Abstract
Introduction
Contrasting findings have been published regarding the effect of human immunodeficiency virus (HIV) on tuberculosis (TB) drug pharmacokinetics (PK).
Objectives
The aim of this systematic review was to investigate the effect of HIV infection on the PK of the first-line TB drugs (FLDs) rifampicin, isoniazid, pyrazinamide and ethambutol by assessing all published literature.
Methods
Searches were performed in MEDLINE (through PubMed) and EMBASE to find original studies evaluating the effect of HIV infection on the PK of FLDs. The included studies were assessed for bias and clinical relevance. PK data were extracted to provide insight into the difference of FLD PK between HIV-positive and HIV-negative TB patients. This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and its protocol was registered at PROSPERO (registration number CRD42017067250).
Results
Overall, 27 studies were eligible for inclusion. The available studies provide a heterogeneous dataset from which consistent results could not be obtained. In both HIV-positive and HIV-negative TB groups, rifampicin (13 of 15) and ethambutol (4 of 8) peak concentration (Cmax) often did not achieve the minimum reference values. More than half of the studies (11 of 20) that included both HIV-positive and HIV-negative TB groups showed statistically significantly altered FLD area under the concentration–time curve and/or Cmax for at least one FLD.
Conclusions
HIV infection may be one of several factors that reduce FLD exposure. We could not make general recommendations with respect to the role of dosing. There is a need for consistent and homogeneous studies to be conducted.
Key Points
| The available studies provide a heterogeneous dataset, and this study exposes the current knowledge gaps regarding the effect of human immunodeficiency virus (HIV) infection on the pharmacokinetics (PK) of first-line tuberculosis drugs (FLDs). |
| There is a need for a consistent and homogeneous approach to studies, and for a uniform quality assessment tool for PK studies. |
| Taking clinical relevance into account, we postulate that HIV infection may increase the risk for low FLD exposure, with potential detrimental consequences for treatment outcomes. |
Introduction
Tuberculosis (TB) is an infectious disease caused by the organism Mycobacterium tuberculosis. Despite concerted efforts, TB has remained a major global health problem [1]. With an estimated 1.8 million TB deaths in 2015, including 0.4 million TB-related deaths among human immunodeficiency virus (HIV) infected persons, TB is a leading infectious killer worldwide [1]. Although improvements have been made in the prevention and treatment of HIV, 2.1 million new HIV infections worldwide were reported in 2015, resulting in a total of 36.7 million people living with HIV globally [2]. The risk of developing TB is 17- to 22-fold higher for people living with HIV, making HIV the most important predisposing factor for TB [3, 4]. TB and HIV are known to act synergistically on the decline of the host immune response, which is fatal if left untreated [5, 6].
The treatment of drug-susceptible TB consists of four first-line TB drugs (FLD): isoniazid (INH), rifampicin (RIF), pyrazinamide (PZA) and ethambutol (EMB) [7]. Due to the limited resources in regions with a high TB burden, the World Health Organization (WHO) advocates standardized treatment with generic, fixed-dose combination (FDC) formulation tablets for reasons of adherence, costs and logistics [7]. The recommended regimen consists of a 2-month intensive phase with all four FLDs, and a 4-month continuation phase with RIF and INH only [7]. Despite the utilization of weight-banded dosing, high pharmacokinetic (PK) variability has been reported for the FLDs in studies investigating the PK of these drugs [8–10]. The hollow-fibre infection model and murine model conducted with the four FLDs showed that their effectiveness is best reflected by the area under the concentration–time curve (AUC)/minimum inhibitory concentration (MIC) ratio [9, 11–13]. Notably, high PK variability and inadequate TB drug exposure are undesirable as high drug concentrations could lead to toxicity, while low drug exposure predisposes to prolonged treatment, treatment failure, relapse, and development of drug resistance [9, 14–17]. Several factors are known to cause interindividual PK variability, including body weight [18], sex [18, 19], pharmacogenomics [20, 21] and comorbid conditions such as diabetes mellitus [19].
Contrasting findings have been published regarding the effect of HIV on TB drug PK variability. Some studies showed reduced FLD exposure in HIV-infected patients [22–24], while others found no impact of HIV co-infection [25, 26]. TB drug concentrations are an important determinant of clinical response to treatment [27] and any potential negative effect of HIV co-infection on the PK of TB drugs is therefore crucial. Despite the WHO recommendation that all individuals living with HIV should be initiated on antiretroviral therapy (ART), resulting in high ART coverage of HIV-infected TB patients, the effect of HIV infection on the PK of FLDs remains relevant. The start of ART does not immediately improve the clinical and immunological condition of the patient, and the high bacterial burden at the start of TB treatment increases the risk of acquired drug resistance if plasma drug concentrations are affected by HIV co-infection.
In high endemic TB areas, drug shortages delay ART initiation and HIV suppression is not always achieved with the available antiretroviral drugs. The aim of this systematic review was to investigate the impact of HIV infection on the PK of RIF, INH, PZA and EMB.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [28]. The protocol was registered at PROSPERO (registration number CRD42017067250).
A specific clinical question was structured according to the population, intervention, comparison, outcome (PICO) approach. In this process, ‘P’ represented HIV-positive patients with TB co-infection; ‘I’ represented treatment of drug-susceptible TB with RIF, INH, PZA and EMB; ‘C’ represented HIV-negative TB patients; and ‘O’ represented the drug concentration of RIF, INH, PZA and EMB.
To retrieve relevant articles, a systematic electronic database search was performed in MEDLINE (through PubMed) and EMBASE on 11 June 2017, and an additional check for new published articles was conducted on 29 August 2018. The searches were assessed to find original studies evaluating the effect of HIV infection on the PK of RIF, INH, PZA and/or EMB. All published studies, without restriction on language and publication date, were eligible. Studies in adult and paediatric populations were included. In cases where healthy volunteers were included as a control group, the study was eligible for inclusion, provided that a group of HIV-infected patients without TB was included to assess the effect of HIV infection on the PK of the FLDs. Studies with HIV-positive patients receiving ART were also eligible for inclusion, provided that the effect of HIV infection on the PK of the FLD was assessed and reported. Studies conducted in HIV-positive TB patients without a comparator HIV-negative TB group were included in the systematic review but were not eligible for in-depth analysis. Reviews, letters, meeting and abstract posters, and correspondence were excluded, as were studies without PK data, drug interaction studies, and nonhuman studies.
The search terms used were: (hiv[mesh] OR hiv infection[mesh] OR hiv[tiab] OR hiv infection[tiab]) AND (tuberculosis[mesh] OR tuberculosis[tiab] OR tb[tiab]) AND ((pharmacokinetics[mesh] AND antitubercular agents[mesh]) OR (pharmacokinetics[tiab] AND (antitubercular[tiab] OR “TB drugs”[tiab] OR antimycobacterial[tiab] OR “antituberculosis drugs”[tiab] OR isoniazid[tiab] OR rifampicin[tiab] OR rifampin[tiab] OR ethambutol[tiab] OR pyrazinamide[tiab])). The studies retrieved from both PubMed and EMBASE were pooled and duplicate articles were removed. First, we screened titles and abstracts for eligibility, and full-text articles were read by the first author (AD) if the abstract was found to be eligible or in case of doubt. When the full-text article met all inclusion and exclusion criteria, it was included in the systematic review. Primary references of the included studies were checked and included if relevant. A second reviewer (LRI) conducted the article selection process independently and any discrepancies were resolved by discussion. In order to identify unpublished studies, the ClinicalTrials.gov website (http://clinicaltrials.gov) was searched.
One researcher (AD) first performed data extraction, using a pre-discussed structured form, and the second researcher (LRI) then independently checked the data extraction. Variables including age group (paediatric or adult), comparator group(s) and the HIV-positive group were noted for the included articles. Dose, AUC, peak drug concentration (Cmax), half-life (t½), time to reach Cmax (Tmax), volume of distribution (Vd) and clearance (CL) were extracted from the included articles if available and were stratified by group. The data were extracted and noted per drug of interest (RIF, INH, PZA and EMB). Corresponding authors were contacted by electronic mail for additional data if relevant data were missing in the included studies. Finally, the possibility of pooling data from included studies was assessed based on the risk of bias assessment, PK calculation strategy and data presentation.
No validated tool for risk of bias assessment of PK studies was available. In the absence of such a tool, we assessed the risk of bias in a study by noting the presence or absence of essential components required for adequate interpretation of results of a PK study. This provided the opportunity to compare the included studies on risk of bias related to the methods and design. The following components were checked: total sample size, inclusion of both HIV-positive and HIV-negative TB groups, proportion of participants with CD4+ < 200 cells/µL or CD4% < 12, proportion of HIV-positive participants receiving ART, presence of an absorption test, report of PK-altering morbidities (gastrointestinal, hepatic or renal), assessment of interacting comedication, calculation of the drug dosage per included group, report of directly observed therapy (DOT), number of plasma samples drawn per participant, description of specimen handling, use of validated analytical methods, method of AUC calculation, AUC calculation, stratification of data by HIV infection, and the number of participants who were lost to follow-up or died during the study period. Studies without a comparator group were only included in the narrative results and were excluded from further analysis. The combination of the number of plasma samples and the AUC calculation method (noncompartmental or model-based) was used to determine whether a study had a high or low risk of bias for the AUC calculation. Five or more plasma samples per patient, as well as utilization of a validated population PK model for all FLDs, were considered low risk.
In addition to a narrative synthesis of the results, the main results per study and the effect of HIV infection on AUC and/or Cmax and additional PK parameters, if available, were displayed in a table. The data from patients at different months of treatment or at different dosing schemes were presented separately. When the AUC and/or Cmax for both HIV-positive and HIV-negative TB groups were available, these results were plotted in histograms for each study, comparing the AUC and/or Cmax between HIV groups. This provided the opportunity to demonstrate an overview of trends. The clinical relevance of our findings was assessed in accordance with European Medicines Agency (EMA) guidelines [29, 30]. EMA guidelines including bioequivalence cut-off values of < 80% and > 125% were also used to estimate the clinical relevance of the reported statistically significant differences. Studies showing a statistically significant difference in AUC with an HIV-positive/HIV-negative ratio of < 80% or > 125% were considered clinically relevant. Only studies reporting data stratified by HIV status were eligible for this analysis.
Results
In total, 282 articles were retrieved from the searches in PubMed and EMBASE. Systematically assessing the retrieved articles resulted in 25 articles being eligible for inclusion. One additional article was a report of a preliminary analysis [31] of the study by Antwi et al. [32] and was therefore excluded. Two further articles were identified by reviewing the references of the first included articles [33, 34], resulting in a total of 27 articles being included in the current systematic review. No relevant unpublished studies were found on the ClinicalTrials.gov website investigating the effect of HIV infection on the PK of the FLDs. A flowchart of the selection process is presented in Fig. 1.
Fig. 1.
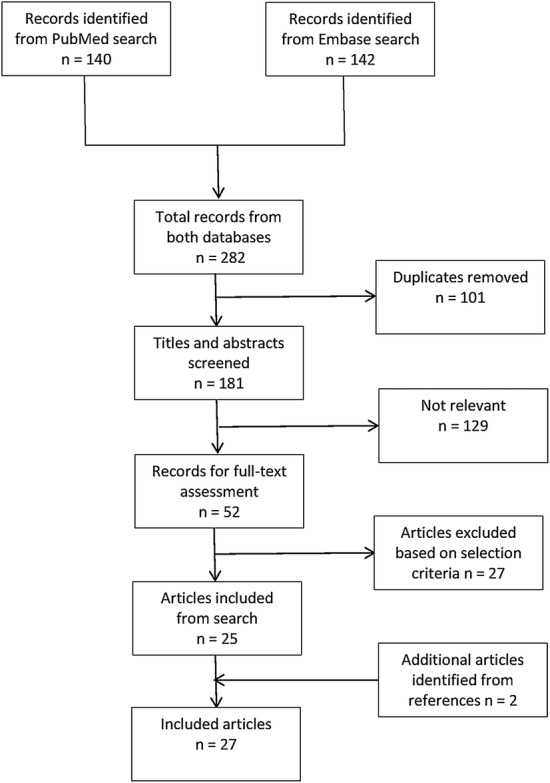
Study search and selection process
All included articles were screened for the presence or absence of essential components as a means of bias risk assessment. Twenty studies were conducted in adults and seven studies were conducted in children. Five studies only included an HIV-positive TB group, whereas a comparator HIV-negative TB group was lacking [34–38]; therefore these studies were excluded for further analysis. Twelve studies only included HIV-positive TB participants not receiving ART [22–24, 26, 32, 33, 39–44], in ten studies a proportion of HIV-positive participants were receiving ART [19, 34, 38, 45–51], and five studies did not provide information on ART use among HIV-positive TB patients [25, 35–37, 52]. In 11 studies, a limited number of fewer than five blood samples were drawn for determination of drug concentrations [19, 25, 34, 36, 37, 40, 41, 43, 46, 47, 52]. Three studies included both an HIV-positive and an HIV-negative TB group but did not provide the AUC and Cmax stratified by HIV status [40, 44, 46]. Two studies only reported Cmax [19, 52], while one study determined the percentage of RIF excreted in urine [23]. The assessment of risk of bias is presented in Table 1.
Table 1.
Risk of bias assessment of the included studies
| Authors | Participants | Study design | Bioanalytical | Endpoints/follow-up | Gradinga | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Both HIV+ and HIV− TB groups included | Total number of participants | HIV and TB confirmation tests described | Number of participants CD4 < 200 cells/µL or CD4% < 12 | Proportion of HIV-positive participants receiving ART | Absorption test conducted | PK-altering comorbidities taken into account | Interacting (non-ART) comedication described | Given dose in mg/kg known per group | DOT | Validated analytical determination | Specimen handling described | Number of plasma samples | Method of AUC calculation | AUC calculation | Cmax calculation | AUC and Cmax data stratified per arm | Number of participants lost to follow-up or died | Risk of bias (high, medium or low) | |
| Antwi et al. [32] | + | 113 | + | +/− | 0/59 | − | − | − | + | + | + | + | 5 | Noncompartmental | +/− | + | + | 6 | Medium |
| Bekker et al. [45] | + | 39 | +/− | 0 | 5/5 | − | − | − | + | + | + | + | 6 | Noncompartmental | +/− | + | + | 2 | Medium |
| Chideya et al. [52] | + | 225 | + | 84 | − | − | − | − | − | + | + | + | 3 | Noncompartmental | − | − | +/− | 17 | High |
| Choudri et al. [39] | + | 29 | +/− | 8 | 0/14 | + | + | + | − | + | + | + | 9 | Noncompartmental | + | + | + | − | Low |
| Conte et al. [40] | + | 80 | +/− | +/− | 0/40 | − | + | + | − | +/− | + | + | 2 | NA | NA | − | − | 0 | High |
| Conte et al. [46] | + | 40 | +/− | − | 10/20 | − | + | + | − | +/− | + | + | 3 | NA | NA | − | − | − | High |
| Denti et al. [41] | + | 100 | + | +/− | 0/50 | − | − | − | − | + | + | + | 3 | Model-based | + | + | − | 8 | Medium |
| Graham et al. [42] | + | 45 | + | − | 0/18 | − | +/− | − | + | + | + | + | 7 | Noncompartmental | + | + | +/− | − | Medium |
| Gurumurthy et al. [22] | + | 41 | +/− | +/− | 0/28 | − | − | − | + | + | + | − | 5 | Noncompartmental | + | + | + | − | Medium |
| Gurumurthy et al. [23] | + | 99 | + | +/− | 0/66 | + | + | + | − | + | + | + | 0 | NA | NA | NA | NA | − | Medium |
| Jaruratanasirikul [35] | − | 8 | +/− | 0 | − | − | + | − | − | − | − | +/− | 14 | Noncompartmental | + | + | − | − | High |
| Jeremiah et al. [43] | + | 100 | + | +/− | 0/50 | − | − | − | − | + | + | + | 3 | Model-based | + | + | + | 8 | Medium |
| Jönsson et al. [44] | + | 189 | − | − | 0/24 | − | − | − | − | + | + | + | 10 | Model-based | − | − | − | 0 | High |
| McIlleron et al. [24] | + | 142 | − | − | 0/9 | − | − | + | + | + | + | + | 10 | Model-based | + | + | + | − | Medium |
| Mukherjee et al. [47] | + | 56 | + | +/− | 19/24 | − | + | − | + | + | + | + | 4 | Noncompartmental | − | + | + | − | Medium |
| Van Oosterhout et al. [48] | + | 47 | + | +/− | 14/30 | − | +/− | − | − | − | + | + | 9 | Model-based | + | + | + | − | Medium |
| Peloquin et al. [34] | − | 26 | + | 23 | 4/26 | − | + | + | + | + | +/− | + | 1 | NA | NA | NA | NA | 0 | High |
| Perlman et al. [36] | − | 48 | + | 36 | − | − | + | + | + | + | + | + | 3 | Noncompartmental | − | + | NA | 5 | Medium |
| Perlman et al. [37] | − | 59 | + | 39 | − | − | + | + | + | + | + | + | 3 | Model-based | − | + | NA | 5 | Medium |
| Ramachandran et al. [38] | − | 77 | + | +/− | 45/77 | − | − | − | + | + | + | + | 5 | Noncompartmental | +/− | + | NA | 5 | Medium |
| Ramachandran et al. [49] | + | 161 | + | +/− | 45/77 | − | − | − | + | + | + | + | 5 | Noncompartmental | +/− | + | + | − | Medium |
| Requena-Mendez et al. [19] | + | 79 | − | +/− | 8/29 | − | + | − | + | + | + | + | 2 | NA | − | + | +/− | 29 | Medium |
| Requena-Mendez et al. [25] | + | 82 | − | − | − | − | + | − | − | + | − | + | 2 | Noncompartmental | − | + | + | 8 | High |
| Rockwood et al. [50] | + | 100 | + | 29 | 50/65 | − | − | − | + | + | + | + | 7 | Model-based | + | + | + | 8 | Low |
| Sahai et al. [33] | + | 48 | − | 24 | 0/36 | + | + | + | + | + | + | + | 13 | Model-based | + | + | + | − | Low |
| Schaaf et al. [51] | + | 60 | +/− | − | 2/21 | − | − | − | − | + | + | + | 5 | Noncompartmental | +/− | + | + | 6 | Medium |
| Taylor and Smith [26] | + | 27 | − | 13 | 0/13 | − | + | + | + | + | + | + | 19 | Noncompartmental | + | + | + | − | Low |
NA not applicable, ART antiretroviral therapy, DOT directly observed therapy, TB tuberculosis, PK pharmacokinetic, AUC area under the concentration–time curve, Cmax peak concentration, + present, − absent or not provided, +/− partially/incomplete
AUC calculation: (1) Model-based and AUC > (0–8 h) = +; (2) noncompartmental and AUC > (0–8 h) and ≥ 5 plasma samples = +; (3) noncompartmental and AUC ≤ (0–8 h) and ≥ 5 plasma samples = +/−; (4) noncompartmental and AUC > (0–8 h) and < 5 plasma samples = +/−; (5) model-based and AUC ≤ (0–8 h) = +/−; (6) noncompartmental and AUC ≤ (0–8 h) and < 5 plasma samples = −
aGrading of the studies was performed based on the risk of bias: 1–6 points, high risk of bias; 7–9 points, medium risk of bias; 10–12 points, low risk of bias. Note: in the absence of a validated risk of bias assessment of pharmacokinetic studies, our strategy was based on the summary of strength and weaknesses of the included studies
Analysis of the extracted data showed that there was clinical, methodological and statistical heterogeneity among the included studies. The clinical heterogeneity consisted of diversity in outcomes, since outcomes were demonstrated as AUC4, AUC6, AUC8, AUC12, AUC24 and AUC∞. The methodological diversity consisted of heterogeneity regarding sampling time points, number of samples collected, calculated AUC range, PK calculation methods and presentation of the results. As a result of the clinical and methodological heterogeneity, the data also showed high statistical heterogeneity as the main outcomes were inconsistent. As a result of the diversity, the data were too heterogeneous to allow pooling. The PK variability within and between studies was high for all four drugs when comparing the mean or median AUC and Cmax. The majority of studies presenting PK data reported AUC (16 of 27 studies) and Cmax (21 of 27 studies). One study reported data on Vd [32], two reported data on CL [32, 46], eight reported data on Tmax [22, 26, 32, 33, 36, 37, 47, 53], and six reported data on t½ [22, 33, 36, 37, 39, 45].
Rifampicin
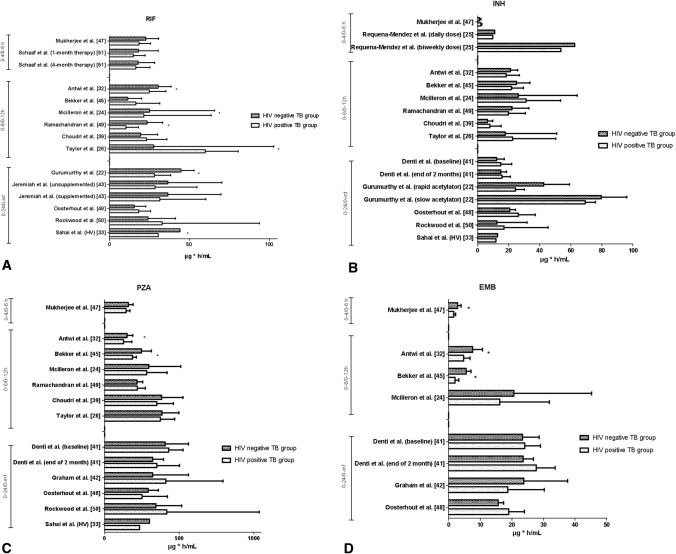
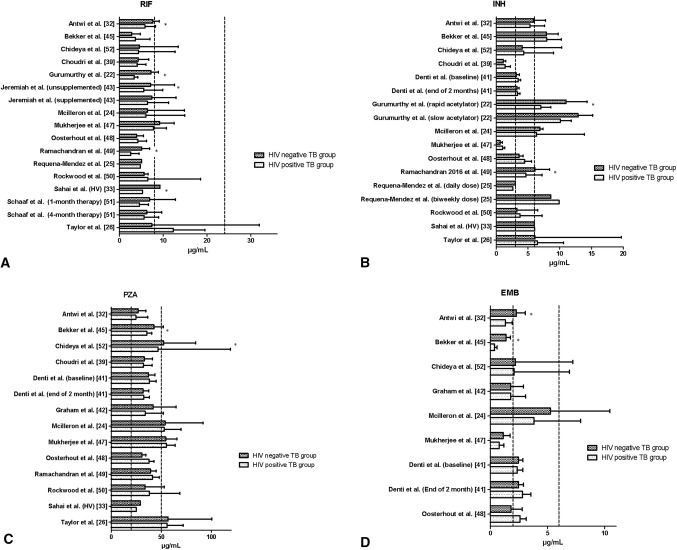
In total, 21 of the included articles assessed the effect of HIV infection on RIF PK. A narrative synthesis of the results is presented in Table 2. Three articles reported a statistically significantly reduced RIF AUC for the HIV-positive TB group compared with the HIV-negative TB group [22, 32, 49]. One article found that the HIV group had a statistically significantly lower RIF AUC value compared with healthy HIV-uninfected volunteers [33], while another study found the RIF AUC was statistically significantly higher in the HIV-positive TB group than in the HIV-negative TB group [26]. Five articles reported a statistically significant reduction of Cmax in the HIV-positive TB group compared with the HIV-negative TB group [22, 32, 43, 49, 52], and one study showed a statistically significantly lower RIF Cmax for the HIV group compared with healthy HIV-uninfected volunteers [33]. One study demonstrated that excretion of RIF was reduced by 27% and 34% in the HIV-positive group with diarrhoea and the HIV TB co-infected group without diarrhoea, respectively, compared with the HIV-uninfected TB group [23]. None of the included articles reported a statistically significant difference in Tmax between the HIV-negative and HIV-positive TB groups. Histograms from studies comparing the HIV-negative and HIV-positive TB groups are plotted in Figs. 2a and 3a in regard to AUC and Cmax, respectively.
Table 2.
Overview of studies investigating the effect of HIV infection on the AUC and Cmax of rifampicin, isoniazid, pyrazinamide and ethambutol
| Authors | Country | Study period | Age group | Comparator (n) | HIV-positive group (n) | Effect on AUC | Effect on Cmax | Additional PK data |
|---|---|---|---|---|---|---|---|---|
| Rifampicin | ||||||||
| Antwi et al. [32] | Ghana | 2012–2015 | Ped | TB (54) | HT (59) | AUC8 decreased 18.3%* | Cmax decreased 23.8%* | |
| Bekker et al. [45] | South Africa | 2014–2015 | Ped | TB (34) | HT (5) | AUC8 ↔# | ↔# | |
| Chideya et al. [52] | Botswana | 1997–2000 | Adult | TB (70) |
HT low CD4+ (84) HT high CD4+ (71) |
– |
↔ for HT group with low CD4+ Cmax increased 24%* for the HT group with high CD4+ |
|
| Choudri et al. [39] | Kenya | 1994–1995 | Adult | TB (15) | HT (14) | AUC12 ↔# | ↔# | |
| Conte et al. [46] | USA | – | Adult | HV (20) | HIV (20) | – | – | HIV status had no effect on C2 and C4 plasma concentrations |
| Gurumurthy et al. [22] | India | 2002 | Adult | TB (13) |
HIV (13) HT (15) |
AUC∞ decreased 52.5%* for the HIV group with low CD4+ AUC∞ decreased 36.8%* for the HT group# |
Cmax decreased 52.8%* for both groups# | |
| Gurumurthy et al. [23] | India | 2001 | Adult | TB (23) |
HIV (40) HT (26) |
– | – | Excretion was reduced 27%* and 34%* for the HIV and HT groups, respectively# |
| Jaruratanasirikul [35] | Thailand | – | Adult | None | HT (8) | – | – | Mean Cmax was 9.81 ± 4.41 µg/mL and mean AUC24 was 60.25 ± 36.88 µg·h/mL# |
| Jeremiah et al. (unsupplemented) [43] | Tanzania | 2010–2011 | Adult | TB (25) | HT (24) | AUC24 ↔ | Cmax decreased 21.8%* | |
| Jeremiah et al. (supplemented) [43] | Tanzania | 2010–2011 | Adult | TB (25) | HT (26) | AUC24 ↔ | ↔ | |
| McIlleron et al. [24] | South Africa | 1999–2002 | Adult | TB (127) | HT (14) | AUC8 decreased 39%* | – | |
| Mukherjee et al. [47] | India | 2009–2013 | Ped | TB (32) | HT (24) | AUC4 ↔# | ↔# | |
| van Oosterhout et al. [48] | Malawi | 2007–2008 | Adult | TB (17) | HT (30) | ↔# | ↔# | HIV did not affect PK parameters |
| Peloquin et al. [34] | USA | 1993–1994 | Adult | TB (lit) | HT (26) | – | – | 2-h serum concentrations were measured |
| Perlman et al. (daily dose) [37] | USA | – | Adult | TB (lit) | HT (34) | – | – | 76% of recipients had lower Cmax values compared with the published range (< 8 µg/mL) |
| Perlman et al. (intermittent dose) [37] | USA | – | Adult | TB (lit) | HT (21) | – | – | 68% of recipients had lower Cmax values compared with the published range (< 8 µg/mL) |
| Ramachandran et al. [38] | India | 2010–2013 | Ped | TB (lit) | HT (77) | – | – | 97% of recipients had lower Cmax values compared with the published range (< 8 µg/mL) |
| Ramachandran et al. [49] | India | 2010–2013 | Ped | TB (84) | HT (77) | AUC8 decreased 55.6%* | Cmax decreased 49.0%* | |
| Requena-Mendez et al. [19] | Peru | 2009 | Adult | TB (50) | HT (29) | – | – | Plasma C2 and C6 ↔ |
| Rockwood et al. [50] | South Africa | 2013–2014 | Adult | TB (35) | HT (65) | AUC24 ↔ | ↔ | HIV was associated with a 21%* decrease in clearance |
| Sahai et al. [33] | Canada | – | Adult | HV (12) |
HIV low CD4+ (24) HIV high CD4+ (12) |
AUC24 decreased 30.8% for the HIV group with low CD4+ * AUC24 decreased 28.8%* for the HIV group with high CD4+ # |
Cmax decreased 42.9%* for the HIV group with low CD4+ Cmax decreased 36.3%* for the HIV group with high CD4+ # |
|
| Schaaf et al. (1-month therapy) [51] | South Africa | 2004–2006 | Ped | TB (33) | HT (21) | AUC6 ↔# | ↔# | |
| Schaaf et al. (4-month therapy) [51] | South Africa | 2004–2006 | Ped | TB (33) | HT (21) | AUC6 ↔# | ↔# | |
| Taylor and Smith [26] | South Africa | 1998 | Adult | TB (14) | HT (13) | AUC12 increased 216%* | ↔ | |
| Isoniazid | ||||||||
| Antwi et al. [32] | Ghana | 2012–2015 | Ped | TB (54) | HT (59) | AUC8 ↔ | ↔ | |
| Bekker et al. [45] | South Africa | 2014–2015 | Ped | TB (34) | HT (5) | AUC8 ↔# | ↔# | |
| Chideya et al. [52] | Botswana | 1997–2000 | Adult | TB (70) |
HT low CD4+ (84) HT high CD4+ (71) |
_ | ↔ for both groups | |
| Choudri et al. [39] | Kenya | 1994–1995 | Adult | TB (15) | HT (14) | AUC12 ↔# | – | |
| Conte et al. [40] | USA | – | Adult | HV (40) |
HIV low CD4+ (4) HIV high CD4+ (36) |
– | – | HIV status had no effect on C1 and C4 plasma concentrations |
| Denti et al. [41] | Tanzania | 2010–2011 | Adult | TB (50) | HT (50) | AUC24 ↔ | ↔ | |
| Gurumurthy et al. (rapid acetylator) [22] | India | 2002 | Adult | TB (5) |
HIV (9) HT (8) |
AUC∞ ↔ for both groups# |
↔ for the HIV group Cmax decreased 36.4%* for the HT group# |
|
| Gurumurthy et al. (slow acetylator) [22] | India | 2002 | Adult | TB (8) |
HIV (4) HT (7) |
AUC∞ ↔ for both groups# | ↔ for both groups# | |
| Gurumurthy et al. [23] | India | 2001 | Adult | TB (23) |
HIV (40) HT (26) |
– | – | Excretion was reduced 24%* and 23%* for the HIV and HT groups, respectively# |
| McIlleron et al. [24] | South Africa | 1999–2002 | Adult | TB (127) | HT (14) | AUC8 ↔ | – | |
| Mukherjee et al. [47] | India | 2009–2013 | Ped | TB (32) | HT (24) | AUC4 ↔# | ↔# | |
| van Oosterhout et al. [48] | Malawi | 2007–2008 | Adult | TB (17) | HT (30) | ↔# | ↔# | HIV did not affect PK parameters |
| Peloquin et al. [34] | USA | 1993–1994 | Adult | TB (lit) | HT (26) | – | – | 2-h serum concentrations were measured |
| Ramachandran et al. [38] | India | 2010–2013 | Ped | TB (lit) | HT (77) | – | – | 28% of recipients had lower Cmax values compared with the published range (< 3 µg/mL) |
| Ramachandran et al. [49] | India | 2010–2013 | Ped | TB (84) | HT (77) | AUC8 ↔ | Cmax decreased 23.0%* | |
| Requena-Mendez et al. (daily dose) [25] | Peru | 2009 | Adult | TB (32) | HT (16) | AUC6 ↔# | ↔# | |
| Requena-Mendez et al. (biweekly dose) [25] | Peru | 2009 | Adult | TB (18) | HT (13) | AUC6 ↔# | ↔# | |
| Rockwood et al. [50] | South Africa | 2013–2014 | Adult | TB (35) | HT (65) | AUC24 ↔ | ↔ | HIV was associated with a 23%* decrease of clearance |
| Sahai et al. [33] | Canada | – | Adult | HV (12) |
HT low CD4+ (24) HT high CD4+ (12) |
AUC24 ↔ for both groups# | ↔ for both groups# | |
| Taylor and Smith [26] | South Africa | 1998 | Adult | TB (14) | HT (13) | AUC12 ↔ | ↔ | |
| Pyrazinamide | ||||||||
| Antwi et al. [32] | Ghana | 2012–2015 | Ped | TB (54) | HT (59) | AUC8 decreased 16.2%* | ↔ | |
| Bekker et al. [45] | South Africa | 2014–2015 | Ped | TB (34) | HT (5) | AUC8 decreased 21%*,# | Cmax decreased 15%*,# | |
| Chideya et al. [52] | Botswana | 1997–2000 | Adult | TB (70) |
HT low CD4+ (84) HT high CD4+ (71) |
– |
Cmax decreased 10.3%* for the HT with low CD4+ group ↔ for the HT with high CD4+ group |
|
| Choudri et al. [39] | Kenya | 1994–1995 | Adult | TB (15) | HT (14) | AUC12 ↔# | ↔# | |
| Denti et al. [41] | Tanzania | 2010–2011 | Adult | TB (50) |
HIV low CD4+ (4) HIV high CD4+ (36) |
AUC24 ↔ | ↔ | |
| Graham et al. [42] | Malawi | 2000–2001 | Ped | TB (9) | HT (18) | AUC24 ↔# | ↔# | |
| Gurumurthy et al. [22] | India | 2002 | Adult | TB (13) |
HIV (13) HT (15) |
– | – | Dosage excreted in urine was reduced 35%* and 48%* for the HIV and HT groups, respectively# |
| McIlleron et al. [24] | South Africa | 1999–2002 | Adult | TB (127) | HT (14) | ↔ | ↔ | |
| Mukherjee et al. [47] | India | 2009–2013 | Ped | TB (32) | HT (24) | AUC4 ↔# | ↔# | |
| van Oosterhout et al. [48] | Malawi | 2007–2008 | Adult | TB (17) | HT (30) | ↔# | Cmax decreased 15%*,# | |
| Peloquin et al. [34] | USA | 1993–1994 | Adult | TB (lit) | HT (26) | – | – | 2-h serum concentrations were measured |
| Perlman et al. (daily dose) [36] | USA | – | Adult | TB (lit) | HT (24) | – | – | 6.4% of recipients had lower Cmax values compared with the published range (< 20 µg/mL) |
| Perlman et al. (intermittent dose) [36] | USA | – | Adult | TB (lit) | HT (23) | – | – | 4.0% of recipients had lower Cmax values compared with the published range (< 25 µg/mL) |
| Ramachandran et al. [38] | India | 2010–2013 | Ped | TB (lit) | HT (77) | – | – | 33% of recipients had lower Cmax values compared with the published range (< 35 µg/mL) |
| Ramachandran et al. [49] | India | 2010–2013 | Ped | TB (84) | HT (77) | AUC8 ↔ | ↔ | |
| Rockwood et al. [50] | South Africa | 2013–2014 | Adult | TB (35) | HT (65) | AUC24 ↔ | ↔ | |
| Sahai et al. [33] | Canada | – | Adult | HV (12) |
HT low CD4+ (24) HT high CD4+ (12) |
AUC24 ↔ for both groups# | ↔# | |
| Taylor and Smith [26] | South Africa | 1998 | Adult | TB (14) | HT (13) | AUC12 ↔ | ↔ | |
| Ethambutol | ||||||||
| Antwi et al. [32] | Ghana | 2012–2015 | Ped | TB (54) | HT (59) | AUC8 decreased 37.1%* | Cmax decreased 41.7%* | |
| Bekker et al. [45] | South Africa | 2014–2015 | Ped | TB (14) | HT (2) | AUC8 decreased 63.0%*,# | Cmax decreased 71.7%*,# | |
| Chideya et al. [52] | Botswana | 1997–2000 | Adult | TB (70) |
HT low CD4+ (84) HT high CD4+ (71) |
– | Cmax ↔ for both groups | |
| Denti et al. [41] | Tanzania | 2010–2011 | Adult | TB (50) | HT (14) | AUC24 ↔ | ↔ | |
| Graham et al. [42] | Malawi | 2000–2001 | Ped | TB (12) | HT (6) | – | ↔ | |
| Gurumurthy et al. [22] | India | 2002 | Adult | TB (13) |
HIV (13) HT (15) |
– | – | Dosage excreted in urine was reduced 43%* and 19%* for the HIV and HT groups, respectively# |
| Jönsson et al. [44] | South Africa | – | Adult | TB (165) | HT (24) | – | – | HIV was associated with a 15% decrease of bioavailability |
| McIlleron et al. [24] | South Africa | 1999–2002 | Adult | TB (127) | HT (14) | AUC8 decreased 27%* | ↔ | |
| Mukherjee et al. [47] | India | 2009–2013 | Ped | TB (32) | HT (24) | AUC4 decreased 44.4%*,# | ↔# | |
| van Oosterhout et al. [48] | Malawi | 2007–2008 | Adult | TB (17) | HT (30) | ↔# | ↔# | |
| Peloquin et al. [34] | USA | 1993–1994 | Adult | TB (lit) | HT (26) | – | – | 2-h serum concentrations were measured |
| Perlman et al. (daily dose) [37] | USA | – | Adult | TB (lit) | HT (48) | – | – | 69% of recipients had lower Cmax values compared with the published range (< 2 µg/mL) |
| Perlman et al. (intermittent dose) [37] | USA | – | Adult | TB (lit) | HT (20) | – | – | 39% of recipients had lower Cmax values compared with the published range (< 4 µg/mL) |
Ped paediatric, n number of participants, TB participants with only tuberculosis, HT TB/HIV co-infected participants, HV healthy volunteers, AUC area under the concentration–time curve, Cmax peak drug concentration, PK pharmacokinetic, low CD4, + < 200 cells/µL, high CD4+ ≥ 200 cells/µL, lit literature, ↔ indicates no statistical significant difference, – indicates no information available
*Statistically significant, all the PK data are expressed as median except for those marked with the # symbol, which represents the mean
Fig. 2.
Histograms of the mean or median area under the concentration–time curve for the HIV-negative and HIV-positive TB groups per study for a rifampicin, b isoniazid, c pyrazinamide and d ethambutol. Asterisk indicates statistical significance; 0–24/0–inf: AUC24 and AUC∞; 0–8/0–12: AUC8 and AUC12; 0–4/0–6; AUC4 and AUC6. The study by Sahai et al. [33] compared HIV-infected individuals without TB with healthy HIV-uninfected volunteers (healthy volunteers). INH isoniazid, RIF rifampicin, PZA pyrazinamide, EMB ethambutol, TB tuberculosis
Fig. 3.
Histograms of the mean or median peak drug concentration for the HIV-negative and HIV-positive TB groups per study for a rifampicin, b isoniazid, c pyrazinamide and d ethambutol. Asterisk indicates statistical significance. The dashed lines represent the generally cited reference ranges by Peloquin [27]: rifampicin 8–24 µg/mL; isoniazid 3–6 µg/mL; pyrazinamide 20–50 µg/mL; ethambutol 2–6 µg/mL. The study by Sahai et al. [33] compared HIV-infected individuals without TB with healthy HIV-uninfected volunteers (healthy volunteers). INH isoniazid, RIF rifampicin, PZA pyrazinamide, EMB ethambutol, TB tuberculosis
Isoniazid
Twenty included articles assessed the effect of HIV on INH PK (Table 2), and none showed statistically significant differences in AUC between the HIV-negative and HIV-positive TB groups. Two studies, both conducted in India, reported a statistically significantly lower Cmax in the HIV-positive TB group compared with the HIV-negative TB group [22, 49], and one study showed a shorter Tmax for the HIV-positive TB group compared with the HIV-negative TB group [32]. In the one study that measured excretion of INH, a significant reduction of the excretion by 24% was found in the HIV-positive group with diarrhoea, and by 23% in the HIV-positive TB group without diarrhoea, compared with the HIV-uninfected TB group [23]. Histograms from studies comparing the HIV-negative and HIV-positive TB groups are plotted in Figs. 2b and 3b in regard to AUC and Cmax, respectively.
Pyrazinamide
Seventeen included articles assessed the effect of HIV on PZA PK (Table 2). Two articles found that the HIV-positive TB group had statistically significantly reduced AUC compared with the HIV-negative TB group [32, 45]. One article reported a statistically significant reduction of Cmax in the HIV-positive TB group compared with the HIV-negative TB group [52], while another study showed a statistically significantly shorter Tmax for the HIV-positive TB group compared with the HIV-negative TB group [32]. Histograms from studies comparing the HIV-negative and HIV-positive TB groups are plotted in Figs. 2c and 3c in regard to AUC and Cmax, respectively
Ethambutol
Twelve included articles assessed the effect of HIV on EMB PK (Table 2). Three articles, all conducted in a paediatric population, showed that the HIV-positive TB group had statistically significantly reduced AUC compared with the HIV-negative TB group [32, 45, 47]. Two of these articles also reported a statistically significant reduction of Cmax in the HIV-positive TB group compared with the HIV-negative TB group [32, 45]. One study showed a statistically significant increase in Tmax for the HIV-positive TB group compared with the HIV-negative TB group [47]. Histograms from studies comparing the HIV-negative and HIV-positive TB groups are plotted in Figs. 2d and 3d in regard to AUC and Cmax, respectively.
Paediatrics
Seven studies were conducted in paediatric populations [32, 38, 42, 45, 47, 49, 51]. One study lacked a comparator TB group [38], but the data were compared with the reference ranges reported by Alsultan and Peloquin [54]. These researchers concluded that the Cmax of RIF, INH and PZA was subtherapeutic in 97%, 28% and 33% of children, respectively. Of the remaining six paediatric studies, four reported that HIV co-infection in children with TB adversely affects the AUC and/or Cmax for at least one of the FLDs [32, 45, 47, 49], and two studies did not detect statistically significant differences between the groups [42, 51].
Clinical Relevance
The ratio in AUC between the HIV-positive and HIV-negative TB groups is shown in Fig. 4. Three of the four studies reporting a statistically significantly reduced RIF AUC for the HIV-positive TB group compared with the HIV-negative TB group were clinically relevantly reduced (≤ 80%) [22, 24, 49]; the fourth study was not considered clinically relevantly reduced [32]. The decrease in RIF AUC reported in HIV-positive patients without TB compared with healthy volunteers [33] was considered clinically relevant. The one study reporting a statistically significant increase in RIF AUC in the HIV-positive TB group compared with the HIV-negative TB group [26] was also considered clinically relevant (≥ 125%). Two studies demonstrated a statistically significantly reduced PZA AUC in the HIV-positive TB group compared with the HIV-negative TB group. One of these studies was considered borderline clinically relevant [45] and the other was not considered clinically relevant [32]. The results of all four studies showing a statistically significantly reduced EMB AUC in the HIV-positive TB group compared with the HIV-negative TB group were considered to be clinically relevant [24, 32, 45, 47].
Fig. 4.
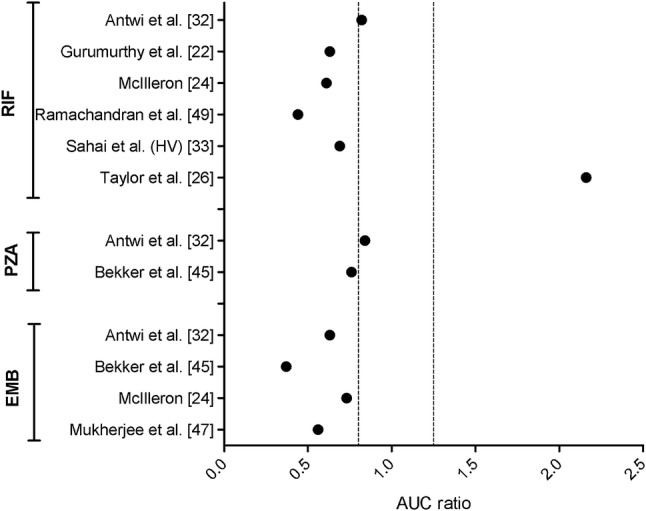
Ratio between the AUCs of HIV-positive and HIV-negative TB patients for studies showing a statistically significant alteration in first-line TB drug AUCs, stratified per drug. The dashed lines represent the 80–125% (0.8–1.25) cut-off values for clinical relevance; all studies with a ratio outside this range were considered clinically relevant. RIF rifampicin, PZA pyrazinamide, EMB ethambutol, TB tuberculosis, AUC area under the concentration–time curve
Discussion
To our knowledge, this is the first systematic review investigating the effect of HIV infection on the PK of FLDs. We found that the published data were heterogeneous and no consistent results emerged from our literature review. We also found that for EMB, and in particular for RIF, both HIV-positive and HIV-negative TB groups often did not achieve the generally accepted threshold (or minimally acceptable) Cmax reference range of 8 µg/mL for RIF and 2 µg/mL for EMB [27]. This phenomenon has already been observed in earlier studies, and research is currently being conducted investigating higher dosages of RIF [55, 56]. Although many studies showed a trend for lower AUC and/or Cmax for at least one FLD in the HIV-positive TB group compared with the HIV-negative TB group, this did not always reach statistical significance. More than half of the studies (11 of 20) that included both HIV-positive and HIV-negative TB groups showed statistically significantly different AUC and/or Cmax for at least one FLD [22, 24, 26, 32, 33, 43, 45, 47–49, 52]. We focused on AUC and/or Cmax since most of the studies reported these as primary endpoints and they are the most relevant PK predictors of clinical outcomes, especially when combined with data on MIC [9, 11–13]. The majority of the articles focused on the PK of RIF and INH, which is justified by the fact that RIF and INH together are the backbone of drug-susceptible TB treatment.
The effect of HIV infection in TB patients on the PK of TB FLDs is an ongoing debate due to lack of consistent study results [54]. There may be several reasons to explain this inconsistency. First, several studies lacked a comparator group, making it difficult to adequately investigate the effect of HIV infection on the PK of the FLDs [34–38]. Instead, these studies compared with the widely cited reference ranges published by Peloquin et al. [27, 57] and Alsultan et al. [54, 58]. However, these reference ranges are not age-, sex-, and weight-matched and often racial and regional differences are not taken into account. Studies have shown that female sex is a determinant of higher RIF, INH and PZA concentrations and lower EMB concentration [18, 24], and older age is a determinant of higher drug levels of all four FLDs [24, 58]. Another study reported that RIF exposure was significantly lower in people of African descent when adjusted for dose and genetic polymorphisms [59]. Although comparing PK data with published reference ranges provides a basic impression, patient characteristics differ highly between different populations, and conclusions from studies that compare PK findings with published reference ranges should therefore be regarded with caution.
Second, we postulate that the effect of HIV infection on the PK of the FLDs might often not have been detected due to a lack of power. Eleven of the studies that included both HIV-positive and HIV-negative TB groups showed statistically significantly that HIV infection adversely affects the PK (mainly AUC and/or Cmax) of at least one of the FLDs [22–24, 32, 33, 43–45, 47–49, 52]. Eight studies with both groups included did not detect a statistically significant difference between the two groups for all four FLDs [19, 25, 39–42, 46, 51], and one study even demonstrated a statistically significantly higher RIF AUC for the HIV-positive TB group [26]. Studies showing statistical differences in drug exposures to any of the FLDs had higher sample sizes and therefore more power compared with the studies that failed to detect such differences.
The third potential contributor to the conflicting results published might be due to inadequate PK sampling and different PK calculation methods used. Studies unable to detect significant differences often had a lower number of collected blood samples for determination of drug concentrations. In addition to the varying numbers of collected blood samples, various different methods for AUC and Cmax estimation were used. Some studies determined Cmax by choosing the highest concentration among two or three blood draws [19, 25, 36, 37]. A more reliable method for estimation of Cmax is fitting a population PK curve to the measured serum concentration–time data using Bayesian estimation [60]. Due to the varying number of blood samples drawn over a certain period of time, and the different methods used (model-based or noncompartmental) for the estimation of the AUC, the curves used to estimate AUCs in the included studies varied from 0 to 4 h [47] to 0 to 24 h [48, 50], thereby leading to potential loss of information. Collecting multiple blood samples over a longer period of time ensures the absorption, distribution, metabolism and elimination phases are adequately captured post-dose, which results in more accurate estimations of the AUC. Another approach is limited sampling strategies (LSS) or computational posteriori estimations using Bayesian methods [60–62]. In this systematic review, we therefore used the combination of the number of blood samples drawn, the use of PK modelling, and the implementation of validated bioanalytical methods for the assessment of risk of bias.
Another explanation for the contrasting results in the included studies is variation in the clinical severity of HIV infection, the degree of immunosuppression, and the use of ART. Several studies have demonstrated that the PK of the FLDs is more adversely altered in cases of more advanced stages of HIV [22, 33, 52]. In the studies that did not find lower drug exposures among HIV-positive TB patients compared with the HIV-negative TB group, the majority of co-infected patients had higher CD4+ cell counts and were receiving ART [41, 45, 48, 50], or data on HIV progression were lacking [24, 42]. It is conceivable that successful ART mitigates the effect of HIV infection on TB drug PK parameters. In 10 studies, a proportion of the included HIV-positive participants was receiving ART [19, 34, 38, 45–51], while five studies did not provide information on ART use among HIV-positive TB patients [25, 35–37, 52]. The simultaneous use of FLD and ART can result in drug–drug interactions [63, 64] and can potentially lead to nonadherence.
Among the included studies, a high interindividual PK variability was found that was not merely attributable to HIV infection. We found that in the majority of studies, both HIV-positive and HIV-negative TB patients had an RIF Cmax below the minimum reference range; the same applied for a proportion of the studies reporting EMB Cmax. This high variability involves the interplay of multiple factors, ranging from drug compounding to the distribution of the drug molecules at the site of infection. Drug formulation [24], pharmacogenomics [20, 21], racial and ethnic differences [20, 59], sex [19, 24], body weight [18], advanced immunosuppression [22, 33], comorbid conditions such as diabetes mellitus [19, 23], comedication [63, 64] and nutritional status [43, 65] are the most investigated and salient factors. It is worth mentioning that a statistically significant reduction of FLD exposure in the HIV-positive TB groups does not necessarily have to be clinically relevant and that this should be explored in future studies that include treatment outcomes. In the absence of such studies at present, the cut-off values of the EMA guidelines (< 80% and > 125%) [29, 30] offer an alternative way to determine the clinical relevance of decreased or increased FLD exposures. Since these cut-off values are based on drug exposure, only studies reporting a statistically significant change in AUC could be included in the assessment. With the exception of the studies by Antwi et al. [32] and Taylor and Smith [26] in regard to RIF, and Antwi et al. [32] in regard to PZA AUC, all studies reporting a statistically significant alteration in FLD AUC were considered clinically relevantly reduced [22, 24, 26, 32, 33, 45, 47, 49]. Taking the risk of bias assessment (Table 1) into account in relation to the studies included in the systematic review, we postulate that in patients prone to low FLD exposure, HIV infection might even further reduce drug exposure [66], leading to poor treatment outcome [9]. While not a substitute for clinical judgement, therapeutic drug monitoring (TDM) could be a powerful tool for identifying patients with subtherapeutic FLD levels at risk of poor treatment outcomes [62, 67, 68]. TDM performed early during TB treatment in patients at risk of subtherapeutic FLD levels may improve treatment response and may also prevent toxicity [68, 69]. For resource-limited settings, dried blood spot analysis combined with LSS or drug concentration measurements in saliva with thin-layer chromatography might provide a solution to address the problems of patients with a burden of blood draws, as well as costs [61, 70, 71].
A recent study by Hiruy et al. reported that HIV-negative children with TB are at risk of subtherapeutic concentrations for all FLDs [72]. Younger age has a considerable impact on TB drug exposure and should be considered in dosing recommendations. This has been attributed to children having a larger liver size and higher hepatic metabolic activity in proportion to body weight [53]. Our findings suggest that RIF and EMB exposures appear to be adversely affected in paediatric HIV-positive TB populations, even after administration of the revised WHO-recommended weight-based dosages. The clinical relevance of such reduced FLD exposures has to be urgently further investigated in paediatric populations.
A broad and comprehensive literature search was conducted systematically that allowed the identification of studies that included data on the effect of HIV infection on the PK of the FLDs. A strength of this systematic review is that it provides a good overview of the available literature and exposes current knowledge gaps. However, the systematic review also has some limitations. Despite the high disease burden, relatively few data were available and with variable quality, increasing the risk of bias. Furthermore, we chose to include all articles that included data on the effect of HIV infection on the PK of the FLDs, to prevent loss of information; therefore, studies lacking a comparator group and with participants receiving ART were included, potentially introducing bias. A more in-depth analysis was restricted to studies that had both an HIV-positive and HIV-negative TB group. Although no registered and unpublished studies were found in the database search, publication bias cannot be completely excluded. A recent study demonstrated that higher MIC values were associated with a greater risk of relapse than lower MIC values [73]. None of the studies included in this systematic review reported data on MIC. Lastly, the published data were too heterogeneous and were reported highly inconsistently, to allow pooling of the data. Due to methodological and statistical heterogeneity, subgroup analyses were also not appropriate.
Conclusion
Relatively few studies have been published investigating the effect of HIV infection on the PK of the FLDs. The available studies provide a heterogeneous dataset from which consistent results could not be obtained. Therefore, we could make no general recommendation with respect to dosing. There is a need for a consistent and homogeneous approach to studies and for a uniform quality assessment tool specifically for PK studies. Taking clinical relevance into account, we postulate that HIV infection may exacerbate a susceptibility to low FLD exposures, with potential detrimental consequences for treatment outcomes. This systematic review may inform further studies investigating the effect of HIV infection on the PK of the FLDs. A population PK analysis may provide a solution for the inability of pooling of the currently available data, as a population PK analysis can adjust for confounders. In addition, a prospective study with both an HIV-positive and HIV-negative TB group, including data on pharmacodynamics and treatment outcome, is needed to provide further insight into the highly complex PK of the FLDs.
Compliance with ethical standards
Funding
No funding was received for this research.
Conflict of interest
Alper Daskapan, Lusiana R. Idrus, Maarten J. Postma, Bob Wilffert, Jos G. W. Kosterink, Ymkje Stienstra, Daniel J. Touw, Aase B. Andersen, Adrie Bekker, Paolo Denti, Agibothu K. Hemanth Kumar, Kidola Jeremiah, Awewura Kwara, Helen McIlleron, Graeme Meintjes, Joep J. van Oosterhout, Geetha Ramachandran, Neesha Rockwood, Robert J. Wilkinson, Tjip S. van der Werf and Jan-Willem C. Alffenaar declare that they have no conflicts of interest.
References
- 1.World Health Organization. Global tuberculosis report 2016; 2016. http://www.who.int/tb/publications/global_report/en/. Accessed 25 June 2017.
- 2.UNAIDS. Global AIDS update 2016; 2016. http://www.unaids.org/en/resources/documents/2016/Global-AIDS-update-2016. Accessed 25 June 2017.
- 3.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 4.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320(9):545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 5.Modjarrad K, Vermund SH. Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis. 2010;10(7):455–463. doi: 10.1016/S1473-3099(10)70093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151(1):129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Treatment of tuberculosis guidelines fourth edition; 2010. http://www.who.int/tb/publications/2010/9789241547833/en/. Accessed 25 June 2017.
- 8.Devaleenal DB, Ramachandran G, Swaminathan S. The challenges of pharmacokinetic variability of first-line anti-TB drugs. Expert Rev Clin Pharmacol. 2017;10(1):47–58. doi: 10.1080/17512433.2017.1246179. [DOI] [PubMed] [Google Scholar]
- 9.Pasipanodya JG, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J Infect Dis. 2013;208(9):1464–1473. doi: 10.1093/infdis/jit352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds J, Heysell SK. Understanding pharmacokinetics to improve tuberculosis treatment outcome. Expert Opin Drug Metab Toxicol. 2014;10(6):813–823. doi: 10.1517/17425255.2014.895813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, et al. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother. 2007;51(11):3781–3788. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumbo T, Louie A, Liu W, Brown D, Ambrose PG, Bhavnani SM, et al. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob Agents Chemother. 2007;51(7):2329–2336. doi: 10.1128/AAC.00185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2003;47(7):2118–2124. doi: 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van’t Boveneind-Vrubleuskaya N, Daskapan A, Kosterink JG, van der Werf TS, van den Hof S, Alffenaar JC. Predictors of prolonged TB treatment in a Dutch outpatient setting. PLoS One. 2016;11(11):e0166030. doi: 10.1371/journal.pone.0166030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J Infect Dis. 2011;204(12):1951–1959. doi: 10.1093/infdis/jir658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta JB, Shantaveerapa H, Byrd RP, Jr, Morton SE, Fountain F, Roy TM. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest. 2001;120(5):1520–1524. doi: 10.1378/chest.120.5.1520. [DOI] [PubMed] [Google Scholar]
- 17.Weiner M, Benator D, Burman W, Peloquin CA, Khan A, Vernon A, Tuberculosis Trials Consortium et al. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin Infect Dis. 2005;40(10):1481–1491. doi: 10.1086/429321. [DOI] [PubMed] [Google Scholar]
- 18.McIlleron H, Rustomjee R, Vahedi M, Mthiyane T, Denti P, Connolly C, et al. Reduced antituberculosis drug concentrations in HIV-infected patients who are men or have low weight: implications for international dosing guidelines. Antimicrob Agents Chemother. 2012;56(6):3232–3238. doi: 10.1128/AAC.05526-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Requena-Mendez A, Davies G, Ardrey A, Jave O, Lopez-Romero SL, Ward SA, et al. Pharmacokinetics of rifampin in Peruvian tuberculosis patients with and without comorbid diabetes or HIV. Antimicrob Agents Chemother. 2012;56(5):2357–2363. doi: 10.1128/AAC.06059-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chigutsa E, Visser ME, Swart EC, Denti P, Pushpakom S, Egan D, et al. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob Agents Chemother. 2011;55(9):4122–4127. doi: 10.1128/AAC.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkin DP, Vandenplas S, Botha FJ, Vandenplas ML, Seifart HI, van Helden PD, et al. Trimodality of isoniazid elimination: phenotype and genotype in patients with tuberculosis. Am J Respir Crit Care Med. 1997;155(5):1717–1722. doi: 10.1164/ajrccm.155.5.9154882. [DOI] [PubMed] [Google Scholar]
- 22.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Padmapriyadarsini C, Swaminathan S, et al. Decreased bioavailability of rifampin and other antituberculosis drugs in patients with advanced human immunodeficiency virus disease. Antimicrob Agents Chemother. 2004;48(11):4473–4475. doi: 10.1128/AAC.48.11.4473-4475.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gurumurthy P, Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Padmapriyadarsini C, Swaminathan S, et al. Malabsorption of rifampin and isoniazid in HIV-infected patients with and without tuberculosis. Clin Infect Dis. 2004;38(2):280–283. doi: 10.1086/380795. [DOI] [PubMed] [Google Scholar]
- 24.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother. 2006;50(4):1170–1177. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Requena-Mendez A, Davies G, Waterhouse D, Ardrey A, Jave O, Lopez-Romero SL, et al. Effects of dosage, comorbidities, and food on isoniazid pharmacokinetics in Peruvian tuberculosis patients. Antimicrob Agents Chemother. 2014;58(12):7164–7170. doi: 10.1128/AAC.03258-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor B, Smith PJ. Does AIDS impair the absorption of antituberculosis agents? Int J Tuberc Lung Dis. 1998;2(8):670–675. [PubMed] [Google Scholar]
- 27.Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs. 2002;62(15):2169–2183. doi: 10.2165/00003495-200262150-00001. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 29.European Medicines Agency (EMA), Committee for Medicinal Product for Human Use. Guideline on the investigation on bioequivalence; 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf. Accessed 18 Dec 2017.
- 30.European Medicines Agency (EMA), Committee for Medicinal Product for Human Use. Guideline on the investigation of drug interactions; 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2012/07/WC500129606.pdf. Accessed 18 Dec 2017.
- 31.Kwara A, Enimil A, Gillani FS, Yang H, Sarfo AM, Dompreh A, et al. Pharmacokinetics of first-line antituberculosis drugs using WHO revised dosage in children with tuberculosis with and without HIV coinfection. J Pediatr Infect Dis Soc. 2016;5(4):356–365. doi: 10.1093/jpids/piv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antwi S, Yang H, Enimil A, Sarfo AM, Gillani FS, Ansong D, et al. Pharmacokinetics of the first-line antituberculosis drugs in Ghanaian children with tuberculosis with or without HIV coinfection. Antimicrob Agents Chemother. 2017;61(2):e01701-16. doi: 10.1128/AAC.01701-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahai J, Gallicano K, Swick L, Tailor S, Garber G, Seguin I, et al. Reduced plasma concentrations of antituberculosis drugs in patients with HIV infection. Ann Intern Med. 1997;127(4):289–293. doi: 10.7326/0003-4819-127-4-199708150-00006. [DOI] [PubMed] [Google Scholar]
- 34.Peloquin CA, Nitta AT, Burman WJ, Brudney KF, Miranda-Massari JR, McGuinness ME, et al. Low antituberculosis drug concentrations in patients with AIDS. Ann Pharmacother. 1996;30(9):919–925. doi: 10.1177/106002809603000901. [DOI] [PubMed] [Google Scholar]
- 35.Jaruratanasirikul S. The pharmacokinetics of oral rifampicin in AIDS patients. J Med Assoc Thai. 1998;81(1):25–28. [PubMed] [Google Scholar]
- 36.Perlman DC, Segal Y, Rosenkranz S, Rainey PM, Peloquin CA, Remmel RP, ACTG 309 Team et al. The clinical pharmacokinetics of pyrazinamide in HIV-infected persons with tuberculosis. Clin Infect Dis. 2004;38(4):556–564. doi: 10.1086/381096. [DOI] [PubMed] [Google Scholar]
- 37.Perlman DC, Segal Y, Rosenkranz S, Rainey PM, Remmel RP, Salomon N, AIDS Clinical Trials Group 309 Team et al. The clinical pharmacokinetics of rifampin and ethambutol in HIV-infected persons with tuberculosis. Clin Infect Dis. 2005;41(11):1638–1647. doi: 10.1086/498024. [DOI] [PubMed] [Google Scholar]
- 38.Ramachandran G, Kumar AK, Bhavani PK, Kannan T, Kumar SR, Gangadevi NP, et al. Pharmacokinetics of first-line antituberculosis drugs in HIV-infected children with tuberculosis treated with intermittent regimens in India. Antimicrob Agents Chemother. 2015;59(2):1162–1167. doi: 10.1128/AAC.04338-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choudhri SH, Hawken M, Gathua S, Minyiri GO, Watkins W, Sahai J, et al. Pharmacokinetics of antimycobacterial drugs in patients with tuberculosis, AIDS, and diarrhea. Clin Infect Dis. 1997;25(1):104–111. doi: 10.1086/514513. [DOI] [PubMed] [Google Scholar]
- 40.Conte JE, Jr, Golden JA, McQuitty M, Kipps J, Duncan S, McKenna E, et al. Effects of gender, AIDS, and acetylator status on intrapulmonary concentrations of isoniazid. Antimicrob Agents Chemother. 2002;46(8):2358–2364. doi: 10.1128/AAC.46.8.2358-2364.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denti P, Jeremiah K, Chigutsa E, Faurholt-Jepsen D, PrayGod G, Range N, et al. Pharmacokinetics of isoniazid, pyrazinamide, and ethambutol in newly diagnosed pulmonary TB patients in Tanzania. PLoS One. 2015;10(10):e0141002. doi: 10.1371/journal.pone.0141002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham SM, Bell DJ, Nyirongo S, Hartkoorn R, Ward SA, Molyneux EM. Low levels of pyrazinamide and ethambutol in children with tuberculosis and impact of age, nutritional status, and human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50(2):407–413. doi: 10.1128/AAC.50.2.407-413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeremiah K, Denti P, Chigutsa E, Faurholt-Jepsen D, PrayGod G, Range N, et al. Nutritional supplementation increases rifampin exposure among tuberculosis patients coinfected with HIV. Antimicrob Agents Chemother. 2014;58(6):3468–3474. doi: 10.1128/AAC.02307-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jonsson S, Davidse A, Wilkins J, Van der Walt JS, Simonsson US, Karlsson MO, et al. Population pharmacokinetics of ethambutol in South African tuberculosis patients. Antimicrob Agents Chemother. 2011;55(9):4230–4237. doi: 10.1128/AAC.00274-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bekker A, Schaaf HS, Draper HR, van der Laan L, Murray S, Wiesner L, et al. Pharmacokinetics of rifampin, isoniazid, pyrazinamide, and ethambutol in infants dosed according to revised WHO-recommended treatment guidelines. Antimicrob Agents Chemother. 2016;60(4):2171–2179. doi: 10.1128/AAC.02600-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conte JE, Golden JA, Kipps JE, Lin ET, Zurlinden E. Effect of sex and AIDS status on the plasma and intrapulmonary pharmacokinetics of rifampicin. Clin Pharmacokinet. 2004;43(6):395–404. doi: 10.2165/00003088-200443060-00003. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee A, Velpandian T, Singla M, Kanhiya K, Kabra SK, Lodha R. Pharmacokinetics of isoniazid, rifampicin, pyrazinamide and ethambutol in Indian children. BMC Infect Dis. 2015;15:126-015-0862-7. doi: 10.1186/s12879-015-0862-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Oosterhout JJ, Dzinjalamala FK, Dimba A, Waterhouse D, Davies G, Zijlstra EE, et al. Pharmacokinetics of antituberculosis drugs in HIV-positive and HIV-negative adults in Malawi. Antimicrob Agents Chemother. 2015;59(10):6175–6180. doi: 10.1128/AAC.01193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramachandran G, Kumar AK, Kannan T, Bhavani PK, Kumar SR, Gangadevi NP, et al. Low serum concentrations of rifampicin and pyrazinamide associated with poor treatment outcomes in children with tuberculosis related to HIV status. Pediatr Infect Dis J. 2016;35(5):530–534. doi: 10.1097/INF.0000000000001069. [DOI] [PubMed] [Google Scholar]
- 50.Rockwood N, Meintjes G, Chirehwa M, Wiesner L, McIlleron H, Wilkinson RJ, et al. HIV-1 coinfection does not reduce exposure to rifampin, isoniazid, and pyrazinamide in south african tuberculosis outpatients. Antimicrob Agents Chemother. 2016;60(10):6050–6059. doi: 10.1128/AAC.00480-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaaf HS, Willemse M, Cilliers K, Labadarios D, Maritz JS, Hussey GD, et al. Rifampin pharmacokinetics in children, with and without human immunodeficiency virus infection, hospitalized for the management of severe forms of tuberculosis. BMC Med. 2009;7:19. doi: 10.1186/1741-7015-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, et al. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV-infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis. 2009;48(12):1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaaf HS, Parkin DP, Seifart HI, Werely CJ, Hesseling PB, van Helden PD, et al. Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Arch Dis Child. 2005;90(6):614–618. doi: 10.1136/adc.2004.052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74(8):839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 55.Boeree MJ, Diacon AH, Dawson R, Narunsky K, du Bois J, Venter A, PanACEA Consortium et al. A dose-ranging trial to optimize the dose of rifampin in the treatment of tuberculosis. Am J Respir Crit Care Med. 2015;191(9):1058–1065. doi: 10.1164/rccm.201407-1264OC. [DOI] [PubMed] [Google Scholar]
- 56.van Ingen J, Aarnoutse RE, Donald PR, Diacon AH, Dawson R, Plemper van Balen G, et al. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis. 2011;52(9):e194–e199. doi: 10.1093/cid/cir184. [DOI] [PubMed] [Google Scholar]
- 57.Peloquin CA, Jaresko GS, Yong CL, Keung AC, Bulpitt AE, Jelliffe RW. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob Agents Chemother. 1997;41(12):2670–2679. doi: 10.1128/AAC.41.12.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alsultan A, Savic R, Dooley KE, Weiner M, Whitworth W, MacKenzie WR, Tuberculosis Trials Consortium et al. Population pharmacokinetics of pyrazinamide in patients with tuberculosis. Antimicrob Agents Chemother. 2017;61(6):e02625-16. doi: 10.1128/AAC.02625-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weiner M, Peloquin C, Burman W, Luo CC, Engle M, Prihoda TJ, et al. Effects of tuberculosis, race, and human gene SLCO1B1 polymorphisms on rifampin concentrations. Antimicrob Agents Chemother. 2010;54(10):4192–4200. doi: 10.1128/AAC.00353-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sturkenboom MG, Mulder LW, de Jager A, van Altena R, Aarnoutse RE, de Lange WC, et al. Pharmacokinetic modeling and optimal sampling strategies for therapeutic drug monitoring of rifampin in patients with tuberculosis. Antimicrob Agents Chemother. 2015;59(8):4907–4913. doi: 10.1128/AAC.00756-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alffenaar JW. Dried blood spot analysis combined with limited sampling models can advance therapeutic drug monitoring of tuberculosis drugs. J Infect Dis. 2012;205(11):1765–1766. doi: 10.1093/infdis/jis270. [DOI] [PubMed] [Google Scholar]
- 62.Zuur MA, Bolhuis MS, Anthony R, den Hertog A, van der Laan T, Wilffert B, et al. Current status and opportunities for therapeutic drug monitoring in the treatment of tuberculosis. Expert Opin Drug Metab Toxicol. 2016;12(5):509–521. doi: 10.1517/17425255.2016.1162785. [DOI] [PubMed] [Google Scholar]
- 63.Semvua HH, Kibiki GS, Kisanga ER, Boeree MJ, Burger DM, Aarnoutse R. Pharmacological interactions between rifampicin and antiretroviral drugs: challenges and research priorities for resource-limited settings. Ther Drug Monit. 2015;37(1):22–32. doi: 10.1097/FTD.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 64.Regazzi M, Carvalho AC, Villani P, Matteelli A. Treatment optimization in patients co-infected with HIV and Mycobacterium tuberculosis infections: focus on drug-drug interactions with rifamycins. Clin Pharmacokinet. 2014;53(6):489–507. doi: 10.1007/s40262-014-0144-3. [DOI] [PubMed] [Google Scholar]
- 65.Saktiawati AM, Sturkenboom MG, Stienstra Y, Subronto YW, Sumardi, Kosterink JG, et al. Impact of food on the pharmacokinetics of first-line anti-TB drugs in treatment-naive TB patients: a randomized cross-over trial. J Antimicrob Chemother. 2016;71(3):703–710. doi: 10.1093/jac/dkv394. [DOI] [PubMed] [Google Scholar]
- 66.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis. 2016;63(7):e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alffenaar JC, Tiberi S, Verbeeck RK, Heysell SK, Grobusch MP. therapeutic drug monitoring in tuberculosis: practical application for physicians. Clin Infect Dis. 2017;64(1):104–105. doi: 10.1093/cid/ciw677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daskapan A, de Lange WC, Akkerman OW, Kosterink JG, van der Werf TS, Stienstra Y, et al. The role of therapeutic drug monitoring in individualised drug dosage and exposure measurement in tuberculosis and HIV co-infection. Eur Respir J. 2015;45(2):569–571. doi: 10.1183/09031936.00142614. [DOI] [PubMed] [Google Scholar]
- 69.Peloquin C. The role of therapeutic drug monitoring in mycobacterial infections. Microbiol Spectr. 2017 doi: 10.1128/microbiolspec.TNMI7-0029-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.L’homme RF, Muro EP, Droste JA, Wolters LR, van Ewijk-Beneken Kolmer NW, Schimana W, et al. Therapeutic drug monitoring of nevirapine in resource-limited settings. Clin Infect Dis. 2008;47(10):1339–1344. doi: 10.1086/592694. [DOI] [PubMed] [Google Scholar]
- 71.Vu DH, Koster RA, Bolhuis MS, Greijdanus B, Altena RV, Nguyen DH, et al. Simultaneous determination of rifampicin, clarithromycin and their metabolites in dried blood spots using LC–MS/MS. Talanta. 2014;121:9–17. doi: 10.1016/j.talanta.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 72.Hiruy H, Rogers Z, Mbowane C, Adamson J, Ngotho L, Karim F, et al. Subtherapeutic concentrations of first-line anti-TB drugs in South African children treated according to current guidelines: the PHATISA study. J Antimicrob Chemother. 2015;70(4):1115–1123. doi: 10.1093/jac/dku478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colangeli R, Jedrey H, Kim S, Connell R, Ma S, Chippada Venkata UD, DMID 01-009/Tuberculosis Trials Consortium Study 22 Teams et al. Bacterial factors that predict relapse after tuberculosis therapy. N Engl J Med. 2018;379(9):823–833. doi: 10.1056/NEJMoa1715849. [DOI] [PMC free article] [PubMed] [Google Scholar]