Abstract
Gaucher disease (GD) is one of the most important lysosomal storage disorders. T-lymphocytes perform and regulate many of the immune processes and play a major role in immune homeostasis. Studies have shown that GD causes impairment in T-lymphocyte functions, although the role and status of T-lymphocytes in GD are still under investigation. It is still not fully known how GD leads to the altered biochemical and immunological cellular functions observed in the disease. Our study aimed to evaluate the variations of regulatory T-lymphocytes (Tregs) in 20 Egyptian children with GD under enzyme replacement therapy, managed in Assiut University Hospitals. Tregs were detected using 3-color flow cytometric immunophenotyping, in which subpopulations of T-lymphocytes and the expression of CD4+ on their surfaces were gated. The expression of CD25+ was assessed on CD4+ cells with different gates to define CD4+CD25, CD4+CD25+high, and CD4+CD25+ low cells. Then, CD4+CD25+highFoxp3+cells and MFI of Foxp3+ expression on CD4+CD25+ high were determined. We found the levels of CD4+CD25+/CD4+, CD4+CD25+high/CD4+, CD4+CD25+highFoxp3+ Tregs, and median fluorescence intensity of Foxp3+ expression on CD4+CD25+high were significantly lower in children with GD compared to healthy controls. In conclusion, our data showed significantly decreased regulatory T-lymphocytes in children with GD. The reduced effect of Tregs may have a role in the pathogenesis of immune dysregulation in children with GD. The relationship of these cells to immune disorders in GD children remains to be determined. Therefore, we recommend further studies to elucidate the role and function of Tregs in GD and its potential role in the disease phenotype, as well as how it is affected by electrical resistivity tomography.
Keywords: Gaucher disease, regulatory T-lymphocytes, children
Introduction
Gaucher disease (GD) is a lysosomal storage disease caused by the deficiency of glucocerebrosidase enzyme, leading to the accumulation of undigested glucosylceramide in the reticuloendothelial system cells mainly the macrophages (Gaucher cells), which accumulate in different organs mainly bone marrow, spleen, and liver giving the disease its distinct clinical picture.1 Although less common, the heart, lungs, and kidneys may be infiltrated by Gaucher cells.2,3 T-lymphocytes perform and regulate many of the immune processes and play a major role in immune homeostasis. Studies have shown that GD causes impairment in T-lymphocyte functions, although the role and status of T-lymphocytes in GD are still under investigation. It is still not fully known how GD leads to the altered biochemical and immunological cellular functions observed in the disease.1–3 Previous studies have shown a significant reduction in CD4+, Tγδ2, and natural killer (NK) T-cells and a significant increase in CD4+ memory cells. While other studies have confirmed decreased numbers of CD4+ and CD8+, others have found an increase in CD8+ and a decrease in CD4+, which was independent of whether patients received enzyme replacement therapy (ERT) or not. On the other hand, increased interferon gamma (IFNγ)-producing T-lymphocytes, a reversal ratio of CD4+/CD8+, and decreased levels of regulatory T-cells (Tregs) were reported by other studies.4–8 The study aimed to evaluate the variations in Tregs in patients with GD under ERT and possible contribution to the disease phenotype.
Patients and Methods
Study Design and Population
This case–control study was carried out in Assiut University Children Hospital. Our Assiut University ethics committee approved our study. Informed written consent was taken from caregivers of all participants.
Patients
Our study included 20 patients with confirmed GD aged 3 to 16 years (12 males). We recruited all patients from the Pediatric Hematology Unit, Assiut University. Twenty sex- and age-matched healthy children were included as controls. All patients with GD received ERT; imiglucerase 45 IU every 2 weeks. All patients are on stable ERT dose for 1 year before the enrolment. We excluded any patient with unconfirmed GD diagnosis, chronic immunosuppressive disease, or received any immunosuppressive drugs, for example, steroids, within 15 days before the study and patients who required a change in their ERT dose 1 year before the study.
Methodology
The medical records of all patients were reviewed, and the following data were collected; anthropometric measurements, the presence or absence of bone pain, neurological manifestations, squint, chest involvement, cardiac involvement, liver span, spleen span, and the presence of Erlenmeyer flask deformity. Venous blood samples were taken from all participants for complete blood count and C-reactive protein (CRP) evaluation, and flow cytometric analysis, to evaluate the levels of Tregs.
Flow Cytometric Detection of Regulatory T-Cells
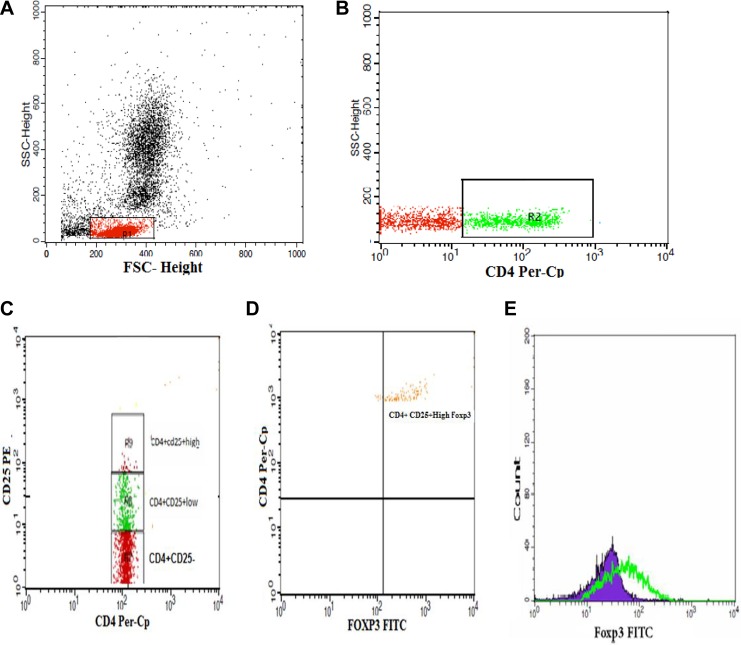
Regulatory T-cells were estimated using fluoroisothiocyanate-conjugated Foxp3 (eBioscience, San Jose, CA), peridinium-chlorophyll-protein-conjugated CD4 (Becton Dickinson Biosciences, San Jose, CA), and phycoerythrin-conjugated CD25 (IQ Product, Rozenburglaan, Groningen Netherlands). Fifty microliters of the blood was incubated with 10 µL of CD25, CD4 in a dark room for 20 minutes. Following incubation and red blood cell lysis and washing with phosphate-buffered saline (PBS), we added a fixed solution to fix the cells and incubated for 10 minutes. The cells were washed with PBS, and then permeabilizing solution and 10 µL of Foxp3 were added and incubated for 30 minutes at room temperature. Flow cytometric analysis was done by fluorescence-activated cell sorter calibur flow cytometry with Cell Quest software (Becton Dickinson Biosciences). We used antihuman immunoglobulin G as an isotype-matched negative control for all samples. Forward and side scatter histogram was used to define the lymphocyte population. Following this, the percentages of CD4+ were assessed on the lymphocyte population. Then the total CD4+CD25+ cells, CD4+CD25-cells, CD4+CD25+lowcells, CD4+CD25+high cells, and CD4+CD25+highFoxp3+ Tregs were evaluated on CD4+ cells. The expression of Foxp3+ on CD4+CD25+high cells was expressed as the geometric mean of fluorescence intensity (MFI), as shown in Figure 1.
Figure 1.
Flow cytometric detection of regulatory T-cells. A, Forward and side scatter histogram was used to define the lymphocytes population (R1). B, The expression of CD4 on the lymphocyte population was then gated. C, The expression of CD25 was then assessed on CD4+lymphocytes, and different gates were drawn to define CD4+ CD25− cells, CD4+CD25+low cells, and CD4+CD25+high cells. D, Then, the percentage of CD4+CD25+high Foxp3+cells was determined. E, The expression of Foxp3+ on CD4+CD25+high cells as a geometric mean of fluorescence intensity.
Statistical analysis
We used SPSS version 18 (SPSS, Inc, Chicago, Illinois) for the analysis of our data. Quantitative data were expressed as mean ± standard deviation, and qualitative data were expressed as number and percentage. We used the Mann–Whitney test for continuous variables, χ2 test for categorical variables, and Spearman correlation coefficient for the examination of correlation among different studied parameters. P value <.05 was used as a sign of a significant difference.
Results
Table 1 shows the main demographic, clinical, and laboratory characteristics of all patients and controls. All patients were on ERT (mean duration of 20 months, range 13-57 months). We found significantly decreased percentages and absolute counts of total CD4+CD25+/CD4+(P = .009 and P = .017, respectively) and CD4+CD25+high/CD4+ (P = .0001 and P = .002, respectively) in GD patient group when compared to healthy children. Furthermore, CD4+CD25+highFoxp3+ Tregs and MFI of Foxp3+ expression on CD4+CD25+high were also significantly lower in patients group than control group (P = .001 and P = .0001, respectively; Table 1). On the other hand, the percentages and absolute counts of CD4+CD25+low/CD4+ were comparable between both groups. The frequencies of CD4+CD25+high Foxp3+ Tregs had significant negative correlations with CRP (r = −0.43, P = .013), total rates of viral infections (r = −0.56, P = .031), and recurrent lower respiratory tract infections (r = −0.70, P = .017). There were no significant correlations between Tregs percentages and patients’ age, sex, age at onset of ERT and bacterial upper respiratory tract, and gastrointestinal infections.
Table 1.
Demographic Characteristics and Laboratory Data in Patients and Controls.
| Patients (20) | Controls (20) | P Value | |
|---|---|---|---|
| Weight (kg) | 32.25 ± 12.6 | 42.1 ± 1.3 | .04a |
| Age (years) | 10.8 ± 2.81 | 11.02 ± 2.87 | NS |
| Gender M/F | 12/8 | 11/9 | NS |
| Liver span/cm (median ± SD) | 13.9 ± 2.3 | 7.6 ± 1.3 | .001a |
| Spleen span/cm (median ± SD) | 11.7 ± 5 | 6.9 ± 1.2 | .003a |
| Bone involvement | |||
| Erlenmeyer flask deformity | 6/20 | – | – |
| Osteopenia | 4/20 | ||
| Fracture | 1/20 | ||
| Kyphoscoliosis | 3/20 | ||
| Pigeon chest | 2/20 | ||
| Avascular necrosis of hip joint | 1/20 | ||
| Neurological manifestation | |||
| Convulsions | 1/20 | – | – |
| Squint | 3/20 | ||
| Chest involvement | |||
| Pulmonary fibrosis | 1/20 | ||
| C-reactive protein | 10.4 ± 4.7 | 0.9 ± 0.4 | .0001a |
| Hemoglobin (g/dL) | 10.14 ± 1.79 | 12.70 ± 2.67 | .004a |
| Platelets (109/L) | 241.05 ± 68.1 | 225.11 ± 73.9 | NS |
| White blood cells | 6.80 ± 1.18 | 10.81 ± 2.14 | .001a |
| CD4+CD25+/CD4+ (%) | 18.36 ± 2.11 | 20.39 ± 2.50 | .009a |
| CD4+CD25low/CD4+ (%) | 13.49 ± 1.63 | 14.03 ± 2.36 | NS |
| CD4+CD25+high/CD4+ (%) | 5.67 ± 0.98 | 7.16 ± 0.94 | .0001a |
| CD4+CD25+highFoxp3+ regulatory T-cells (%) | 1.36 ± 0.73 | 2.12 ± 0.65 | .001a |
| MFI of Foxp3+ expression on CD4+CD25+high | 84.71 ± 19.18 | 117.08 ± 15.77 | .0001a |
Abbreviations: F, female; M, male; MFI, mean of fluorescence intensity; NS, non-significant; SD, standard deviation.
aIndicates significant.
Discussion
Although evidence suggests immune dysregulation plays a pivotal part in the clinical picture of GD, the exact role and status of T-lymphocytes in GD is still under investigation, and little is known about it, especially in children.8 In our study, we evaluated the variations of Tregs in children with GD under ERT and its impact on the disease phenotype. Regulatory T-cells were detected using flow cytometry in which the population of lymphocytes and the expression of CD4 on their surfaces was gated. The expression of CD25+ was assessed on CD4+ lymphocytes with different gates to define CD4+CD25− cells, CD4+CD25+high cells, and CD4+CD25+low cells. Then, CD4+CD25+high Foxp3+cells and MFI of Foxp3+ expression on CD4+CD25+high were determined. Our findings could demonstrate that the levels of CD4+CD25+/CD4+, CD4+CD25+high/CD4+, CD4+CD25+highFoxp3+ Tregs and MFI of Foxp3+ expression on CD4+CD25+high were significantly lower in GD children compared to normal controls. This may suggest that the reduced effect of Tregs may have a role in the pathogenesis of immune dysregulation in children with GD. In line with our results, Sotiropoulos et al4 has reported significant reductions in CD3+/CD4+ T-helper cells (P = .002) and the CD4+ Tregs compartment with significantly lower levels of absolute numbers and percentages of CD4+CD25high T-cells (P = .039), and CD4+CD25high Foxp3+ Tregs (P = .036) in patients with GD when compared to healthy subjects. In addition, they reported a significantly high percentages of IFNγ-producing CD4+ (P = .0003), and CD8+ T-lymphocytes (P = .023). Rodic et al7 found a significantly lower count of leucocytes, T-lymphocytes, CD4+, CD8+ T-cells, and NK cells in 30 patients with GD when compared to healthy controls.
Moreover, they reported significantly decreased levels of CD4+CD25high Foxp3+ Tregs (1.4%) and CD4+CD25dim Foxp3+ Tregs (2.3%) in patients with GD as compared to healthy control.7 Another study5 reported a decreased proportion of CD4+ cells and an increased proportion of CD8+ cells in patients with GD with or without ERT. They suggested that these changes might be the result of increased apoptotic signals after the activation of TCD4+ cells.5 Other researchers found significant decreased absolute numbers of CD4+ and CD8+ T-cells among patients with GD with marked bone manifestations.6 However, we could not approve this correlation in our patients, which may be due to the younger age of our patient group. Our study and the previous researches confirmed that patients with GD show primarily quantitative and functional abnormalities of the adaptive immunity. The CD4+ cells and CD4+CD25+highFoxp3+ Tregs compartments are the most affected subpopulations. Impairment of the functions of T-helper cells seems to be a significant feature in patients with GD. T-helper cells play a crucial role in fighting infections, and therefore, their reduction as part of the GD itself or as a result of ERT is an essential aspect of the disease and or its treatment process that needs to be addressed.3 The increased incidence of infections seen in our patient population on ERT might be attributed to the reduction in the CD4+CD25 found in this population.
Moreover, CD4+ CD25+ Tregs are critical for the preservation of immune tolerance,9,10 which suggests an explanation for why autoimmune diseases and malignancies were reported in GD cases.7,11
Our study has some limitations. First, we investigated a low number of children with GD from a single center. Second, we were unable to investigate the impact of treatment on Tregs abnormalities since all patients in our study were under ERT 1 year before the study.
In conclusion, our data showed a significant decreased Tregs in children with GD. The reduced effect of Tregs may have a role in the pathogenesis of immune dysregulation in children with GD. The relationship of these cells to immune disorders in GD children remains to be determined. Therefore, we recommend further studies to elucidate the role and function of Tregs in GD and its potential role in the disease phenotype, as well as how it is affected by ERT.
Footnotes
Authors’ Note: K.S., K.E., A.M.A, and M.Y. designed the study and followed the analysis of patients. A.Z., S.F.D, M.A.A. performed all laboratory investigations in the study. A.A., E.M.E., R.R. analyzed the data and drafted the manuscript. All authors were involved in the critical analysis of the final version of the manuscript. All authors approved the manuscript as submitted and agreed to be accountable for all aspects of the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Khaled Saad  https://orcid.org/0000-0002-8473-6116
https://orcid.org/0000-0002-8473-6116
Esraa M. Eloseily  https://orcid.org/0000-0002-4770-7383
https://orcid.org/0000-0002-4770-7383
References
- 1. Zahran AM, Eltayeb AA, Elsayh KI, Saad K, Ahmad FA, Ibrahim AIM. Activated and memory T lymphocytes in children with Gaucher disease. Arch Immunol Ther Exp (Warsz). 2017;65(3):263–269. [DOI] [PubMed] [Google Scholar]
- 2. Martins AM, Valadares ER, Porta G, et al. Recommendations on diagnosis, treatment, and monitoring for Gaucher disease. J Pediatr. 2009;155(4 suppl):S10–S18. [DOI] [PubMed] [Google Scholar]
- 3. Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6(4):353–360. [DOI] [PubMed] [Google Scholar]
- 4. Sotiropoulos C, Theodorou G, Repa C, et al. Severe impairment of regulatory T-cells and Th1-lymphocyte polarization in patients with Gaucher disease. JIMD Rep. 2015;18:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balreira A, Lacerda L, Miranda CS, Arosa FA. Evidence for a link between sphingolipid metabolism and expression of CD1d and MHC-class II: monocytes from Gaucher disease patients as a model. Br J Haematol. 2005;129(5):667–676. [DOI] [PubMed] [Google Scholar]
- 6. Lacerda L, Arosa FA, Lacerda R, et al. T cell numbers relate to bone involvement in Gaucher disease. Blood Cells Mol Dis. 1999;25(2):130–138. [DOI] [PubMed] [Google Scholar]
- 7. Rodic P, Kraguljac Kurtovic N, Suvajdzic Vukovic N, et al. Flow cytometric assessment of lymphocyte subsets in Gaucher type 1 patients. Blood Cells Mol Dis. 2014;53(4):169–170. [DOI] [PubMed] [Google Scholar]
- 8. Matta MC, Soares DC, Kerstenetzky MS, Freitas AC, Kim CA, Torres LC. CD4+CD25 high Foxp3+ Treg deficiency in a Brazilian patient with Gaucher disease and lupus nephritis. Hum Immunol. 2016;77(2):196–200. [DOI] [PubMed] [Google Scholar]
- 9. Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30(5):616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. [DOI] [PubMed] [Google Scholar]
- 11. Cajaiba MM, Reyes-Múgica M. Gaucher or pseudo-Gaucher? The challenge of several diseases colliding in a pediatric patient. Hum Pathol. 2009;40(4):594–598. [DOI] [PubMed] [Google Scholar]