Abstract
Thrombocytopenia (less than 100 × 109/L platelets) presents in around one quarter of patients with nonalcoholic fatty liver disease (NAFLD), the hepatic component of insulin resistance (IR). It is unknown whether IR, by itself, associates with thrombocytopenia. Persons with NAFLD and/or IR were prospectively accrued in the study after February 2018. Insulin resistance was defined by assessing α hydroxybutyrate, lynoleoyl glycerolphosphocoline, oleic acid, and insulin (Quantose IR), whereas the presence of NAFLD was defined by serologic determinations (Fibromax) and liver transient elastography (Fibroscan). In 78 patients with NAFLD, thrombocytopenia was identified in 22 (28%), whereas in 19 persons with IR, 14 (73%) were found to have NAFLD. In persons with IR + NAFLD, thrombocytopenia presented in 9 (64%). In the subset of patients with IR, the prevalence of thrombocytopenia was 52%. There was only 1 patient with IR/without NAFLD who displayed thrombocytopenia. Significant statistical association between NAFLD and thrombocytopenia was found (odds ratio [OR]: = 13, confidence interval [CI]: 1.5-162, P = .05), whereas there was no association between IR and thrombocytopenia (OR = 0.38, CI: 0.06-2.3, P = .61). Insulin resistance, by itself, was not found to be associated with diminished platelet counts. The presence of NAFLD, one of the consequences of IR, seems to be required to lead into thrombocytopenia.
Keywords: NAFLD, platelets, thrombocytopenia, liver, insulin resistance
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver injury worldwide, being the hepatic component of insulin resistance (IR).1 It encompasses a wide spectrum of liver disorders ranging from steatosis through steatohepatitis to liver cirrhosis. Thrombocytopenia (less than 100 × 109/L platelets) has been described in around 25% of cases of NAFLD.2–6 The definite diagnosis of NAFLD is ideally done by means a liver biopsy, an invasive procedure not free from potential complications; however, several noninvasive diagnostic strategies have been employed as diagnostic alternatives to the biopsy, each with different sensitivities and accuracies.7–11 Several studies have demonstrated the predictive value and a better benefit to risk ratio than biopsy of combinations of simple serum biochemical markers (Fibromax)7 and/or liver transient elastography (TE; Fibroscan).8–11 In previous studies, we have shown that NAFLD, as defined by Fibromax5 and/or Fibroscan,6 is associated with thrombocytopenia in the absence of overt liver cirrhosis. Since NAFLD may be a consequence of IR, we decided to explore if this metabolic condition, by itself, could be associated with a low platelet count, excluding other conditions leading into thrombocytopenia.
Material and Methods
Patients
Individuals were prospectively included in the study after February 2018. Insulin resistance was defined by Quantose IR,12 whereas NAFLD was defined by Fibroscan and/or Fibromax.7–11 The study was approved by the Ethics Committee of the Clinica Ruiz and informed consent was obtained from all the patients. Individuals with autoimmune thrombocytopenia, autoimmune diseases, hepatitis B, hepatitis C, chronic cholestatic diseases, overt liver cirrhosis, or alcoholism were excluded from the analysis.
Insulin Resistance
The Quantose IR index was defined by a single fasting blood sample assessing α hydroxybutyrate (α-HB), lynoleoyl glycerolphosphocoline (L-GPC), oleic acid, and insulin.12 All α-HB, L-GPC, and oleic acid were measured by high-performance liquid chromatography–mass spectrometry, whereas insulin was measured by chemiluminescent microparticles immunoassay.12 The Quantose IR index ranges between 0 and 120 points, and IR was defined by its value above 63 points.12
Fibroscan
To asses TE, a Fibroscan 502 Touch (Echosens, Paris, France) instrument was employed, with the XL probe.10,11 The ultrasonic controlled attenuation parameter (CAP) defines steatosis when the measurement is above 200 dB/mW (S1-S3), whereas liver stiffness measurement (LSM) defines fibrosis when being above 7.5 kPa (F2-F4).10 In this study, patients with a CAP value above 200 dB/mW, coupled with an LSM below 7.5 kPa, were defined as individuals with liver steatosis.10,11
Fibromax
Alpha-2 macroglobulin, haptoglobin, apolipoprotein A, bilirubin, γ glutamyl transpeptidase, alanine aminotransferase, aspartate aminotransferase, glucose, cholesterol, and triglycerides were measured in all patients; these biochemical markers were analyzed in various ways to define: FibroTest for the quantitative assessment of fibrosis, SteatoTest for the quantitative assessment of steatosis, ActiTest for the quantitative assessment of necroinflammatory activity in chronic viral hepatitis, and NashTest for the categorical diagnosis of nonalcoholic steatohepatitis.7–9 Patients with a score above 50% in either SteatoTest or NashTest, coupled with a score below 50% in the FibroTest, were defined as individuals with NAFLD.7–9
Aspartate Aminotransferase to Platelet Index
Aspartate aminotransferase to platelet index was calculated in all the patients included in the study.
Results
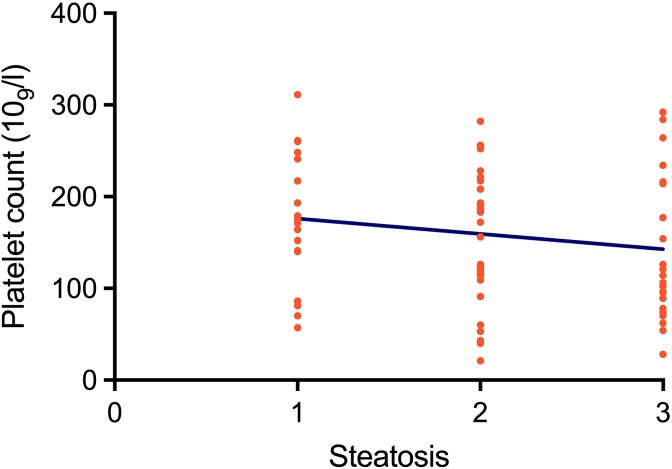
In 78 consecutive patients with NAFLD, thrombocytopenia (less than 100 × 109/L platelets) was identified in 22 (28%) patients. On the other hand, in 19 persons with IR, 14 (73%) were found to have NAFLD. In those with IR + NAFLD, thrombocytopenia presented in 9 (64%); accordingly, the relative risk of having thrombocytopenia in patients with NAFLD is 2.3 times higher in the presence of IR (P = .0085). In the subset of patients with IR, the incidence of thrombocytopenia was 52%; there was only 1 patient with IR without NAFLD who displayed thrombocytopenia; the remaining 4 persons with IR and no NAFLD had normal platelet counts. Significant statistical association between NAFLD and thrombocytopenia was found (odds ratio [OR]: = 13, confidence interval [CI]: 1.5-162, P = .05), whereas there was no association between IR and thrombocytopenia (OR = 0.38, CI: 0.06-2.3, P = .61). Table 1 depicts the salient features of the patients with IR + NAFLD + thrombocytopenia. The salient data of persons with IR (n = 19), IR + NAFLD (n = 14), and IR + NAFLD + thrombocytopenia (n = 9) are given in Table 2. The median platelet counts in these groups were 245, 219, and 109, respectively (P < .0001). There was an association of the IR score with NAFLD and thrombocytopenia: It was higher in persons with IR + NAFLD + thrombocytopenia than in those with IR + NAFLD. Figure 1 depicts the association between the degree of fatty infiltration of the liver and the platelet count in the group of 78 patients with NAFLD, with or without IR.
Table 1.
Salient Features of the Patients With Nonalcoholic Fatty Liver Disease, Insulin Resistance, and Thrombocytopenia.
| Patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Reference Values | |
| Sex, M/F | F | F | M | F | M | M | F | M | F | |
| Age, years | 44 | 19 | 55 | 50 | 65 | 54 | 66 | 66 | 70 | |
| BMI | 28.5 | 27.9 | 28.1 | 23.1 | 28.1 | 28.5 | 27.6 | 27.7 | 23.4 | 18.5-24.9 |
| Fibroscan steatosis | S3 | S3 | S3 | S2 | S2 | S3 | S3 | S3 | S3 | S0 |
| APRI | 0.6 | 3.1 | 0.6 | 1.3 | 0.7 | 1.6 | 0.6 | 1.6 | 0.9 | <1.5 |
| Alpha2M, g/L | 1.42 | 1.56 | 2.42 | 2.35 | 3.2 | 4.22 | 6.35 | 1.64 | 1.49 | 1.3-3 |
| Haptoglobin, g/L | 1.59 | 1.57 | 1.51 | 133 | 0.51 | 3.41 | 2.59 | 1.06 | 0.79 | 0.3-2 |
| Apo A1, g/L | 1.57 | 1.19 | 1.36 | 1.53 | 1.56 | 1.23 | 1.6 | 1.54 | 1.56 | 1.23-2.15 |
| Bilirubin, mg/dL | 10.26 | 0.4 | 9.41 | 0.83 | 15.05 | 3.08 | 13.34 | 11 | 21.03 | 0.2-1.2 |
| γ GT, IU | 165 | 58 | 17 | 43 | 24 | 77 | 23 | 124 | 114 | 0-42 |
| ALT, IU | 67 | 237 | 40.5 | 25.7 | 48.5 | 119.6 | 16 | 101 | 60.7 | 7-56 |
| AST, IU | 36 | 116 | 29.4 | 22.3 | 40.5 | 84.2 | 19.9 | 82 | 41 | 5-40 |
| Glucose, mg/dL | 221.6 | 92 | 90.8 | 102.3 | 108 | 119.4 | 383.8 | 118.9 | 119 | 70-100 |
| Triglycerides, mg/dL | 305.4 | 183 | 72.8 | 564.5 | 163.4 | 215.2 | 261.1 | 276.3 | 240.5 | <150 |
| Cholesterol, mg/dL | 199.9 | 236 | 144.6 | 205 | 235 | 331 | 288 | 204.2 | 144 | <200 |
| Quantose IR | 97 | 87 | 83 | 75 | 83 | 104 | 67 | 82 | 89 | <63 |
| CBC | ||||||||||
| Hb, g/dL | 14.4 | 14 | 16.7 | 14 | 17 | 15.8 | 12.4 | 15.2 | 13.5 | 12.5-15.5 |
| Htc, % | 43.3 | 42.1 | 47.9 | 40.3 | 52 | 46.2 | 38.1 | 44.8 | 38.7 | 46-56 |
| WBC, ×109/L | 7.6 | 7.2 | 6.8 | 10.8 | 8.1 | 5.6 | 8.3 | 5.6 | 7.6 | 4-12 |
| Platelets, ×109/L | 137 | 93 | 119 | 43 | 143 | 131 | 89 | 123 | 106 | 150-450 |
| MPV, fL | 9.2 | 9.3 | 8.4 | 9.2 | 9.1 | 10.5 | 9.7 | 9.9 | 9.8 | 7.4-10.4 |
Abbreviations: Alpha2M, α-2 macroglobulin; ALT, alanine transaminase; Apo A1, apolipoprotein A1; APRI, AST to platelets ratio index; AST, aspartate transaminase; BMI, body mass index; γ GT, γ-glutamyl transpeptidase; F, female; Hb, hemoglobin; Htc, hematocrit; M, male; MPV, mean platelet volume; WBC, white blood cell count.
Table 2.
Some Features of Patients With Insulin Resistance (IR) Nonalcoholic Fatty Liver Disease (NAFLD) and Thrombocytopenia.
| IR, n = 19 | IR + NAFLD, n = 14 | IR + NAFLD + Thrombocytopenia, n = 9 | P | |
|---|---|---|---|---|
| Age (SD) | 35.5 (20.35) | 56.2 (5.6) | 54.3 (15.8) | .103 |
| BMI (SD) | 27.1 (6.6) | 28 (2.7) | 27 (2.1) | .877 |
| Quantose IR points (SD) | 76.5 (11.03) | 78.2 (2.5) | 85.2 (10.9) | .246 |
| Fibroscan score (SD) | 0 (0) | 2 (1) | 2.7 (0.4) | .068 |
| Fibromax score (SD) | 0 (0) | 1.2 (0.4) | 1.3 (0.5) | .620 |
| Platelet count ×109/L (SD) | 245.7 (66.7) | 219.8 (34) | 109.3 (31.1) | <.0001a |
Abbreviations: BMI, body mass index; SD, standard deviation.
aStatistical significance.
Figure 1.
Association between the degree of fatty infiltration of the liver as defined by Fibromax and the platelet count expressed in platelets ×109/L (r = −0.15, P = .18, CI 95%).
Discussion
Nonalcoholic fatty liver disease is considered to be the hepatic component of the metabolic syndrome as its features are similar to those of metabolic disorders such as obesity, inflammation, IR, and type 2 diabetes mellitus (T2DM).13 Insulin resistance is one of the hallmarks of NAFLD, being pivotal in the pathogenesis of the disease as associated with obesity and being an early important factor in the development of T2DM, which may be present for years before the emergence of any changes in glycemic control.13 Several lines of evidence suggest that increased oxidative stress and changes in several molecular factors, including adipokines, chemokines, and pro-inflammatory or anti-inflammatory cytokines, are mainly involved in the development and progression of NAFLD.1,13
We have previously shown that persons with NAFLD, as defined by Fibromax5 and/or Fibroscan,6 may have diminished platelet counts in the absence of liver cirrhosis, an association that had been suggested previously.3,4 The salient features of the NAFLD-associated thrombocytopenia are (1) it presents in around one quarter of patients; (2) it is associated with overweight; (3) it is usually mild, above 40 × 109/L; (4) it is not associated with mucocutaneous bleeding; and (5) it does not require treatment. A deficiency of thyroid peroxidase (TPO) has been mentioned as a possible cause of the thrombocytopenia of individuals with NAFLD3–6 and there is information about the usefulness of TPO mimetics in the treatment of several liver diseases. It is thus possible that TPO agonists could be useful in the treatment of the thrombocytopenia of NAFLD, when necessary.4–6
Since IR may lead to NAFLD, we decided to analyze if IR, by itself, could be associated with diminished platelets counts. We found a significant association between NAFLD and thrombocytopenia (OR = 13, CI: 1.5-162, P = .05), whereas there was no association between IR and thrombocytopenia (OR = 0.38, CI: 0.06-2.3, P = .61). Accordingly, our findings suggest that the liver damage secondary to the IR is needed to induce the thrombocytopenia. In addition, we found a relationship between the degree of steatosis and the platelet count in the patients with NAFLD, therefore, the more severe the steatosis, the lower the platelet count (r = −0.15, P = .18, 95% CI = 0.37 to 0.05), see Figure 1).
In summary, we have confirmed our previous observations about thrombocytopenia being present in around one quarter (24% in our previous paper6 and 28% in this one) of patients with NAFLD. In addition, we have shown that the liver damage is needed in the setting of IR to produce the association with thrombocytopenia and that the degree of thrombocytopenia is related to the degree of the fatty infiltration of the hepatic tissue. Further studies are needed to explain the mechanisms underlying the development of thrombocytopenia in the setting of NAFLD.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Guillermo J. Ruiz-Argüelles  https://orcid.org/0000-0002-9335-0653
https://orcid.org/0000-0002-9335-0653
References
- 1. Ibrahim MA, Kelleni M, Geddawy A. Nonalcoholic fatty liver disease: current and potential therapies. Life Sci. 2013;92(2):114–118. [DOI] [PubMed] [Google Scholar]
- 2. Mawatari H, Yoneda M, Kirikoshi H, Maeda S, Nakajima A, Saito S. Thrombocytopenia is more severe in patients with chronic hepatitis C than in patients with nonalcoholic fatty liver disease. J Gastroenterol. 2012;47(5):606–607. [DOI] [PubMed] [Google Scholar]
- 3. Dasanu CA, Lamana S, Trikudanathan G. Thrombocytopenia, NAFLD, and metabolic syndrome: is there a link?. South Med J. 2010;103(10):1071. [DOI] [PubMed] [Google Scholar]
- 4. Dasanu CA, Lamana S, Trikudanathan G. Thrombocytopenia in NAFLD: is thrombopoietin involved?. South Med J. 2010;103(12):1278–1279. [DOI] [PubMed] [Google Scholar]
- 5. Ruiz-Argüelles GJ, Velázquez-Sanchez-de-Cima S, Zamora-Ortiz G, Hernández-Reyes J, Ruiz-Delgado GJ. Nonalcoholic fatty liver disease may cause thrombocytopenia. Acta Haematol. 2014;132(2):159–162. [DOI] [PubMed] [Google Scholar]
- 6. Olivares-Gazca JC, Núñez-Cortés AK, Méndez-Huerta MA, Cantero-Fortiz Y, Orea-Martínez JG, Ruiz-Argüelles GJ. More on the thrombocytopenia of the non-alcoholic fatty liver disease. Hematology. 2017;22(5):316–319. [DOI] [PubMed] [Google Scholar]
- 7. Morra R, Munteanu M, Imbert-Bismut F, Messous D, Ratziu V, Poynard T. FibroMAX: towards a new universal biomarker of liver disease?. Expert Rev Mol Diagn. 2007;7(5):481–490. [DOI] [PubMed] [Google Scholar]
- 8. Festi D, Schiumerini R, Marzi L, et al. Review article: the diagnosis of non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;37(4):392–400. [DOI] [PubMed] [Google Scholar]
- 9. Kaswala DH, Lai M, Afdhal NH. Fibrosis assessment in nonalcoholic fatty liver disease (NAFLD) in 2016. Dig Dis Sci. 2016;61(5):1356–1364. [DOI] [PubMed] [Google Scholar]
- 10. Lupsor-Platon M, Badea R. Noninvasive assessment of alcoholic liver disease using unidimensional transient elastography (Fibroscan®). World J Gastroenterol. 2015;21(42):11914–11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong LH. Transient elastography: kill two birds with one stone. World J Hepatol. 2013;5(5):264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cobb J, Gall W, Adam KP, et al. A novel fasting blood test for insulin resistance and prediabetes. J Diabetes Sci Technol. 2013;7(1):100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. 2017;9(4):387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]