Short abstract
Background
The objective of this study was to investigate the dynamic brain activity following auricular point acupressure (APA) in chemotherapy-induced neuropathy (CIN).
Methods
Participants received 4 weeks of APA in an open-pilot trial with repeated observation. Along with the clinical self-reported CIN outcomes, objective outcomes were measured over the course of the treatment by physiological changes in pain sensory thresholds from quantitative sensory testing (QST) and repeated functional magnetic resonance imaging scans.
Results
After 4 weeks of APA, participants had reported clinically significant improvements (ie, ≥30%) in a reduction of CIN symptoms (including pain, numbness, tingling, and stiffness) in lower extremity stiffness (32%), reduced foot sensitivity (13%), and higher pain threshold (13%). Across the 11 intrinsic brain networks examined, there was a trend toward significance of the connectivity of the basal ganglia network (BGN) to the salience network (SAL), which was decreased pre-APA versus immediate-APA (effect size [ES] = 1.04, P = .07). The BGN also demonstrated decreased connectivity with the language network pre-APA versus delayed imaging post-APA (ES = −0.92, P = .07). Furthermore, there was increased executive control network (ECN) and SAL within-network connectivity comparing pre-APA to delayed imaging post-APA, trending toward significance (ES = 0.41, P = .09 and ES = 0.17, P = .09, respectively).
Conclusion
The changes in connectivity and activity within or between the ECN, SAL, and BGN from pre- to post-APA suggest ongoing alterations in brain functional connectivity following APA, particularly in the insula, anterior cingulate, and dorsolateral prefrontal cortices, which play significant roles in pain, memory, and cognitive function.
Keywords: auricular point acupressure, quantitative sensory testing, functional magnetic resonance imaging, chemotherapy-induced peripheral neuropathy
Chemotherapy-induced peripheral neuropathy (CIN)—pain, numbness, and/or tingling distributed in the hands and feet in a stocking-and-glove distribution—is a severe adverse result of chemotherapeutic agents such as platinum drugs, vinca alkaloids, bortezomib, and taxanes.1–4 With improved cancer treatments and longer survival, the burdensome effects on sensation and balance from CIN arise in more than 80% of patients receiving treatment5 and persist in up to 50% of cancer survivors as they continue to suffer CIN 6 years after their initial treatments.6
Currently, there is no established treatment that effectively and consistently manages CIN.6 While Duloxetine, the only recommended drug by the American Society of Clinical Oncology,6 demonstrated more successful effects when compared to placebo, it only improved CIN by 0.73 points on a 0 to 10 scale.7 One of the few suggested treatments for CIN is exercise, yet its ES is <0.50 and therefore cannot adequately manage CIN symptoms.8 Although opioids alleviate neuropathic pain,9,10 long-term usage presents a serious risk of opioid overuse as well as other psychological and physical side effects.11 Prolonged CIN increases the risk of dangerous falls 1.8-fold,12 which can lead to a significant loss of functional abilities and overall poorer quality of life (QOL).3,4 The impact of CIN remains extensive, as it may lead to treatment delays, dose reductions, and discontinuation of therapy, which can then adversely affect survival rates and raise health-care costs.13
The pathophysiology of CIN is not well-understood,5 as underlying mechanisms leading to the development of CIN appear to be medication specific.14 For example, platinum agent-induced formation of platinum-DNA adducts leads to apoptosis, whereas taxane agent-induced microtubule depolymerization prompts mitotic arrest.14 Such variety in CIN pathophysiology may explain the lack of effective pharmacological treatments. Recent studies have identified dorsal root ganglion (DRG) neurons playing an active role in the development of neuropathic pain.15,16 Given that DRG neurons are not protected by the blood–brain barrier,17 they remain especially susceptible to damage from chemotherapeutic agents16 and subsequently provoke symptoms of CIN such as pain, numbness, and even sensory ataxia.17 Based on this assumption, interventions that do not damage DRG neurons or repair DRG neuron function could potentially prevent and manage CIN.
Auricular point acupressure (APA), a noninvasive and nonpharmacological treatment, presents a promising solution for patients suffering from CIN. Derived from traditional Chinese medicine auricular acupuncture, Dr Nogier, a French neurosurgeon, theorized a somatotopic map of the human body onto the ear to show that auricular points correspond to specific organs and regions of the body.18–20 Functional magnetic resonance imaging (fMRI) studies corroborate changes in brain activity correlating with auricular acupuncture,21,22 body symptoms can be treated by the stimulation of auricular points, via needle, electricity, or acupressure, to elicit a therapeutic effect.23,24 Original findings, using self-reported outcomes, and meta-analyses25,26 studying the analgesic effects of APA consistently reveal a decrease in pain intensity, a reduction in pain medication usage, and a recommendation to implement APA as an adjunct therapy to current pain management strategies.27–33
fMRI34 has provided an objective method for measuring pain-related brain activity and acquiring details related to the neural mechanisms involved in pain processing. Recent neuroimaging studies have enhanced our understanding that acupuncture can affect cortical thickness or restore normal functional connectivity related to pain reduction35–37 and elucidated the effect of placebo (sham acupuncture).38,39 In the only 2, small published fMRI auricular acupuncture studies,21,22 evidence supports the existence of a body–ear–brain somatotopical connection, suggesting neuroimaging may be a useful tool to determine the correlations of body structures and auricular points. However, we cannot assume that the beneficial effects of body or auricular acupuncture for pain relief will simply translate into APA because the stimulation between needle and acupressure may differ. APA has shown the immediate and lasting pain relief in chronic low back pain28–30 and breast cancer patients with pain.27,33 However, mechanisms of APA on pain relief have yet to be clearly explained. We have examined APA on CIN management.40,41 In this article, using self-reported pain outcomes, quantitative sensory testing (QST) battery, and multiple fMRI scans, we report the findings of alterations in connectivity and/or activity within the brain’s cortical pain processing system following APA.
Methods
Complete details of the study design, sample, and data collection are provided in our previous manuscripts.40,41 A subgroup of the study participants from the main study40,41 underwent an fMRI scan. An open-pilot trial with repeated observation study design was used. Participants received a 4-week APA treatment protocol to manage their CIN. The recorded data included self-report clinical assessments and sensory function measured by QST battery. A detailed description of the questionnaires and QST battery are explained in previous reports. After baseline data were collected, participants received a pre-APA fMRI scan (hereafter, pre-), followed by 10 minutes of APA. Immediately after the treatment, a repeat fMRI was performed (hereafter, immediate-; see Procedure in detail). Participants received another fMRI after completion of 4 weeks of APA (hereafter, post-).
Participants and Setting
Potential participants were referred from a study of scrambler therapy for CIN and from oncology outpatient clinics at Johns Hopkins Hospital. Participants were eligible for the study if they (1) were cancer patients who were 18 years of age or older, (2) were able to read and write in English, (3) had CIN due to receiving neurotoxic chemotherapy for cancer, (4) had average intensity of pain/or numbness/or tingling due to CIN ≥ 4 on a 11-point numerical pain scale in the previous week, (5) pain/or numbness/or tingling > 3 months duration attributed to CIN. Participants were excluded from the study if they reported any of the following: (1) use of an investigational agent for pain control concurrently or within the past 30 days; (2) prior celiac plexus block or other neurolytic pain control treatment; (3) other identified causes of painful paresthesia existing prior to chemotherapy (eg, radiation or malignant plexopathy, lumbar or cervical radiculopathy, pre-existing peripheral neuropathy of another etiology); (4) allergy to latex (the tapes for the APA include latex); and (5) any MRI contraindications.
APA Treatment Protocol
The APA treatment protocol has been described in detail in our previous publications.40,41 In brief, auricular points for the CIN included (1) points corresponding to the body pain location (ear points selected for treatment were individualized to the specified CIN bodily location such as toes, sole, or heel), (2) 4 points known for alleviating stress and pain (ie, shenmen, sympathetic, cingulate gyrus, and nervous subcortex), and (3) 2 points related to brain (ie, brain and spinal cord sensory neurons).
Participants received 4 weekly APA treatments. Each week, after seed placement by the first author (CHY), participants were instructed to evenly press the tape and seed covering each ear point without rubbing (to avoid skin irritation and infection at the acupuncture point) for 3 minutes, 3 times daily (9 min total), even if they did not experience pain. A 2-second pause occurred between each of the 2 pressings. The optimal pressure was achieved when the participant felt localized tingling or mild discomfort. The tape and seeds remained on the ear points for 5 days. Participants were instructed to remove both at the end of the fifth day and let the ears rest for 2 days to regain their sensitivity.
Measures
Study outcomes include self-report surveys, QST, and fMRI. fMRI scans were collected at pre-, immediate-, and post- 4-week APA. Self-report and QST were collected at pre- and post- 4-week APA.
Self-report Survey
CIN symptoms. CIN symptoms were used to assess 4 common CIN symptoms (ie, pain, numbness, tingling, and stiffness).40 Participants were asked to rate their CIN symptoms on their lower extremities location (ie, toes, soles, feet, and ankles). Scores for each symptom severity and body locations ranged from 0 to 10. The average of the total score from each location was used, with higher scores indicating greater symptom intensity.
- Physical function. Physical functioning was measured by the following questionnaires:
- The subscale of Physical Dysfunction from The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)42 version 3.1 was used to assess physical function (17 items). Each item scored from 1 “none” to 5 “extreme difficulty.” WOMAC has been widely used to assess nonosteoarthritic pain, particularly for lower extremity function with demonstrated psychometrics.43
- BPI-CIN-Symptoms-Interferences40 (wording was revised from BPI-sf) was used to measure the impact of CIN on physical function for the following 7 items: general activity, mood, walking ability, normal work, relations with other persons, sleep, and enjoyment of life. Participants rated these items from 0, “does not interfere” to 10, “completely interferes.” Their scores were subsequently summed to determine the level of interference, ranging from 0 to 70.
Quality of Life. PROMIS-29 v2.0 was used to measure 5 subscales of health QOL on each domain: anxiety, depression, fatigue, sleep disturbance, and satisfaction with participation in social roles. Each subscale includes 4 items of a 5-point Likert scale plus a pain intensity scale using a 0 to 10 numeric rating item. PROMIS-29 has established reliability and validity44,45 and is widely used in the United States. Scores were summed to determine each domain’s health QOL, ranging from 0 to 20. The higher the score on a subscale, the better the quality of each domain. PROMIS-29 has shown satisfactory reliability (Cronbach’s alpha coefficients ranged from .86 in sleep disturbance to .96 fatigue).
Quantitative Sensory Testing
The QST battery included the following: (1) mechanical QST (ie, mechanical detection threshold and mechanical pain threshold), (2) cold pressor testing and conditioned pain modulation, and (3) pressure pain threshold. Detailed QST procedures were described in our previous publication.41
fMRI Data Acquisition
All MRIs were acquired on a 3.0 Tesla Siemens Prisma System (Siemens Medical Solutions, Erlangen, Germany) using a 12-channel head matrix coil. Structural images were obtained utilizing a 3-dimenstional T1 sequence (TR = 2300 ms, TI = 900 ms, TE =3.5 ms, flip angle = 9 degrees, field of view = 24 cm, acquisition matrix 256 × 256 × 176, slice thickness =1 mm. Functional T2*-weighted blood-oxygen-level-dependent (BOLD) images for resting state fMRI (rs-fMRI) were acquired using 2-dimensional gradient echo echo-planar imaging (TR = 2000 ms, TE = 30 ms, flip angle 90 degrees, field of view 24 cm, acquisition matrix 64 × 64 × 33, slice thickness = 4 mm, slice gap = 1 mm, interleaved acquisition). Moreover, 300 volumes (10 min) of rs-fMRI data were acquired. Participants were instructed to stay as motionless as possible, not think of anything in particular, and keep eyes open for the duration of the scan.
Procedure
After consent was obtained and all of the survey and QST data were collected (hereafter, pre-), participants received the first fMRI scan (10 min; pre-). After completion of the first scan, participants were removed from the scanner but remained on the table. The first author (CHY) instructed the participants to keep their head still and administered the APA to the participants on ear points corresponding to the lower extremity points (including toes, soles, and feet; see Yeh et al.40; Figure 1). The whole procedure took up to 10 minutes, including seed placements and pressing. Then, participants were returned into the scanner for a repeated fMRI scan (immediate-APA; 10 min). The whole procedure took up to 1 hour. After completion of both scans, seeds/tapes were removed and reapplied according to the CIN treatment protocol. After 4 weeks of APA, participants received another fMRI scan (10 min; post-).
Figure 1.
Group Functional Connectivity Matrices of APA Effects. Connectivity matrices of (A) pre-APA, (B) immediate-APA, and (C) post-APA. Z score normalized mean connectivity is shown within (diagonal of matrix) and between (off-diagonal of matrix) networks. Positive values (toward red) denote positive correlations, negative values (toward blue) denote negative correlations, and values near zero (green) denote absence of correlation. The black boxes outline connectivity of the ECN versus SAL in the 3 different conditions, demonstrating progressive negative connectivity from A to C. ATT, attention network; AUD, auditory network; BGN, basal ganglia network; DMN, default mode network; ECN, executive control network; LAN, language network; LIM, limbic network; SAL, salience network; SMN, sensorimotor network; THA, thalamic network; VIZ, visual network.
fMRI Data Analyses
Descriptive statistics were conducted for the demographic characteristics of the participants and clinical outcomes of APA effects on CIN. To examine the study outcomes and consideration of small sample size, the Wilcoxon signed-rank test, nonparametric statistics, was used to compare the differences of mean ranks on the pair outcomes.
Imaging data were processed using Statistical Parametric Mapping (SPM) version 12 (Wellcome Department of Imaging Neuroscience, University College, London, UK) and custom MATLAB (Mathworks, Natick Massachusetts) scripts. fMRI underwent slice timing correction and motion correction. The ArtRepair toolbox was then used to detect outlier fMRI volumes (based on global signal mean intensity, affected by scan to scan motion) which were tagged for subsequent removal from analysis.46 The fMRI data were linearly normalized to a Montreal Neurological Institute (MNI) 152 brain template following coregistration to T1-weighted images. Images were detrended, and physiological nuisance data were removed from the fMRI utilizing the CompCor method.47 Bandpass filtering was performed at 0.01 to 0.1 Hz and then spatial smoothing performed with a 6 mm full width at half maximum Gaussian kernel. The outlier volumes tagged by ArtRepair were then removed.
For this exploratory analysis of functional connectivity, 11 intrinsic brain networks were analyzed: the attention network (ATT), default mode network (DMN), executive control network (ECN), salience network (SAL), auditory network (AUD), basal ganglia network (BGN), language network (LAN), limbic network (LIM), sensorimotor network (SMN), thalamic network (THA), and the visual network (VIZ). Seed regions of interest (ROIs) were previously generated from a cohort of healthy volunteers48 as per Sair et al.49 There were 79 total 6 mm in radius seed ROIs comprising the 11 different networks. For each ROI, the eigenvariate of the time course was extracted across the fMRI acquisition. Pearson correlation coefficients were calculated for each ROI pair. Within-network connectivity was calculated for each intrinsic network as the mean of the correlation of all seeds within the network. Between-network connectivity was calculated as the mean correlation between all ROIs in network pairs. Correlations were z-score normalized to allow for between-subject comparison.
Results
Characteristics of the Participants
Eight participants were enrolled in the study (6 females and 2 males; 2 Africa-Americans and 6 Caucasians). The mean age of the participants was 57.15 years (standard deviation [SD] = 9.74, range, 40–70). Cancer diagnosis included breast cancer (n = 3), leukemia (n = 1), non-Hodgkin lymphoma (n = 1), endometrial cancer (n = 1), and colon cancer (n = 1). Average body mass index was 27.68 (SD = 5.68, range, 21.97–38.57). Two participants dropped out due to the time strain of study appointments (n = 1) and kidney transplant (n = 1).
Self-report of Clinical Outcomes
Table 1 presents the basic descriptive CIN findings (ie, mean and standard deviation) and percent change from pre-APA. After 4 weeks of APA, participants had reported clinical significant improvements (ie, ≥30%) in CIN symptoms (including pain, numbness, tingling, and stiffness, measured by the CIN symptoms), and lower extremity stiffness (32%). The Wilcoxon signed-ranks test indicated that post-APA ranks were statistically significantly lower than pre-APA on pain intensity (z = −2.02, P = .04) and numbness (z = −2.01, P = .04) in the feet (z = −2.03, P = .04; see Table 1 for details).
Table 1.
Impact of Auricular Point Acupressure on Clinical Outcomes of Chemotherapy-Induced Neuropathy.
| Pre-APA (n = 6) | Post-APA (n = 6) | Change | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | % | |
| CIN | |||
| Pain | 5.56 ± 2.44 | 2.00 ± 1.90 | −64* |
| Numbness | 4.20 ± 3.26 | 2.55 ± 2.77 | −39* |
| Tingling | 4.65 ± 3.47 | 2.40 ± 1.91 | −48 |
| Stiffness | 3.60 ± 2.55 | 1.90 ± 1.46 | −47 |
| Physical function | |||
| CIN inference | |||
| Function (WOMAC) | 21.60 ± 13.07 | 17.80 ± 10.92 | −18 |
| QuickDash | 35.00 ± 16.82 | 25.45 ± 8.10 | −27 |
| QST parameter | |||
| MDT plantar | 3.21 ± 0.93 | 2.96 ± 0.92 | −8 |
| MDT dorsal | 3.05 ± 1.14 | 2.10 ± 0.96 | −31 |
| MDT total foot | 3.18 ± 0.97 | 2.77 ± 0.92 | −13 |
| MPT total hand | 2.30 ± 1.37 | 3.13 ± 2.28 | 44 |
| CPM/PPT | 16.39 ± 8.55 | 18.52 ± 11.00 | 13 |
Abbreviations: CIN, chemotherapy-induced neuropathy; CPM, conditional pain modulation; MDT, mechanical detection threshold; MPT, mechanical pain threshold; PPT, pressure pain threshold; QST, quantitative sensory test; QuickDash, Disabilities of the Arm, Shoulder and Hand Score; SD, standard deviation; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
*P < .05.
Pain Sensitivity and Threshold Outcome
Mechanical detection threshold (MDT)
Compared to pre-APA, the mean scores of the monofilament for each tested site on the feet decreased, indicating a reduction in numbness in the participant’s feet (Table 1). For the plantar side of the foot, the mean percentage change was 8%, whereas the dorsal side of the food showed the largest decrease of 31% post-APA. In general, the effects of APA on the entire foot resulted in a mean percentage change of 13%.
Mechanical pain threshold (MPT)
The suprathreshold pinprick stimuli mean scores for the total hand increased from pre-APA to post-APA, indicating increased pain threshold or reduced sensitivity (Table 1). After the 4-week APA, the mean percentage change score increased by 44% for the entire hand compared to the pre-APA intervention. Total hand scores were calculated by calculating the average of all tested sites on the hand including index finger, thumb, middle finger, hypothenar, and dorsum.
Conditional pain modulation (CPM)
The pressure pain thresholds for thumb and trapezius increased by 13% post-APA while the participant underwent a series of cold pressor tasks. The change in pressure pain thresholds between cold pressor tasks indicated an increase in the pain threshold for the thumb and trapezius, meaning enhanced endogenous pain modulation (Table 1).
Resting-State Functional Connectivity Results
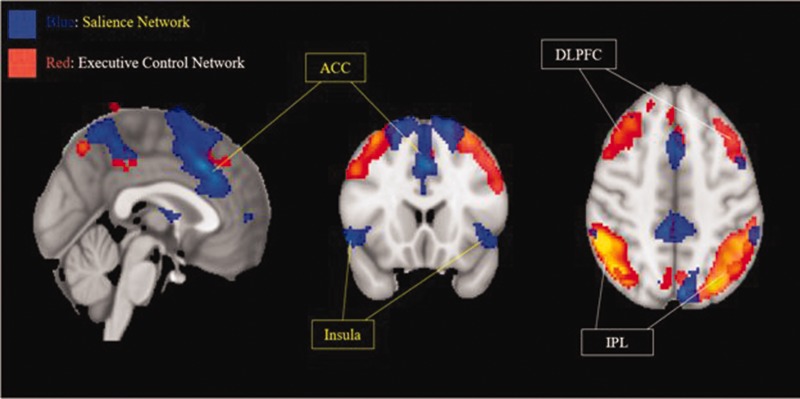
Figure 1 illustrates the group mean within-network and between-network connectivity across all network pairs for pre-, immediate-, and post- 4-week APA. For between-network connectivity, during pre-APA versus immediate-APA, the following pairs demonstrated a trend toward significance: SAL-BGN (Pearson correlation coefficients change from 0.41 [pre-] to −0.01 [immediate-], P = .06), LAN-BGN (Pearson correlation coefficients change from 0.12 [pre-] to −0.17 [immediate-], P = .07). During immediate-APA versus post-APA, the pair of SAL-BGN demonstrated trend toward significance (Pearson correlation coefficients change from −0.01 [immediate-] to 0.05 [post-], P = .07). During pre- versus post-APA, the pair of SAL-BGN demonstrated decreased trend toward significance from 0.41 (pre-) to 0.05 (post-) (P = .07). For within network connectivity, the Pearson correlation coefficient of ECN (pre- = 0.82; immediate- = 0.94; and post- = 1.12, P = .82 [pre- vs immediate-] or 0.09 [pre- vs post-]) and SAL (pre- =1.48; immediate- = 1.52; and post- = 1.65, P = .5 [pre- vs immediate-] or .09 [pre- vs immediate-]) demonstrated a trend toward increased connectivity between pre-APA and immediate-APA as well as continued increased connectivity after 4 weeks of APA. However, BGE demonstrated a trend toward decreased connectivity after the APA (pre- = 1.45; immediate- = 1.20; and post- = 1.20, P = .4 [pre- vs immediate-] or .99 [pre- vs post-]). Figure 2 provides a representative depiction of the anterior cingulate cortex and insula of the SAL and the dorsolateral prefrontal cortex and inferior parietal lobule of the ECN from a group independent component analysis of the resting state fMRI post-APA treatment.
Figure 2.
Representative Depiction of the Executive Control Network (Red) and the Salience Network (Blue), Generated From Group Independent Component Analysis of the Resting State fMRI Data. Networks are overlaid onto a standard T1-weighted MNI template brain (MNI coordinates x: 0 y: 18 z: 46). ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule.
Discussion
We report a comprehensive and novel investigation of APA effects on CIN using fMRI to examine the dynamic functional connectivity change patterns in cancer patients with CIN. After 4 weeks of APA, patients who showed a decrease in CIN-related symptoms concurrently demonstrated an increase in sensation and pain thresholds. The functional connectivity change patterns revealed (1) a decreased activity within-network of BGN and between-network of SAL and BGN and (2) increased activity within-network of ECN and SAL, respectively. Although the functional connectivity results did not reach statistical significance, imaging of the SAL, ECN, and BGN does provide an objective method for the assessment of APA-induced CIN relief. These findings suggested that CIN improvement is associated with network connectivity between the BGN and SAL. The alteration of brain activity in different networks following APA treatment suggests that a correlation may exist between APA and alterations in brain functional connectivity.
Based on the fMRI findings, we speculate that APA-induced CIN relief may result from direct or indirect effects. For direct effects, the stimulation of ear points may take advantage of trigeminal and vagus nerve innervation distributed on the ear. The stimulation of these cranial nerves may elicit changes in brain connectivity and thereby alter pain processing in the central nervous system; for example, the changes seen in SAL, ECN, and BGN after APA. Given the role of SAL,50–53 ECN,54,55 and BGN56,57 in the central pain processing pathway, the possible modification of cortical activity and/or connectivity within these brain regions involved in cerebral top-down, inhibitory mechanisms of pain processing following APA reveals that APA can be an exciting and promising treatment for CIN.
For indirect effects, we recognize the intentional stimulation of ear points as a conscience behavior and speculate it consequently results in an altered awareness of pain, specifically through an interaction with SAL and ECN. Directing attention and cognitive behaviors (eg, stimulating auricular points) to respond to CIN with simultaneous SAL, ECN or BGN activity, cortical areas associate with affective-,58,59 attention-,60,61 and pain-related processing57,62 might explain CIN symptom reduction in APA. Possible normalization of brain activity in the salience, executive control, and BGNs following APA may provide a new direction for the treatment of CIN. The potential interaction between APA and these brain networks certainly requires further investigation, but it may also provide insight for future therapeutic targets for pharmacological approaches as well as behavioral or psychological interventions.
Given the small sample size of this pilot study and the relatively uncertain pathophysiology of CIN, it remains difficult to pin point a specific physiological mechanism for the observed analgesic effect of APA. Although our initial analysis did not reach statistical significance, our findings show promise in revealing the possibility of a relationship between APA-induced analgesic effects and the cortical pain processing pathway. A large clinical trial is needed to determine the specific neural pathway by which APA modulates CIN-related symptoms.
In summary, we find that APA can (1) reduce CIN symptoms40 and (2) improve pain sensitivity (evaluated by pinprick sensibility41 and Semmes-Weinstein monofilaments41). Our previous studies have shown that APA can modulate neurotransmitter levels by blocking cytokine production (decreased pro-inflammatory cytokines [IL-1β,27,63,64 IL-12,27 IL-6,27,64 and TNF-α63,64]) or inhibiting cytokine release (increased anti-inflammatory cytokines [IL-1327 and IL-463]). In conjunction with these findings, fMRI data suggest that APA may alter pain processing in the central nervous system through (1) top-down regulation of brain regions involved in sensory discrimination, the cognitive and affective dimensions of pain, and the regulation of nociceptive information to modulate CIN relief; and (2) bottom-up detection of a salient events by modulating A-δ and A-β sensitivity and/or directing the individual’s attention to respond to CIN (i.e., change of threat after 4 weeks of APA). CIN patients improve not only their CIN (self-reported data) but also their pain sensitivity (evaluated by pinprick sensibility on their hand and Semmes-Weinstein monofilaments on their foot). These findings indicate that APA may impact peripheral and central neuronal signaling by modulating nerve sensitivity via A-δ fibers (findings from pinprick test) and/or A-β fibers (findings from Semmes-Weinstein monofilaments). Larger randomized clinical trials with the consideration of the place effects are needed to confirm our hypotheses of APA on CIN relief.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the Johns Hopkins Discovery Award (to CHY).
ORCID iD
Chao Hsing Yeh https://orcid.org/0000-0001-5326-6660
References
- 1.Shah A, Hoffman EM, Mauermann MLet al. Incidence and disease burden of chemotherapy-induced peripheral neuropathy in a population-based cohort. J Neurol Neurosurg Psychiatry. 2018;89(6):636–641. [DOI] [PMC free article] [PubMed]
- 2.Park SB. Chemotherapy-induced peripheral neuropathy: highlighting unmet needs. J Neurol Neurosurg Psychiatry. 2018;89(6):558. [DOI] [PubMed] [Google Scholar]
- 3.Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 2017; 81(6):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seretny M, Currie GL, Sena ESet al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014; 155(12):2461–2470. [DOI] [PubMed] [Google Scholar]
- 5.Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 2014; 10(12):694–707. [DOI] [PubMed] [Google Scholar]
- 6.Hershman DL, Lacchetti C, Dworkin RHet al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014; 32(18):1941–1967. [DOI] [PubMed] [Google Scholar]
- 7.Smith E, Pang H, Cirrincione Cet al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA Int Med. 2013; 309(13):1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleckner IR, Kamen C, Gewandter JSet al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer. 2018; 26(4):1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005; 352(13):1324–1334. [DOI] [PubMed] [Google Scholar]
- 10.Gilron I, Tu D, Holden RR, Jackson AC, DuMerton-Shore D. Combination of morphine with nortriptyline for neuropathic pain. Pain. 2015; 156(8):1440–1448. [DOI] [PubMed] [Google Scholar]
- 11.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. 2016; 315(15):1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winters-Stone KM, Horak F, Jacobs PGet al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol. 2017; 35(23):2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother Res Pract. 2012; 2012:913848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addington J, Freimer M. Chemotherapy-induced peripheral neuropathy: an update on the current understanding. F1000Research. 2016;5:F1000 Faculty Rev-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krames ES. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med (Malden, Mass). 2014; 15(10):1669–1685. [DOI] [PubMed] [Google Scholar]
- 16.Cavaletti G, Fabbrica D, Minoia C, Frattola L, Tredici G. Carboplatin toxic effects on the peripheral nervous system of the rat. Ann Oncol. 1998; 9(4):443–447. [DOI] [PubMed] [Google Scholar]
- 17.Berta T, Qadri Y, Tan PH, Ji RR. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin Ther Targets. 2017; 21(7):695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nogier P. Handbook to Auriculotherapy. 1st ed. Moulins-les-Metz, France: Maisonneuve; 1981. [Google Scholar]
- 19.Nogier R. How did Paul Nogier establish the map of the ear? Med Acupunct. 2014; 26(2):76–83. [Google Scholar]
- 20.Nogier P. Points Reflexes Auricularis. Moulin Les-Metz, France: Maisonneuve SA; 1987. [Google Scholar]
- 21.Alimi D, Geissmann A, Gardeur D. Auricular acupuncture stimulation measured on functional magnetic resonance imaging. Med Acupunct. 2002; 13(2):18–21. [Google Scholar]
- 22.Romoli M, Allais G, Airola Get al. Ear acupuncture and fMRI: a pilot study for assessing the specificity of auricular points. Neurol Sci. 2014; 35 Suppl 1:189–193. [DOI] [PubMed] [Google Scholar]
- 23.Oleson T. Auriculotherapy Manual: Chinese and Western Systems of Ear Acupuncture. 4th ed Edinburgh, England: Churchill Livingstone, Elsevier; 2014. [Google Scholar]
- 24.Huang LC. Auricular Medicine: A Complete Manual of Auricular Diagnosis and Treatment. 1st ed. Orlando, FL: Auricular International Research & Training; 2005. [Google Scholar]
- 25.Yeh CH, Chiang YC, Hoffman Set al. Efficacy of auricular therapy for pain management: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2014;2014:934670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asher GN, Jonas DE, Coeytaux RRet al. Auriculotherapy for pain management: a systematic review and meta-analysis of randomized controlled trials. J Alternat Complement Med. 2011; 16(10):1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh CH, Lin WC, Suen LKPet al. Auricular point acupressure to manage arthralgia related to aromatase inhibitors in breast cancer survivors. Oncol Nurs Forum. 2017; 44(4):476–487. [DOI] [PubMed] [Google Scholar]
- 28.Yeh CH, Suen LKP, Chien LCet al. Day-to-day changes of auricular point acupressure to manage chronic low back pain: a 29-day randomized control study. Pain Med. 2015; 16(10):1857–1869. [DOI] [PubMed] [Google Scholar]
- 29.Yeh CH, Morone NE, Chien LCet al. Auricular point acupressure to manage chronic low back pain in older adults: a randomized controlled pilot study. Evid Based Complement Alternat Med. 2014; 2014:375173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh CH, Chien LC, Balaban Det al. A randomized clinical trial of auricular point acupressure for chronic low back pain: a feasibility study. Evid Based Complement Alternat Med. 2013; 2013:196978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh CH, Chien LC, Chiang YC, Huang LC. Auricular point acupressure for chronic low back pain: a feasibility study for 1-week treatment. Evid Based Complement Alternat Med. 2012; 2012:383257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh CH, Chien LC, Chiang YC, Suen L, Ren D. Analgesic effect of auricular point acupressure as an adjunct treatment for cancer patients with pain. Pain Manag Nurs. 2015; 16(3):285–293. [DOI] [PubMed] [Google Scholar]
- 33.Yeh CH, Chien LC, Lin WC, Bovbjerg DH, van Londen G. Pilot randomized controlled trial of auricular point acupressure to manage symptom clusters of pain, fatigue, and disturbed sleep in breast cancer patients. Cancer Nursing. 2016; 39(5):402–410. [DOI] [PubMed] [Google Scholar]
- 34.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. NeuroImage. 2008; 39(4):1666–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Spaeth RB, Retzepi K, Ott D, Kong J. Acupuncture modulates cortical thickness and functional connectivity in knee osteoarthritis patients. Sci Rep. 2014; 4:6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Zhang JH, Yi T, Tang WJ, Wang SW, Dong JC. Acupuncture treatment of chronic low back pain reverses an abnormal brain default mode network in correlation with clinical pain relief. Acupunct Med. 2014; 32(2):102–108. [DOI] [PubMed] [Google Scholar]
- 37.Lang PM, Stoer J, Schober GM, Audette JF, Irnich D. Bilateral acupuncture analgesia observed by quantitative sensory testing in healthy volunteers. Anesth Analg. 2010; 110(5):1448–1456. [DOI] [PubMed] [Google Scholar]
- 38.Cai RL, Shen GM, Wang H, Guan YY. Brain functional connectivity network studies of acupuncture: a systematic review on resting-state fMRI. J Integr Med. 2018; 16(1):26–33. [DOI] [PubMed] [Google Scholar]
- 39.Huang W, Pach D, Napadow Vet al. Characterizing acupuncture stimuli using brain imaging with FMRI—a systematic review and meta-analysis of the literature. PLoS One. 2012; 7(4):e32960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh CH, Lukkahatai N, Campbell Cet al. Preliminary effectiveness of auricular point acupressure on chemotherapy-induced neuropathy: part 1 self-reported outcomes. Pain Mang Nurs. 2019; 20(6):614–622. [DOI] [PubMed] [Google Scholar]
- 41.Yeh CH, Lukkahatai N, Campbell Cet al. Preliminary effectiveness of auricular point acupressure on chemotherapy-induced neuropathy: part 2 laboratory-assessed and objective outcomes. Pain Manag Nurs. 2019; 20(6):623–632. [DOI] [PubMed] [Google Scholar]
- 42.Bellamy N. WOMAC Osteoarthritis Index User Guide. Version V; Brisbane, Australia; 2002. [Google Scholar]
- 43.Pinsker E, Inrig T, Daniels TR, Warmington K, Beaton DE. Reliability and validity of 6 measures of pain, function, and disability for ankle arthroplasty and arthrodesis. Foot Ankle Int. 2015; 36(6):617–625. [DOI] [PubMed] [Google Scholar]
- 44.Hays RD, Spritzer KL, Schalet BD, Cella D. PROMIS((R))-29 v2.0 profile physical and mental health summary scores. Qual Life Res. 2018; 27(7):1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hays RD, Revicki DA, Feeny D, Fayers P, Spritzer KL, Cella D. Using linear equating to map PROMIS((R)) global health items and the PROMIS-29 V2.0 profile measure to the health utilities index mark 3. Pharmaco Economics. 2016; 34(10):1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazaika P, Hoeft FH, Glover GL, Reiss A. Methods and software for fMRI analysis of clinical subjects. Neuroimage. 2009; 47(Suppl 1):S58. [Google Scholar]
- 47.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007; 37(1):90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Landman BA, Huang AJ, Gifford Aet al. Multi-parametric neuroimaging reproducibility: a 3-T resource study. NeuroImage. 2011; 54(4):2854–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sair HI, Hannawi Y, Li Set al. Early functional connectome integrity and 1-year recovery in comatose survivors of cardiac arrest. Radiology. 2018; 287(1):247–255. [DOI] [PubMed] [Google Scholar]
- 50.Starr CJ, Sawaki L, Wittenberg GFet al. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci. 2009; 29(9):2684–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci USA. 2005; 102(36):12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003; 126(Pt 5):1079–1091. [DOI] [PubMed] [Google Scholar]
- 53.Mufson EJ, Mesulam MM. Insula of the old world monkey. II: afferent cortical input and comments on the claustrum. J Comparat Neurol. 1982; 212(1):23–37. [DOI] [PubMed] [Google Scholar]
- 54.Brown CA, Huneke NT, Jones AK. Pain Syndromes. Brain Mapping. Amsterdam, the Netherlands: Elsevier Inc; 2015:1135–1141. [Google Scholar]
- 55.Morton DL, Sandhu JS, Jones AK. Brain imaging of pain: state of the art. J Pain Res. 2016; 9:613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chudler EH, Dong WK. The role of the basal ganglia in nociception and pain. Pain. 1995; 60(1):3–38. [DOI] [PubMed] [Google Scholar]
- 57.Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia–insights gained through human functional imaging. Mol Pain. 2010; 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levens SM, Phelps EA. Insula and orbital frontal cortex activity underlying emotion interference resolution in working memory. J Cognit Neurosci. 2010; 22(12):2790–2803. [DOI] [PubMed] [Google Scholar]
- 59.Menon V. Salience network In: Toga AW, ed. Brain Mapping: An Encyclopedic Reference. Vol 2 Cambridge, MA: Academic Press: Elsevier; 2015:597–611. [Google Scholar]
- 60.Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J Cognit Neurosci. 2006; 18(5):766–780. [DOI] [PubMed] [Google Scholar]
- 61.Voytek B, Davis M, Yago E, Barcelo F, Vogel EK, Knight RT. Dynamic neuroplasticity after human prefrontal cortex damage. Neuron. 2010; 68(3):401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freund W, Klug R, Weber F, Stuber G, Schmitz B, Wunderlich AP. Perception and suppression of thermally induced pain: a fMRI study. Somatosens Motor Res. 2009; 26(1):1–10. [DOI] [PubMed] [Google Scholar]
- 63.Lin WC, Yeh CH, Chien LC, Morone NE, Glick RM, Albers KM. The anti-inflammatory actions of auricular point acupressure for chronic low back pain. Evid Based Complement Alternat Med. 2015;2015:103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeh CH, Lukkahataia N, Campbell Cet al. The preliminary effectiveness of auricular point acupressure on chemotherapy-induced neuropathy: part 2 laboratory-assessed and objective outcomes. Pain Manag Nurs. 2019; 20(6):623–632. [DOI] [PubMed] [Google Scholar]