Abstract
Intermediate-risk pulmonary embolism (PE) has variable outcomes. Current risk stratification models lack the positive predictive value to identify patients at highest risk of PE-related mortality. We identified intermediate-risk PE patients who underwent catheter-based interventions and right heart catheterization (RHC) and identified those with low cardiac index (CI < 2.2 L/min/m2). We utilized regression models to identify echocardiographic predictors of low CI and Kaplan Meier curve to evaluate PE-related mortality when stratified by the echocardiographic predictor. Of 174 intermediate-risk PE patients, 41 underwent RHC. Within this cohort, 46.3% had low CI. Univariable linear regression identified right ventricular outflow tract velocity time integral (RVOT VTI), right/left ventricular ratio, S prime, inferior vena cava diameter, and pulmonary artery systolic pressure as potential predictors of low CI. Multivariable linear regression identified RVOT VTI as significant predictor of low CI (β coefficient 0.124, 95% confidence interval [CI]: 0.01-0.24, P = .034). Right ventricular outflow tract velocity time integral <9.5 cm was associated with increased PE-related mortality, P = .002. A substantial proportion of intermediate-risk PE patients referred for catheter-based interventions had low CI despite normotension. Right ventricular outflow tract velocity time integral was a significant predictor of low CI. Low RVOT VTI was associated with increased PE-related mortality.
Keywords: pulmonary embolism, thrombolysis, cardiac index, cardiogenic shock
Introduction
Acute pulmonary embolism (PE) is a heterogeneous disease process with variable presentation and outcomes. Initial risk stratification is performed using clinical, imaging, and laboratory parameters such as systemic blood pressure (BP), computed tomography (CT), and echocardiographic evidence of right ventricular (RV) dysfunction, as well as biomarker evidence of RV strain.1 Using this strategy, PE may be divided into low-risk, intermediate-low, intermediate-high, and high-risk PE.1 Although anticoagulation alone has been the mainstay treatment of normotensive patients with acute PE,2 there is less certainty regarding which intermediate-risk subgroups may benefit from escalation of therapy.2,3 Currently available risk stratification models lack the positive predictive value needed to identify specific patients who are at risk of early PE-related adverse events.4 It is thus clinically desirable to further risk-stratify patients who present with intermediate-risk PE.
Acute RV failure with resultant decrease in cardiac index is the primary cause of death in patients presenting with high risk PE.1 Therefore, having the ability to identify this high-risk cohort with simple and reliable markers may aid in patient selection for closer monitoring and potential escalation of therapy. Given the widespread use of transthoracic echocardiography (TTE) in risk assessment of acute PE, we sought to identify the echocardiographic predictors of low cardiac index (CI) in these patients.
Methods
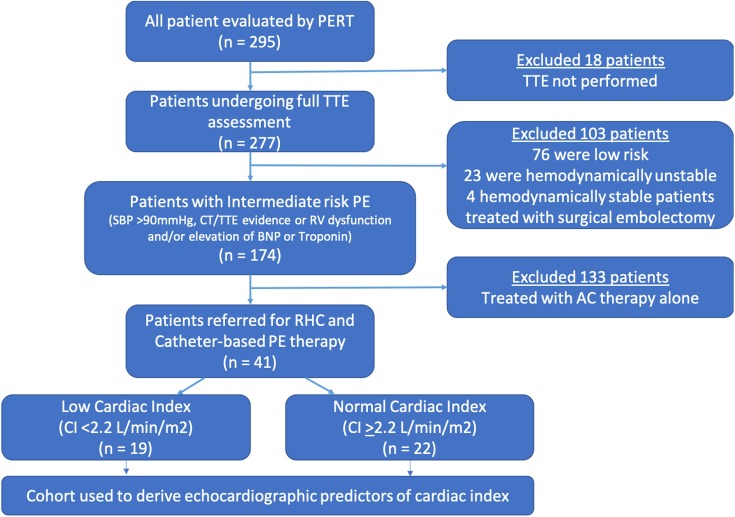
This was a single-center retrospective analysis of all consecutive patients included in our institutional Pulmonary Embolism Response Team (PERT) registry between March 2016 and December 2018. From this patient cohort, we selected all patients who had intermediate risk PE—defined as acute PE, systolic BP >90 mm Hg, and evidence of RV dysfunction by TTE or CT, or evidence of elevation of troponin I and/or BNP values (normal values: troponin I [<0.04 ng/mL], BNP [<100 pg/mL]). We also identified a subgroup of patients who underwent catheter-based intervention and right heart catheterization (RHC) during the study period. Patients were selected to undergo catheter-based procedures based on PE risk assessment, bleeding risk, and shared decision-making with the patient. All of the included patients underwent a TTE prior to catheter-based PE intervention. Finally, the patients were divided into 2 categories based on CI: those with reduced CI (<2.2 L/min/m2) and those with normal CI (>2.2 L/min/m2).5 The patient selection flow diagram is shown in Figure 1.
Figure 1.
Flow diagram of the methodology of the study.
We collected baseline clinical characteristics, laboratory data, and patient outcomes by reviewing the relevant electronic medical records. Right heart catheterization was performed at the time of catheter-based procedure and prior to any intervention. We utilized 6F Swan-Ganz catheter (Edwards Lifesciences, Irvine, California) and a fluid-filled transducer to measure the right atrial (RA), RV, and pulmonary artery pressures. Pulmonary capillary wedge pressure was not obtained in the majority of cases. Cardiac output (CO) was obtained using the assumed Fick method after determining the mixed venous saturation. Cardiac output was indexed to body surface area to obtain CI.
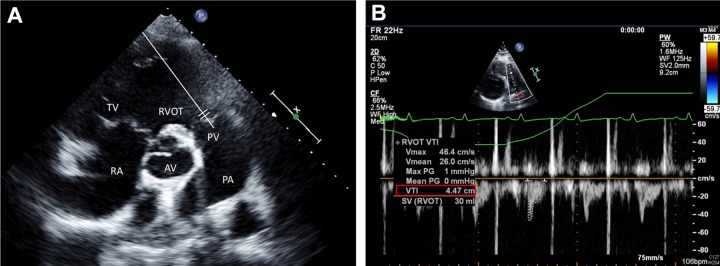
Patients in whom the initial TTE was performed after intervention, was not performed at all, or was not available for analysis for other reasons were excluded. Both RV and left ventricular (LV) measurements on echocardiography were obtained based on the current guidelines.6,7 Right ventricular/LV ratio was obtained using the RV and LV internal diameter in diastole (1 cm apical to atrio-ventricular valves) from the apical 4 chamber view. S prime (S′) was assessed using tissue Doppler velocity of the lateral tricuspid annulus in the apical 4 chamber view. Inferior vena cava (IVC) diameter was measured in millimeters from the subcostal window. Right atrial pressure was estimated as 3 mm Hg, 8 mm Hg, or 15 mm Hg based on IVC diameter and concomitant respiro-phasic changes.6 Pulmonary artery systolic pressure (PASP) was estimated by modified Bernoulli principle utilizing the tricuspid regurgitation velocity (4V2) and adding the estimate of RA pressure. Systolic right ventricular outflow tract velocity time integral (RVOT VTI) was obtained utilizing pulse wave doppler at the level of the RVO immediately adjacent to the pulmonic valve (parasternal short axis window), Figure 2. The study was approved by the Institutional Review Board at Loyola University Medical Center.
Figure 2.
A, Echocardiographic parasternal short axis view at the base of the heart with a pulse wave doppler at the level of the pulmonic valve (PV; as demonstrated by illustrative white line) (B) representative example of the RVOT spectral doppler with low VTI. PA indicates pulmonary artery; PV, pulmonic valve; RA, right atrium, TV, tricuspid valve, RVOT, right ventricular outflow tract, VTI, velocity time integral.
Statistical Analysis
Patient demographics and clinical characteristics were presented using descriptive statistics and compared using independent samples t test for continuous and χ2 test for categorical variables. A univariable linear regression analysis was used to evaluate for potential echocardiographic predictors of low CI within the cohort of intermediate-risk PE patients selected to undergo catheter-based PE intervention (P value of <.10 was used as a cutoff for statistical significance in selection of univariate variables to include in the multivariable model). Predictive variables entered into the model included the following: RVOT VTI, RV/LV ratio, IVC diameter, S′, and PASP. We then developed a multivariable linear regression model to adjust for relevant echocardiographic parameters. A receiver operative characteristic (ROC) curve was constructed to illustrate the sensitivity and specificity of the predictor identified in our analysis. The cohort was divided into normal and low CI, using 2.2 L/min/m2 as a cut point for the ROC analysis. Youden J index was used to identify the optimum cut point to optimize sensitivity and specificity of the test.8
Left ventricular outflow tract (LVOT) VTI was measured on TTE, but not included in the regression model. Since LVOT VTI and RVOT VTI are colinear variables that correlate highly with each other (further discussion under Sensitivity Analysis section of Results), inclusion of both in the regression model may result in derivation of unreliable predictors.9 We also performed a sensitivity analysis to evaluate the effect of LVOT VTI on the regression model (LVOT VTI included and RVOT VTI excluded from the model), Supplementary Table 1. Tricuspid annular plane systolic excursion (TAPSE) variable was not included in the regression model as it was not measured reliably in our cohort (we did, however, include S′ which is a tissue doppler-derived measurement of longitudinal excursion of RV free wall similar to TAPSE)6
Kaplan Meier survival curves were constructed for the entire cohort of PE patients (all patients included in PERT database) to evaluate PE-related mortality as well as all-cause mortality, utilizing the echocardiographic predictor as a discriminator of the 2 groups. Log-rank test was used to ascertain statistical significance. Pulmonary embolism-related mortality was defined as mortality directly resulting from RV failure, shock, and/or respiratory failure, as ascertained by treating physician.
A P value of <.05 was considered to be statistically significant. Analyses were performed using IBM SPSS version 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Macintosh, Version 25.0, Armonk, New York: IBM Corp).
Results
Of 295 patients available for this study, 174 (59%) patients were deemed to have intermediate risk PE (Figure 1). Within the intermediate risk PE cohort, 41 patients were referred for catheter-based PE intervention and had a complete RHC and TTE assessment. Baseline clinical characteristics of patients with intermediate risk PE who underwent intervention or were treated with anticoagulation therapy alone are presented in Table 1. Patients who underwent catheter intervention had higher BMI (38 vs 32.5, P = .002) and lower incidence of cancer (17.1% vs 41.3%, P = .003) when compared to patients treated with anticoagulation therapy alone. Echocardiographically, patients undergoing intervention had higher RV/LV ratio (1.4 vs 0.99, P < .001), lower RVOT VTI (10 vs 13.8, P < .001), lower S′ (10.8 vs 13.5, P = .02), and higher PASP (56.8 mm Hg vs 49.7 mm Hg, P = .03), compared to patients treated with anticoagulation alone (Table 2).
Table 1.
Baseline Clinical Characteristics.
| Variable | Intermediate-Risk PE—Intervention Cohort n = 41 (%) | Intermediate-Risk PE—Nonintervention Cohort n = 133 (%) | P Value |
|---|---|---|---|
| Age (+SD) | 59.7 + 13.2 | 63.5 + 15.7 | .16 |
| Female | 19 (46.3) | 70 (52.6) | .30 |
| Race | |||
| White | 26 (63.6) | 80 (60.2) | .79 |
| Black | 13 (31.7) | 42 (31.6) | |
| Hispanic | 2 (4.9) | 8 (6) | |
| Other | 0 (0) | 3 (2.3) | |
| BMI (kg/m2; ±SD) | 38 + 10.9 | 32.5 + 9.5 | .002 |
| PESI score (±SD) | 111 + 38 | 118 + 48 | .39 |
| Hypertension | 23 (56.1) | 79 (61.7) | .32 |
| Diabetes | 14 (34.1) | 25 (20.2) | .06 |
| CKD | 7 (17.1) | 13 (10.6) | .20 |
| Cancer | 7 (17.1) | 52 (41.3) | .003 |
| CHF | 4 (10) | 27 (21.6) | .08 |
| CAD | 2 (4.9) | 26 (20.8) | .12 |
| COPD | 2 (4.9) | 13 (10.4) | .23 |
| Prior stroke | 3 (7.3) | 16 (12.6) | .27 |
| Prior PE | 8 (19.5) | 16 (12.6) | .19 |
| Acute DVT | 29 (70.7) | 88 (67.7) | .44 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DVT, deep vein thrombosis; PE, pulmonary embolism; PESI, Pulmonary Embolism Severity Index.
Table 2.
Baseline Echocardiographic Parameters.
| Echocardiographic Variable | Intermediate- Risk PE INTERVENTION Cohort n = 41 (SD) |
Intermediate- Risk PE Nonintervention Cohort n = 133 (SD) | P Value |
|---|---|---|---|
| LVIDD (mm) | 36.4 (7.5) | 42.6 (6.7) | <.001 |
| RVIDD (mm) | 49.3 (6.9) | 41.4 (8.5) | <.001 |
| RV/LV ratio | 1.4 (0.33) | 0.99 (0.27) | <.001 |
| RVOT VTI (cm) | 10 (3.6) | 13.8 (4.8) | <.001 |
| LVOT VTI (cm) | 15.9 (4.7) | 18.6 (5.9) | .01 |
| IVC diameter (mm) | 19.8 (5.6) | 17.8 (6.6) | .11 |
| S′ (cm/s) | 10.8 (3.7) | 13.5 (4.4) | .02 |
| PASP (mm Hg) | 56.8 (16.6) | 49.7 (15.4) | .03 |
| LVEF (%) | 58.7 (9.4) | 56 (10.7) | .18 |
Abbreviations: IVC, inferior vena cava; LVIDD, left ventricular internal diastolic diameter; LVEF, left ventricular ejection fraction; LVOT VTI, left ventricular outflow tract velocity time integral; RVIDD, right ventricular internal diastolic diameter; RV/LV, right ventricular to left ventricular ratio; RVOT VTI, right ventricular outflow tract velocity time integral; S′, tissue velocity of lateral tricuspid anulus; PASP, pulmonary artery systolic pressure.
Among patients who underwent intervention, 46.3% had a low CI (CI < 2.2 L/min/m2). The average CI in patients with low CI (low CI group) was 1.8 L/min/m2 as compared to CI of 2.9 L/min/m2 in the normal CI group (P < .001). Patients with low CI had higher BNP levels (776 vs 260 pg/mL, P = .021), but similar lactate (2.5 vs 1.7 mmol/L, P = .10), and troponin (1.04 vs 0.50 ng/mL, P = .13) levels. Mean arterial pressure was similar between the 2 groups 83.4 mm Hg versus 82.1 mm Hg, P = .74.
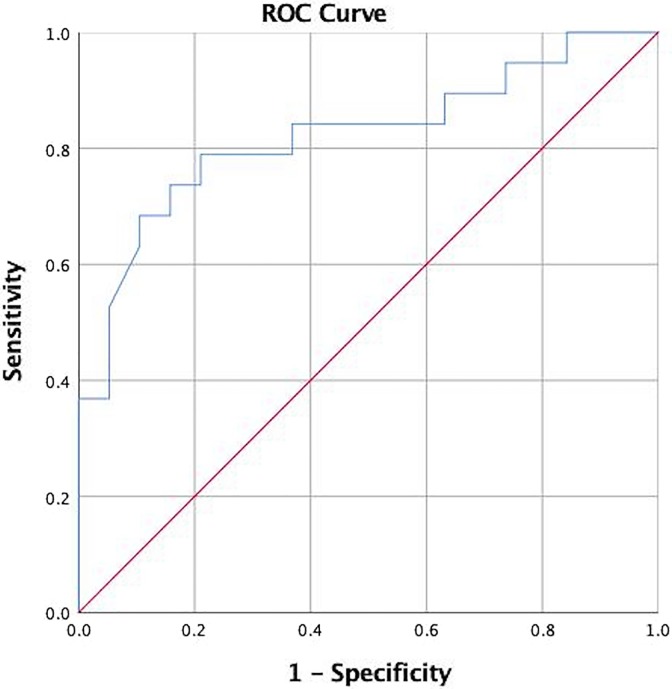
Univariable linear regression analysis identified RVOT VTI, RV/LV ratio, S′, IVC diameter, and PASP as potentially significant predictors of low CI, Table 3. Multivariable linear regression analysis identified RVOT VTI as the only significant predictor of low CI (β coefficient 0.124 95% confidence interval [CI]: 0.01-0.24, P = .034). Right ventricular outflow tract velocity time integral cutoff value of 9.5 cm was associated with sensitivity of 74% and specificity of 79% for identifying patients with low CI. Area under the curve (AUC) was 0.78 (95% CI: 0.62-0.94), P = .006, Figure 3. Mean CI was 2.99 L/min/m2 for RVOT VTI >9.5 cm, and 1.99 L/min/m2 for low RVOT VTI <9.5 cm (P = .003).
Table 3.
Univariable and Multivariable Linear Regression Model to Identify Predictors of Cardiac Index.
| Risk Factor | Unadjusted β Coefficient (95% CI) | P Value | Adjusted β Coefficient (95% CI) | P Value |
|---|---|---|---|---|
| RVOT VTI | 0.13 (0.06 to 0.19) | <.01 | 0.124 (0.01 to 0.24) | .03 |
| RV/LV ratio | −0.98 (−1.73 to −0.22) | .01 | −0.79 (−1.7 to 0.12) | .08 |
| IVC diameter | −0.05 (−0.11 to −0.001) | .05 | −0.024 (−0.09 to 0.042) | .45 |
| S′ | 0.07 (−0.01 to 0.14) | .08 | 0.006 (−0.11 to 0.12) | .92 |
| PASP | −0.02 (−0.03 to −0.002) | .03 | −0.013 (−0.034 to 0.009) | .23 |
Abbreviations: CI, confidence interval; PASP, PASP, pulmonary artery systolic pressure; RV/LV, right ventricular to left ventricular ratio; RVOT VTI, right ventricular outflow tract velocity time integral.
Figure 3.
Receiver operating characteristic (ROC) curve for RVOT VTI for the diagnosis of low cardiac index (CI < 2.2 L/min/m2). Area under the curve (AUC) is 0.78, P = .006 Cutoff RVOT VTI (right ventricular outflow tract velocity time integral) of 9.5 cm provides sensitivity of 74% and specificity of 77%.
Overall mortality among the 295 patients within our PERT registry was 20% (59/295) over the study period of 33 months, with a median follow-up of 276 days. Pulmonary embolism-related mortality was 3.3% (10/295). The mortality rate of the cohort of patients with intermediate-risk PE was 26.4% (46/174): 17% (7/41) of patients died within the intervention group and 29.3% (39/133) of patients died in the anticoagulation alone group. Pulmonary embolism-related mortality in the intermediate-risk PE group was 1.7% (3/174). The cause of death of all patients is included in Supplementary Table 1.
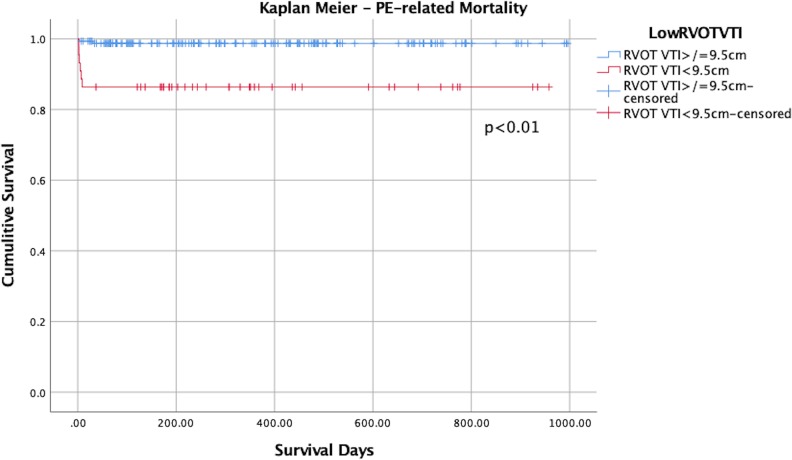
Kaplan-Meier analysis of the entire PERT registry cohort demonstrated that RVOT VTI of < 9.5 cm had an association with increased PE-related mortality as compared to patients with RVOT VTI values > 9.5, P < 0.01 Figure 4. Among the patients who had adequate RVOT VTI measurement, 13.6% (6/44) had PE-related mortality in the low RVOT VTI group as compared to 1.28% (2/156) in the normal RVOT VTI group (P = .002). There was no significant difference in all-cause mortality between the 2 groups, Figure 1A in Online Appendix.
Figure 4.
Kaplan-Meier curve of PE-related mortality. Right ventricular outflow tract velocity time integral of 9.5 cm serves as a discriminator between the 2 groups. Right ventricular outflow tract velocity time integral indicates right ventricular outflow tract velocity time integral.
Sensitivity Analyses
Right ventricular outflow tract velocity time integral and LVOT VTI correlated significantly with each other (r = 0.71, P < .01). Therefore, only one of these 2 variables (RVOT VTI or LVOT VTI) can be included in the final multivariable model as to avoid collinearity.
Receiver operative characteristic analysis evaluating the association between LVOT VTI and CI yielded AUC of 0.73 (95% CI: 0.6-0.9, P = .02), Supplementary Figure 3A. LVOT VTI < 18.3 cm was associated with 50% sensitivity and 88% specificity to detect low CI. By contrast, ROC analysis evaluating the associated between RVOT VTI and low CI yielded a better AUC of 0.78 (95% CI: 0.62-0.94), P = .006, Figure 3. Right ventricular outflow tract velocity time integral cutoff <9.5 cm was associated with sensitivity of 74% and specificity of 79% for detecting patients with low CI. Right ventricular outflow tract velocity time integral was therefore included in the final multivariable regression model. Furthermore, when LVOT VTI is used in the multivariable regression analysis instead of RVOT VTI, the LVOT VTI was not found to be a significant predictor of low CI (Supplementary Table 1A).
Discussion
We found that a substantial proportion of patients who were selected to undergo advanced therapies for management of acute PE had low CI despite normotension. Echocardiographic assessment of RVOT VTI appears to be a significant predictor of low CI. Furthermore, decreased RVOT VTI may also have a role in identifying those patients who are more likely to die from an acute PE.
Forty-six percent of all acute intermediate risk PE patients who were referred for interventional therapies in our study had a reduced baseline CI. Prior studies in which patients with intermediate risk PE underwent baseline invasive hemodynamic assessment, have not specifically stratified patients by CI.10–12 Therefore, comparative outcomes of this patient subgroup are not currently known. Nevertheless, reduced CI has been associated with worse prognosis in patients with pulmonary arterial hypertension13 and may thus portend a worse outcome in the setting of acute PE. Currently available risk stratification models for patients with acute intermediate risk PE, rely on factors such as Pulmonary Embolism Severity Index, elevation of cardiac biomarkers, RV dysfunction, and presence of concomitant lower extremity deep vein thrombosis. However, even when considered together, these factors had a positive predictive value of only 21.2% for predicting death from any cause, hemodynamic collapse or recurrent PE at 30 days.4 Future studies will be necessary to determine whether consideration of baseline CI as part of the initial risk stratification, will improve the accuracy of the currently available models.
Echocardiographic RVOT VTI analysis was identified as a significant predictor of low CI. Right ventricular outflow tract velocity time integral is identified on TTE from the RV outflow view and can be used to estimate the RV stroke volume when used in combination with the RVOT diameter14 (Figure 2).
Although echocardiography-derived CO using LVOT VTI has been previously shown to correlate well with invasive CO in other patient populations,15 in our cohort of patients with intermediate risk PE, RVOT VTI was the only significant predictor of low CI. Whereas the traditional assessment of CI requires RHC, performance of an invasive hemodynamic study is not feasible in most patients presenting with PE. Transthoracic echocardiography on the other hand is a much more readily available modality which can be performed early in the hospital course. In fact, out of the 36 000 patients with acute PE enrolled in the Registro Informatizado de Enfermedad TromboEmbólica (RIETE Registry), 42.8% of patients underwent an early TTE assessment.16 Although RVOT VTI was not assessed in the RIETE registry, this measurement was successfully obtained in 88% of all patients within our study cohort.
We found that reduced RVOT VTI (<9.5 cm) had an association with increased PE-related mortality ( Figure 4 ). Right ventricular outflow tract velocity time integral is a surrogate echocardiographic marker of RV stroke volume. Given that acute RV failure with subsequent decrease in cardiac index is the primary cause of death in patients with acute PE, identifying patients with reduced RVOT VTI may aid in early risk stratification in acute PE by identifying patients at high risk of PE-related mortality. Although our study is not powered to evaluate the role of RVOT VTI as an independent predictor of PE-related mortality, nonetheless, this is a novel finding which will need to be explored in future studies.
Limitations
Our study has several limitations. (1) Evaluation of the hemodynamic and echocardiographic parameters were performed exclusively within the smaller intervention cohort. Ideally invasive and noninvasive measurements should be performed in all PE patients; however, this is not feasible from clinical or ethical standpoint. However, when stratifying all patients within our PERT database by RVOT VTI, there is a stepwise progression of VTI values from the highest in low-risk PE cohort to lowest in high-risk PE cohort, Figure 2A in Online Appendix. This finding gives further support to the use of RVOT VTI in a broader PE population. (2) Echocardiography and RHC were not performed at the same time. The mean delay between TTE and RHC was 1.2 days (range 1-6 days). Nevertheless, 87.5% of all TTEs were performed within 2 days of RHC. Furthermore, TTE assessment was performed prior to RHC in all patients. (3) Finally, there is always a potential for selection bias in the intervention group and for measured and unmeasured confounders in relation to outcome.
Conclusion
A substantial proportion of patients with acute intermediate risk PE that are selected to undergo invasive therapies had low CI despite normotension. Echocardiographic assessment of RVOT VTI is a significant predictor of low CI which is also associated with increased PE-related mortality. Measuring the RVOT VTI may thus identify patients that will benefit from advanced therapies. Further studies are required to prospectively ascertain the true utility of RVOT VTI and validate our findings in the general intermediate risk PE patient population.
Supplemental Material
Supplemental Material, Figure_1A_Kaplan_Meier_All_Cause for Right Ventricular Outflow Doppler Predicts Low Cardiac Index in Intermediate Risk Pulmonary Embolism by Yevgeniy Brailovsky, Vladimir Lakhter, Ido Weinberg, Katerina Porcaro, Jeremiah Haines, Stephen Morris, Dalila Masic, Erin Mancl, Riyaz Bashir, Mohamad Alkhouli, Kenneth Rosenfield, Verghese Mathew, John Lopez, Carlos F. Bechara, Cara Joyce, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, Figure_2A for Right Ventricular Outflow Doppler Predicts Low Cardiac Index in Intermediate Risk Pulmonary Embolism by Yevgeniy Brailovsky, Vladimir Lakhter, Ido Weinberg, Katerina Porcaro, Jeremiah Haines, Stephen Morris, Dalila Masic, Erin Mancl, Riyaz Bashir, Mohamad Alkhouli, Kenneth Rosenfield, Verghese Mathew, John Lopez, Carlos F. Bechara, Cara Joyce, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, LVOT_VTI_Supplemmentary_Figur_3A for Right Ventricular Outflow Doppler Predicts Low Cardiac Index in Intermediate Risk Pulmonary Embolism by Yevgeniy Brailovsky, Vladimir Lakhter, Ido Weinberg, Katerina Porcaro, Jeremiah Haines, Stephen Morris, Dalila Masic, Erin Mancl, Riyaz Bashir, Mohamad Alkhouli, Kenneth Rosenfield, Verghese Mathew, John Lopez, Carlos F. Bechara, Cara Joyce, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, Supplemental_Figure_Legend for Right Ventricular Outflow Doppler Predicts Low Cardiac Index in Intermediate Risk Pulmonary Embolism by Yevgeniy Brailovsky, Vladimir Lakhter, Ido Weinberg, Katerina Porcaro, Jeremiah Haines, Stephen Morris, Dalila Masic, Erin Mancl, Riyaz Bashir, Mohamad Alkhouli, Kenneth Rosenfield, Verghese Mathew, John Lopez, Carlos F. Bechara, Cara Joyce, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, Supplemmentary_table_1A_Sensitivity_analysis for Right Ventricular Outflow Doppler Predicts Low Cardiac Index in Intermediate Risk Pulmonary Embolism by Yevgeniy Brailovsky, Vladimir Lakhter, Ido Weinberg, Katerina Porcaro, Jeremiah Haines, Stephen Morris, Dalila Masic, Erin Mancl, Riyaz Bashir, Mohamad Alkhouli, Kenneth Rosenfield, Verghese Mathew, John Lopez, Carlos F. Bechara, Cara Joyce, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funding from Division of Cardiology and Cardiovascular Research Institute, Loyola University Chicago Stritch School of Medicine and Loyola University Medical Center, Maywood, Illinois.
ORCID iD: Yevgeniy Brailovsky  https://orcid.org/0000-0002-4811-5267
https://orcid.org/0000-0002-4811-5267
Jawed Fareed  https://orcid.org/0000-0003-3465-2499
https://orcid.org/0000-0003-3465-2499
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: the task force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC)endorsed by the European Respiratory Society (ERS). Eur Heart J. 2014;35(43):3033–3069. [DOI] [PubMed] [Google Scholar]
- 2. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: chest guideline and expert panel report. Chest. 2016;149(2):315–352. doi:10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 3. Secemsky E, Chang Y, Jain CC, et al. Contemporary management and outcomes of patients with massive and submassive pulmonary embolism. Am J Med. 2018;131(12):1506–1514.e0. doi:10.1016/j.amjmed.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 4. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718–726. doi:10.1164/rccm.201311-2040OC. [DOI] [PubMed] [Google Scholar]
- 5. Van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136(16):e232–e268. doi:10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 6. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23(7):685–713. doi:10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015. doi:10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 8. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. [DOI] [PubMed] [Google Scholar]
- 9. Kleinbaum DG, Klein M. Logistic regression: a self-learning text, chapter 8; pages 270-275. Statist Biol Health. 2010. doi:10.1007/978-1-4419-1742-3. [Google Scholar]
- 10. Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014. doi:10.1161/circulationaha.113.005544. [DOI] [PubMed] [Google Scholar]
- 11. Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the Seattle II study. JACC Cardiovasc Interv. 2015. doi:10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 12. Sharifi M, Bay C, Skrocki L, Rahimi F, Mehdipour M. Moderate pulmonary embolism treated with thrombolysis (from the ‘mOPETT’ trial). Am J Cardiol. 2013. doi:10.1016/j.amjcard.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 13. Galie N, Humbert M, Vachiery JL, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the internet. Eur Heart J. 2009. doi:10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 14. Porter TR, Shillcutt SK, Adams MS, et al. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015. doi:10.1016/j.echo.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 15. Mercado P, Maizel J, Beyls C, et al. Transthoracic echocardiography: an accurate and precise method for estimating cardiac output in the critically ill patient. Crit Care. 2017. doi:10.1186/s13054-017-1737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bikdeli B, Lobo JL, Jiménez D., et al. Early use of echocardiography in patients with acute pulmonary embolism: findings from the RIETE registry. J Am Heart Assoc. 2018. doi:10.1161/JAHA.118.009042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Figure_1A_Kaplan_Meier_All_Cause for Right Ventricular Outflow Doppler Predicts Low Cardiac Index in Intermediate Risk Pulmonary Embolism by Yevgeniy Brailovsky, Vladimir Lakhter, Ido Weinberg, Katerina Porcaro, Jeremiah Haines, Stephen Morris, Dalila Masic, Erin Mancl, Riyaz Bashir, Mohamad Alkhouli, Kenneth Rosenfield, Verghese Mathew, John Lopez, Carlos F. Bechara, Cara Joyce, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, Figure_2A for Right Ventricular Outflow Doppler Predicts Low Cardiac Index in Intermediate Risk Pulmonary Embolism by Yevgeniy Brailovsky, Vladimir Lakhter, Ido Weinberg, Katerina Porcaro, Jeremiah Haines, Stephen Morris, Dalila Masic, Erin Mancl, Riyaz Bashir, Mohamad Alkhouli, Kenneth Rosenfield, Verghese Mathew, John Lopez, Carlos F. Bechara, Cara Joyce, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, LVOT_VTI_Supplemmentary_Figur_3A for Right Ventricular Outflow Doppler Predicts Low Cardiac Index in Intermediate Risk Pulmonary Embolism by Yevgeniy Brailovsky, Vladimir Lakhter, Ido Weinberg, Katerina Porcaro, Jeremiah Haines, Stephen Morris, Dalila Masic, Erin Mancl, Riyaz Bashir, Mohamad Alkhouli, Kenneth Rosenfield, Verghese Mathew, John Lopez, Carlos F. Bechara, Cara Joyce, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, Supplemental_Figure_Legend for Right Ventricular Outflow Doppler Predicts Low Cardiac Index in Intermediate Risk Pulmonary Embolism by Yevgeniy Brailovsky, Vladimir Lakhter, Ido Weinberg, Katerina Porcaro, Jeremiah Haines, Stephen Morris, Dalila Masic, Erin Mancl, Riyaz Bashir, Mohamad Alkhouli, Kenneth Rosenfield, Verghese Mathew, John Lopez, Carlos F. Bechara, Cara Joyce, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material, Supplemmentary_table_1A_Sensitivity_analysis for Right Ventricular Outflow Doppler Predicts Low Cardiac Index in Intermediate Risk Pulmonary Embolism by Yevgeniy Brailovsky, Vladimir Lakhter, Ido Weinberg, Katerina Porcaro, Jeremiah Haines, Stephen Morris, Dalila Masic, Erin Mancl, Riyaz Bashir, Mohamad Alkhouli, Kenneth Rosenfield, Verghese Mathew, John Lopez, Carlos F. Bechara, Cara Joyce, Jawed Fareed and Amir Darki in Clinical and Applied Thrombosis/Hemostasis