Abstract
Unfractionated heparin is the first anticoagulant drug and has been successfully used clinically for over 80 years. Heparin and its analogues are used during surgery and dialysis and are often used to coat indwelling catheters and other devices where the vascular system is exposed. Most of the heparins used clinically are derived from porcine intestinal mucosa. However, heparins have also been manufactured from tissues of other mammalian species such as cows and sheep. Recently there have been attempts to generate bioengineered heparin in order to overcome contamination and antigenicity problems. Currently there are some concerns about the shortage of the porcine heparins as they are widely used in the manufacturing of the low-molecular-weight heparins. Moreover, due to cultural and religious reasons in some countries, alternative sources of heparins are needed. The Food and Drug Administration and other regulatory agencies have considered alternative sourcing of heparin for potential substitution of porcine heparin and are currently reviewing this matter. Numerous studies are ongoing to understand the structure-activity relationships of these various heparins. In this article, heparins from different animal sources were studied to determine the extent of biosimilarity between them. For these investigations, 10 batches each of bovine mucosal heparin (BMH), ovine mucosal heparin (OMH), and porcine mucosal heparin (PMH) were studied. These studies have demonstrated that OMH and PMH have comparable anticoagulant and antiproteases activities. However, BMH exhibited somewhat a lower potency compared to OMH and PMH in functional assays.
Keywords: porcine, bovine, ovine, biosimilarity, heparin, anticoagulants
Background
Heparin was first used as an anticoagulant in the early part of the 20th century.1 The controversy surrounding its discovery stems back to 1916 when a medical student named Jay McLean isolated a fat soluble anticoagulant compound (phosphatides) from the liver of dogs.1 This took place in the laboratory of William Henry Howell, who later presented findings related to the discovery of a water soluble carbohydrate compound which was distinct from the discovery by McLean.1 Since then, the pharmaceutical industry has worked to optimize the process of its purification.
Heparin is a highly sulfated glycosaminoglycan that consists of repeating disaccharide units, containing iduronic acid (or glucuronic acid) and glucosamine, exhibiting variable degrees of sulfation.2 Glycosaminoglycan heparin is processed to pharmaceutical grade heparin, which can undergo controlled depolymerization to produce low-molecular-weight heparins (LMWHs) whose molecular weights (MWs) are approximately one-third that of the parent heparin.3 Different LMWHs have distinct therapeutic and pharmacological properties due to differences in their chemical structure and degree of depolymerization.4 Heparin’s mechanism of action depends in large part on a specific pentasaccharide sequence that enables binding to antithrombin (AT). This binding results in a conformational change in AT which facilitates inactivation of thrombin (Factor IIa) and Factor Xa, leading to a significant increase in anticoagulant activity.2
Although most of the heparin used globally is obtained from porcine mucosa,5 bovine and ovine heparins have also been used in non-Western markets.6 Worldwide there are an estimated 1.4 billion cattle, 1.9 billion sheep and goats, and 980 million pigs. Both cows and sheep provide alternate sources of heparin which are not widely used primarily due to concerns of possible transmission of bovine spongiform encephalopathy (BSE) and scrapie through the parental use of heparin coming from infected animals.7 The use of primarily porcine heparin has led to shortages and an increased cost.8 Ultimately, due to the poorly regulated supply originating mainly from China, contamination with a semisynthetic over sulfated chondroitin sulfate took place, leading to what is understood as the heparin contamination crisis of 2007 and early 2008.9,10 This led to approximately 100 deaths in America as a result of a rapid anaphylactic reaction.9 The source of the contamination was never established, but could have taken place at any point during the production process, ranging from the isolation of the crude heparin from the intestine of pigs in China to the commercial distribution of heparin in the United States.11 Also of concern is the possibility of disease limiting the size of the pig population and thus the amount of raw material available for heparin production.11
Ovine heparin has not been widely used because of it has had a perceived lower potency than porcine and bovine heparins.12 This potency, however, was based on a study published in 1942 that used protamine and toluidine blue tests to estimate the potency of heparin. Sheep heparin required a higher dose to obtain the same clotting times measured by a coagulometer when compared to porcine, bovine, and canine heparins.12 At that time, the authors hypothesized that the difference in potency was attributed to the length of the heparin chain and not due to differences in the sulfur, nitrogen, carbohydrate, or acid components.12
With the advances in technology and manufacturing processes, higher quality bovine and ovine heparins are now available. In addition, the biologic contaminants such as BSE are eliminated.13 Currently bovine and ovine heparins are used in various parts of the world and have been shown to exhibit similar biologic and clinical profiles.6,14 The US Food and Drug Administration, European Medicines Agency, and other regulatory agencies have identified the importance of alternate resourcing of heparin for potential substitution of porcine mucosal heparin (PMH) and are currently reviewing this matter. Several Brazilian and Chinese manufacturers provide bovine and ovine heparins for clinical use.15 The purpose of this article is to determine the extent of biosimilarity between bovine, ovine, and porcine heparins.
Materials and Methods
Test Agents
Ten samples of PMH were obtained from Medefil Inc, Glendale Heights, Illinois. Ten samples of bovine mucosal heparin (BMH) were obtained from KinMaster Indústrias Químicas, Passo Fundo, Brazil. Ten samples of ovine mucosal heparin (OMH) were obtained from Ronnsi Pharmaceutical, China. The 10 samples of each origin were pooled to form 3 heparin pools. Each of the individual pool, namely porcine mucosal pool (PMH), bovine mucosal pool (BMH), and ovine mucosal pool (OMH) were prepared from 10 individual batches of the heparins from the same origin. The average MW of PMH, BMH, and OMH batches were 18.5 kDa, 21 kDa, and 17.5 kDa, respectively, as determined by high-performance liquid chromatography. All heparin samples were obtained as white powders which were stored at room temperature in a desiccator. These samples were weighed on a Mettler balance and 0.9% NaCl was used as a diluent to make stock solutions of 10 mg/mL for the in vivo and in vitro testing.
Proteins/Enzymes
Heparinase-I (EC 4.2.2.7) was obtained from IBEX Pharmaceuticals, Montreal, Canada. The material was provided as a solution and was stored at –70°C until use. Protamine sulfate was obtained from Sigma (St. Louis, Missouri) in powder form and reconstituted in normal physiologic saline at a concentration of 100 μg/mL. Human AT was obtained from Sekisui Diagnostics (Stamford, Connecticut) in vials containing 2.5 IU. The product was stored at 4°C prior to reconstitution with 1 mL of saline.
Blood Products
Normal human citrate phosphate dextrose adenine 1 plasma was obtained from the Blood Bank, Department of Pathology, Loyola University Medical Center, Maywood, Illinois. This plasma was obtained from blood donated by normal healthy human volunteers who tested negative for acquired immunodeficiency syndrome (AIDS) and hepatitis B and C viruses. The fresh frozen plasma packs (n = 5), not more than 30 days old, were thawed at 37°C, and then the pooled. Pooled plasma was aliquoted in volumes of 10 mL and refrozen at –70°C for a period of not more than 2 months.
Sample Analysis
The USP potency was determined using commercially available assay kits in accordance to the specifications and directions provided by the manufacturer (HYPHEN BioMed, Neuville-sur-Oise, France). Anti-Xa and anti-IIa activities were determined using in-house amidolytic assays9 employing human thrombin or bovine factor Xa (Enzyme Research Laboratories, South Bend, Indiana) and Spectrozyme TH or Spectrozyme Xa (Biomedical Diagnostics, Windsor, Nova Scotia, Canada). Assays were performed on an ACL ELITE (Werfen, Bedford, Massachusetts). Activated partial thromboplastin time (aPTT) was determined using TriniClot reagents (Tcoag, Wicklow, Ireland). A standard method used in the Hemostasis and Thrombosis Research Lab was employed to determine the anti-Xa and anti-IIa activities in a plasma-free AT supplemented system.16 Thrombin time (TT) was measured using a clot-based assay. Heparinase-I (HP) digestion studies were performed on the heparin pools where activity was determined, prior to and after the digestion using TT, aPTT, anti-Xa and anti-IIa assays. Protamine sulfate (PS) neutralization studies were carried out on the heparin pools. The pools were supplemented into blood bank plasma (BBP) to obtain final concentrations ranging from 0 to 10 μg/mL. Protamine sulfate was added to the heparin supplemented plasma to obtain a final protamine concentration of 10 μg/mL. Anticoagulant activity was determined using the aPTT and the TT assays. Antiprotease activity was determined using anti-Xa and anti-IIa assays.
Statistical Analysis
The experimental data are presented as the mean ± standard deviation. USP potency, IC50, the anticoagulant and the antiprotease assays were carried out on the individual pools and their respective components. The final concentrations of each preparation ranged from 0.0 to 10 μg/mL to obtain a concentration response curve for the calculation of various parameters. Each experiment was performed in 3 replicates (n = 3). Neutralization studies (Protamine sulfate and HP) were carried out on the individual pools and each experiment was performed in 3 replicates (n = 3). The results obtained from the biochemical experiments were analyzed using a 2-way analysis of variance (ANOVA) followed by a Bonferroni multiple comparison test to determine statistically significant differences between the effects of various heparins. Potencies (IC50) were compared using a 1-way ANOVA followed by a Tukey multiple comparison test. Other data were analyzed using one of the following statistical tests as such: Student t test (unpaired), 1-way ANOVA with a Tukey multiple comparison test, Pearson χ2 test, and Fisher exact test. In all cases a P value < .05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism for Windows (GraphPad Software, San Diego, California).
Results
Porcine mucosal heparin and OMH pools showed comparable anti-Xa and anti-IIa potencies while the potency of the BMH pool was notably lower. The USP potency of PMH pool as measured by anti-Xa assay was 190 ± 1.4 U/mg, whereas the OMH pool exhibited comparable potency of 197 ± 2.8 U/mg. In contrast, the USP potency of the BMH pool was significantly lower (135 ± 2.1 U/mg; P = .00531) as shown in Figure 1A and B. The USP potency as measured by anti-IIa assay showed the same trend. Ovine mucosal heparin and PMH exhibited comparable potencies of 200 ± 1.2 U/mg and 196 ± 1.4 U/mg respectively. However, the BMH pool exhibited significantly lower potency of 131 ± 1.3 U/mg (P = .0063) compared to others (Figure 1C and D).
Figure 1.
Comparative USP potencies as measured by the chromogenic anti-Xa and anti-IIa assays (Hyphen Kits). Anti-Xa of heparin pools (A) and heparins mean (B). Anti-IIa of heparin pools (C) and heparins mean (D). The USP potency of PMHs and OMHs were comparable. In contrast, the USP potency of the BMHs were significantly lower compared to others (N = 3, *P < .05). BMHs indicates bovine mucosal heparins; OMHs, ovine mucosal heparins; PMHs, porcine mucosal heparins.
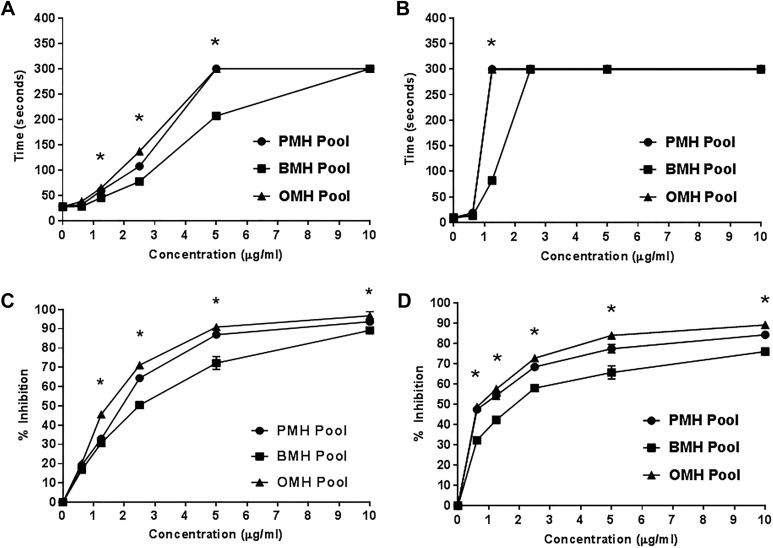
The anticoagulant and the antiprotease effects of PMH, OMH, and BMH pools as measured by aPTT, TT, anti-Xa, and anti-IIa assays are shown in Figure 2. All agents were supplemented into BBP over a concentration range of 0 to 10 μg/mL. In aPTT assay, OMH and PMH produced a significantly longer clotting time at concentrations above 2.5 μg/mL compared to BMH. At concentrations above 1.25 μg/mL, BMH produced significantly longer clotting times compared to baseline values. While OMH and PMH produced comparable prolongations in clotting time, BMH exhibited lower activity (Figure 2A). In TT assay, at concentrations above 1.25 μg/mL, the anticoagulant effects of OMH and PMH were comparable. The overall anticoagulant effects of BMH in the 5U Ca2+ TT assay was lower compared to OMH and PMH at concentrations less than 2.5 μg/mL (Figure 2B).
Figure 2.
Comparative anticoagulant and antiprotease effects in blood bank plasma (BBP) as measured by TT, aPTT, Anti-Xa, and anti-IIa assays. (A) Anticoagulant activity was determined by measuring the activated partial thromboplastin time (aPTT), (B) Anticoagulant activity was determined by measuring the thrombin time (TT), (C) Anti-Xa activities were measured using amidolytic assays, and (D) anti-IIa activities were measured using amidolytic assays. Porcine mucosal heparins and OMHs produced comparable anticoagulant and antiprotease activities, however, BMHs showed lower anticoagulant and antiprotease activities compare to others (N = 3, *P < .03). BMHs indicates bovine mucosal heparins; OMHs, ovine mucosal heparins.
Antiprotease actions of these agents were determined by amidolytic assays, which utilized substrates for factor Xa (Spectrozyme Xa) and thrombin (Spectrozyme TH). Optical density changes were used to determine the percent inhibition by each agent as compared to unsupplemented BBP.
Porcine mucosal heparin and OMH pools, when supplemented in BBP, exhibited comparable anti-Xa activity at concentrations higher than 2.5 μg/mL. Porcine mucosal heparin and OMH inhibited factor Xa at the highest concentration (10 μg/mL) by 93.7% ± 1.2% and 97% ± 1.5% respectively. In contrast, BMH pool exhibited lower anti-Xa activity compared to other pools and inhibited factor Xa at its highest concentration by 89.2% ± 0.4% (Figure 2C). In anti-IIa assays, at the concentration of 10 μg/mL, PMH and OMH pools inhibited factor IIa by 84.2% ± 0.7% and 89% ± 0.5%, respectively. While the BMH inhibited factor IIa by 76% ± 1.3% at the same concentration (Figure 2D).
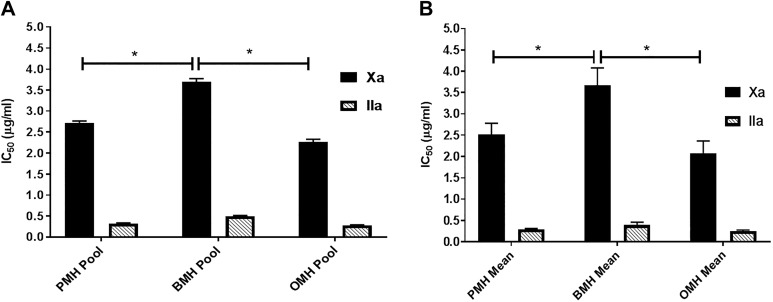
Heparins were supplemented in a purified AT system over a concentration range of 0 to 10 μg/mL and were mixed with a known amount of thrombin or factor Xa. The potency of each agent is presented in terms of IC50 values; the concentration required to produce 50% inhibition. Porcine mucosal heparin and OMH pools exhibited the highest anti-Xa potency with IC50 values of 2.72 ± 0.04 μg/mL and 2.26 ± 0.06 μg/mL, respectively, while BMH pool was significantly weaker to inhibit factor Xa with IC50 value of 3.7 ± 0.07 μg/mL (Figure 3A). In terms of anti-IIa potency, PMH and OMH pools showed the highest anti-IIa potency with IC50 values of 0.32 ± 0.02 μg/mL and 0.28 ± 0.01 μg/mL, respectively, while the BMH pool was significantly weaker at inhibiting factor IIa with an IC50 value of 0.5 ± 0.01 μg/mL (Figure 3A). The same trend was noted with the various heparin batches as shown in Figure 3B.
Figure 3.
Potency (IC50) comparison of various heparin agents when supplemented in purified AT system as measured by the amidolytic anti-Xa and anti-IIa assays. Heparin pools (A) and heparins mean (B). All agents were supplemented in the purified AT over a concentration range of 0 to 10 μg/mL. Porcine mucosal heparins and OMHs showed comparable IC50 values and both were more potent inhibitors of factor Xa and thrombin than BMHs (N = 3, P < .03). AT indicates antithrombin; BMHs bovine mucosal heparin; OMHs, ovine mucosal heparin.
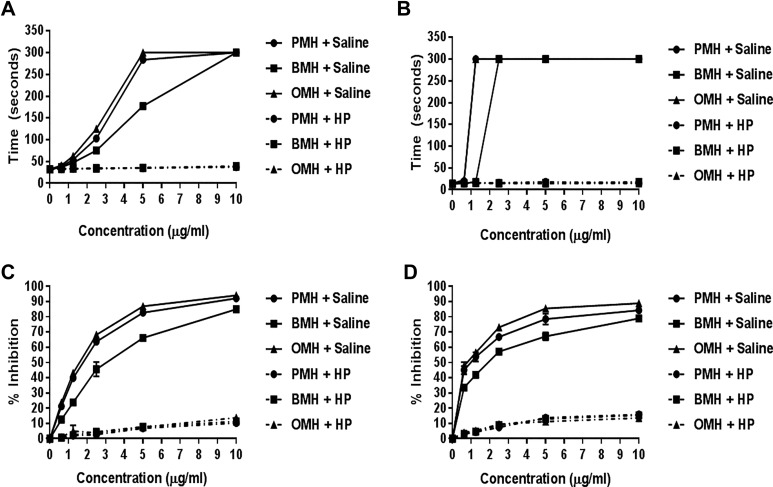
The neutralization of various heparin pools by HP digestion, as measured by the aPTT and TT assays, is shown in Figure 4A and B. When determined by the aPTT assay, HP strongly neutralized the anticoagulant effects of all heparin pools. The same trend was noted for TT assay. Heparinase-I was able to completely neutralize all heparin pools at all concentration points as determined by the amidolytic anti-Xa and anti-IIa assays (Figure 4C and D).
Figure 4.
Comparative neutralization profile of various heparin pools using heparinase-I (HP) as determined by the anticoagulant and antiprotease assays. aPTT assay (A), TT assay (B), Anti-Xa assay (C), and Anti-IIa (D). Heparinase-I (1 U/mL final concentration) was incubated with various heparin pools (10 mg/mL, pH = 5.0) at 37°C for 30 minutes. Heparinase-I completely neutralized all heparin pools at all concentration points in all assays (N = 3).
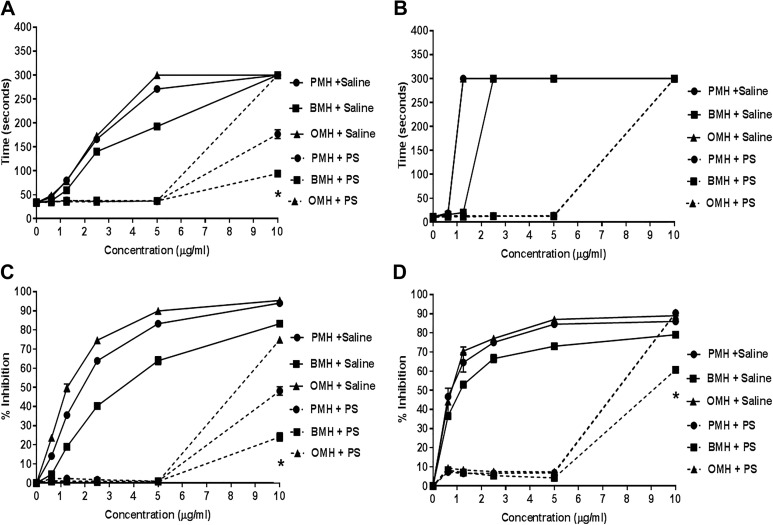
Protamine (10 μg/mL) was able to completely neutralize the anticoagulant effects of all heparins at concentrations at or below 5 μg/mL (Figure 5A and B). In TT assay, PS failed to neutralize the anticoagulants effects of all heparins at 10 μg/mL. The same trend was observed for the amidolytic anti-Xa and anti-IIa assays. Protamine sulfate was able to completely neutralize the different heparin pools at concentrations at or below 5 μg/mL. However, at concentration (10 μg/mL), PS partially neutralized PMH and BMH and failed to neutralize OMH (Figure 5C and D).
Figure 5.
Comparative neutralization profile of various heparin pools using protamine sulfate (PS) as determined the anticoagulant and antiprotease assays. aPTT assay (A), TT assay (B), Anti-Xa assay (C), and Anti-IIa (D). All agents were supplemented in BBP over a concentration range of 0 to 10 (μg/mL). Protamine sulfate was added to the heparin supplemented plasma to obtain a final PS concentration of 10 (μg/mL). Protamine sulfate completely neutralized all heparin pools at or below 5 (μg/mL) in all assays (N = 3, *P <.05).
Discussion
Heparin is a heterogeneous drug in terms of its MW, structural composition, and its biological actions. Preparations of heparin can contain fractions that vary in MW from 1.5 kDa to 30 kDa.17 Most unfractionated heparins (UFHs) are derived from porcine intestinal mucosa, however, heparin has also been manufactured from other mammalian sources such as bovine (cow) and ovine (sheep) tissues.8,18 Nowadays, there are some concerns about a shortage of porcine heparin. The FDA and other regulatory agencies are currently considering the introduction of bovine UFH for parenteral indications and ovine heparin is being developed in non-US markets. As heparins derived from different source material would ideally be used clinically as interchangeable drugs, it is important to fully understand the similarities and differences of various heparins. In this article, the differences among bovine, ovine, and porcine heparins were investigated by comparing the biochemical and pharmacological effects of these heparins in a variety of in vitro assays.
The therapeutic effects of heparins are predominately due to their interactions with a number of plasma proteins including factor Xa and thrombin. The ability of heparins to bind to and inhibit such serine proteases is predominately mediated by cofactors such as AT and Heparin Co-factor II (HCII).
The anticoagulant and antiprotease actions of BMH, OMH, and PMH were studied following supplementation in BBP. The anticoagulant and antiprotease effects were measured using the aPTT, TT, anti-Xa, and anti-IIa assays. In the aPTT assay, the anticoagulant effects of OMH and PMH heparins were comparable and both exhibited significant anticoagulant effects at concentrations above 2.5 μg/mL compared to BMH. At concentrations above 5 μg/mL, BMH produced significantly longer clotting times compared to baseline values. The concentrations of heparin needed to double the aPTT for OMH (1.21 ± 0.08 μg/mL) and PMH (1.25 ± 0.11 μg/mL) were significantly lower (P = .05) compared to the BMH (1.9 ± 0.09 μg/mL). In the TT assay, OMH and PMH showed comparable anticoagulant effect and both exhibited significant clotting time prolongation at the 1.25 μg/mL concentration, while BMH exhibited significant anticoagulant effects at a higher concentration (2.5 μg/mL).
The antiprotease activities of the heparins were measured in terms of the inhibition of factor Xa and thrombin at concentration ranging from 0 to 10 μg/mL. Porcine mucosal heparin and OMH exhibited comparable anti-Xa activity at all concentrations. In contrast, BMH exhibited a lower anti-Xa activity compared to the other heparins, and its maximal inhibition of factor Xa (89.2% ± 0.4%) was lower than that observed for PMH (93.7% ± 1.2%) and OMH (97% ± 1.5%).The same pattern was noted with anti-IIa activity. At a concentration of 10 μg/mL, PMH and OMH inhibited thrombin by 84.2% (±0.7%) and 89% (±0.5%), respectively. While BMH inhibited thrombin by 76% (±1.3%) at the same concentration. The overall results from these global anticoagulants and amidolytic antiprotease studies demonstrate that the BMH was consistently weaker than PMH and OMH samples.
The USP potencies of the heparins were compared using chromogenic anti-Xa and anti-IIa assays. The USP potency of PMH batches ranged from 189 to 190 U/mg, whereas the OMH batches exhibited comparable potencies in the range of 191 to 197 U/mg. In contrast, the USP potency of the BMH batches were much lower, ranging from 135 to 138 U/mg. The anti-Xa/IIa ratios for all heparins were comparable. Overall results from these studies showed that BMH has lower potency compared to OMH and PMH.
In assays measuring AT-dependent inhibition of factor Xa ad thrombin, the IC50 values for OMH and PMH were significantly lower than that of BMH, as measured by both assays. These studies suggest that OMH and PMH are more potent than BMH. A possible explanation of this observation could be that BMH has a lower AT binding content compared to OMH and PMH which alters its ability to inhibit factor Xa and thrombin. This observation has been reported in an earlier publication.19
Heparin, and its analogues, are used during surgery and dialysis, and are often used to coat indwelling catheters and other devices where the vascular system is exposed. As such, the ability to reverse the biological effects of heparins is of considerable importance. Presently, the anticoagulant and bleeding effects of heparin are neutralized by protamine sulfate. The objective of these studies was to determine the neutralization profiles of various heparin antagonists, such as HP and PS. Heparinase-I is an enzyme purified and characterized from Flavobacterium heparinum. 20 The cleavage of heparin by HP occurs at the linkage regions between the GlcNS6S (1→4) IdoA2S and GlcNS3S6S (1→4) IdoA2S residues.21 Xiao et al demonstrated that the GlcNS3S6S (1→4) IdoA2S linkage, which is found mainly within the AT binding site, is more susceptible to cleavage by HP than the major heparin disaccharide repeating unit GlcNS6S (1→ 4) IdoA2S.
These studies showed that the anticoagulant and antiprotease activities of all heparins (BMH, OMH, and PMH) were completely neutralized by HP to almost the same degree. This is due to the exhaustive digestion of the chains present in these heparins.
The interaction of protamine with heparin and its analogs have been extensively studied.22 In the aPTT and TT assays, PS was able to completely neutralize the anticoagulant effects of the different heparins at concentrations at or below 5 μg/mL. In antiprotease assays, PS was able to completely neutralize the different heparins at concentrations at or below 5 μg/mL. However, at 10 μg/mL, the neutralization effects of PS were variable. These results suggested that heparins from different animal sources could be neutralized by PS. This is probably because OMH, BMH, and PMH have chain lengths containing greater than 18 saccharides, which is essential for optimal PS binding to heparin and its neutralizing effects.23
The studies reported in this communication clearly suggest that bovine and ovine heparins maybe substituted for porcine heparin for anticoagulation purposes. As the supply of the porcine heparin is strained by several factors, resourcing of heparin from other animal sources is a viable alternate. Recently, an outbreak of African swine fever has reduced the availability of porcine tissues due to profound reduction in big populations. Hundreds of millions of animals have died or expected to die due to disease or culling resulting in mark reduction of pig population.24 Currently Chinese authorities and manufactures are exploring the options to solve this problem.
As the US heparin supply is dependent on Chinese manufacturers and up to 70% of the US heparin is imported from China, the US government is concerned on the dependence on one source and the potential impact of its shortage on patient usage.25 Open heart surgery, hemodialysis, and interventional procedures are totally dependent on UFH. Moreover, the LMWHs used in the United States are mainly derived from porcine heparin. For these reasons, bovine and ovine heparins provide feasible options to keep the supply of this anticoagulant to meet the clinical needs.
The studies reported in this manuscript have certain limitations in terms of number of batches of individual heparins used, numbers of replicates carried out in various matrices including citrated plasma preparation obtained from Loyola blood bank. Population-based variations in heparin responses are known due to the endogenous composition in-terms of levels of coagulation factors and inhibitors. Nevertheless, these studies were carried out in replicates of 3 for each assay used, which is the standard practice for the in-vitro evaluation of the anticoagulant and antiprotease effects of heparin.
Conclusions
The global anticoagulant and antiprotease effects of the BMH are lower than PMH and OMH samples. Ovine and porcine heparins exhibit comparable anticoagulant and antiprotease activities. The chromogenic antiprotease assays using Biomed kits confirm that the USP potency of OMH and PMH are comparable and range from 180 to 210 U/mg. In contrast, the USP potency of BMH is much lower and ranges from 110 to 150 U/mg. The anti-Xa/IIa ratio for all agents are comparable. In the purified AT supplemented system, the OMH and PMH preparations show lower IC50 values for both the thrombin and F-Xa inhibition in contrast to the BMH. The neutralization studies show that all heparins are neutralized by protamine sulfate and HP to similar degrees. Although, some functional differences are noted between the BMH, OMH, and PMH, the results from this investigation clearly demonstrate the comparability of OMH and PMH. The BMH consistently exhibited lowered potency in all assays. Potency adjusted BMH may exhibit comparable anticoagulant effects to OMH and PMH. Additional studies are warranted to validate this hypothesis.
Acknowledgments
The authors are thankful to the staff of the Hemostasis Research Laboratory for their skillful assistance in completing this study. Special thanks to Mr Jonas Kingo of Aniara (Mason, Ohio) for helpful suggestions for the USP assays and the anti-Xa and anti-IIa kits used in these studies. The authors are also grateful to Ms Valentina Baigorria of Kin Master, Brazil, for providing the bovine mucosal heparin samples and to Mr Yimimg Yao of Ronnsi Pharmaceutical, China, for the supply of ovine heparins.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The studies were supported by internal research funds and the Hemostasis Research Laboratory and Cardiovascular Institute, Health Science Division, Loyola University Chicago.
ORCID iDs: Ahmed Kouta  https://orcid.org/0000-0001-7579-9572
https://orcid.org/0000-0001-7579-9572
Debra Hoppensteadt  https://orcid.org/0000-0001-8235-3624
https://orcid.org/0000-0001-8235-3624
Jawed Fareed  https://orcid.org/0000-0003-3465-2499
https://orcid.org/0000-0003-3465-2499
References
- 1. Wardrop D, Keeling D. The story of the discovery of heparin and warfarin. Br J Haematol. 2008;141(6):757–763. [DOI] [PubMed] [Google Scholar]
- 2. Christopher ML, Elizabeth L, Frank L. Laboratory monitoring of heparin therapy; partial thromboplastin time or anti-Xa assay. Labmed. 2009;40(1):47–51. [Google Scholar]
- 3. Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost. 1999;25(suppl 3):5–16. [PubMed] [Google Scholar]
- 4. Bisio A, Mantegazza A, Vecchietti D, et al. Determination of the molecular weight of low-molecular-weight heparins by using high-pressure size exclusion chromatography on line with a triple detector array and conventional methods. Mole. 2015;20(3):5085–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulloy B, Gray E, Barrowcliffe TW. Characterization of unfractionated heparin: comparison of materials from the last 50 years. Thromb Haemost. 2000. 84(6):1052–1056. [PubMed] [Google Scholar]
- 6. Jasper JP, Zhang F, Russell BP, Linhardt RJ. Stable isotopic analysis of porcine, bovine, and ovine heparins. J Pharm Sci. 2015;104(2):457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Possible Implications of Bovine Spongiform Encephalopathy for the Safety of Bovine Heparin. www.fda.gov/downloads/AdvisoryCommittees. Accessed June, 2014.
- 8. Fu L, Li G, Yang B, et al. Structural characterization of pharmaceutical heparins prepared from different animal tissues. J Pharm Sci. 2013;102(5):1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu H, Zhang Z, Linhardt RJ. Lessons learned from the contamination of heparin. Nat Prod Rep. 2009;26(3):313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Szajek AY, Chess E, Johansen K, et al. The US regulatory and pharmacopeia response to the global heparin contamination crisis. Nat Biotechnol. 2016;34(6):625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fairclough G. How heparin maker in China tackles risk. Wall Street Journal; 2015. Accessed March 8, 2010. [Google Scholar]
- 12. Jaques LB, Waters ET, Charles AF. A comparison of the heparins of various mammalian species. J Biol Chem. 1942;144:229–235. [Google Scholar]
- 13. Asher DM. Bovine sera used in the manufacture of biologicals: current concerns and policies of the US Food and Drug Administration regarding the transmissible spongiform encephalopathies. Dev Biol Stand. 1999;99:41–44. [PubMed] [Google Scholar]
- 14. Hoppensteadt D, Maia P, Silva A, et al. Resourcing of heparin and low molecular weight heparins from bovine, ovine and porcine origin. Studies to demonstrate the biosimilarities. Blood. 2015;126(3):4733. [Google Scholar]
- 15. Monakhova YB, Diehl BWK, Fareed J. Authentication of animal origin of heparin and low molecular weight heparin including ovine, porcine and bovine species using 1D NMR spectroscopy and chemometric tools. J Pharmaceut Biomed Anal. 2018;149:114–119. [DOI] [PubMed] [Google Scholar]
- 16. Hoppensteadt DA, Walenga JM, Fareed J. Validity of serine protease inhibition tests in the evaluation and monitoring of the effect of heparin and its fractions. Semin Thromb Hemost. 1985;11(2):112–120. [DOI] [PubMed] [Google Scholar]
- 17. Nader HB, McDuffie NM, Dietrich CP. Heparin fractionation by electrofocusing: presence of 21 components of different molecular weights. Biocheml Biophys ResCommu. 1974;57(2):488–493. [DOI] [PubMed] [Google Scholar]
- 18. Watt DK, Yorke SC, Slim GC. Comparison of ovine, bovine and porcine mucosal heparins and low molecular weight heparins by disaccharide analyses and 13C NMR. Carbohydrate Polym. 1997;33(1):5–11. [Google Scholar]
- 19. Guerrini M, Rudd TR, Mauri L, et al. Differentiation of generic enoxaparins marketed in the United States by employing NMR and multivariate analysis. Anal Chem. 2015;87(16):8275–8283. [DOI] [PubMed] [Google Scholar]
- 20. Lohse DL, Linhardt RJ. Purification and characterization of heparin lyases from flavobacterium heparinum. J Biol Chem. 1992;267(34):24347–24355. [PubMed] [Google Scholar]
- 21. Xiao Z, Zhao W, Yang B, et al. Heparinase 1 selectivity for the 3, 6-di-O-sulfo-2-deoxy-2-sulfamido-α-D-glucopyranose (1, 4) 2-O-sulfo-α-L-idopyranosyluronic acid (GlcNS3S6S-IdoA2 S) linkages. Glycobiology. 2010;21(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Racanelli A, Fareed J, Walenga JM, Coyne E. Biochemical and pharmacologic studies on the protamine interactions with heparin, its fractions and fragments. Semin Thromb Hemost. 1985;11(2):176–189. [DOI] [PubMed] [Google Scholar]
- 23. Harenberg J, Gnasso A, De Vries JX, Zimmermann R, Augustin J. Inhibition of low molecular weight heparin by protamine chloride in vivo. Thromb Res. 1985;38(1):11–20. [DOI] [PubMed] [Google Scholar]
- 24. https://edition.cnn.com/2019/07/26/business/african-swine-fever-china-pork/index.htmlChinahasanewplantosolveitsporkproblem. Accessed July 26, 2019.
- 25. Bipartisan E&C Leaders Request FDA Briefing on Threat to U.S. Heparin Supply. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-suppl. Accessed July 30, 2019.