Abstract
Infectious diseases, such as Helicobacter pylori, which produce systemic inflammation may be one key factor in the onset of autoimmunity. The association between H. pylori and antinuclear antibodies (ANA), a marker of autoimmunity, has been understudied. Data from the 1999–2000 National Health and Nutrition Examination Survey were used to evaluate the cross-sectional association between H. pylori seroprevalence and ANA positivity in US adults aged ≥20 years. ANA was measured in a 1:80 dilution of sera by indirect immunofluorescence using HEp-2 cells (positive ⩾3). H. pylori immunoglobulin G enzyme-linked immunosorbent assays were used to categorise individuals as seropositive or seronegative. H. pylori seropositivity and ANA positivity were common in the adult US population, with estimated prevalences of 33.3% and 9.9%, respectively. Both were associated with increasing age. H. pylori seropositivity was associated with higher odds of ANA (prevalence odds ratio = 1.89, 95% confidence interval = 1.08–3.33), adjusted for age, sex, race/ethnicity, educational attainment and body mass index. H. pylori infection may be one key factor in the loss of self-tolerance, contributing to immune dysfunction.
Key words: Antinuclear antibodies, autoimmunity, epidemiology, H. pylori
Introduction
Loss of self-tolerance is a hallmark of autoimmune disease, but less well understood are the drivers of self-tolerance loss in the immune system. Antinuclear antibodies (ANA), representing humoral immunity to cellular components, are one indicator of autoimmunity that occurs in the context of the failure of self-tolerance mechanisms. ANA are a feature of Systemic Lupus Erythematosus [1] and other systemic autoimmune diseases. The prevalence of ANA in the general population ranges from 12% to 16%, is higher in women, and increases with age [2].
Autoimmunity is thought to result from the interactions of environmental and genetic risk factors [3, 4]. Infectious agents may be important environmental exposures resulting in autoimmunity. In particular, chronic infections that have evolved extensive immune evasion mechanisms are of interest as potential drivers of self-tolerance loss. One such infectious agent is Helicobacter pylori, a bacterium often contracted in childhood, which colonises the mucosal layer of the gastric epithelium [3]. H. pylori infection is common, with the prevalence being approximately 50% worldwide [5, 6], and is well-known for its causative role in gastritis and peptic ulcer disease [7]. Importantly, H. pylori has evolved a variety of immune evasion tactics, including circumventing recognition by the innate immune system, inhibition of phagocytic killing, modulation of antigen-presenting cell functions and manipulation of host T-cell responses [8]. Without antibiotic treatment, H. pylori infection may remain for many years, if not the entire life of the individual, as the infection is often asymptomatic [7].
Previous studies have examined the role of H. pylori in autoimmune disease due to its common occurrence and influence on the immune system [3]. However, the relationship between H. pylori and ANA in the general population is unknown. Using data from the 1999–2000 National Health and Nutrition Examination Survey (NHANES), we evaluated the cross-sectional association between H. pylori seroprevalence and ANA positivity in the adult US population.
Materials and methods
Study population
Data were from NHANES, which is a multistage, nationally representative survey sample of the non-institutionalised US population [9]. The NHANES protocol was approved by the human subjects Institutional Review Board of the US Centers for Disease Control and Prevention, and written informed consent was obtained from all participants. Only the 1999–2000 NHANES cycle had existing laboratory data on both H. pylori seropositivity and ANA. Individuals with information on laboratory and demographic covariates of interest were included in this study, resulting in a final sample size of 1005 adults aged 20 years or older.
H. pylori seropositivity
As detailed in the NHANES protocol [10], serum samples from NHANES participants (N = 7493) were collected via venepuncture and stored at −80 °Celsius until they were tested at the University of Washington. H. pylori-specific immunoglobulin G (IgG) was measured using Wampole Laboratories (Cranbury, NJ) H. Pylori IgG Enzyme-Linked Immunosorbent Assay (ELISA). Standard ELISA cut-offs were used to categorise participants into seropositive (optical density (OD) value ≥1.1) or seronegative (OD value <0.9) to H. pylori. Equivocal values (0.9–1.1) were categorised as seronegative to produce conservative estimates of serostatus.
ANA positivity
Autoantibody testing was previously conducted on stored sera from a representative subsample (N = 4754) of survey participants [11]. Standard indirect immunofluorescence was used to measure ANA in serum specimens, based on commercial HEp-2 ANA slides (Inova Diagnostics) with 1:80 dilutions of sera and staining with DyLight 488-conjugated donkey anti-human IgG antibodies (Jackson ImmunoResearch), as previously reported [2]. Staining intensities were graded from 0 to 4 relative to a standard reference gallery, with values of 3 and 4 considered indicative of ANA positivity [2]. Two independent raters agreed on >95% of the readings for overall intensity ratings, and differences were resolved by consensus or adjudicated by a third reviewer.
Covariates
Covariates considered for this analysis include age, sex, race/ethnicity, educational attainment and body mass index (BMI). These covariates were chosen based on a priori knowledge of their relationships with both H. pylori serostatus and ANA positivity. Age was measured in years and categorised into approximate tertiles (20–34 years, 35–59 years and 60 years or older) for age-stratified analyses. Sex was dichotomised into male or female. Race was categorised into non-Hispanic White, non-Hispanic Black or Other. Educational attainment was used as a proxy for socioeconomic status as it is established early in life, not modified by chronic disease and contributes to the development of health capital [12, 13]. Educational attainment was categorised as less than high school, high school or more than high school. BMI was calculated by dividing the weight in kg by the height in m2 and then classified as normal (<25 kg/m2), overweight (25 to <30 kg/m2) or obese (≥30 kg/m2). A dichotomous variable representing medical history of ulcers was ascertained by asking participants ‘Have you ever been told by a doctor or other health professional that you had an ulcer (stomach, duodenal or peptic)?’ Current use of omeprazole, lansoprazole, rabeprazole, pantoprazole, esomeprazole or dexlansoprazole was coded as using vs. not using proton pump inhibitor medication. No individuals reported taking H. pylori eradication agents within the last month, including bismuth subsalicylate, metronidazole, tetracycline, amoxicillin and/or clarithromycin. Individuals with anti-extractable nuclear antigen (ENA) antibodies (measured using previously described immunoprecipitation methods among the ANA positive [2]) or self-reported autoimmune disease (thyroid problems, rheumatoid arthritis or type 1 diabetes) were classified as having possible autoimmune disease [14].
Statistical analyses
Analyses were conducted with SAS version 9.4 (SAS Institute, Inc., Cary, NC) using SURVEY procedures and the Taylor series variance estimation to account for the complex survey design. Medical exam unit sampling weights were revised for participation in the substudy as previously described [2]. Bivariate relationships between ANA status, H. pylori seropositivity and covariates were assessed using design-based Rao–Scott χ2 statistics [15]. The association between H. pylori seropositivity and ANA was modelled using multiple logistic regression. The continuous age-adjusted prevalence odds ratio (POR) and 95% confidence interval (CI) were estimated first, followed by estimates that also adjusted for sex and race/ethnicity. Finally, a third set of estimates additionally adjusted for educational attainment and BMI. Several sensitivity analyses were performed, including an examination of the association between H. pylori and ANA within strata specified by age or sex, as well as one that additionally adjusted for current use of proton pump inhibitors, medical history of ulcer or likely autoimmune disease.
Results
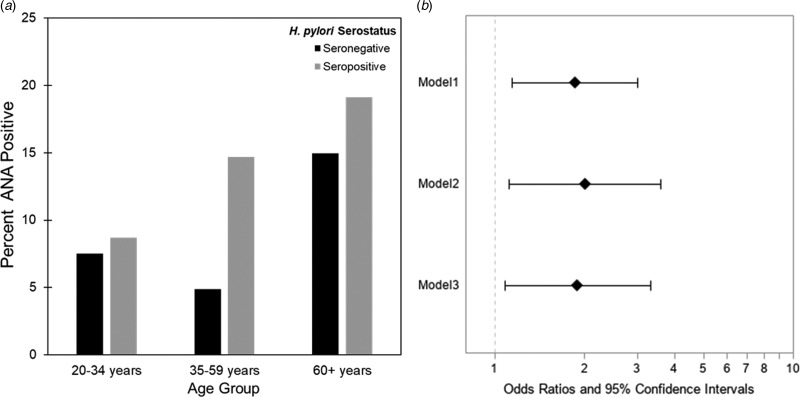
The estimated weighted prevalence of ANA positivity was 9.9% in the adult population and the proportion seropositive for H. pylori was 33.3%. The weighted mean age was 45.5 years (range 20–85 years) and 49.8% were female. A majority reported non-Hispanic White race/ethnicity (71%), some college education or above (51%) and normal BMI (38%). Ten percent of participants indicated ever having an ulcer and of those, 21% reported the occurrence of an ulcer in the past 12 months. Prevalence of ANA varied by sex and age, with females and older individuals more likely to be ANA positive, as expected (Table 1). Higher seroprevalence of H. pylori was observed with increasing age, and among minorities, those with less than high school educational attainment, and those who self-reported medical history of ulcer (Table 1). The rising prevalence of ANA positivity among H. pylori seropositive individuals by age group is depicted in Figure 1a. The greatest difference of ANA positivity by serostatus was observed in the 35–59-year-old age group.
Table 1.
Characteristics and weighted proportions by ANA status and H. pylori serostatus in the adult US population 20+, NHANES 1999–2000
| ANA status | H. pylori Serostatus | ||||||
|---|---|---|---|---|---|---|---|
| Overall | Negative | Positive | Negative | Positive | |||
| Characteristic | n (%) | n (%) | n (%) | P-valuea | n (%) | n (%) | P-valuea |
| 1005 | 899 (90.1) | 116 (9.9) | 541 (66.7) | 464 (33.3) | |||
| Sex | <0.0001 | 0.59 | |||||
| Female | 503 (49.8) | 421 (84.7) | 82 (15.3) | 277 (65.8) | 226 (34.2) | ||
| Male | 502 (50.2) | 468 (95.4) | 34 (4.6) | 264 (67.5) | 238 (32.5) | ||
| Age (years) | 0.009 | 0.004 | |||||
| 20–34 | 251 (30.6) | 226 (92.2) | 25 (7.8) | 171 (75.4) | 80 (24.6) | ||
| 35–59 | 393 (47.5) | 354 (91.8) | 39 (8.2) | 202 (65.6) | 191 (34.4) | ||
| 60+ | 361 (21.9) | 309 (83.3) | 52 (16.7) | 168 (56.9) | 193 (43.1) | ||
| Race/ethnicity | 0.78 | <0.0001 | |||||
| Non-Hispanic White | 461 (71.1) | 412 (90.1) | 49 (9.9) | 337 (76.6) | 124 (23.4) | ||
| Non-Hispanic Black | 167 (9.1) | 148 (87.9) | 19 (12.1) | 78 (50.4) | 89 (49.6) | ||
| Other | 377 (19.8) | 329 (90.7) | 48 (9.3) | 126 (38.5) | 251 (61.5) | ||
| BMI (kg/m2) | 0.29 | 0.40 | |||||
| Normal (<25) | 340 (38.1) | 291 (88.3) | 49 (11.7) | 191 (66.6) | 149 (33.4) | ||
| Overweight (25 to <30) | 363 (33.7) | 328 (89.9) | 35 (10.1) | 190 (64.2) | 173 (35.8) | ||
| Obese (30+) | 302 (28.2) | 270 (92.5) | 32 (7.5) | 160 (69.7) | 142 (30.3) | ||
| Education | 0.04 | <0.0001 | |||||
| Less than high school | 382 (22.9) | 338 (90.2) | 44 (9.8) | 137 (47.8) | 245 (52.2) | ||
| High school | 221 (25.7) | 189 (85.8) | 32 (14.2) | 131 (67.6) | 90 (32.4) | ||
| More than high school | 402 (51.4) | 362 (92.1) | 40 (7.9) | 273 (74.6) | 129 (25.4) | ||
| Ever had ulcer (stomach, duodenal or peptic), N = 1004 | 0.64 | 0.03 | |||||
| No | 903 (89.8) | 797 (90.2) | 106 (9.8) | 492 (68.0) | 411 (32.0) | ||
| Yes | 101 (10.2) | 91 (80.6) | 10 (11.4) | 48 (55.0) | 53 (45.0) | ||
| Proton pump inhibitor medication, N = 997 | 0.20 | 0.45 | |||||
| No | 952 (95.6) | 843 (90.3) | 109 (9.7) | 513 (67.1) | 439 (32.9) | ||
| Yes | 45 (4.4) | 39 (84.1) | 6 (15.9) | 23 (59.7) | 22 (40.3) | ||
Rao–Scott χ2.
Fig. 1.
(a) Weighted percent of participants who were ANA positive by age group among H. pylori seropositives and seronegatives, NHANES 1999–2000 (N = 1005). (b) Association between H. pylori seropositivity and ANA positivity, ages 20+, NHANES 1999–2000, N = 1005. Model 1 adjusted for age; Model 2 adjusted for age, sex and race/ethnicity; Model 3 adjusted for age, sex, race/ethnicity, BMI and education. Additional adjustment for peptic ulcer, proton pump inhibitor medication use and ENA or autoimmune illness did not meaningfully alter results.
Results from multiple logistic regressions are provided in Figure 1b. H. pylori seropositive individuals had almost twice the age-adjusted odds of ANA positivity as H. pylori seronegative individuals (POR: 1.88, 95% CI 1.19–2.99). This association was robust to adjustment for sex, race/ethnicity, educational attainment and BMI (POR: 1.99, 95% CI 1.18–3.35). When stratified by sex (Supplementary Table S1) or age (Supplementary Table S2), the association between H. pylori and ANA appeared to be stronger in males (P-value interaction: 0.11) and in the 35–59-year-old age group. Sensitivity analyses adjusting for proton pump inhibitor medication use, self-reported history of an ulcer or likely autoimmune disease (ENA or autoimmune illness) did not meaningfully alter results.
Discussion
In this cross-sectional study of a sample of the general US population, we found that H. pylori seropositivity was associated with ANA. Previous studies have documented the presence of potentially damaging autoantibodies to self-epitopes in response to H. pylori infection [8], including anti-platelet autoantibodies in immune thrombocytopenia (ITP) [16], anti-parietal cell autoantibodies present in the stomach [17] and anti-gastric autoantibodies in sera [18]. In exploring infectious determinants of autoimmune disease, research has shown an association between H. pylori and ANA in Europe and Latin America [19]. Here we extend this analysis to the US population.
H. pylori has evolved extensive mechanisms to evade recognition by the immune system as well as to establish persistent infection through manipulating the innate and adaptive immune systems [8]. The long-term consequences of immune evasion, suppression and induction activities by H. pylori beyond gastric health effects are still being uncovered. Many of the negative health outcomes associated with H. pylori are thought to be a consequence of systemic effects produced by the chronic inflammatory state triggered by this infection [8]. H. pylori may play a pathogenic role in the development of autoimmunity and autoimmune disease [3] as its immune evasion mechanisms have been shown to induce autoantibodies, likely through inflammatory mechanisms [20]. For example, H. pylori upregulates interferon (INF)-γ, a cytokine that mediates the host response to bacterial infection and autoimmune disease [21]. INF-γ treatment for cancer has been shown to induce ANA [22], and upregulation of INF-γ by H. pylori could potentially induce ANA positivity.
In our study, the 35–59-year-old age group exhibited the strongest association between H. pylori seropositivity and ANA. Previous studies of age trends in ANA positivity have posited that differential exposure to factors related to ANA development by age may be driving non-linear trends [2]. In this study, the 35–59-year-old age group made up the greatest proportion of individuals who were H. pylori seropositive. This age range may represent a critical point in the life course where individuals have lived long enough to not only be exposed to H. pylori, but also experience pathophysiological consequences of the infection, such as systemic inflammation and other downstream health effects.
Sensitivity analyses also revealed that the association between H. pylori and ANA may be stronger in men than in women; however, the analysis was underpowered due to relatively few ANA seropositive males. Further studies of sex-specific effects are needed to better understand important differences in ANA and other autoimmunity markers by sex.
A previous study showed omeprazole use, a common proton pump inhibitor medication for ulcers, was associated with higher risk of ANA positivity [14]. Prior to this study, omeprazole was not thought to induce autoimmunity nor was it linked with autoimmune disease. The association of H. pylori and ANA in the present study was robust to adjustment for proton pump inhibitor medication use (POR = 2.05, 95% CI 1.19–3.54), therefore, it is possible omeprazole use was a proxy for H. pylori infection in the previous analysis of medications and ANA.
Our study has several limitations, including the exclusion of institutionalised adults by NHANES and the cross-sectional study design. We do not know the timing of H. pylori infection nor do we know the onset date of ANA. However, H. pylori is often acquired in childhood [5] when ANA prevalence is the lowest [2] so it is likely that H. pylori infection preceded ANA positivity. Another limitation is that we do not know if individuals have active or previous H. pylori infection, only that individuals have been exposed to H. pylori and mounted an immune response. Importantly, no individuals reported taking H. pylori eradication medications in the last month, meaning that H. pylori exposure had at least a 1-month lag time to induce an inflammatory response before ANA assessment. Future research with longer medication ascertainment and longitudinal measurement of both H. pylori and ANA is needed to clarify the temporal relationship, as well as better understand whether ANA are transient or permanent, especially in relation to treatment with H. pylori eradication medication. For example, studies of ITP have shown that H. pylori eradication treatment results in the resolution of autoimmune disease [23, 24]. If future research supports a causal association between H. pylori and ANA, screening for H. pylori and treatment of the infection may play a role in reducing autoimmunity and potentially prevent progression to autoimmune disease. Finally, we are limited to data from 1999–2000 as both ANA and H. pylori have not been measured in more recent NHANES cycles; however, the results remain relevant as a previous study demonstrated H. pylori seroprevalence levels were consistent over 10 years from NHANES III to 1999–2000 [5] and although treatment for H. pylori infection is available, many individuals do not experience symptoms and do not seek treatment, indicating H. pylori seroprevalence is unlikely to have changed meaningfully in the USA over time.
Despite these limitations, our study extends previous research on the association of H. pylori and ANA to a large, representative sample of the adult US population. We observed a strong and biologically plausible association that was robust to adjustment for multiple factors. Not fully understanding the drivers of autoimmunity is a key research gap. Infectious diseases, such as H. pylori, that produce systemic inflammation may be one key factor in the loss of self-tolerance. Future work exploring the enduring consequences of immune evasion and suppression activities by H. pylori over the life course is warranted. Specifically, future research should examine possible mechanisms by which altered immune function resulting from persistent infection, including autoimmunity and inflammation, influence subsequent chronic disease aetiology and progression.
Acknowledgements
We acknowledge Regina Manansala, MPH and the UWM Center for Aging and Translational Research's NHANES working group.
Conflict of interest
The authors have no known conflicts of interest.
Author contributions
Conceived of and designed the study: HM, FM and CP. Acquired the data and performed the analysis: CC and HM. Drafted manuscript: HM. Consulted on analytic strategy and critically revised manuscript: CP, FM, CW and GD.
Financial support
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences under project ES101074 and contract HHSN273201600011C to Social & Scientific Systems, and in part by the Shaw Scientist Award from the Greater Milwaukee Foundation.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268820000126.
click here to view supplementary material
References
- 1.Arbuckle MR et al. (2003) Development of autoantibodies before the clinical onset of systemic lupus erythematosus. The New England Journal of Medicine 349, 1526–1533. [DOI] [PubMed] [Google Scholar]
- 2.Satoh M et al. (2012) Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis and Rheumatism 64, 2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasni SA (2012) Role of Helicobacter pylori infection in autoimmune diseases. Current Opinion in Rheumatology 24, 429–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller FW (2011) Environmental agents and autoimmune diseases. Advances in Experimental Medicine and Biology 711, 61–81. [DOI] [PubMed] [Google Scholar]
- 5.Grad YH, Lipsitch M and Aiello AE (2012) Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. American Journal of Epidemiology 175, 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pounder RE and Ng D (1995) The prevalence of Helicobacter pylori infection in different countries. Alimentary Pharmacology & Therapeutics 9(suppl. 2), 33–39. [PubMed] [Google Scholar]
- 7.McColl KE (2010) Clinical practice. Helicobacter pylori infection. The New England Journal of Medicine 362, 1597–1604. [DOI] [PubMed] [Google Scholar]
- 8.Lina TT et al. (2014) Immune evasion strategies used by Helicobacter pylori. World Journal of Gastroenterology 20, 12753–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtin LR et al. (2012) The National Health and Nutrition Examination Survey: sample design, 1999–2006. Vital Health Statistics 2, 1–39. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 1999–2001 Data Documentation, Codebook, and Frequencies – H. pylori Available at https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/LAB11.htm (Accessed 5 November 2018).
- 11.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 1999–2001 Data Documentation, Codebook, and Frequencies: Autoantibodies – Immunofluorescence & Immunoprecipitation Analyses. Available at https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/SSANA_A.htm (Accessed 5 November 2018).
- 12.Meier HCS et al. (2016) Early life socioeconomic position and immune response to persistent infections among elderly Latinos. Social Science & Medicine 166, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobak M et al. (2000) Own education, current conditions, parental material circumstances, and risk of myocardial infarction in a former communist country. Journal of Epidemiology and Community Health 54, 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinse GE et al. (2018) Prescription medication use and antinuclear antibodies in the United States, 1999–2004. Journal of Autoimmunity 92, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao JNK and Scott AJ (1987) On simple adjustments to chi-squared tests with survey data. Annals of Statistics 5, 385–397. [Google Scholar]
- 16.Kuwana M (2014) Helicobacter pylori-associated immune thrombocytopenia: clinical features and pathogenic mechanisms. World Journal of Gastroenterology 20, 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basso D et al. (2000) Antigastric autoantibodies in Helicobacter pylori infection: role in gastric mucosal inflammation. International Journal of Clinical Laboratory Research 30, 173–178. [DOI] [PubMed] [Google Scholar]
- 18.Faller G et al. (2000) Mucosal production of antigastric autoantibodies in Helicobacter pylori gastritis. Helicobacter 5, 129–134. [DOI] [PubMed] [Google Scholar]
- 19.Ram M et al. (2013) Helicobacter pylori serology in autoimmune diseases – fact or fiction? Clinical Chemistry and Laboratory Medicine 51, 1075–1082. [DOI] [PubMed] [Google Scholar]
- 20.Amedei A et al. (2003) Molecular mimicry between Helicobacter pylori antigens and H+, K+–adenosine triphosphatase in human gastric autoimmunity. Journal of Experimental Medicine 198, 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez-Arellano L and Maldonado-Bernal C (2014) Helicobacter pylori and neurological diseases: married by the laws of inflammation. World Journal of Gastrointestinal Pathophysiology 5, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wandl UB et al. (1992) Lupus-like autoimmune disease induced by interferon therapy for myeloproliferative disorders. Clinical Immunology and Immunopathology 65, 70–74. [DOI] [PubMed] [Google Scholar]
- 23.Frydman GH et al. (2015) Helicobacter pylori eradication in patients with immune thrombocytopenic purpura: a review and the role of biogeography. Helicobacter 20, 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanegas YAM and Vishnu P (2019) Management of Helicobacter pylori in patients with immune thrombocytopenia. Hamostaseologie 39, 279–283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268820000126.
click here to view supplementary material