Abstract
The increased mortality reported with intensive glycaemic control has been attributed to an increased risk of treatment-related hypoglycaemia. This study investigated the relationships of haemoglobin (Hb) A1c, anti-hyperglycaemic treatment, and potential risks of adverse effects with all-cause mortality in patients with type 2 diabetes. Patients (n = 15,773) were stratified into four categories according to baseline HbA1c and then assigned to three target categories, based on whether HbA1c was ≤0.5% below or above (on-target), >0.5% below (below-target) or >0.5% above (above-target) their HbA1c goal, personalized according to the number of potential risks among age > 70 years, diabetes duration > 10 years, advanced complication(s), and severe comorbidity (ies). The vital status was retrieved for 15,656 patients (99.26%). Over a 7.4-year follow-up, mortality risk was increased among patients in the highest HbA1c category (≥8.5%) (adjusted hazard ratio, 1.34 (95% confidence interval, 1.22–1.47), p < 0.001) and those above-target (1.42 (1.29–1.57), p < 0.001). Risk was increased among individuals in the lowest HbA1c category (<6.5%) and those below-target only if treated with agents causing hypoglycaemia (1.16 (1.03–1.29), p = 0.01 and 1.10 (1.01–1.22), p = 0.04, respectively). These data suggest the importance of setting both upper and lower personalized HbA1c goals to avoid overtreatment in high-risk individuals with type 2 diabetes treated with agents causing hypoglycaemia.
Keywords: type 2 diabetes, HbA1c all-cause mortality, adverse treatment effects, hypoglycaemia
1. Introduction
The impact of strict glycaemic control on excess morbidity and mortality from cardiovascular disease (CVD) in individuals with type 2 diabetes is still a matter of debate [1]. Indeed, the post-trial follow-up of the United Kingdom Prospective Diabetes Study (UKPDS) showed a significant reduction of macrovascular outcomes, together with the persistence of microvascular benefits, in patients originally randomized to intensive treatment, thus supporting the need for strict glycaemic control since the early stage of the disease [2]. Conversely, other landmark intervention trials designed to achieve more ambitious glycaemic targets, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) [3], the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) [4], and the Veterans Affairs Diabetes Trial (VADT) [5], were successful in reducing the burden from microvascular disease, but failed to detect significant effects on the primary composite CVD outcome. In addition, in the intensive treatment arm, the ACCORD reported an increase in all-cause and CVD mortality, which led to the anticipated end of the trial [3], whereas the VADT showed a non-significant increment of the death rate [5]. These contrasting results have been related to differences in patients’ baseline clinical features between the UKPDS and the other three trials, i.e., newly diagnosed versus longstanding type 2 diabetes, younger versus older age, better versus worse glycaemic control, and low versus high CVD risk [6,7].
Altogether, the above findings prompted a substantial paradigm shift in glycaemic targets for patients with type 2 diabetes, moving from “one size fits all” to “personalized goals”. As a consequence, the recommended haemoglobin (Hb) A1c level of <7.0% for all individuals was changed to HbA1c values ranging from <6.5% to <8.5%, based on the presence and extent of several factors which may render the patient vulnerable to aggressive treatment, thus decreasing the benefits and increasing the harm from intensive glycaemic control [8]. The factors to be considered include age/life expectancy, disease duration, established complications, important comorbidities, risks associated with hypoglycaemia, individual attitude and expected treatment efforts, and resources and support systems.
However, the increased all-cause and CVD mortality reported among the ACCORD (and VADT) participants assigned to the intensive treatment group remains poorly understood. The extent (and velocity) of HbA1c reduction from baseline and the rate of severe hypoglycaemia were higher in the intensive arms of the ACCORD and VADT than in other trials [6,7], thus prompting the hypothesis that the increased mortality was attributable to hypoglycaemic episodes associated with (rapid) achievement of more stringent HbA1c targets in vulnerable individuals. However, although severe hypoglycaemia was associated with increased mortality, the risk of death associated with severe hypoglycaemia was relatively greater in the standard than in the intensive group of the ACCORD [9], ADVANCE [10], and VADT [11]. In addition, a post hoc analysis of the ACCORD trial showed that higher, not lower average HbA1c was associated with greater risk of death, which was higher with the intensive than with the standard strategy only when average HbA1c was >7.0% and when little or no decrease in HbA1c followed treatment initiation [12]. These findings suggest that patients with the smaller response on glycaemic control and, hence, with persistently higher HbA1c requiring more aggressive treatment, including insulin, are those at higher risk of hypoglycaemia and death, pointing to a more complex relationship among hypoglycaemia, achieved HbA1c, and treatment intensity.
This study aimed to investigate the relationships of on-treatment HbA1c levels, type of anti-hyperglycaemic treatment, and potential risks of adverse treatment effects with all-cause mortality in individuals with type 2 diabetes. To this end, we analysed the data from participants in the Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicentre Study, who were evaluated at baseline in the years 2006–2008, when the recommended HbA1c target was <7.0% for all patients.
2. Materials and Methods
2.1. Design
The RIACE is an observational, prospective, multicentre, cohort study on the impact of estimated glomerular filtration rate (eGFR) on morbidity and mortality in patients with type 2 diabetes [13]. The study was conducted in accordance with the Declaration of Helsinki. It was approved by the locally appointed ethics committees, and participants gave informed consent. Trial Registration: NCT00715481; www.ClinicalTrials.gov.
2.2. Subjects
The study population included 15,773 Caucasian patients (after excluding 160 individuals with missing or implausible values), consecutively attending 19 hospital-based, tertiary referral Diabetes Clinics of the National Health Service throughout Italy (see Supplementary Materials) in the years 2006–2008. Exclusion criteria were dialysis or renal transplantation. Traditional CVD risk factors and complications were determined as part of the baseline assessment using a standardized protocol across participating centres [13].
2.3. All-Cause Mortality
The vital status of study subjects on 31 October 2015 was verified by interrogating the Italian Health Card database (http://sistemats1.sanita.finanze.it/wps/portal/), which provides updated and reliable information on all current Italian residents [14].
2.4. Traditional Cardiovascular Disease (CVD) Risk Factors
The study subjects underwent a structured interview in order to collect the following information: age at the time of the interview, smoking status, known diabetes duration, co-morbidities, and current glucose-, lipid-, and blood pressure (BP)-lowering therapy [13].
Body mass index (BMI) was calculated from weight and height. Waist circumference was measured in 4618 subjects and estimated in the remaining 11,155 individuals from the log-transformed BMI values, as previously described [15]. BP was measured with a sphygmomanometer with the patients seated with the arm at the heart level.
HbA1c was measured by high-performance liquid chromatography (HPLC) using Diabetes Control and Complications Trial (DCCT)-aligned methods; triglycerides and total and high-density lipoprotein (HDL) cholesterol were determined in fasting blood samples by colorimetric enzymatic methods; non-HDL cholesterol was calculated by the formula: total cholesterol–HDL cholesterol; and low-density lipoprotein (LDL) cholesterol was calculated by the Friedewald formula. HbA1c variability was calculated for each patient as the intra-individual standard deviation (HbA1c-SD) of 3-to-5 (4.52 ± 0.76) HbA1c values obtained in 8252 individuals from 9 centres during the 2-year period preceding recruitment, including the enrolment visit [14].
2.5. Complications
The presence of diabetic kidney disease (DKD) was assessed by measuring albuminuria and serum creatinine, as previously detailed [13,16]. Albumin excretion rate was obtained from 24-h urine collections or calculated from the albumin-to-creatinine ratio in early-morning, first-voided urine samples, using a conversion formula developed in patients with type 1 diabetes and preliminarily validated in a subgroup of RIACE participants. Albuminuria was measured in fresh urine samples by immunonephelometry or immunoturbidimetry, in the absence of interfering clinical conditions. One-to-three measurements for each patient were obtained; in cases of multiple measurements, the geometric mean of 2–3 values was used for analysis. In individuals with multiple measurements, the concordance rate between the first value and the geometric mean was >90% for all albuminuria categories [16]. Serum (and urine) creatinine was measured by the modified Jaffe method and eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration equation [13]. Patients were then classified into Kidney Disease: Improving Global Outcomes categories of albuminuria (A1 to A3) and eGFR (G1 to G5) and assigned to one of the following DKD phenotypes: no DKD (i.e., A1G1-A1G2), albuminuria alone (albuminuric DKD with preserved eGFR, i.e., A2G1-A2G2-A3G1-A3G2), reduced eGFR alone (non-albuminuric DKD, i.e., A1G3-A1G4-A1G5), or both albuminuria and reduced eGFR (albuminuric DKD with reduced eGFR, i.e., A2G3-A2G4-A2G5-A3G3-A3G4-A3G5), as previously reported [13].
In each centre, the presence of diabetic retinopathy (DR) was assessed by an expert ophthalmologist by dilated fundoscopy. Patients with mild or moderate non-proliferative DR were classified as having non-advanced DR, whereas those with severe non-proliferative DR, proliferative DR, or maculopathy were grouped into the advanced DR category. DR grade was assigned based on the worse eye [17].
Previous major acute CVD events, including myocardial infarction; stroke; foot ulcer/gangrene/amputation; and coronary, carotid, and lower limb revascularization, were adjudicated based on hospital discharge records by an ad hoc committee in each centre [18].
2.6. Categorization of Patients
Patients were stratified into the following HbA1c categories according to their baseline HbA1c value: <6.5% (C1); 6.5–7.49% (C2); 7.5–8.49% (C3); and ≥8.5% (C4).
In addition, patients were arbitrarily assigned the following personalized HbA1c goals: <6.5% (G0); <7.0% (G1); <7.5% (G2); <8.0% (G3); and <8.5% (G4). HbA1c goal were personalized according to the number of potential risks of treatment adverse effects among age > 70 years; known diabetes duration > 10 years; presence of advanced complication(s), i.e., advanced DKD (eGFR < 30 mL/min/1.73 m2 and/or macroalbuminuria) and/or advanced DR (severe non-proliferative, proliferative, or maculopathy) and/or history of major acute CVD event(s) (myocardial infarction, stroke, foot ulcer/gangrene/amputation, and coronary, carotid and lower limb revascularization); the presence of severe comorbidity(ies) (chronic obstructive pulmonary disease, chronic liver disease and/or cancer). Specifically, the HbA1c goal was set at <6.5% if none of above risks was present (G0) and was increased by 0.5% for each of these four risks (i.e., from <7.0% (G1), if only one was present, to <8.5% (G4), if all four were present).
Patients were then assigned to three target categories, based on whether their baseline HbA1c value was ≤0.5% below or above (on-target, T1), >0.5% below (below-target, T2), or >0.5% above (above-target, T3) their personalized HbA1c goal.
Finally, patients from each HbA1c category (C1–C4) or target category (T1–T3) were further classified based on whether they (a) were treated with anti-hyperglycaemic drugs causing hypoglycaemia (n = 9830), i.e., insulin and/or insulin secretagogues (sulfonylureas or glinides), either alone or combined with other anti-hyperglycaemic drugs, or (b) were not treated with these agents (n = 5826) and were either on lifestyle measures only (n = 2123, 36.4%), i.e., diet and physical activity, or receiving drugs not causing hypoglycaemia (n = 3703, 63.6%), i.e., acarbose, pioglitazone and/or metformin, the latter taken by the vast majority of these individual (97.3%).
The distribution of patients according to personalized HbA1c goals and target categories is reported in Table S1.
2.7. Statistical Analysis
Data are expressed as mean ± standard deviation (SD) or median (interquartile range), for continuous variables, and number of cases (percentage), for categorical variables. Comparisons among groups were performed by one-way analysis of variance (ANOVA) or Kruskal–Wallis test, according to the parametric or non-parametric distribution of continuous variables, followed by Bonferroni correction or Mann–Whitney test, respectively, for post-hoc comparisons, and by Pearson’s χ2 test for categorical variables.
Kaplan–Meier survival curves for all-cause mortality were calculated according to both HbA1c categories (C1–C4) and target categories (T1–T3). Differences in survival rates were analysed using the log-rank statistic. Survival analyses were performed by Cox proportional hazards regression according to HbA1c categories and target categories using C2 and T1, respectively, as reference category. Analyses by HbA1c categories were adjusted for baseline age and gender (model 1), age and gender plus CVD risk factors, i.e., smoking habits, diabetes duration, BMI, triglycerides, total and HDL cholesterol, lipid-lowering treatment, systolic and diastolic BP, and anti-hypertensive treatment (model 2), and age, gender, and CVD risk factors plus complications, i.e., DKD phenotypes, DR grade, history of major acute CVD events, and comorbidities (model 3). Analyses by HbA1c target categories were adjusted for gender and the CVD risk factors smoking habits, BMI, triglycerides, total and HDL cholesterol, lipid-lowering treatment, systolic and diastolic BP, anti-hypertensive treatment (model 1), and gender, the above CVD risk factors, and the factors considered for patients’ stratification into target categories, i.e., age, diabetes duration, complications, and comorbidities (model 2). In the subgroup of patients with 3-to-5 HbA1c values obtained during the 2-year period before enrolment, all the above models were also adjusted for HbA1c-SD. In addition, the above analyses were conducted separately for individuals treated or not with agents causing hypoglycaemia and further dividing patients not on these agents in those treated with lifestyle measures only and those on drugs not causing hypoglycaemia. The results of these analyses were expressed as hazard ratios (HRs) and their 95% confidence intervals (CIs).
All p values were two-sided, and a p < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA).
3. Results
Valid information on vital status was retrieved for 15,656 patients (99.26% of the original cohort). At the time of the census, 12,054 (76.99%) patients were alive, whereas 3602 (23.01%) patients were deceased; the follow-up duration was 7.42 ± 2.05 years [19].
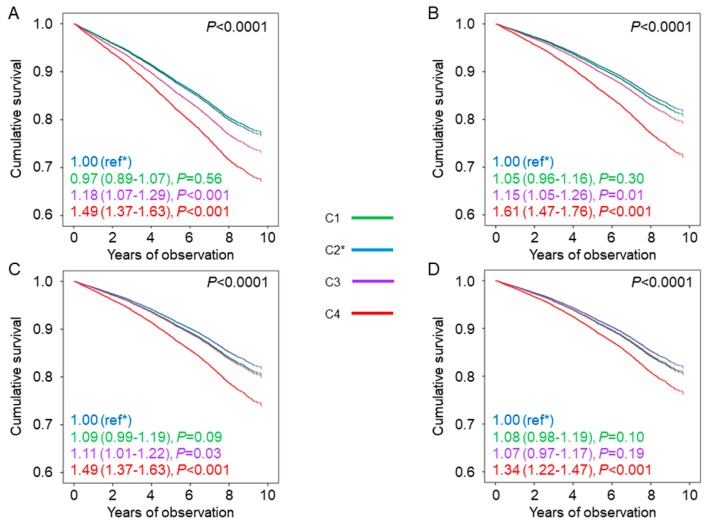
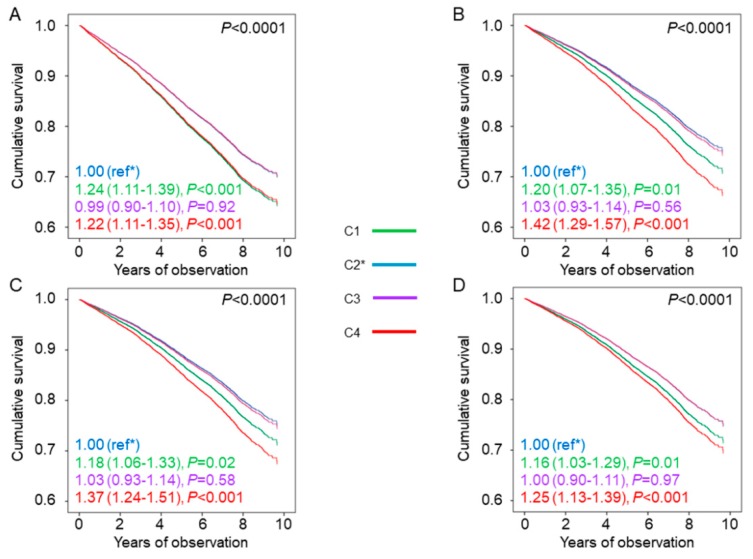
The baseline clinical features by HbA1c categories are reported in Table 1 and Table S2. The general HbA1c goal of <7.0% was met by 6287 participants (40.2%), whereas 3645 (23.3%) patients achieved the more stringent HbA1c goal of <6.5%. Individuals in the lowest HbA1c category were younger and more frequently males, had lower diabetes duration and prevalence of complications and a more favourable CVD risk profile, and were less frequently current smokers and on anti-hyperglycaemic, lipid-lowering, and anti-hypertensive treatment (including therapy with agents causing hypoglycaemia) than those in the other HbA1c categories (Table 1). In each HbA1c category, age, diabetes duration, and prevalence of complications/comorbidities were higher, whereas the lipid and BP profiles were better in patients who were on agents causing hypoglycaemia than in those who were not (Table S2). Kaplan–Meier estimates (Figure S1A), and unadjusted HRs (Figure 1A) for all-cause mortality increased with increasing HbA1c category, with similar values for C1 and C2; after adjustment for age and gender and further adjustment for CVD risk factors and complications/comorbidities, the HR for C3 became progressively similar to those of C1 and C2, whereas that for C4 remained significantly higher (Figure 1B–D). The same trend was observed among patients not treated with agents causing hypoglycaemia (Figure S1B and Table 2). Conversely, among those on treatment with these drugs, Kaplan-Meier estimates and unadjusted and adjusted HRs were highest in individuals in the lowest (C1) and highest (C4) HbA1c categories (Figure S1B and Figure 2) and the HRs remained significantly higher versus the reference category C2 after adjustment for all confounders in both C1 and C4 (1.16 (1.03–1.29), p = 0.01, and 1.25 (1.13–1.39), p < 0.001, respectively). When analysed separately, mortality risk was higher in patients on insulin (alone or in combination) than in those on insulin secretagogues in each HbA1c category, whereas it was not increased in patients treated with agents not causing hypoglycaemia and falling in the lowest HbA1c category, as compared with those on lifestyle measures only. Inclusion of HbA1c-SD as a covariate in model 3 showed that HbA1c variability was independently associated with an increased mortality risk (1.30 (1.20–1.40), p < 0.001, and 1.26 (1.19–1.36), p < 0.001, without and with patients’ stratification for type of anti-hyperglycaemic treatment, respectively), but the HRs for HbA1c categories and HbA1c target categories were not affected.
Table 1.
Baseline clinical features of study participants by HbA1c categories.
| Variables | C1 | C2 | C3 | C4 | p-Value |
|---|---|---|---|---|---|
| N (%) | 3645 (23.3) | 5081 (32.5) | 3608 (23.0) | 3322 (21.2) | |
| Deaths, n (%) | 737 (20.2) | 1049 (20.6) | 858 (23.8) | 958 (28.8) | <0.001 |
| Age, years | 65.6 ± 10.6 | 67.1 ± 9.9 | 67.1 ± 10.2 | 66.3 ± 10.8 | <0.001 |
| Male gender, n (%) | 2233 (57.1) | 2903 (55.9) | 2018 (52.6) | 1748 (56.9) | <0.001 |
| Smoking, n (%) | <0.001 | ||||
| Never | 2021 (55.4) | 2850 (56.1) | 2090 (57.9) | 1888 (56.8) | |
| Former | 1107 (30.4) | 1472 (29.0) | 960 (26.6) | 868 (26.1) | |
| Current | 517 (14.2) | 759 (14.9) | 558 (15.5) | 566 (17.0) | |
| Diabetes duration, years | 9.0 ± 8.9 | 12.5 ± 9.9 | 15.7 ± 10.1 | 16.1 ± 10.2 | <0.001 |
| HbA1c, % | 5.93 ± 0.46 | 6.97 ± 0.29 | 7.93 ± 0.28 | 9.77 ± 1.31 | <0.001 |
| (mmol·mol−1) | (41.3 ± 5.0) | (52.7 ± 3.2) | (63.2 ± 3.1) | (83.3 ± 14.3) | |
| BMI, kg·m−2 | 28.6 ± 4.9 | 28.6 ± 4.9 | 29.1 ± 5.2 | 29.7 ± 5.5 | <0.001 |
| Waist circumference, cm | 101.8 ± 10.0 | 101.9 ± 10.0 | 102.7 ± 10.6 | 104.0 ± 11.1 | <0.001 |
| Triglycerides, mmol·L−1 | 1.46 ± 0.94 | 1.49 ± 0.85 | 1.56 ± 0.89 | 1.84 ± 1.27 | <0.001 |
| Total cholesterol, mmol L−1 | 4.74 ± 0.96 | 4.77 ± 0.95 | 4.75 ± 0.96 | 4.89 ± 1.09 | <0.001 |
| HDL cholesterol, mmol·L−1 | 1.31 ± 0.36 | 1.31 ± 0.35 | 1.28 ± 0.34 | 1.24 ± 0.35 | <0.001 |
| LDL cholesterol, mmol L−1 | 3.43 ± 0.92 | 3.46 ± 0.91 | 3.47 ± 0.92 | 3.65 ± 1.05 | 0.01 |
| Non-HDL cholesterol, mmol L−1 | 2.78 ± 0.84 | 2.79 ± 0.83 | 2.76 ± 0.82 | 2.83 ± 0.90 | <0.001 |
| Systolic BP, mmHg | 136.3 ± 17.5 | 137.9 ± 17.7 | 139.6 ± 18.2 | 138.6 ± 18.7 | <0.001 |
| Diastolic BP, mmHg | 79.0 ± 9.4 | 78.6 ± 9.2 | 78.9 ± 9.5 | 78.7 ± 9.7 | 0.16 |
| Pulse pressure, mmHg | 57.3 ± 15.3 | 59.3 ± 15.6 | 60.8 ± 16.0 | 60.0 ± 15.8 | <0.001 |
| Anti-hyperglycaemic treatment, n (%) | |||||
| Lifestyle | 1017 (27.9) | 762 (15.0) | 192 (5.3) | 142 (4.3) | <0.001 |
| Insulin | 440 (12.1) | 913 (18.0) | 1062 (29.4) | 1509 (45.4) | <0.001 |
| Non-insulin agents | 2188 (60.0) | 3406 (67.0) | 2354 (65.2) | 1671 (50.3) | <0.001 |
| Metformin | 1746 (47.9) | 2840 (55.9) | 2206 (61.1) | 1853 (55.8) | <0.001 |
| Pioglitazone | 87 (2.4) | 167 (3.3) | 163 (4.5) | 137 (4.1) | <0.001 |
| Acarbose | 36 (1.0) | 43 (0.8) | 47 (1.3) | 44 (1.3) | 0.09 |
| Sulfonylureas | 820 (22.5) | 1677 (33.0) | 1472 (40.8) | 1281 (38.6) | <0.001 |
| Repaglinide | 335 (9.2) | 537 (10.6) | 371 (10.3) | 282 (8.5) | 0.01 |
| Agents causing hypoglycaemia, n (%) | 1538 (42.2) | 2944 (57.9) | 2646 (73.3) | 2702 (81.3) | <0.001 |
| Lipid-lowering treatment, n (%) | 1572 (43.1) | 2389 (47.0) | 1728 (47.9) | 1549 (46.6) | <0.001 |
| Anti-hypertensive treatment, n (%) | 2509 (68.8) | 3611 (71.1) | 2601 (72.1) | 2351 (70.8) | <0.001 |
| Albuminuria, mg·day−1 | 53.8 ± 225.5 | 68.7 ± 373.2 | 74.4 ± 329.7 | 96.0 ± 293.1 | <0.001 |
| Serum creatinine, μmol·L−1 | 81.3 ± 38.0 | 80.4 ± 35.4 | 80.4 ± 31.8 | 82.2 ± 31.8 | 0.27 |
| eGFR, mL·min−1·1.73 m−2 | 81.6 ± 21.0 | 80.3 ± 20.1 | 79.9 ± 20.5 | 79.2 ± 22.6 | <0.001 |
| DKD phenotype, n (%) | <0.001 | ||||
| No DKD | 2554 (70.1) | 3385 (66.6) | 2269 (62.9) | 1776 (53.5) | |
| Albuminuric DKD with preserved eGFR | 532 (14.6) | 877 (17.3) | 691 (19.2) | 866 (26.1) | |
| Non-albuminuric DKD | 321 (8.8) | 465 (9.2) | 360 (10.0) | 330 (9.9) | |
| Albuminuric DKD with reduced eGFR | 238 (6.5) | 354 (7.0) | 288 (8.0) | 350 (10.5) | |
| DR, n (%) | <0.001 | ||||
| No DR | 3178 (87.2) | 4185 (82.4) | 2658 (73.7) | 2168 (63.5) | |
| Non-advanced DR | 242 (6.6) | 503 (9.9) | 586 (16.2) | 616 (18.5) | |
| Advanced DR | 225 (6.2) | 393 (7.7) | 364 (10.1) | 538 (16.2) | |
| CVD, n (%) | |||||
| Any | 699 (19.2) | 1086 (21.4) | 906 (25.1) | 929 (28.0) | <0.001 |
| Myocardial infarction | 354 (9.7) | 514 (10.1) | 445 (12.3) | 429 (12.9) | <0.001 |
| Coronary revascularization | 295 (8.1) | 487 (9.6) | 411 (11.4) | 386 (11.6) | <0.001 |
| Stroke | 126 (3.5) | 143 (2.8) | 118 (3.3) | 126 (3.8) | 0.09 |
| Carotid revascularization | 120 (3.3) | 240 (4.7) | 235 (6.5) | 261 (7.9) | <0.001 |
| Ulcer/gangrene/amputation | 92 (2.5) | 167 (3.3) | 131 (3.6) | 166 (5.0) | <0.001 |
| Lower limb revascularization | 65 (1.8) | 135 (2.7) | 121 (3.4) | 129 (3.9) | <0.001 |
| Comorbidities, n (%) | |||||
| Any | 682 (18.7) | 872 (17.2) | 620 (17.2) | 613 (18.5) | 0.15 |
| COPD | 173 (4.7) | 201 (4.0) | 142 (3.9) | 158 (4.8) | <0.001 |
| Chronic liver disease | 299 (8.2) | 415 (8.2) | 326 (9.0) | 321 (9.7) | 0.06 |
| Cancer | 279 (7.7) | 342 (6.7) | 215 (6.0) | 195 (5.9) | <0.001 |
HbA1c = haemoglobin A1c; C1 = HbA1c <6.5%; C2 = HbA1c 6.5–7.49%; C3 = HbA1c 7.5–8.49%; C4 = HbA1c ≥8.5%; BMI = body mass index; HDL = high-density lipoprotein; LDL = low-density lipoprotein; BP = blood pressure; ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; eGFR = estimated glomerular filtration rate; DKD = diabetic kidney disease; DR = diabetic retinopathy; CVD = cardiovascular disease; COPD = chronic obstructive pulmonary disease.
Figure 1.
Mortality risk by HbA1c categories. Cox proportional hazards regression according to HbA1c categories, unadjusted (A) and adjusted for age and gender (B), plus cardiovascular disease (CVD) risk factors (C), plus complications/comorbidities (D). HRs (95% CI) for mortality are shown for each HbA1c category. HbA1c = haemoglobin A1c; HR = hazard ratio; CI = confidence interval; C1 = HbA1c <6.5%; C2 = HbA1c 6.5%–7.49%; C3 = HbA1c 7.5%–8.49%; C4 = HbA1c ≥8.5%; ref*, the asterisk indicates the reference category.
Table 2.
Mortality risk by HbA1c categories among participants not treated with agents causing hypoglycaemia.
| HbA1c Target Categories | Unadjusted | Adjusted | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| C2 (ref) | 1.00 | - | <0.001 | 1.00 | - | <0.001 | 1.00 | - | <0.001 | 1.00 | - | <0.001 |
| C1 | 0.92 | 0.78–1.10 | 0.36 | 1.01 | 0.85–1.20 | 0.94 | 1.03 | 0.87–1.23 | 0.70 | 1.03 | 0.87–1.22 | 0.74 |
| C3 | 1.36 | 1.12–1.66 | 0.01 | 1.34 | 1.10–1.63 | 0.01 | 1.28 | 1.05–1.55 | 0.02 | 1.26 | 1.04–1.54 | 0.02 |
| C4 | 1.67 | 1.35–2.07 | <0.001 | 1.72 | 1.39–2.13 | <0.001 | 1.59 | 1.28–1.97 | <0.001 | 1.50 | 1.21–1.86 | <0.001 |
Cox proportional hazards regression according to HbA1c categories plus use of agents causing hypoglycaemia, unadjusted and adjusted for age and gender (model 1), plus CVD risk factors (model 2), plus complications/comorbidities (model 3). HRs (95% CI) for mortality are shown for each HbA1c category. HbA1c = haemoglobin A1c; HR = hazard ratio; CI = confidence interval; C1 = HbA1c < 6.5%; C2 = HbA1c 6.5–7.49%; C3 = HbA1c 7.5–8.49%; C4 = HbA1c ≥ 8.5%.
Figure 2.
Mortality risk by HbA1c categories and use of agents causing hypoglycaemia. Cox proportional hazards regression according to HbA1c categories among participants treated with agents causing hypoglycaemia, unadjusted (A) and adjusted for age and gender (B), plus CVD risk factors (C), plus complications/comorbidities (D). HRs (95% CI) for mortality are shown for each HbA1c category. HbA1c = haemoglobin A1c; HR = hazard ratio; CI = confidence interval; C1 = HbA1c < 6.5%; C2 = HbA1c 6.5–7.49%; C3 = HbA1c 7.5–8.49%; C4 = HbA1c ≥ 8.5%.
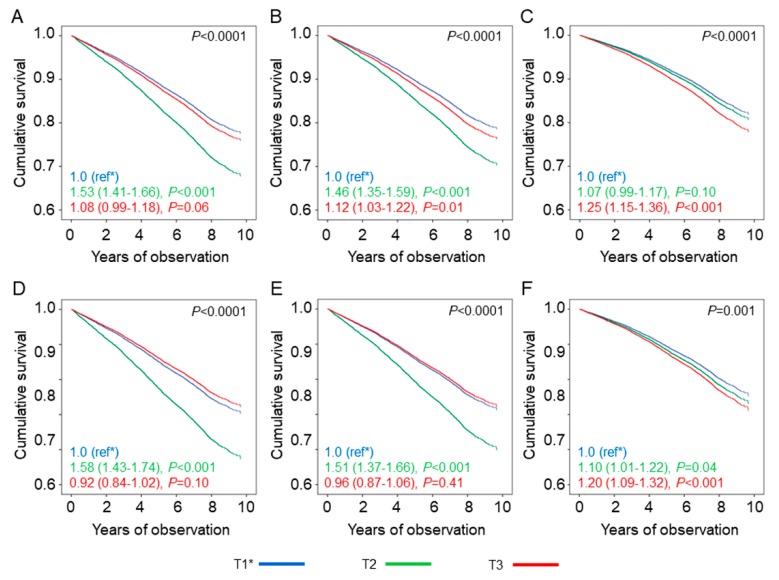
As expected, the great majority of participants (75.4%) had at least one potential risk (age ≥ 70 years: 38.7%; diabetes duration > 10 years: 50.3%; presence of advanced complication (s): 32.0%; and presence of severe comorbidity (ies): 17.8%) and, hence, were assigned to a higher HbA1c goal. The baseline clinical features by HbA1c target categories are reported in Table 3 and Table S3. Participants with above-target HbA1c values were 6,046 (38.6%), whereas the remaining 9610 patients (61.4%) achieved their personalized HbA1c goal; however, of them, 4989 (31.9%) were on-target, whereas 4621 (29.5%) were below-target, i.e., showed HbA1c values > 0.5% lower than their personalized goal. As expected, individuals who were below-target had a higher prevalence of potential risks (especially older age and higher prevalence of complications/comorbidities) and, hence, were more frequently assigned the highest personalized HbA1c goals (i.e., <7.5% or higher, G2–G4), than both on-target and above-target patients (59.6% versus 40.7% and 37.0%, respectively) (Table S1). In addition, below-target individuals were more frequently males and less frequently on insulin and agents causing hypoglycaemia and showed a better CVD risk profile than above-target patients (Table 3). In each HbA1c target category, potential risks, i.e., age, diabetes duration, and prevalence of complications/comorbidities, were higher in patients who were on agents causing hypoglycaemia than in those who were not (Table S3). Kaplan–Meier estimates (Figure S1C) and unadjusted HRs (Figure 3A) for all-cause mortality were lowest in patients on-target, intermediate in those above-target, and highest in those below-target. The increased risk in below-target individuals was progressively attenuated by adjustment for CVD risk factors and potential risks, whereas it became significantly elevated in above-target patients (Figure 3B,C). All the other covariates included in model 3 were significantly associated with mortality, except BMI and systolic BP, and sensitivity analysis showed that, among potential risks, age had the highest weight. The same trend was observed among patients not treated with agents causing hypoglycaemia (Figure S1D and Table 4). Conversely, among patients on treatment with these drugs, the increased risk of death versus the reference category T1 remained after adjustment for CVD risk factors and potential risks in both T2 (1.10 (1.01–1.22), p = 0.04) and T3 (1.20 (1.09–1.32), p < 0.001) individuals. Again, when analysed separately, mortality risk was higher in individuals on insulin (alone or in combination) than in those on insulin secretagogues in each HbA1c target category and was not increased in patients treated with agents not causing hypoglycaemia and falling in the below-target category, as compared with those on lifestyle measures only. Results did not change when including HbA1c-SD among the covariates or when HbA1c goals were personalized using different cut-offs for age (>75 years) and diabetes (>15 years).
Table 3.
Baseline clinical features of study participants by HbA1c target categories.
| Variables | T1 | T2 | T3 | p-Value |
|---|---|---|---|---|
| n (%) | 4989 (31.9) | 4621 (29.5) | 6046 (38.6) | |
| Deaths, n (%) | 991 (19.9) | 1330 (28.8) | 1281 (21.2) | <0.001 |
| Age, years | 66.7 ± 9.9 | 69.4 ± 10.1 | 64.4 ± 10.3 | <0.001 |
| Male gender, n (%) | 2833 (56.8) | 2818 (61.0) | 3251 (53.8) | <0.001 |
| Smoking, n (%) | <0.001 | |||
| Never | 2837 (56.9) | 2581 (55.9) | 3431 (56.7) | |
| Former | 1435 (28.8) | 1455 (31.5) | 1517 (25.1) | |
| Current | 717 (14.4) | 585 (12.7) | 1098 (18.2) | |
| Diabetes duration, years | 12.7 ± 10.1 | 13.3 ± 10.7 | 13.6 ± 9.8 | <0.001 |
| HbA1c, % | 7.13 ± 0.57 | 6.22 ± 0.66 | 8.90 ± 1.40 | <0.001 |
| (mmol·mol−1) | (54.4 ± 6.2) | (44.5 ± 7.2) | (73.8 ± 15.3) | |
| BMI, kg·m−2 | 28.8 ± 5.0 | 28.2 ± 4.7 | 29.7 ± 5.5 | <0.001 |
| Waist circumference, cm | 102.1 ± 10.1 | 101.1 ± 9.7 | 103.9 ± 11.0 | <0.001 |
| Triglycerides, mmol·L−1 | 1.51 ± 0.89 | 1.42 ± 0.86 | 1.74 ± 1.14 | <0.001 |
| Total cholesterol, mmol·L−1 | 4.78 ± 0.95 | 4.69 ± 0.96 | 4.86 ± 1.04 | <0.001 |
| HDL cholesterol, mmol·L−1 | 1.30 ± 0.34 | 1.32 ± 0.37 | 1.26 ± 0.35 | <0.001 |
| LDL cholesterol, mmol·L−1 | 3.47 ± 0.91 | 3.37 ± 0.91 | 3.61 ± 1.00 | <0.001 |
| Non-HDL cholesterol, mmol·L−1 | 2.79 ± 0.82 | 2.73 ± 0.83 | 2.83 ± 0.88 | <0.001 |
| Systolic BP, mmHg | 138.3 ± 17.9 | 137.5 ± 17.9 | 138.2 ± 18.2 | 0.06 |
| Diastolic BP, mmHg | 79.0 ± 9.2 | 78.3 ± 9.5 | 78.9 ± 9.6 | 0.01 |
| Pulse pressure, mmHg | 59.4 ± 15.6 | 59.2 ± 15.8 | 59.3 ± 15.7 | 0.87 |
| Anti-hyperglycaemic treatment, n (%) | ||||
| Lifestyle | 810 (16.2) | 927 (20.1) | 376 (6.2) | <0.001 |
| Insulin | 972 (19.5) | 860 (18.6) | 2092 (34.6) | <0.001 |
| Non-insulin agents | 3207 (64.3) | 2834 (61.3) | 3578 (59.2) | <0.001 |
| Metformin | 2784 (55.8) | 2195 (47.5) | 3666 (60.6) | <0.001 |
| Pioglitazone | 181 (3.6) | 92 (2.0) | 281 (4.6) | <0.001 |
| Acarbose | 49 (1.0) | 47 (1.0) | 74 (1.2) | 0.41 |
| Sulfonylureas | 1610 (32.3) | 1314 (28.4) | 2326 (38.5) | <0.001 |
| Repaglinide | 461 (9.2) | 510 (11.0) | 554 (9.2) | 0.01 |
| Agents causing hypoglycaemia, n (%) | 2835 (56.8) | 2557 (55.3) | 4438 (73.4) | <0.001 |
| Lipid-lowering treatment, n (%) | 2314 (46.4) | 2148 (46.5) | 2776 (45.9) | 0.82 |
| Anti-hypertensive treatment, n (%) | 3504 (70.2) | 3449 (74.6) | 4119 (68.1) | <0.001 |
| Albuminuria, mg·day−1 | 64.1 ± 285.4 | 80.3 ± 377.0 | 73.0 ± 290.3 | 0.04 |
| Serum creatinine, μmol·L−1 | 79.6 ± 31.8 | 85.7±42.4 | 78.7±29.2 | <0.001 |
| eGFR, mL·min−1·1.73 m−2 | 81.0±19.7 | 76.6±21.7 | 82.5±21.0 | <0.001 |
| DKD phenotype, n (%) | <0.001 | |||
| No DKD | 3348 (67.1) | 2859 (61.9) | 3777 (62.5) | |
| Albuminuric DKD with preserved eGFR | 863 (17.3) | 767 (16.6) | 1336 (22.1) | |
| Non-albuminuric DKD | 461 (9.2) | 523 (11.3) | 492 (8.1) | |
| Albuminuric DKD with reduced eGFR | 317 (6.4) | 472 (10.2) | 441 (7.3) | |
| DR, n (%) | <0.001 | |||
| No DR | 4064 (81.5) | 3695 (80.0) | 4430 (73.3) | |
| Non-advanced DR | 529 (10.6) | 444 (9.6) | 974 (16.1) | |
| Advanced DR | 396 (7.9) | 482 (10.4) | 642 (10.6) | |
| CVD, n (%) | ||||
| Any | 1004 (20.1) | 1457 (31.5) | 1159 (19.2) | <0.001 |
| Myocardial infarction | 480 (9.6) | 703 (15.2) | 559 (9.2) | <0.001 |
| Coronary revascularization | 447 (9.0 | 617 (13.4) | 515 (8.5) | <0.001 |
| Stroke | 137 (2.7) | 231 (5.0) | 145 (2.4) | <0.001 |
| Carotid revascularization | 250 (5.0) | 308 (6.7) | 298 (4.9) | <0.001 |
| Ulcer/gangrene/amputation | 158 (3.2) | 217 (4.7) | 181 (3.0) | <0.001 |
| Lower limb revascularization | 122 (2.4) | 171 (3.7) | 157 (2.6) | <0.001 |
| Comorbidities n (%) | ||||
| Any | 756 (15.2) | 1261 (27.3) | 770 (12.7) | <0.001 |
| COPD | 167 (3.3) | 322 (7.0) | 185 (3.1) | <0.001 |
| Chronic liver disease | 385 (7.7) | 570 (12.3) | 406 (6.7) | <0.001 |
| Cancer | 276 (5.5) | 504 (10.9) | 251 (4.2) | <0.001 |
HbA1c = haemoglobin A1c; T1 = HbA1c on-target (≤0.5% below or above personalized goal); T2 = HbA1c below-target (>0.5% below personalized goal); T3 = HbA1c above-target (>0.5% above personalized goal); BMI = body mass index; HDL = high-density lipoprotein; LDL = low-density lipoprotein; BP = blood pressure; ACE-I = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; eGFR = estimated glomerular filtration rate; DKD = diabetic kidney disease; DR = diabetic retinopathy; CVD = cardiovascular disease; COPD = chronic obstructive pulmonary disease.
Figure 3.
Mortality risk by HbA1c target categories and use of agents causing hypoglycaemia. Cox proportional hazards regression according to HbA1c target categories in the whole cohort (A–C) and among participants treated with agents causing hypoglycaemia (D–F), unadjusted (A,D) and adjusted for CVD risk factors (B,E), complications/comorbidities (C,F). HRs (95% CI) for mortality are shown for each HbA1c target category. HbA1c = haemoglobin A1c; HR = hazard ratio; CI = confidence interval; T1 = HbA1c on-target (≤0.5% below or above personalized goal); T2 = HbA1c below-target (>0.5% below personalized goal); T3 = HbA1c above-target (>0.5% above personalized goal).
Table 4.
Mortality risk by HbA1c target categories among participants not treated with agents causing hypoglycaemia.
| HbA1c Target Categories | Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||||
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| T1 (ref) | 1.00 | - | <0.001 | 1.00 | - | <0.001 | 1.00 | - | <0.001 |
| T2 | 1.54 | 1.31–1.82 | <0.001 | 1.49 | 1.26–1.75 | <0.001 | 1.08 | 0.92–1.28 | 0.34 |
| T3 | 1.11 | 0.92–1.33 | 0.290 | 1.14 | 0.94–1.37 | 0.19 | 1.21 | 1.01–1.46 | 0.04 |
Cox proportional hazards regression according to HbA1c target categories plus use of agents causing hypoglycaemia, unadjusted and adjusted for CVD risk factors (model 1) and complications/comorbidities (model 2). HRs (95% CI) for mortality are shown for each HbA1c target category. HbA1c = haemoglobin A1c; HR = hazard ratio; CI = confidence interval; T1 = HbA1c on-target (≤0.5% below or above personalized goal); T2 = HbA1c below-target (>0.5% below personalized goal); T3 = HbA1c above-target (>0.5% above personalized goal).
4. Discussion
This analysis of individuals with type 2 diabetes from the RIACE cohort provides evidence that achieving HbA1c levels close to normal values (i.e., <6.5%) is not associated with increased mortality, unless patients are on treatment with drugs causing hypoglycaemia. These finding are consistent with the positive relationship between severe hypoglycaemia and increased mortality reported in the ACCORD [9], ADVANCE [10], and VADT [11] and with the observation that, in the ACCORD, risk of death was higher in the intensive than in the conventional group only in patients requiring more aggressive treatment, including insulin, because of little or no decrease in HbA1c following treatment initiation [12]. However, unmeasured factors other than hypoglycaemia may be involved in the increased mortality in patients on treatment with agents causing hypoglycaemia, including poor adherence, depression, cognitive impairment, education, socio-economic status, etc. The results of our study are also consistent with the increased mortality reported in older people with type 2 diabetes from the Fremantle Diabetes Study Phase II who were treated with sulfonylurea and/or insulin and had HbA1c levels < 7.0%, but not with the increased mortality observed in those who were treated with metformin and had HbA1c levels < 6.5%, a finding which the authors attributed to confounding by indication [20]. Conversely, our data are in contrast with previous reports from the UK General Practice Research Database [21] and the Kaiser Permanente Diabetes Registry of Northwest [22] and Northern California [23] showing a U-shape relationship between achieved HbA1c and mortality in diabetic individuals, with both higher and lower HbA1c values associated with increased all-cause mortality. However, Currie et al. analysed patients whose treatment had been intensified from oral monotherapy to combination therapy with oral agents or to regimens that included insulin; in addition, those with lower mean HbA1c levels were older and had worse renal function than those with higher mean HbA1c values [21]. Indeed, the 2008 American Diabetes Association (ADA) guidelines recommended less stringent HbA1c goals when the incremental but small benefit from lowering HbA1c from 7.0% to 6.0% [24,25] may be outweighed by the increased risk of hypoglycaemia and the great effort required to achieve near-normoglycaemia [26]. Subsequently, based on the results of the ACCORD [3], ADVANCE [4], and VADT [5], the 2012 Position Statement of the ADA and the European Association for the Study of Diabetes recommended a patient-centred approach with setting of personalized HbA1c goals according to several potential risks of adverse treatment effects [8].
When patients from the RIACE cohort were arbitrarily assigned to five different HbA1c goals (from <6.5% to <8.5%) according the number (0 to 4) of potential risks among age > 70 years, diabetes duration >10 years, presence of advanced complication and severe comorbidities, 44.8% of patients were reclassified to a higher target, whereas 24.6% of patients were reclassified to a lower target than the general goal of <7.0%. As a consequence, the proportion of individuals with HbA1c values less than the personalized goal (61.4%) was higher than that of patients with HbA1c values less than the general < 7.0% goal (40.2%) and much higher than that of patients meeting the more stringent HbA1c goal of <6.5% (23.3%), consistent with previous reports from the National Health and Nutrition Examination Survey (NHANES) [27,28]. In addition, about half of the RIACE participants with HbA1c levels less than their personalized goal (29.5% of the whole cohort) were well below this threshold (i.e., >0.5%), as the personalized goal for the majority of them was higher than the general < 7.0% goal which was recommended at the time patients underwent baseline evaluation (i.e., in the years 2006–2008). These findings are consistent with a cross-sectional analysis of the data from older (≥65 years) NHANES participants with diabetes from 2001 through 2010, which showed that a substantial proportion of patients were potentially overtreated, i.e., had an HbA1c < 7.0% irrespective of health status and type of treatment, with no change over the 10-year study period [29]. In addition, a cross-sectional analysis of 42,669 outpatients with type 2 diabetes from the Diabetes Collaborative Registry showed that one-fourth were tightly controlled with medications that confer a high risk of hypoglycaemia, thus suggesting potential overtreatment of a substantial proportion of people [30]. Finally, a retrospective cohort study using data from the US Veterans Health Administration showed that only one-fourth of the older patients whose treatment resulted in very low levels of HbA1c or BP underwent de-intensification of anti-hyperglycaemic or anti-hypertensive therapy [31].
The most intriguing and original finding of our study is that below-target patients showed a mortality risk higher than that of on-target individuals (i.e., with HbA1c values ≤ 0.5% below or above their personalized goal) and similar to that of individuals above-target (i.e., with HbA1c values > 0.5% above their personalized goal). Excess risk was observed in the whole cohort and in patients on treatment with anti-hyperglycaemic drugs causing hypoglycaemia, but not in patients not treated with these agents, and was attenuated, though not completely in those on “hypoglycaemic” agents, after adjusting for CVD risk factors and the potential risks considered for patients’ stratification into target categories.
Our findings that, in patients on treatment with agents causing hypoglycaemia, HbA1c values < 6.5% or ≥0.5% less than the personalized goal are associated with increased mortality risk have important implications for clinical practice. First, these data support the importance of setting a personalized HbA1c range rather than an upper HbA1c threshold (e.g., 7.0–7.5% instead of <7.5%) when the use of agents causing hypoglycaemia is required for achieving the glycaemic goal. This approach would be in line with that adopted by the recent guidelines of the European Society of Cardiology (in collaboration with the European Association for the Study of Diabetes (EASD)) for the treatment of hypertension in diabetic patients, which recommend a systolic BP goal of <130 mmHg, but not <120 mmHg, in younger individuals and of <140 mmHg, but not <130 mmHg, in older individuals and a diastolic BP goal of <80 mmHg, but not <70 mmHg in all patients [32]. Second, our results provide further support to the current guideline recommendations to set higher glycaemic goals when the risks of lower HbA1c targets may outweigh the potential benefits and to modify the treatment regimen accordingly [33]. Treatment modification may include de-intensification of pharmacologic therapy with drugs causing hypoglycaemia in patients with HbA1c levels well below the individual HbA1c goal. At the time patients underwent baseline evaluation, only a few therapeutic options (i.e., metformin, pioglitazone, and acarbose) were available in addition to insulin and insulin secretagogues which, therefore, were used by a substantial proportion of the RIACE participants (62.8%). However, during the last few decades, several new classes of anti-hyperglycaemic agents that do not cause hypoglycaemia have been made available, possibly allowing more stringent HbA1c goals even in high-risk patients, provided that combination therapy with insulin and/or insulin secretagogues is not required for achieving these targets.
The strengths of this study include the large size of the cohort, the analysis of a contemporary real-life dataset, the assessment of a wide range of clinical parameters, and the completeness of baseline and follow-up data. However, this study has several limitations. First, the analysis is based on baseline HbA1c levels and treatments, which have likely changed during the follow-up for several reasons, including disease progression, guideline change, and availability of new drugs, the use of which was however very limited at the time of the census. Second, no data are available on the number and severity of hypoglycaemic episodes as well as on individual attitude and expected treatment efforts and resources and support systems. Third, the score (0 to 4) used for personalized HbA1c goals may not mirror the severity of complications and comorbidities, as it does not distinguish one complication or comorbidity from another and individuals with one or multiple complications or comorbidities. Fourth, the study findings may not be applicable to the general ambulatory diabetes population, as only part of the individuals with type 2 diabetes attend tertiary referral outpatients Diabetes Clinics in Italy. Fifth, the observational design makes causal interpretation impossible. Sixth, there are potential methodological limitations, which have been extensively addressed in previous RIACE reports [13,14,15,16,17,18,19].
5. Conclusions
This analysis of the RIACE cohort indicates that near-normal HbA1c levels or HbA1c values well below the personalized goal are associated with an increased risk of death only if achieved with the use of agents causing hypoglycaemia. These findings support the importance of avoiding overtreatment with these drugs in high-risk individuals by setting both upper and lower personalized HbA1c goals and of using anti-hyperglycaemic agents that do not cause hypoglycaemia for safely achieving more stringent HbA1c goals.
Acknowledgments
The Authors thank the RIACE Investigators for participating in this study (see the complete list in the Supplementary Materials).
Abbreviations
| CVD | cardiovascular disease |
| UKPDS | United Kingdom Prospective Diabetes Study |
| ACCORD | Action to Control Cardiovascular Risk in Diabetes |
| ADVANCE | Action in Diabetes and Vascular Disease |
| VADT | Veterans Affairs Diabetes Trial |
| HbA1c | haemoglobin A1c |
| RIACE | Renal Insufficiency And Cardiovascular Events |
| eGFR | estimated glomerular filtration rate |
| BP | blood pressure |
| BMI | body mass index |
| DCCT | Diabetes Control and Complications Trial |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| DKD | diabetic kidney disease |
| DR | diabetic retinopathy |
| HR | hazard ratio |
| CI | Confidence interval |
| ADA | American Diabetes Association |
| NHANES | National Health And Nutrition Examination Survey |
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/1/246/s1,: The RIACE Study Group: the RIACE Steering Committee and Participating Diabetes Centres, Figure S1: Kaplan–Meier survival curves for all-cause mortality by HbA1c and HbA1c target categories, Table S1: Distribution of study participants according to personalized HbA1c goals and target categories, Table S2: Baseline clinical features of study participants by HbA1c categories plus use of agents causing hypoglycaemia, Table S3: Baseline clinical features of study participants by HbA1c target categories and use of agents causing hypoglycaemia.
Author Contributions
Conceptualization: E.O., E.B., A.S., G.P. (Giuseppe Penno), and G.P. (Giuseppe Pugliese); Data curation: E.O., G.P. (Giuseppe Penno), and G.P. (Giuseppe Pugliese); Formal analysis: E.O., A.N., G.P. (Giuseppe Penno), and G.P. (Giuseppe Pugliese); Funding acquisition: G.P. (Giuseppe Pugliese); Investigation: E.O., E.B., A.S., C.F., R.T., M.V., F.C., G.Z., S.M., G.P. (Giuseppe Penno), and G.P. (Giuseppe Pugliese); Methodology: E.O., E.B., A.S., A.N., G.P. (Giuseppe Penno), and G.P. (Giuseppe Pugliese); Project administration: G.P. (Giuseppe Pugliese); Resources: A.N., G.P. (Giuseppe Penno), and G.P. (Giuseppe Pugliese); Software: A.N. and G.P. (Giuseppe Penno); Supervision: G.P. (Giuseppe Pugliese); Validation: E.O., E.B., A.S., A.N., G.P. (Giuseppe Penno), and G.P. (Giuseppe Pugliese); Visualization: E.O., E.B., A.S., A.N., G.P. (Giuseppe Penno), and G.P. (Giuseppe Pugliese); Writing—original draft: E.O. and G.P. (Giuseppe Pugliese); Writing—review and editing: E.B., A.S., C.F., R.T., M.V., F.C., G.Z., S.M., A.N., and G.P. (Giuseppe Penno). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Foundation of the Italian Diabetes Society (Diabete Ricerca) and the Diabetes, Endocrinology and Metabolism (DEM) Foundation, and by unconditional grants from Eli Lilly, Sigma-Tau, Takeda, Chiesi Farmaceutici, and Boehringer Ingelheim.
Conflicts of Interest
E.O.: consultant fees from Eli Lilly and Novo Nordisk; E.B.: consultant fees from Abbot, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Bruno Farmaceutici, Eli Lilly, Janssen, Johnson&Johnson, Merck Sharp & Dohme, MundiPharma, Novartis, Novo Nordisk, Roche, Sanofi-Aventis, Servier, and Takeda, and research grants from AstraZeneca, Genzyme, Menarini Diagnostics, Novo Nordisk, Roche, and Takeda; A.S.: consultant fees from AstraZeneca, Boehringer Ingelheim, and Sanofi-Aventis, and lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and MundiPharma; C.F.: lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk and travel grants from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Sanofi-Aventis, and Takeda; R.T.: consultant fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi-Aventis, and lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk; M.V.: lecture fees from Lifescan and Novo Nordisk; F.C.: lecture fees from AstraZeneca, Sanofi-Aventis, and Takeda; G.Z.: research grants from NTC Pharma and Omikron Italia; S.M.: lecture fees from AstraZeneca, Sanofi-Aventis, and Takeda; A.N.: consultant fees from AstraZeneca, Pikdare, and Roche, lecture fees from AstraZeneca, Boehringer Ingelheim, Medtronic, and Novo Nordisk, and research grants from Aboca, AstraZeneca, Eli Lilly, Novo Nordisk, Sanofi-Aventis, and Theras; G.P. (Giuseppe Penno): lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, Sigma-Tau, and Takeda, and travel grants from AstraZeneca, Novo Nordisk, and Takeda; G.P. (Giuseppe Pugliese): consultant fees from AstraZeneca, Boehringer Ingelheim, and Eli Lilly, lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, MundiPharma, Novartis, Novo Nordisk, Sigma-Tau, Takeda, and travel grants from AstraZeneca, Laboratori Guidotti, Sanofi-Aventis, and Takeda. The funding sponsors had no role in the choice of research project; design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Data Availability
Data are available upon request from the corresponding author.
References
- 1.Rodriguez-Gutierrez R., Lipska K.J., McCoy R.G. Intensive glycemic control in type 2 diabetes mellitus—A balancing act of latent benefit and avoidable harm: A teachable moment. JAMA Intern. Med. 2016;176:300–301. doi: 10.1001/jamainternmed.2015.8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 3.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein H.C., Miller M.E., Byington R.P., Goff D.C., Jr., Bigger J.T., Buse J.B., Cushman W.C., Genuth S., Ismail-Beigi F., et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008;8:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advance Collaborative Group. Patel A., MacMahon S., Chalmers J., Neal B., Billot L., Woodward M., Marre M., Cooper M., Glasziou P., et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 5.Duckworth W., Abraira C., Moritz T., Reda D., Emanuele N., Reaven P.D., Zieve F.J., Marks J., Davis S.N., Hayward R., et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 6.Del Prato S. Megatrials in type 2 diabetes. From excitement to frustration? Diabetologia. 2009;52:1219–1226. doi: 10.1007/s00125-009-1352-5. [DOI] [PubMed] [Google Scholar]
- 7.Hill D., Fisher M. The effect of intensive glycaemic control on cardiovascular outcomes. Diabetes Obes. Metab. 2010;12:641–647. doi: 10.1111/j.1463-1326.2010.01199.x. [DOI] [PubMed] [Google Scholar]
- 8.Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M., Peters A.L., Tsapas A., Wender R., Matthews D.R., et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonds D.E., Miller M.E., Bergenstal R.M., Buse J.B., Byington R.P., Cutler J.A., Dudl R.J., Ismail-Beigi F., Kimel A.R., Hoogwerf B., et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: Retrospective epidemiological analysis of the ACCORD study. BMJ. 2010;340:b4909. doi: 10.1136/bmj.b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoungas S., Patel A., Chalmers J., de Galan B.E., Li Q., Billot L., Woodward M., Ninomiya T., Neal B., MacMahon S., et al. Severe hypoglycemia and risks of vascular events and death. N. Engl. J. Med. 2010;363:1410–1418. doi: 10.1056/NEJMoa1003795. [DOI] [PubMed] [Google Scholar]
- 11.Davis S.N., Duckworth W., Emanuele N., Hayward R.A., Wiitala W.L., Thottapurathu L., Reda D.J., Reaven P.D., Investigators of the Veterans Affairs Diabetes Trial Effects of severe hypoglycemia on cardiovascular outcomes and death in the Veterans Affairs Diabetes Trial. Diabetes Care. 2019;42:157–163. doi: 10.2337/dc18-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riddle M.C., Ambrosius W.T., Brillon D.J., Buse J.B., Byington R.P., Cohen R.M., Goff D.C., Jr., Malozowski S., Margolis K.L., Probstfield J.L., et al. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care. 2010;33:983–990. doi: 10.2337/dc09-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penno G., Solini A., Bonora E., Fondelli C., Orsi E., Zerbini G., Trevisan R., Vedovato M., Gruden G., Cavalot F., et al. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J. Hypertens. 2011;29:1802–1809. doi: 10.1097/HJH.0b013e3283495cd6. [DOI] [PubMed] [Google Scholar]
- 14.Orsi E., Solini A., Bonora E., Fondelli C., Trevisan R., Vedovato M., Cavalot F., Gruden G., Morano S., Nicolucci A., et al. Haemoglobin A1c variability is a strong, independent predictor of all-cause mortality in patients with type 2 diabetes. Diabetes Obes. Metab. 2018;20:1885–1893. doi: 10.1111/dom.13306. [DOI] [PubMed] [Google Scholar]
- 15.Penno G., Solini A., Bonora E., Fondelli C., Orsi E., Zerbini G., Trevisan R., Vedovato M., Gruden G., Laviola L., et al. Gender differences in cardiovascular disease risk factors, treatments and complications in patients with type 2 diabetes: The RIACE Italian multicentre study. J. Intern. Med. 2013;274:176–191. doi: 10.1111/joim.12073. [DOI] [PubMed] [Google Scholar]
- 16.Pugliese G., Solini A., Fondelli C., Trevisan R., Vedovato M., Nicolucci A., Penno G., Renal Insufficiency And Cardiovascular Events (RIACE) Study Group Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the Renal Insufficiency and Cardiovascular Events (RIACE) Study. Nephrol. Dial. Transplant. 2011;26:3950–3954. doi: 10.1093/ndt/gfr140. [DOI] [PubMed] [Google Scholar]
- 17.Penno G., Solini A., Zoppini G., Orsi E., Zerbini G., Trevisan R., Gruden G., Cavalot F., Laviola L., Morano S., et al. Rate and determinants of association between advanced retinopathy and chronic kidney disease in patients with type 2 diabetes: The Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicenter Study. Diabetes Care. 2012;35:2317–2323. doi: 10.2337/dc12-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solini S., Penno G., Bonora E., Fondelli C., Orsi E., Arosio M., Trevisan R., Vedovato M., Cignarelli M., Andreaozzi F., et al. Diverging association of reduced glomerular filtration rate and albuminuria with coronary and noncoronary events in patients with type 2 diabetes: The Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicentre Study. Diabetes Care. 2012;35:143–149. doi: 10.2337/dc11-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penno G., Solini A., Orsi E., Bonora E., Fondelli C., Trevisan R., Vedovato M., Cavalot F., Lamacchia O., Scardapane M., et al. Non-albuminuric renal impairment is a strong predictor of mortality in individuals with type 2 diabetes: The Renal Insufficiency and Cardiovascular Events (RIACE) Italian multicentre study. Diabetologia. 2018;61:2277–2289. doi: 10.1007/s00125-018-4691-2. [DOI] [PubMed] [Google Scholar]
- 20.Bruce D.G., Davis W.A., Davis T.M.E. Glycaemic control and mortality in older people with type 2 diabetes: The Fremantle Diabetes Study Phase II. Diabetes Obes. Metab. 2018;20:2852–2859. doi: 10.1111/dom.13469. [DOI] [PubMed] [Google Scholar]
- 21.Currie C.J., Peters J.R., Tynan A., Evans M., Heine R.J., Bracco O.L., Zagar T., Poole C.D. Survival as a function of HbA(1c) in people with type 2 diabetes: A retrospective cohort study. Lancet. 2010;375:481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 22.Nichols G.A., Joshua-Gotlib S., Parasuraman S. Glycemic control and risk of cardiovascular disease hospitalization and all-cause mortality. J. Am. Coll. Cardiol. 2013;62:121–127. doi: 10.1016/j.jacc.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 23.Huang E.S., Liu J.Y., Moffet H.H., John P.M., Karter A.J. Glycemic control, complications, and death in older diabetic patients: The diabetes and aging study. Diabetes Care. 2011;34:1329–1336. doi: 10.2337/dc10-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diabetes Control and Complications Trial Research Group. Nathan D.M., Genuth S., Lachin J., Cleary P., Crofford O., Davis M., Rand L., Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 25.Stratton I.M., Adler A.I., Neil H.A., Matthews D.R., Manley S.E., Cull C.A., Hadden D., Turner R.C., Holman R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(Suppl. 1):S12–S54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 27.Ali M.K., Bullard K.M., Saaddine J.B., Cowie C.C., Imperatore G., Gregg E.W. Achievement of goals in U.S. diabetes care, 1999–2010. N. Engl. J. Med. 2013;368:1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 28.Carls G., Huynh J., Tuttle E., Yee J., Edelman S.V. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther. 2017;8:863–873. doi: 10.1007/s13300-017-0280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipska K.J., Ross J.S., Miao Y., Shah N.D., Lee S.J., Steinman M.A. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern. Med. 2015;175:356–362. doi: 10.1001/jamainternmed.2014.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold S.V., Lipska K.J., Wang J., Seman L., Mehta S.N., Kosiborod M. Use of intensive glycemic management in older adults with diabetes mellitus. J. Am. Geriatr Soc. 2018;66:1190–1194. doi: 10.1111/jgs.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sussman J.B., Kerr E.A., Saini S.D., Holleman R.G., Klamerus M.L., Min L.C., Vijan S., Hofer T.P. Rates of deintensification of blood pressure and glycemic medication treatment based on levels of control and life expectancy in older patients with diabetes mellitus. JAMA Intern. Med. 2015;175:1942–1949. doi: 10.1001/jamainternmed.2015.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosentino F., Grant P.J., Aboyans V., Bailey C.J., Ceriello A., Delgado V., Federici M., Filippatos G., Grobbee D.E., Hansen T.B., et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association 6. Glycemic Targets: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(Suppl. 1):S66–S76. doi: 10.2337/dc20-S006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the corresponding author.