study by Lanuke et al.6 The difference was that, in their study, all patients received prophylactic pneumatic sequential compression stockings and subcutaneous unfractionated heparin, but patients in our center all had IPC device combined with physiotherapy, and heparin was only used for those with VTE. This interesting result strongly suggest the key role of physiotherapy in VTE prevention. Just as van Stralen et al.49 concluded that regular sports activities could reduce the risk of VTE.
There is sound pathophysiological evidence to support physiotherapy in VTE prevention. According to the classical theory,50 sluggish venous blood flow, hypercoagulability, and blood vessel wall injury are the 3 major contributors to thrombosis. The rationale of this physiotherapy program is to intervene 2 of the 3 factors. First, activating calf muscle pump and thoracic pump to increase blood velocity to prevent venous stasis. Stein et al51 demonstrated that ankle exercise increased venous blood velocity no matter in supine or sitting positions. Caldwell et al52 found upper body exercise could also increase lower extremity venous blood flow in healthy volunteers and in patients with acute DVT. Second, alterations in plasma coagulation factors to decrease blood clotting. Jahangard et al53 found short-term aerobic training could reduce fibrinogen, von Willebrand factor antigen, plasminogen activator inhibitor-1 activity and antigen, and increase prothrombin time, partial thromboplastin time, tissue plasminogen activator activity, and antigen in sedentary postmenopausal women. Moreover, Chen et al54 found that low-load resistance exercise plays an advantageous role in preventing thrombogenesis by reducing inflammatory process.
In addition to the effectiveness, this systematic physiotherapy program has several obvious advantages compared with other prophylactic measures. First, all hospitalized patients can easily master the key points, so it provides better compliance and higher practicability. Second, it can bring positive psychological benefits. These series of upper and lower extremity exercises under the instruction and encouragement of the treating physician or nurse have produced positive psychological feedback to the patients. They could feel a sense of control over their disease, still being an active part in the recovery process and being in close and active contact with the physicians and the nurses. Third, in some countries, some heparin drugs specific for VTE therapy are not easily accessible and can be costly, but this strategy is almost free, which means better social economic benefits.
In summary, this retrospectively observational study demonstrated the safety and feasibility, and potential beneficial effects of physiotherapy program integrating upper and lower extremity exercises on the prevention of VTE among patients having PC treated with CRS + HIPEC. The study also demonstrated the key role of early exercises after surgery in thromboprophylaxis. Randomized trials are warranted to validate this physiotherapy program.
Conclusions
Patients treated with CRS + HIPEC are at highest risk of developing VTE. The systematic physiotherapy program including dorsiflexion–plantarflexion exercise of the feet, and chest-expanding exercises is safe and feasible to prevent VTE in such patients. Randomized controlled trials are warranted to evaluate the efficacy of the systematic physiotherapy program.
Footnotes
Authors’ Note: Xin-Bao Li and Kai-Wen Peng contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Special Fund for the Capital Characteristic Clinical Medicine Development Project (Z161100000516077), Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20180701), Beijing Municipal Grant for Medical Talents Group on Peritoneal Surface Oncology (2017400003235J007), Special Fund for Key Academic Discipline Development of Beijing Shijitan Hospital, Capital Medical University (201601fmzlwk), and Beijing Natural Science Foundation (7172108); Health Science Promotion Project of Beijing (2018-TG-27).
ORCID iD: Yan Li  https://orcid.org/0000-0001-6018-6538
https://orcid.org/0000-0001-6018-6538
Ethical approval: Ethical approval to report this case series was obtained from Beijing Shijitan Hospital, Capital Medical University.
Informed Consent: Written informed consent was obtained from the patients for their anonymized information to be published in this article.
References
- 1. De Martino RR, Goodney PP, Spangler EL, et al. Variation in thromboembolic complications among patients undergoing commonly performed cancer operations. J Vasc Surg. 2012;55(4):1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cronin-Fenton DP, Sondergaard F, Pedersen LA, et al. Hospitalization for venous thromboembolism in cancer patients and the general population: A population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–3743. [DOI] [PubMed] [Google Scholar]
- 5. van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378:230–240. [DOI] [PubMed] [Google Scholar]
- 6. Lanuke K, Mack LA, Temple WJ. A prospective evaluation of venous thromboembolism in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Can J Surg. 2009;52(1):18–22. [PMC free article] [PubMed] [Google Scholar]
- 7. Vukadinovic V, Chiou JD, Morris DL. Clinical features of pulmonary emboli in patients following cytoreductive surgery (peritonectomy) and hyperthermic intraperitoneal chemotherapy (hipec), a single center experience. Eur J Surg Oncol. 2015;41(5):702–706. [DOI] [PubMed] [Google Scholar]
- 8. Tang L, Mei LJ, Yang XJ, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of gastric cancer with peritoneal carcinomatosis: Evidence from an experimental study. J Transl Med. 2011;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang CQ, Yang XJ, Yu Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for patients with peritoneal carcinomatosis from colorectal cancer: A phase II study from a Chinese center. PLoS One. 2014;9(9):e108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun JH, Ji ZH, Yu Y, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat advanced/recurrent epithelial ovarian cancer: Results from a retrospective study on prospectively established database. Transl Oncol. 2016;9(2):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu HT, Peng KW, Ji ZH, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: Results from a Chinese center. Eur J Surg Oncol. 2016;42(7):1024–1034. [DOI] [PubMed] [Google Scholar]
- 12. Mei LJ, Yang XJ, Tang L, et al. Establishment and identification of a rabbit model of peritoneal carcinomatosis from gastric cancer. BMC Cancer. 2010;10:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shao LH, Liu SP, Hou JX, et al. Cathepsin B cleavable novel prodrug Ac-Phe-Lys-PABC-ADM enhances efficacy at reduced toxicity in treating gastric cancer peritoneal carcinomatosis: an experimental study. Cancer. 2012;118(11):2986–2996. [DOI] [PubMed] [Google Scholar]
- 14. Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18(6):1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caprini JA. Risk assessment as a guide to thrombosis prophylaxis. Curr Opin Pulm Med. 2010;16(5):448–452. [DOI] [PubMed] [Google Scholar]
- 16. Yang XJ, Li Y, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: results from a Chinese center. J Surg Oncol. 2010;101(6):457–464. [DOI] [PubMed] [Google Scholar]
- 17. Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221(1):29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol. 2001;27(3):239–243. [DOI] [PubMed] [Google Scholar]
- 19. Consensus Committee on Tumor and Thromboembolism from Chinese Society of Clinical Oncology. Guideline on prevention and treatment of tumor-associated venous thromboembolism in China. Chin J Pract Internal Med. 2015;35(11):907–920. [Google Scholar]
- 20. Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013;11(1):56–70. [DOI] [PubMed] [Google Scholar]
- 21. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. [DOI] [PubMed] [Google Scholar]
- 22. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. [DOI] [PubMed] [Google Scholar]
- 23. Trinh VQ, Karakiewicz PI, Sammon J, et al. Venous thromboembolism after major cancer surgery: temporal trends and patterns of care. JAMA Surg. 2014;149(1):43–49. [DOI] [PubMed] [Google Scholar]
- 24. Sparks DS, Morris B, Xu W, et al. Cytoreductive surgery and heated intraperitoneal chemotherapy for peritoneal carcinomatosis secondary to mucinous adenocarcinoma of the appendix. Int Surg. 2015;100(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang Y, Alzahrani NA, Liauw W, Morris DL. Learning curve for cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis. ANZ J Surg. 2017;87(1-2):49–54. [DOI] [PubMed] [Google Scholar]
- 26. Witkamp AJ, de Bree E, Kaag MM, van Slooten GW, van Coevorden F, Zoetmulder FA. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxoma peritonei. Br J Surg. 2001;88(3):458–463. [DOI] [PubMed] [Google Scholar]
- 27. Ahmad SA, Kim J, Sussman JJ, et al. Reduced morbidity following cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion. Ann Surg Oncol. 2004;11(4):387–392. [DOI] [PubMed] [Google Scholar]
- 28. Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–3292. [DOI] [PubMed] [Google Scholar]
- 29. Kusamura S, Younan R, Baratti D, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer. 2006;106(5):1144–1153. [DOI] [PubMed] [Google Scholar]
- 30. Moran BJ, Mukherjee A, Sexton R. Operability and early outcome in 100 consecutive laparotomies for peritoneal malignancy. Br J Surg. 2006;93(1):100–104. [DOI] [PubMed] [Google Scholar]
- 31. Smeenk RM, Verwaal VJ, Zoetmulder FA. Toxicity and mortality of cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei-a report of 103 procedures. Eur J Surg Oncol. 2006;32(2):186–190. [DOI] [PubMed] [Google Scholar]
- 32. Hagendoorn J, van Lammeren G, Boerma D, van der Beek E, Wiezer MJ, van Ramshorst B. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal and gastrointestinal origin shows acceptable morbidity and high survival. Eur J Surg Oncol. 2009;35(8):833–837. [DOI] [PubMed] [Google Scholar]
- 33. Kerscher AG, Mallalieu J, Pitroff A, Kerscher F, Esquivel J. Morbidity and mortality of 109 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC) performed at a community hospital. World J Surg. 2010;34(1):62–69. [DOI] [PubMed] [Google Scholar]
- 34. Canda AE, Sokmen S, Terzi C, et al. Complications and toxicities after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2013;20(4):1082–1087. [DOI] [PubMed] [Google Scholar]
- 35. Votanopoulos KI, Swords DS, Swett KR, et al. Obesity and peritoneal surface disease: Outcomes after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for appendiceal and colon primary tumors. Ann Surg Oncol. 2013;20(12):3899–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wagner PL, Austin F, Maduekwe U, et al. Extensive cytoreductive surgery for appendiceal carcinomatosis: Morbidity, mortality, and survival. Ann Surg Oncol. 2013;20(4):1056–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jimenez W, Sardi A, Nieroda C, et al. Predictive and prognostic survival factors in peritoneal carcinomatosis from appendiceal cancer after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2014;21(13):4218–4225. [DOI] [PubMed] [Google Scholar]
- 38. Konigsrainer I, Horvath P, Struller F, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in recurrent epithelial ovarian cancer with peritoneal metastases: a single center experience. Langenbeck’s Arch Surg. 2014;399(5):589–594. [DOI] [PubMed] [Google Scholar]
- 39. Shen P, Thomas CR, Fenstermaker J, Aklilu M, McCoy TP, Levine EA. Phase II trial of adjuvant oral thalidomide following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal surface disease from colorectal/appendiceal cancer. J Gastroint Cancer. 2014;45(3):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Spiliotis JD, Halkia E, Boumis VA, Vassiliadou DT, Pagoulatou A, Efstathiou E. Cytoreductive surgery and HIPEC for peritoneal carcinomatosis in the elderly. Int J Surg Oncol. 2014;2014:987475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beckert S, Struller F, Horvath P, Falcke A, Königsrainer A, Königsrainer I. Overall morbidity but not mortality is increased in elderly patients following cytoreductive surgery and HIPEC. Langenbeck’s Arch Surg. 2015;400(6):693–698. [DOI] [PubMed] [Google Scholar]
- 42. Coccolini F, Campanati L, Catena F, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: A multicenter prospective observational study. J Gynecol Oncol. 2015;26(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lord AC, Shihab O, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Recurrence and outcome after complete tumor removal and hyperthermic intraperitoneal chemotherapy in 512 patients with pseudomyxoma peritonei from perforated appendiceal mucinous tumors. Eur J Surg Oncol. 2015;41(3):396–399. [DOI] [PubMed] [Google Scholar]
- 44. Randle RW, Ahmed S, Levine EA, et al. Significance of diabetes on morbidity and mortality following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2015;111(6):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Baumgartner JM, Kwong TG, Ma GL, Messer K, Kelly KJ, Lowy AM. A novel tool for predicting major complications after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23(5):1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dagbert F, Thievenaz R, Decullier E, et al. Splenectomy increases postoperative complications following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2016;23(6):1980–1985. [DOI] [PubMed] [Google Scholar]
- 47. Martin AS, Abbott DE, Hanseman D, et al. Factors associated with readmission after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2016;23(6):1941–1947. [DOI] [PubMed] [Google Scholar]
- 48. Sargant N, Roy A, Simpson S, et al. A protocol for management of blood loss in surgical treatment of peritoneal malignancy by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Transfus Med. 2016;26(2):118–122. [DOI] [PubMed] [Google Scholar]
- 49. van Stralen KJ, Le Cessie S, Rosendaal FR, Doggen CJ. Regular sports activities decrease the risk of venous thrombosis. J Thromb Haemost. 2007;5(11):2186–2192. [DOI] [PubMed] [Google Scholar]
- 50. Virchow R. Phlogose und thrombose in gefassystem. Gesammelte Abhandlungen zur Wissenschaftlichen Medicin. 1856;9:458. [Google Scholar]
- 51. Stein PD, Yaekoub AY, Ahsan ST, et al. Ankle exercise and venous blood velocity. Thromb Haemost. 2009;101(6):1100–1103. [PubMed] [Google Scholar]
- 52. Caldwell K, Prior SJ, Kampmann M, et al. Upper body exercise increases lower extremity venous blood flow in deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2013;1(2):126–133. [DOI] [PubMed] [Google Scholar]
- 53. Jahangard T, Torkaman G, Ghoosheh B, Hedayati M, Dibaj A. The effect of short-term aerobic training on coagulation and fibrinolytic factors in sedentary healthy postmenopausal women. Maturitas. 2009;64(4):223–227. [DOI] [PubMed] [Google Scholar]
- 54. Chen YW, Apostolakis S, Lip GY. Exercise-induced changes in inflammatory processes: implications for thrombogenesis in cardiovascular disease. Ann Med. 2014;46(7):439–455. [DOI] [PubMed] [Google Scholar]