Abstract
Purpose:
The aim of the study was to exploit the feasibility of thermoluminescent dosimeters (TLDs) in radiation therapy techniques in which high dose per fraction is involved.
Methods:
Dose–response of TLD-100 (LiF: Mg, Ti) was investigated in both 6-MV photon and 6-MeV electron beams. The element correction factor (ECF) generation method was applied to check the variability of the TLDs response. Two batches of 50 TLDs were divided into groups and exposed in the dose range 0 to 30 Gy. Regression analysis was performed with both linear and quadratic models. For each irradiation beam, the calibration curves were obtained in 3 dose range 0 to 8 Gy, 0 to 10 Gy, and 0 to 30 Gy. The best-fitting model was assessed by the Akaike Information Criterion test.
Results:
The ECF process resulted a useful tool to reduce the coefficients of variation from original values higher than 5% to about 3.5%, for all the batches exposed. The results confirm the linearity of dose–response curve below the dose level of 10 Gy for photon and electron beam and the supralinear trend above.
Conclusion:
The TLDs are suitable dosimeters for dose monitoring and verification in radiation treatment involving dose up to 30 Gy in a single fraction.
Keywords: TLD, calibration, correction factor, hypofractionated radiotherapy
Introduction
Conventional external beam radiation therapy (RT) involves the use of a total radiation dose delivered to the tumor with a fractionation scheme of 1.5 to 2.0 Gy per fraction.1
However, with increases in understanding of radiobiology of both tumor and normal tissues, several altered fractionation regimens (hyperfractionated, accelerated hyperfractionated, hypofractionation, and combinations of these) have been tested in clinical trials and came into clinical routine.2 Modern RT techniques (ie, stereotactic body radiation therapy, stereotactic radiosurgery, intraoperative radiation therapy) along with unconventional fractionation RT schemes imply the use of modified total number of fractions as well as daily treatment doses as much as 30 Gy.3-6 Accordingly, miss-irradiation can have a great impact on patient safety, and accurate in vivo dosimetry protocols have to be implemented in order to verify the correct delivery of irradiation.
In this framework, thermoluminescent dosimeters (TLDs) are well established for off-line in vivo dosimetry, and they are widely used to determine patient dose in radiation diagnostic and external beam RT.7-9 Their characteristics match ideal requirements for the standard use in clinic dosimetry: small dimensions, ease of handling, reusability, several formats, and types of materials.
In particular, the TLD 100 dosimeter, based on Lithium Fluoride doped with Magnesium and Titanium (LiF: Mg, Ti), is routinely used for dosimetry in RT because of its close tissue equivalence (effective atomic number 8.2, compared to 7.4 for tissue), low signal fading (5%-10% per year), wide linear response range (10 µGy-10 Gy), and high sensitivity.10 The TLDs need a calibration procedure to enable their use as dosimeters in the range of interest,11 and while several studies have assessed the supralinear behavior of TLDs-100 above 10 Gy in photon beams,12,13 very few works reported calibration curves of TLDs in high-dose range with electron beams.14
The aim of the present study was to investigate the feasibility of TLDs to verify the patient dose distribution, especially when high dose per fraction are involved, in order to guarantee the best safety of the RT treatment. To this end, we evaluated the dose–response of TLD-100 up to 30 Gy, with both 6-MV photon beam and 6-MeV electron beam.
In addition, in order to provide accurate TLDs readouts and to compensate the response variations among the TLD batches, the method of element correction factor (ECF) generation proposed by Plato and Miklos15 was applied. Indeed, due to the very small size of TLD, the amount of TL materials on each element can vary slightly and accordingly, a variation in element response occurs among a TLD’s batch. The method also ensures that changes in calibration of the TLD reader do not affect the ECFs of the dosimeters.
Materials and Methods
Irradiation Facilities
In this study, calibration curves of TLDs-100 were obtained in 6 MV photon beam and 6 MeV electron beam.
Photon irradiation was performed by a linear accelerator Primus (Siemens Germany) delivering X-rays beam of nominal energy of 6 MV and dose rate of 200 MU/min.
Electron irradiation was performed by 6 MeV electron beam produced by TrueBeam STx (Varian Medical System, Palo Alto, CA). Both linear accelerators are installed at the Radiation Oncology Unit of the University of Naples “Federico II”, Italy.
Dosimeters, Annealing, and Readout Procedures
The irradiation experiments were performed using LiF: Mg, Ti (TLD-100; Harshaw Chemical Company) chips, having sizes of 3.2 × 3.2 × 0.89 mm3, spatial resolution of 2 mm, density of 2.64 g/cm3 (“href="http://Thermoscientific.comwebsite”).16
A total of 100 TLDs from 2 different batches were available (50 TLDs from batch A and 50 from batch B).
Prior to each irradiation, TLDs were annealed in air at 400°C for 1 hour, followed by a 2 hours annealing at 100°C and by rapid cooling to room temperature.9 The readout of TLDs was performed by a Harshaw model 3500 manual TLD reader installed at the Laboratory of Radioactivity, Department of Physics of the University of Naples Federico II. The TLDs have been read at 300°C using a heating rate of 10°C/s to optimize the TL signal-to-background ratio in the high-temperature region. A continuous nitrogen flow was used to reduce chemiluminescence and spurious signals not related to the irradiation.
The reading of the TLD was controlled through a computer by using the Thermo Scientific WinREMS Software.
Element Correction Factor Generation
The ECF generation method proposed by Plato and Miklos15 was applied in order to reduce the measurement uncertainty of TLD responses. According to this approach, each batch of TLD was further divided in 2 classes: reference dosimeters and field dosimeters. Each dosimeter response was normalized to the mean ECF-corrected element response of reference dosimeters. The role of reference dosimeters was to provide a mean response to which the response of field dosimeters is normalized to produce ECFs. The process of ECF generation ensures that, when ECFs are applied, the response of each field dosimeter is the same as the mean response of the reference dosimeters.
As first step of the process of the ECF generation, all the reference dosimeters were exposed to an equal dose (2 Gy). The ECFs were calculated by the following equation:
| 1 |
where ECF(i) is the ECF for the dosimeter “i,” e(i) is the response of dosimeter “i,” and EMr is the mean response of all reference TLDs.
Along with ECFs, the measurement of response variability among each TLD set was evaluated by the coefficient of variation (CV) calculated by:
| 2 |
After examining the ECFs and CV distributions, we identified the TLDs in which the ECF resulted within 20% of unity (ie, within 0.8-1.20) and a CV% <5% as recommended in Plato and Miklos.15
As second step of the process, the field dosimeters were irradiated with the group of the reference ones, and the ECFs were generated. The reading by each dosimeter was calculated after compensation for ECF; therefore, Equation 1 was applied substituting e(i) with the response of field dosimeter “i” and EMr with the mean response of group of reference dosimeters. After each reading, the response of a given TLD was divided by its ECF to obtain the corrected response. Each of the 2 steps of the process was repeated several times to provide a mean response.
Both batches were used for ECF generation process with 6-MV photon beam. According to ECF generation method, from batch A, 10 TLDs were used as reference and 30 as field dosimeters, while from batch B 20 TLDs were used as reference and 30 as field dosimeters.
The batch A was also used for ECF generation process with 6-MeV electron beam, 10 TLDs were used as reference and 40 as field dosimeters.
Irradiation of TLD: Experimental Setup
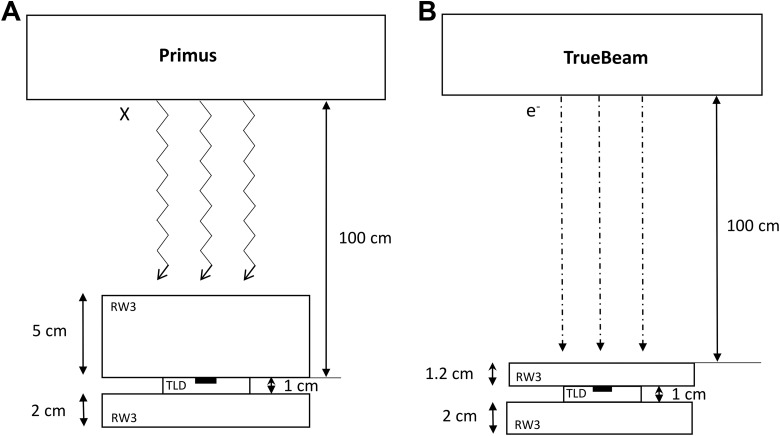
For photon beam irradiation, TLDs were placed at a distance of 100 cm from the source (source-to-axis distance technique) at a depth of 5 cm in a water-equivalent slab phantom (RW3 slab phantom) and irradiated with a beam size of 10 × 10 cm2.
For electron beam irradiation, the TLDs were placed at the depth of maximum dose of electron energy (z max = 1.2 cm). The distance between the source and the upper surface of the phantom was of 100 cm (source surface distance technique) and irradiated with a beam size of 10 × 10 cm2.
The TLDs were placed in a specially designed cavity on a plexiglass slab in the RW3 phantom at depth of maximum dose to reproduce the adequate conditions of electronic equilibrium. Figure 1A and B shows the irradiation set up for both photon and electron irradiation.
Figure 1.
Schematic diagram showing the thermoluminescent dosimeter (TLD)-100 calibration setup using water-equivalent RW3 slab phantom for photon (A) and electron irradiations (B).
Following the recommendation of International Atomic Energy Agency,17 before the irradiation, the beam output (Gy/UM) was verified using a Farmer chamber (PTW type 30001, Freiburg, Germany).
During each TLD irradiation session, the delivered dose was separately measured by an ionization chamber to verify the accuracy of the delivered dose.
In order to have an acceptable statistic for the ECFs values, the reference dosimeters were irradiated 3 times, and the ECFs mean values were calculated. Then, the reference and field TLDs were irradiated together to calculate the ECFs of the field dosimeters; the process was repeated 2 times to derive the average value of ECF for each TLD. Similarly, for the electron irradiation, the reference dosimeters were irradiated 2 times, and the field dosimeters were irradiated 1 time.
Once the ECFs were computed, they were applied to the response of each dosimeter to correct the measurement and to evaluate the error reduction via the CV value.
The subsequent calibration curves for 6-MV photon and 6-MeV electron beams were obtained exposing dosimeters of batch A.
For 6-MV photon beam curves, 11 groups of field dosimeters, each containing at least 3 TLDs, were exposed at different doses from 2 to 30 Gy. Dose steps varied between 2 to 4 Gy: step of 2 Gy in the range 0 to 14 Gy and step of 4 Gy in the range 14 to 30 Gy. Seven dosimeters were not exposed to radiation and were used to measure the background signal (readings for the 0 cGy dose).
For 6 MeV electron beam curves, 50 fields dosimeters were arranged in 9 groups, each having at least 3 TLDs and were irradiated to 0 to 30 Gy in steps of 2 Gy in the range 0 to 10 Gy and in steps of 4 Gy in the range 10 to 30 Gy. A group of 9 TLDs, not exposed to radiation, was used to measure the background signal.
After each irradiation session, the ECF for each TLD reading was applied and the TLD response versus the absorbed dose plotted.
Statistical Analysis
Statistical analysis was performed with OriginLab (OriginLab Corporation, Northampton, Massachusetts). For each dose-energy measurements, the mean response and standard error for the TLD’s group were calculated.
For all beams, regression analysis was performed on TLD response as a function of delivered doses. In order to determine the best trend of the calibration curves, fits were performed by both linear and second-order polynomial functions in 3 different dose range: 0 to 8 Gy, 0 to 10 Gy, and 0 to 30 Gy.
The goodness of fit was assessed by the R 2 coefficient. The Akaike Information Criterion (AIC) test was used to evaluate the probability of the better model.18
Results
Element Correction Factors
The distributions of ECFs of TLDs for the batch A and batch B, irradiated with 6-MV photon beam, and for batch A irradiated with 6-MeV electron beam are reported in Figure 2. It can be observed that all values of ECFs of TLDs of batch A were within 10% of unit (ie, within 0.90-1.10), and all values of ECFs of TLDs of batch B are within 20% of unit (ie, within 0.80 -1.20). The distributions of the ECFs of the TLD irradiated with electrons show that all values are within a range of 20%.
Figure 2.
Distribution of element correction factors (ECFs) generated for thermoluminescent dosimeters (TLDs) exposed to 6-MeV photon beam (A and B) and 6-MeV electron beam (C).
Once the ECFs were applied, to photon measurements, the %CV was reduced from 5.2% to 3.7% for batch A and from 4.7% to 3.4% for batch B. Thus, consistent results were obtained by the application of ECF approach. Similarly, the %CV of the TLDs irradiated with electron was reduced from 7.3% to 3.5%.
Dose–Response Curves
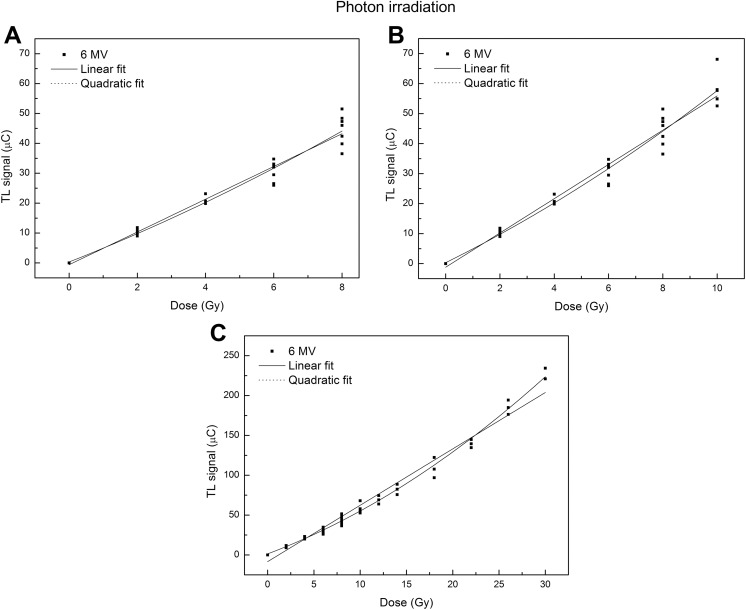
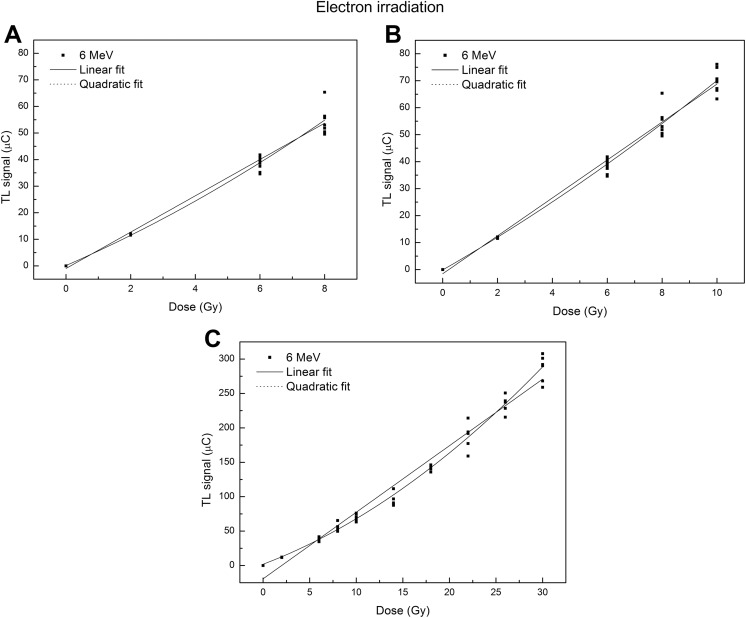
The dose–response curves obtained in the 3 dose ranges for both photon and electron beams are shown in Figures 3 and 4.
Figure 3.
Calibration curves of thermoluminescent dosimeters (TLDs) exposed to 6-MV photon beam in different dose range: 0-8 Gy (A), 0-10 Gy (B), and 0-30 Gy (C). The solid line represents the linear fit, and the dot line represents the quadratic fit.
Figure 4.
Calibration curves of thermoluminescent dosimeters (TLDs) exposed to 6-MeV electron beam in different dose range: 0-8 Gy (A), 0-10 Gy (B), and 0-30 Gy (C). The solid line represents the linear fit, and the dot line represents the quadratic fit.
In each case, the linear and polynomial fitting functions were compared using the Akaike’s weight (Aw).
The best-fit regression coefficients and the AIC for photon and electron beams are reported in Table 1 and Table 2, respectively.
Table 1.
Best-Fit Regression Coefficients and Statistical Parameters for Linear and Quadratic Dose–Response Models for the TLD-Set Exposed to 6-MV Photon Beam.
| Dose Range, Gy | Equation Model | Equation Model | ||||
|---|---|---|---|---|---|---|
| Linear | Quadratic | |||||
| y = α + β0 x | y = α + β0 x + β1 x 2 | |||||
| Parameter | Value | SE | Parameter | Value | SE | |
| 0-8 | α | −0.6 | 0.9 | α | 0 | 1 |
| β0 | 5.5 | 0.2 | β0 | 4.5 | 0.7 | |
| β1 | 0.12 | 0.08 | ||||
| Adjusted R 2 | .967 | Adjusted R 2 | .968 | |||
| Aw | 0.51 | Aw | 0.49 | |||
| 0-10 | α | −1 | 1 | α | 0 | 1 |
| β0 | 5.7 | 0.2 | β0 | 4.5 | 0.6 | |
| β1 | 0.13 | 0.06 | ||||
| Adjusted R 2 | .969 | Adjusted R 2 | .973 | |||
| Aw | 0.20 | Aw | 0.79 | |||
| 0-30 | α | −8 | 2 | α | 1 | 1 |
| β0 | 7.07 | 0.15 | β0 | 4.4 | 0.3 | |
| β1 | 0.10 | 0.01 | ||||
| Adjusted R 2 | .977 | Adjusted R 2 | .992 | |||
| Aw | 0 | Aw | 1 | |||
Abbreviations: SE, standard error; TLD, thermoluminescent dosimeters.
Table 2.
Best-Fit Regression Coefficients and Statistical Parameters for Linear and Quadratic Dose–Response Models for the TLD-Sets Exposed to 6-MeV Electron Beam.
| Dose Range, Gy | Equation Model | Equation Model | ||||
|---|---|---|---|---|---|---|
| Linear | Quadratic | |||||
| y = α + β0 x | y = α + β0 x + β1 x 2 | |||||
| Parameter | Value | SE | Parameter | Value | SE | |
| 0-8 | α | −1 | 1 | α | 0.1 | 1.1 |
| β0 | 6.8 | 0.2 | β0 | 5.3 | 0.7 | |
| β1 | 0.2 | 0.1 | ||||
| Adjusted R 2 | .979 | Adjusted R 2 | .981 | |||
| Aw | 0.28 | Aw | 0.72 | |||
| 0-10 | α | −1 | 1 | α | 0 | 1 |
| β0 | 7.03 | 0.15 | β0 | 5.8 | 0.5 | |
| β1 | 0.12 | 0.05 | ||||
| Adjusted R 2 | .982 | Adjusted R 2 | .984 | |||
| Aw | 0.17 | Aw | 0.83 | |||
| 0-30 | α | −19 | 3 | α | 2 | 3 |
| β0 | 9.7 | 0.2 | β0 | 5.1 | 0.5 | |
| β1 | 0.147 | 0.015 | ||||
| Adjusted R 2 | .973 | Adjusted R 2 | .989 | |||
| Aw | 0 | Aw | 1 | |||
Abbreviations: SE, standard error; TLD, thermoluminescent dosimeters.
The values of Akaike’s weights, reported in Tables 1 and 2, show that the calibration curves exhibit a linear behavior for doses ≤8 Gy (Aw linear > Aw polynomial). Moving to higher doses, the quadratic model performs better than the linear model (Aw linear < Aw polynomial).
Discussion and Conclusion
Dosimetry based on TLD is routinely used in standard external beam RT to verify absorbed dose calculations at specific sites in a radiation field, either directly on patients or in a phantom. The field of use of this kind of dosimeters is very wide. They are routinely used for photon than for electron than for neutron beams.19 The TLDs can be used to validate TPS calculation20 and to verify and validate the in vivo dose distribution during the first treatment fraction to detect potential dose heterogeneity and to allow for an individual dose optimization with adjustment of the monitor units.21
New studies on the efficiency of RT are increasingly pushing toward protocols that use hypofractionation of the dose, with the administration of a few fractions of high dose (10 Gy) or even single fraction of a dose between 25 and 30 Gy. As a consequence, the knowledge of the behavior of TLDs when exposed to high dose per fraction or at high-dose rate beams is necessary.
This study investigated the dose–response of TLD-100 in the photon and electron beams, in the dose range useful for hypofractionated RT application.
Several studies exploited the behavior of the TLDs under different energies and radiation beams (photons, electrons, and protons) for several radiotherapeutic techniques. However, to the best of our knowledge, there aren’t investigations focusing on the energies and dose levels here investigated.
In a previous work,14 we obtained calibration curves with a batch of TLD-100 exposed to photon and high dose per pulse electron beams in the dose range 0 to 10 Gy. In continuity with our study,14 we derived calibration curve for TLD-100 in a wide dose range from 0 to 30 Gy applying the ECF generation method,15 in order to check the response variability among the TLDs of each batch.
As results of the element correction, the TLD sets used for the irradiation experiments show a narrow distribution of the ECFs, within the 20% of unit, as it is desirable. After the compensation of the TLD readouts with ECFs, the spread of %CV distribution resulted within the tolerance range of 5%, for each TLD batch both in photon and in electron irradiation. In particular, the application of ECF method resulted useful for batch A exposed to electron beam, since they benefitted from a reduction in the response variation from a value of 7.3% to 3.5%.
From the calibration curves for 6-MV photon beam (Figure 3), we observed that the fitting curve has a linear behavior under the level of 10 Gy, as expected. This result was in line with the well-known published results22,23 and with the findings of our previous work.14 In addition, a recent study was carried out by Bahreyni Toossi et al24 about calibration of TLDs with 6-MV photon and 6-MeV electron irradiation but restricted to the dose range 0 to 2 Gy. The results of the study showed that for dose up to 120 cGy the TLD response is linear and for higher dose supralinear, for both types of radiation beams.
In our study, the investigation of the TLD dose–response was performed in a wider dose range, up to 30 Gy. The investigation focused on 3 different dose regions through 3 different regression analyses. The most interesting result was that in the range of 0 to 30 Gy, a quadratic function fits the dose–response data better than a linear one (Figure 3C and Tables 1 and 2) both for photon and for electron beams. By the analysis of the estimated model parameters, the R 2 values indicated that the quadratic model was the best representation of the dose–response relationship (R 2 = .992 vs R 2 = .977 for photon beam and R 2 = .989 vs R 2 = .973 for electron beam in the range 0-30 Gy). Similarly, the Akaike test for photon and electron irradiations shows that the Aw values were close to 1 for quadratic fit and close to 0 for the linear fit.
The findings of the present study could have a potential impact on dosimetry in clinical settings, especially in most modern RT treatments of high complexity25,26 for which in vivo dosimetry is recommended. In vivo measurements provide an accurate and independent verification of the overall treatment procedure assessing clinically relevant differences between planned and delivered dose to individual patients. In particular, the small dimensions of TLDs allow verifying that the prescription dose is distributed homogenously over the treatment volume of the patient, when a high dose is applied for a single fraction.
In the performed analysis, we did not consider the effect of the calibration conditions on the TLD dose–response curves. Calibration conditions such as field size, beam energy, angle of beam incidence, and air gap may indeed affect the calibration curves.24,27 Consequently, those factors and their effects should be evaluated in order to get a more complete description of TLDs performance. In particular, according to the available literature, to increase the accuracy of measurement by TLD, it is important to consider the field size correction factor.28,29 In this framework, a possible future analysis can be focused on the dependence of the calibration curves on field sizes and beam energies.
In conclusion, we obtained the calibration curves of TLD-100 in conventional photon beam by linear accelerator Primus and electron field by the advanced linear accelerator TrueBeam. The TrueBeam RT system was designed to treat targets with enhanced speed and accuracy having the power to not only treat quickly but also deliver highly precise dose rates delivering localized high dose in a single fraction in the region of the tumor. Therefore, the importance of our results lies in the feasibility to use TLDs-100 to perform an accurate dosimetry in high-dose region (up to 30 Gy) as required in the nonconventional RT treatments.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the National Institute for Nuclear Physics, (INFN); project name: MoVe-IT (Modeling and Verification for Ion beam Treatment planning).
ORCID iD: Vittoria D’Avino  https://orcid.org/0000-0001-8925-1022
https://orcid.org/0000-0001-8925-1022
References
- 1. Laine AM, Pompos A, Timmerman R, et al. The role of hypofractionated radiation therapy with photons, protons, and heavy ions for treating extracranial lesions. Front Oncol. 2015;5:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed KA, Correa CR, Dilling TJ, et al. Altered fractionation schedules in radiation treatment: a review. Semin Oncol. 2014;41(6):730–750. [DOI] [PubMed] [Google Scholar]
- 3. D’Andrea M, Strolin S, Ungania S, et al. Radiobiological optimization in lung stereotactic body radiation therapy: are we ready to apply radiobiological models? Front Oncol. 2017;7:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Hezewijk M, Creutzberg CL, Putter H, et al. Efficacy of a hypofractionated schedule in electron beam radiotherapy for epithelial skin cancer: analysis of 434 cases. Radiother Oncol. 2010;95(2):245–249. [DOI] [PubMed] [Google Scholar]
- 5. Pilar A, Gupta M, Ghosh Laskar S, Laskar S. Intraoperative radiotherapy: review of techniques and results. Ecancermedicalscience. 2017;11:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cella L, Liuzzi R, Salvatore M. The Italian affair: the employment of parallel-plate ionization chambers for dose measurements in high dose-per-pulse IORT electron beams. Med Phys. June 2010;37(6):2918–2924. [DOI] [PubMed] [Google Scholar]
- 7. Kron T. Thermoluminescence dosimetry and its applications in medicine—part 1: physics, materials and equipment. Australas Phys Eng Sci Med. 1994;17(4):175–199. [PubMed] [Google Scholar]
- 8. Kron T. Thermoluminescence dosimetry and its applications in medicine—part 2: history and applications. Australas Phys Eng Sci Med. March 1995;18(1):1–25. [PubMed] [Google Scholar]
- 9. Rudén BI. Evaluation of the clinical use of TLD. Acta Radiol Ther Phys Biol. 1976;15(5):447–464. [DOI] [PubMed] [Google Scholar]
- 10. Horowitz YS, Oster L, Datz H. The thermoluminescence dose-response and other characteristics of the high-temperature TL in LiF: Mg, Ti (TLD-100). Radiat Prot Dosimetry. 2007;124(2):191–205. [DOI] [PubMed] [Google Scholar]
- 11. Podgoršak EB. International Atomic Energy Agency. Radiation Dosimeters. Radiation Oncology Physics: A Handbook for Teachers and Students. Vienna, Austria: International Atomic Energy Agency; 2005:657. [Google Scholar]
- 12. Chen R, Leung P. Nonlinear dose dependence and dose-rate dependence of optically stimulated luminescence and thermoluminescence. Radiat Meas. 2001;33(5):475–481. [Google Scholar]
- 13. Massillon-JL G, Gamboa-deBuen I, Brandan ME. Onset of supralinear response in TLD-100 exposed to 60Co gamma-rays. Phys D Appl Phys. 2006;39(2):262–268. [Google Scholar]
- 14. Liuzzi R, Savino F, D’Avino V, Pugliese M, Cella L. Evaluation of LiF: Mg, Ti (TLD-100) for intraoperative electron radiation therapy quality assurance. PLoS One. 2015;10(10):e0139287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Plato P, Miklos J. Production of element correction factors for thermoluminescent dosimeters. Health Phys. 1985;49(5):873–881. [DOI] [PubMed] [Google Scholar]
- 16. Thermoscientific.com website. http://www.thermoscientific.com/en/product/tld-100-thermoluminescent-dosimetry-material.html. Accessed October 28, 2019.
- 17. International Atomic Energy Agency. Absorbed Dose Determination in External Beam Radiotherapy: An International Code of Practice for Dosimetry Based on Standards of Absorbed Dose to Water. Vienna, Austria: International Atomic Energy Agency; 2001. [Google Scholar]
- 18. Glatting G, Kletting P, Reske SN, Hohl K, Ring C. Choosing the optimal fit function: comparison of the Akaike Information Criterion and the F-test. Med Phys. 2007;34(11):4285–4292. [DOI] [PubMed] [Google Scholar]
- 19. Tsai WC, Huang CK, Jiang SH. QA measurement of gamma-ray dose and neutron activation using TLD-400 for BNCT beam. Appl Radiat Isot. 2018;137:73–79. [DOI] [PubMed] [Google Scholar]
- 20. Taheri H, Tavakoli MB, Akhavan A. Radiobiological evaluation of three common clinical radiotherapy techniques including combined photon-electron, tangential beams and electron therapy in left-sided mastectomy patients. Adv Biomed Res. 2018;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schuttrumpf L, Neumaier K, Maihoefer C, et al. Dose optimization of total or partial skin electron irradiation by thermoluminescent dosimetry. Strahlenther Onkol. 2018;194(5):444–453. [DOI] [PubMed] [Google Scholar]
- 22. Li K, Kliauga P, Rossi HH. Microdosimetry and thermoluminescence. Radiat Res. 1984;99(3):465–475. [PubMed] [Google Scholar]
- 23. Chen R, Mckeever SWS. Characterization of nonlinearities in the dose dependence of thermoluminescence. Radiat Meas. 1994;23(4):667–673. [Google Scholar]
- 24. Bahreyni Toossi TM, Noghreiyan AV, Gholamhosseinian H. Assessment of the Effects of Radiation Type and Energy on the Calibration of TLD-100. Iran J Med Phys. 2018;15(3):140–145. [Google Scholar]
- 25. Cella L, Lomax A, Miralbell R. New techniques in hadrontherapy: intensity modulated proton beams. Phys Med. 2001;17(suppl 1):100–102. [PubMed] [Google Scholar]
- 26. Pacelli R, Caroprese M, Palma G, et al. Technological evolution of radiation treatment: Implications for clinical applications. Semin Oncol. 2019;46(3):193–201. [DOI] [PubMed] [Google Scholar]
- 27. Troncalli AJ, Chapman J. TLD linearity vs. beam energy and modality. Med Dosim. 2002;27(4):295–296. [DOI] [PubMed] [Google Scholar]
- 28. Moafi M, Geraily G, Shirazi AR, Teimouri J. Analysis of TLD-100 calibration and Correction factor in different field sizes under low dose conditions irradiated with two systems: Gamma knife 4C and Theratron 780-C. Front Biom Technol. 2015;2(4):227–236. [Google Scholar]
- 29. Apipunyasopon L, Srisatit S, Phaisangittisakul N. An investigation of the depth dose in the build-up region, and surface dose for a 6-MV therapeutic photon beam: Monte Carlo simulation and measurements. J Radiat Res. 2013;54(2):374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]