Abstract
Many studies have explored the association between n-3 fatty acids and depression, but research on the associations of n-6 fatty acids and n-6:n-3 ratio with depression is more scarce, and the results are controversial. Therefore, we conducted this cross-sectional study to explore the associations of n-3 and n-6 fatty acid intakes and n-6:n-3 ratio with the risk of depressive symptoms using data from National Health and Nutrition Examination Survey (NHANES) 2009–2016. Dietary data on n-3 and n-6 fatty acids were obtained through two 24-h dietary recall interviews, and were adjusted by energy. Depressive symptoms were measured by PHQ-9 (nine-item Patient Health Questionnaire). We applied logistic regression and restricted cubic spline models to assess the relationships of n-3 and n-6 fatty acids intake and n-6:n-3 ratio with the risk of depressive symptoms. A total of 17,431 individuals over 18 years old were enrolled in this study. In the multivariate-adjusted model 2, compared with the lowest category, the highest odd ratios (ORs) with 95% confidence intervals (CIs) for n-3 fatty acid intake and n-6:n-3 ratio were 0.71 (0.55–0.92) and 1.66 (1.10–2.50), and middle OR (95% CI) for n-6 fatty acid intake was 0.72 (0.56–0.92), respectively. Our study suggests that n-3 and n-6 fatty acids intake were inversely associated with the risk of depressive symptoms, while the n-6:n-3 ratio was positively associated with the risk of depressive symptoms.
Keywords: depressive symptoms, n-3 fatty acids, n-6 fatty acids, n-6:n-3 ratio, dose-response
1. Introduction
According to the WHO (World Health Organization), more than 300 million people suffer from depression worldwide [1]. Moreover, depression has been ranked as one of the largest contributors to global disability and the risk of mortality [2]. Many studies have suggested that depression is often comorbid with many chronic diseases [3,4,5], which may gradually worsen people’s health. Thus, it is indispensable to investigate the adjustable risk factors to prevent depression.
Epidemiologic studies have shown that depression is related to genetic and environmental factors, especially dietary factors [6,7,8,9]. For instance, vegetables [10], fruits [10], fish [11], and dietary fiber [12] have been reported to reduce the risk of depression, and some nutrients [13,14] (e.g., magnesium, zinc, iron, copper, and selenium) can also reduce the risk of depression.
As essential nutrients for human body, polyunsaturated fatty acids (mainly n-3 and n-6 fatty acids) have also been reported to be associated with depression [15,16,17,18]. Observational and experimental studies have indicated that a higher consumption of n-3 fatty acids is associated with lower risk of depression [17,18,19,20,21,22,23,24,25,26,27,28], suggesting that n-3 fatty acids have a protective effect on depression. However, few studies have explored the association between n-6 fatty acids and depression, and the results are inconsistent [16,17,18,29]. The Nurses’ Health Study [16] performed among American women indicated that a higher intake of linoleic acid (the major component of n-6 fatty acids) may increase the risk of depression, while the Japan Public Center-based Prospective Study (JPHC study) [17] and Shika’s study [18] reported no association in American and Japanese populations. Moreover, studies on the association of n-6:n-3 ratio and depressive symptoms are also controversial [16,17,18,23,30,31]. A cross-sectional study [23] and two cohort studies [16,30] suggested a positive association, whereas several other studies reported no association [17,18,31]. Furthermore, few studies have investigated the dose–response relationship between n-3 fatty acids and depression [17], and no study has explored the dose–response relationship between dietary n-6 fatty acids and n-6:n-3 ratio and depression. Therefore, we explored the associations and dose–response relationships of total n-3 fatty acids, total n-6 fatty acids, and n-6:n-3 ratio with the risk of depressive symptoms in US adults based on data from the National Health and Nutrition Examination Survey (NHANES) 2009–2016.
2. Materials and Methods
2.1. Study Population
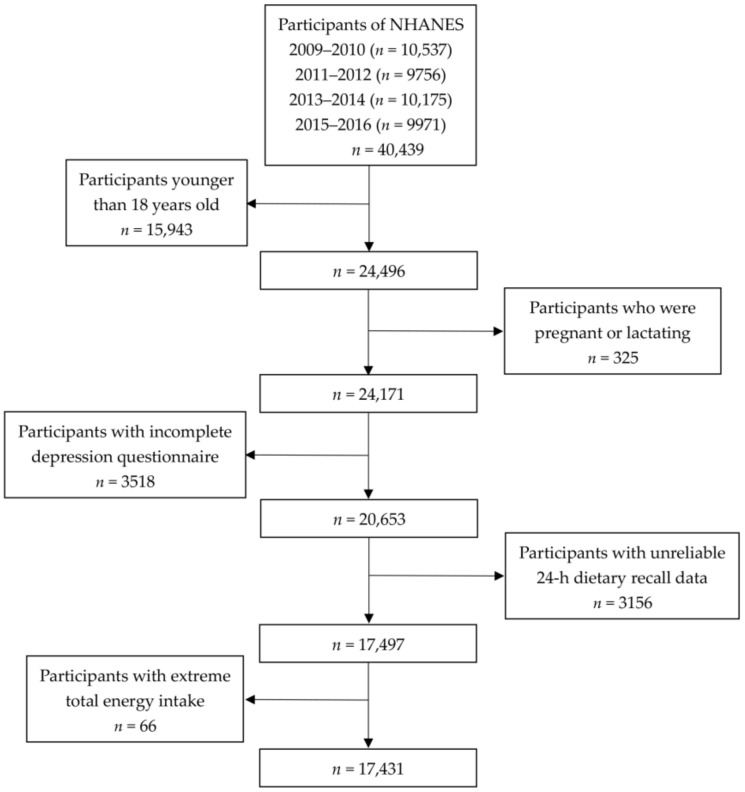
The NHANES is an ongoing, 2-year-cycle program under administration of the Centers for Disease Control and Prevention (CDC) of the US. The detailed procedures of NHANES data collection are described in the literature [32]. The study protocol is approved by National Centers for Health Statics (NCHS), and all participants gave informed consent. In this study, public data from four cycles of NHANES (2009–2010, 2011–2012, 2013–2014, and 2015–2016) were used. A total of 40,439 people participated in the NHANES from 2009 to 2016, and our analyses were restricted to 24,496 individuals over 18 years old. Among them, we excluded 325 pregnant and lactating women, 3518 participants with incomplete depression questionnaires, 3156 individuals with incomplete 24-h recall data, and 66 individuals with extreme total energy intake (<500 kcal/day for both men and women, >5000 kcal/day for women, and >8000 kcal/day for men). Finally, a total of 17,431 participants were included in the present study (Figure 1).
Figure 1.
Flow chart of the screening process for the selection of eligible participants.
2.2. Assessment of Depressive Symptoms
Depressive symptoms were assessed by the PHQ-9 (nine-item Patient Health Questionnaire) which integrates DSM-IV depression diagnostic criteria and was found to be a reliable and effective screening instrument in both clinical and research settings [33,34]. The total scores of PHQ-9 range from 0 to 27, and a score of 10 was used as the cut-off point to identify depression according to Kroenke et al. [35].
2.3. Dietary n-3 and n-6 Fatty Acid Intakes
Dietary n-3 and n-6 fatty acids intake were assessed by two 24-h dietary recall interviews. The subtypes of n-3 and n-6 fatty acids in this study are consistent with our previous studies [36]. The daily average n-3 and n-6 fatty acids intake were adjusted by energy.
2.4. Covariates
The following covariates were included in this study: age, gender, race, marital status, education level, annual household income, smoking status, alcohol consumption status, work activity, recreational activity, body mass index (BMI), hypertension, diabetes, coronary heart disease, and energy intake. The classifications of covariates were based on our previous studies [13,14,37] and are shown in Table S1.
2.5. Statistical Analysis
Kolmogorov–Smirnov normality tests were used to test the normality of continuous variables. The normally distributed variables were described by mean ± standard deviation, and non-normally distributed variables were described by median (interquartile range). According to the characteristics of variables, t-tests or chi-square tests were applied to compare the differences between the depressive symptoms group and the non-depressive-symptoms group. The adjusted dietary n-3 and n-6 fatty acids intake were divided into three groups based on tertiles; the lowest tertile was the reference. In view of the large number of people whose dietary n-6:n-3 ratio was less than 10 in our study, and because studies have proved that the average value of dietary n-6:n-3 ratio is about 15 [38], we divided the n-6:n-3 ratio into three groups (group 1: <10; group 2: ≥10 to 15; group 3: >15), where group 1 was the reference.
Binary logistic regression analyses were conducted to explore the associations between dietary n-3 and n-6 fatty acids, n-6:n-3 ratio, and the risk of depressive symptoms. Only age and gender were adjusted in model 1, and all the covariates were adjusted in model 2. In addition, stratified analyses were performed to test whether these associations differed by age and gender. To further explore the dose–response relationships between dietary n-3 and n-6 fatty acids intake and n-6:n-3 ratio and the risk of depressive symptoms, we applied restricted cubic spline with three knots at the 5th, 50th, and 95th percentiles of the exposure distribution in the multivariate-adjusted model 2. In order to perform nationally representative estimates, the analyses were weighted in this study. All statistical analyses were conducted using Stata 15.0. A two-sided p-value less than 0.05 was considered statistically significant.
3. Results
The characteristics of NHANES participants by depressive symptoms are shown in Table 1. The prevalence of depressive symptoms was 8.87%. Compared with the non-depressive-symptoms group, the participants with depressive symptoms tended to be older, women, single or living alone, obese, smokers, with lower education level, lower income, and lower recreation activity. The prevalence of depressive symptoms was high in the subjects with hypertension and diabetes. Moreover, the energy and n-3 and n-6 fatty acids intake in participants with depressive symptoms were lower than those without depressive symptoms, while the n-6:n-3 ratio in in participants with depressive symptoms was higher than those without depressive symptoms.
Table 1.
Characteristics of National Health and Nutrition Examination Survey (NHANES) participants by depressive symptoms.
| With Depressive Symptoms (PHQ-9 ≥ 10) |
Without Depressive Symptoms (PHQ-9 < 10) | p-Value | |
|---|---|---|---|
| Number of subjects (%) | 1546 (8.87) | 15,885 (91.13) | |
| Age group (%) 1 | <0.01 | ||
| 18–44 years | 622 (40.2) | 6964 (43.8) | |
| 44–59 years | 480 (31.0) | 3724 (23.4) | |
| ≥60 years | 444 (28.7) | 3613 (22.7) | |
| Sex (%) 1 | <0.01 | ||
| Male | 553 (35.8) | 8002 (50.4) | |
| Female | 993 (64.2) | 7883 (48.6) | |
| Race (%) 1 | <0.01 | ||
| Mexican American | 232 (15.0) | 2329 (14.6) | |
| Other Hispanic | 214 (13.8) | 1574 (9.9) | |
| Non-Hispanic White | 648 (41.9) | 6789 (42.7) | |
| Non-Hispanic Black | 334 (21.6) | 3392 (21.4) | |
| Other race | 118 (7.6) | 1810 (11.4) | |
| Marital status (%) 1 | <0.01 | ||
| Married/Living with partner | 650 (42.0) | 9135 (57.5) | |
| Windowed/Divorced/Separated/Never married | 838 (54.2) | 5875 (37.0) | |
| Education level (%) 1 | <0.01 | ||
| Below high school | 527 (34.1) | 3379 (21.3) | |
| High school | 370 (23.9) | 3669 (23.1) | |
| Above high school | 649 (42.0) | 8825 (55.6) | |
| Household income (%) 1 | <0.01 | ||
| <$20,000 | 579 (37.5) | 2948 (18.6) | |
| $20,000–$44,999 | 529 (34.2) | 5003 (31.50) | |
| $50,000–$74,999 | 208 (13.5) | 2946 (18.5) | |
| ≥$75,000 | 162 (10.5) | 4280 (26.9) | |
| Body mass index (%) 1 | <0.01 | ||
| <25 kg/m2 | 377 (24.4) | 4740 (29.8) | |
| 25 to <30 kg/m2 | 365 (23.6) | 5201 (32.7) | |
| ≥30 kg/m2 | 783 (50.6) | 5801 (36.5) | |
| Smoking at least 100 cigarettes in life (%) 1 | <0.01 | ||
| Yes | 891 (57.6) | 6473 (40.7) | |
| No | 627 (40.6) | 8961 (56.4) | |
| Have at least 12 alcoholic drinks/year (%) 1 | 0.175 | ||
| Yes | 1117 (72.3) | 11,196 (70.5) | |
| No | 410 (26.5) | 4460 (28.1) | |
| Work activity (%) 1 | 0.124 | ||
| Vigorous activity | 294 (19.0) | 2982 (18.8) | |
| Moderate activity | 287 (18.6) | 3420 (21.5) | |
| Other | 963 (62.3) | 9477 (59.7) | |
| Recreation activity (%) 1 | <0.01 | ||
| Vigorous activity | 178 (11.5) | 3852 (24.2) | |
| Moderate activity | 3231 (20.9) | 4360 (27.4) | |
| Other | 1045 (67.6) | 7669 (48.3) | |
| Hypertension (%) 1 | <0.01 | ||
| Yes | 732 (47.3) | 5519 (34.7) | |
| No | 812 (52.5) | 10,352 (65.2) | |
| Diabetes (%) 1 | <0.01 | ||
| Yes | 316 (20.4) | 1911 (12.0) | |
| No | 1226 (79.3) | 13,968 (87.9) | |
| Coronary heart disease (%) 1 | <0.01 | ||
| Yes | 961 (62.2) | 10,812 (68.1) | |
| No | 171 (11.1) | 924 (5.8) | |
| Total energy intake (kcal/d) 2 | 1952.5 (814.4) | 2049.8 (799.4) | <0.01 |
| Adjusted n-3 fatty acid intake (mg/kcal/day) 2 | 0.83 (0.38) | 0.89 (0.41) | <0.01 |
| Adjusted n-6 fatty acid intake (mg/kcal/day) 2 | 7.46 (2.83) | 7.77 (2.73) | <0.01 |
| n-6:n-3 ratio 2 | 9.72 (3.51) | 9.43 (3.37) | <0.05 |
1 Chi-square and 2 t-tests were applied to compare the differences between the depressive-symptoms group and the non-depressive-symptoms group. PHQ-9: nine-item Patient Health Questionnaire.
The odds ratios (ORs) with 95% confidence intervals (CIs) of depressive symptoms with n-3 and n-6 fatty acids intake and n-6:n-3 ratio are shown in Table 2. In the unadjusted model of binary logistic regression analyses, the ORs (95% CIs) were 0.67 (0.55–0.82), 0.78 (0.65–0.95), and 1.43 (1.08–1.91) for the highest group versus the reference, respectively. In model 1, n-3 and n-6 fatty acids intake and n-6:n-3 ratio were still associated with the risk of depressive symptoms. In model 2, compared with the reference, the ORs of highest group of n-3 fatty acids and n-6:n-3 ratio were 0.70 (0.55–0.92) and 1.66 (1.10–2.50), and the ORs of second group of n-6 fatty acids was 0.72 (0.56–0.92).
Table 2.
The ORs (95% CIs) for depressive symptoms by adjusted dietary n-3 and n-6 fatty acids intake and n-6:n-3 ratio, NHANES 2009–2016 (n = 17,431).
| Crude | Model 1 | Model 2 | |
|---|---|---|---|
| Adjusted n-3 fatty acids intake (mg/kcal/day) | |||
| <0.68 | 1.00 | 1.00 | 1.00 |
| 0.68 to <0.95 | 0.70 (0.58–0.85) ** | 0.68 (0.56–0.82) ** | 0.70 (0.55–0.89) ** |
| ≥0.95 | 0.67 (0.55–0.82) ** | 0.64 (0.52–0.78) ** | 0.71 (0.55–0.92) * |
| Adjusted n-6 fatty acids intake (mg/kcal/day) | |||
| <6.39 | 1.00 | 1.00 | 1.00 |
| 6.39 to <8.57 | 0.68 (0.56–0.82) ** | 0.66 (0.55–0.80) ** | 0.72 (0.56–0.92) * |
| ≥8.57 | 0.78 (0.65–0.95) * | 0.75 (0.62–0.90) ** | 0.84 (0.66–1.07) |
| n-6:n-3 ratio | |||
| < 10 | 1.00 | 1.00 | 1.00 |
| 10 to <15 | 1.19 (0.96–1.49) | 1.19 (0.96–1.49) | 1.15(0.86–1.55) |
| ≥15 | 1.43 (1.08–1.91) * | 1.47 (1.10–1.96) ** | 1.66(1.10–2.50) * |
* p < 0.05; ** p < 0.01.
In stratified analyses by age and gender, the results are shown in Table 3 and Table 4, respectively. In stratified analyses by age, n-3 and n-6 fatty acids intake and n-6:n-3 ratio were associated with the risk of depressive symptoms for participants over 60 years old. The ORs (95%CIs) in model 2 were 0.42 (0.28–0.61), 0.42 (0.29–0.62), and 3.83 (1.53–9.54), respectively. In stratified analyses by gender, n-3 fatty acids intake was inversely associated with the risk of depressive symptoms among men, and the n-6:n-3 ratio was positively associated with the risk of depressive symptoms among women; the corresponding ORs (95%CIs) were 0.57 (0.36–0.91) and 1.84(1.05–3.22) in model 2.
Table 3.
The ORs (95%CIs) of depressive symptoms by adjusted dietary n-3 and n-6 fatty acid intakes and n-6:n-3 ratio, stratified by age, NHANES 2009–2016 (n = 17,431).
| 18 ≤ Age < 45 Years | 45 ≤ Age < 60 Years | Age ≥ 60 Years | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude | Model 1 | Model 2 | Crude | Model 1 | Model 2 | Crude | Model 1 | Model 2 | |
| Adjusted n-3 fatty acids intake (mg/kcal/day) | |||||||||
| <0.68 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 0.68 to <0.95 | 0.61 (0.48–0.77) ** |
0.58 (0.46–0.74) ** |
0.74 (0.54–1.02) |
0.75 (0.51–1.12) |
0.73 (0.50–1.08) |
0.69 (0.43–1.10) |
0.85 (0.53–1.35) |
0.83 (0.52–1.33) |
0.58 (0.35–0.95) * |
| ≥0.95 | 0.66 (0.49–0.90) ** |
0.62 (0.46–0.84) * |
0.82 (0.57–1.18) |
0.82 (0.59–1.14) |
0.77 (0.56–1.07) |
0.78 (0.50–1.19) |
0.53 (0.37–0.76) |
0.50 (0.35–0.72) ** |
0.42 (0.28–0.61) ** |
| Adjusted n-6 fatty acids intake (mg/kcal/day) | |||||||||
| <6.39 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6.39 to <8.57 | 0.81 (0.60–1.09) |
0.78 (0.58–1.05) |
0.95 (0.68–1.33) |
0.70 (0.50–0.98) * |
0.69 (0.49–0.97) * |
0.67 (0.44–1.01) |
0.42 (0.29–0.62) ** |
0.42 (0.28–0.61) ** |
0.42 (0.27–0.64) ** |
| ≥8.57 | 0.79 (0.59–1.06) |
0.75 (0.56–1.01) |
0.94 (0.65–1.35) |
0.89 (0.64–1.25) |
0.85 (0.61–1.20) |
0.99 (0.66–1.50) |
0.61 (0.42–0.88) * |
0.58 (0.40–0.84) ** |
0.42 (0.29–0.62) ** |
| n-6:n-3 ratio | |||||||||
| <10 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10 to <15 | 1.24 (0.96–1.60) |
1.25 (0.96–1.62) |
1.26 (0.90–1.77) |
1.01 (0.68–1.51) |
1.01 (0.68–1.50) |
0.96 (0.57–1.64) |
1.36 (0.84–2.20) |
1.42 (0.88–2.30) |
1.13 (0.65–1.96) |
| ≥15 | 1.45 (0.91–2.31) |
1.49 (0.93–2.39) |
1.33 (0.73–2.42) |
1.22 (0.67–2.22) |
1.25 (0.70–2.26) |
1.51 (0.70–1.78) |
1.74 (0.79–3.86) |
1.83 (0.81–4.12) |
3.83 (1.53–9.54) ** |
* p < 0.05; ** p < 0.01.
Table 4.
The ORs (95%CIs) of depressive symptoms by adjusted dietary n-3 and n-6 fatty acids intake and n-6:n-3 ratio, stratified by gender. NHANES 2009–2016 (n = 17,431).
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Crude | Model 1 | Model 2 | Crude | Model 1 | Model 2 | |
| Adjusted n-3 fatty acids intake (mg/kcal/day) | ||||||
| <0.68 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 0.68 to <0.95 | 0.56 (0.38–0.83) ** | 0.56 (0.38–0.83) ** | 0.57 (0.36–0.91) * | 0.75 (0.58–0.95) * | 0.76 (0.58–0.99) * | 0.80 (0.56–1.14) |
| ≥0.95 | 0.66 (0.48–0.92) * | 0.67 (0.48–0.93) * | 0.77 (0.51–1.18) | 0.62 (0.49–0.79) ** | 0.63 (0.49–0.80) ** | 0.71 (0.50–1.01) |
| Adjusted n-6 fatty acids intake (mg/kcal/day) | ||||||
| <6.39 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6.39 to <8.57 | 0.57 (0.41–0.80) ** | 0.57 (0.41–0.80) ** | 0.66 (0.41–1.04) | 0.73 (0.59–0.90) ** | 0.73 (0.59–0.90) ** | 0.76 (0.56–1.04) |
| ≥8.57 | 0.67 (0.51–0.88) ** | 0.67 (0.51–0.88) ** | 0.82 (0.55–1.22) | 0.80 (0.61–1.05) | 0.81 (0.61–1.06) | 0.89 (0.61–1.30) |
| n-6:n-3 ratio | ||||||
| <10 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10 to <15 | 1.29 (0.92–1.81) | 1.29 (0.92–1.80) | 1.39 (0.92–2.12) | 1.17 (0.90–1.52) | 1.14 (0.87–1.48) | 1.02 (0.74–1.39) |
| ≥15 | 1.56 (0.94–2.16) | 1.56 (0.92–2.63) | 1.43 (0.73–2.79) | 1.43 (0.98–2.10) | 1.41 (0.96–2.06) | 1.84 (1.05–3.22) * |
* p < 0.05; ** p < 0.01.
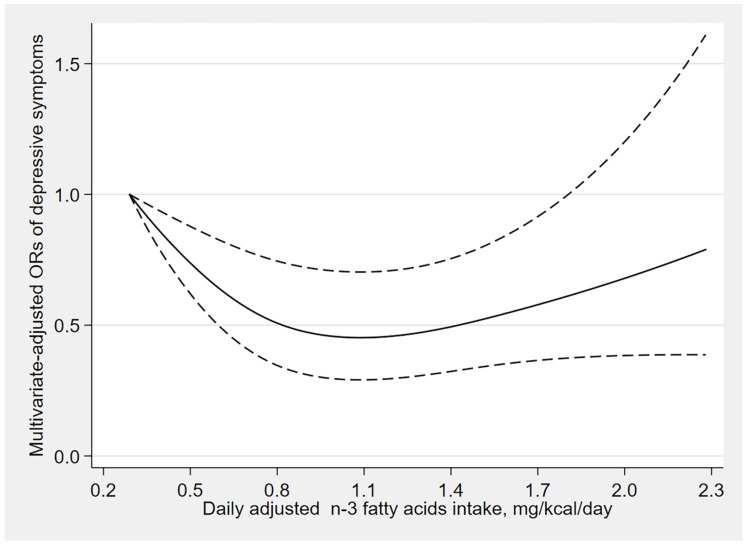
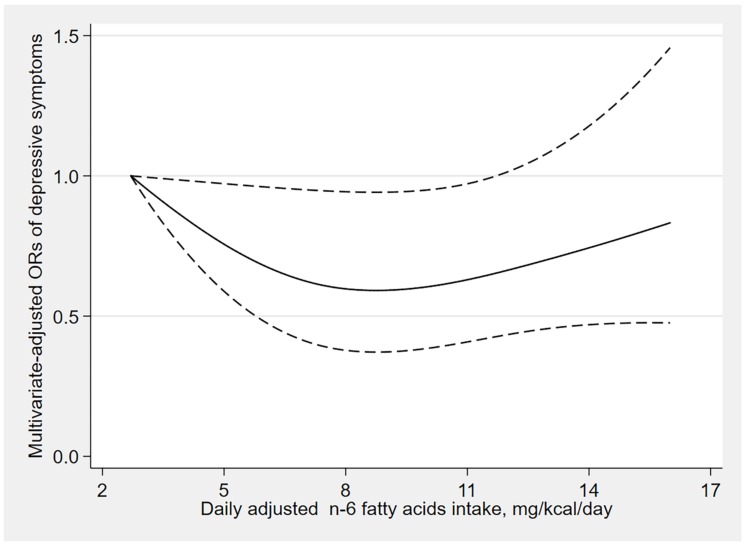
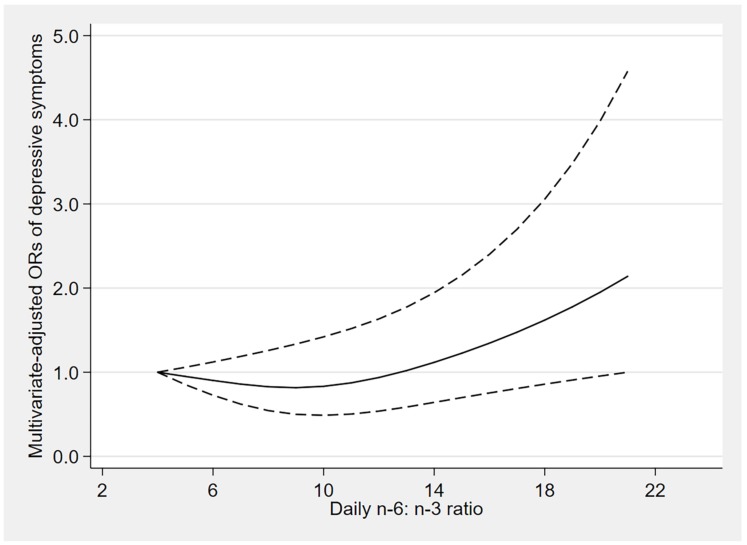
The results of dose–response relationships are shown in Figure 2, Figure 3 and Figure 4. There was a U-shaped association between n-3 fatty acids intake and the risk of depressive symptoms (p for nonlinearity = 0.006). When the n-3 fatty acids intake was 1.1 mg/kcal/day, the OR value tended to the lowest (OR: 0.45; 95% CI: 0.29–0.70), and there was no significant association between the risk of depressive symptoms and n-3 fatty acids intake when the consumption was beyond 1.8 mg/kcal/day (OR: 0.62; 95% CI: 0.38–1.01). Similarly, a U-shaped association between n-6 fatty acids intake and the risk of depressive symptoms was also found. When the n-6 fatty acids intake was 8.8 mg/kcal/day, the OR value tended to the lowest (OR: 0.59; 95% CI: 0.37–0.94), and there was no significant association between the risk of depressive symptoms and n-6 fatty acids intake when the consumption was beyond 11.9 mg/kcal/day (OR: 0.66; 95% CI: 0.43–1.01). However, the n-6:n-3 ratio was linearly positively associated with the risk of depressive symptoms (p for nonlinearity = 0.069).
Figure 2.
Dose–response relationship between n-3 fatty acids intake and the risk of depressive symptoms. The solid line represents the OR values and dashed lines represent the 95% confidence intervals.
Figure 3.
Dose–response relationship between n-6 fatty acids intake and the risk of depressive symptoms. The solid line represents the OR values and dashed lines represent the 95% confidence intervals.
Figure 4.
Dose–response relationship between n-6:n-3 ratio and the risk of depressive symptoms. The solid line represents the OR values and dashed lines represent the 95% confidence intervals.
4. Discussion
In this study, we found that the intakes of n-3 and n-6 fatty acids were inversely associated with the risk of depressive symptoms, and dietary n-6:n-3 ratio was positively associated with the risk of depressive symptoms, and these associations were stronger among the elderly. Furthermore, the dose–response relationship analysis indicated that the n-3 and n-6 fatty acids intake had nonlinear and U-shaped associations with the risk of depressive symptoms, while n-6:n-3 ratio was linearly positively associated with the risk of depressive symptoms.
Our findings about n-3 fatty acids intake are consistent with other studies, including cross-sectional [18,23,24], prospective cohort [17,25,26,27], meta-analysis [19,28] and experimental studies [20]. The results from a cross-sectional study [23] indicated that higher n-3 fatty acids intake was inversely associated with the risk of evaluated depressive symptoms. Chika Horikawa et al. [27] also found the protective effect of n-3 fatty acids on depression through an established cohort. Moreover, a recent meta-analysis [19] explored the dose–response relationship between n-3 fatty acids and depression, and a reverse J-shaped effect was found. The inverse association between n-3 fatty acids and the risk of depression could be explained by several possibilities. First, research has shown that depression is related to inflammatory response [39], and n-3 fatty acids possess anti-inflammatory properties that reduce the risk of depression [40]. Second, n-3 fatty acids promote neurotransmitter binding and intracellular signaling [41].
We also found that n-6 fatty acids intake was inversely associated with the risk of depressive symptoms, which was inconsistent with previous studies. The NHANES Epidemiologic Followup Study (NHEFS) [29] found the opposite results: greater n-6 fatty acids intake was positively associated with severe depressive symptoms among men, while Shika’s study [18] in an elderly Japanese population indicated that the intake of n-6 fatty acids was not associated with depression. However, the reasons for the different outcomes remain to be explored, and the mechanism of this association is unclear. Therefore, further research is required to explore the relationship between n-6 fatty acids intake and the risk of depression.
In addition, a positive association between n-6:n-3 ratio and depressive symptoms was found in our study, which is consistent with several cohort studies [16,23,30]. However, other studies reported no association between n-6:n-3 ratio and depression [17,18]. Although the mechanisms between n-6:n-3 ratio and the increased risk of depression are not fully understood, several possibilities have been proposed. Primarily, a high n-6:n-3 ratio promotes the production of arachidonic acid (AA) derived from n-6 eicosanoids, and AA has been shown to increase the production of proinflammatory factors, which may increase the incidence of depression [42]. Another possible mechanism is that a high n-6:n-3 ratio is related to catecholaminergic or serotonergic neurotransmission [43].
There are several notable strengths of our study. Firstly, we simultaneously explored the associations of n-3 and n-6 fatty acids intake and n-6:n-3 ratio with the risk of depressive symptoms, and the dose–response relationships were also investigated. Secondly, we explored age and gender differences in the association between n-3 and n-6 fatty acids intake, n-6:n-3 ratio, and the risk of depressive symptoms, respectively. Finally, we used a large nationally representative sample that could increase the statistical power and provide a more reliable and accurate result.
However, there are several limitations to our study. First, since our study was cross-sectional, it was difficult to determine causality. Second, depressive symptoms were assessed by the PHQ-9 self-reported scale, and misclassification bias could not be completely avoided, which might affect the results. Third, dietary data were obtained through two 24-h dietary recall interviews, which may have uncertainties in estimating the long-term average consumption. However, some studies have shown that two 24-h recalls are sufficient to assess the daily dietary intake [44].
5. Conclusions
Our study indicates that the intakes of n-3 and n-6 fatty acids were inversely associated with the risk of depressive symptoms, and the n-6:n-3 ratio was positively associated with the risk of depressive symptoms. Further research is needed to verify whether these relationships are consistent.
Acknowledgments
We acknowledge the staff at the National Center for Health Statistics at the CDC, who design, collect, administer the NHANES data and release the data available for public use. We are thankful to all study participants for their cooperation.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/1/240/s1, Table S1: The classifications of categorical covariates.
Author Contributions
D.Z. and R.Z. conceived and designed the study; R.Z., J.S., and Y.L. analyzed the data; D.Z. and R.Z. wrote the paper and reviewed the manuscript; D.Z. had primary responsibility for the final content. All authors provided critical revisions of the manuscript and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization Depression and Other Common Mental Disorders. [(accessed on 29 November 2019)]; Available online: https://www.who.int/mental_health/management/depression/
- 2.Chesney E., Goodwin G.M., Fazel S. Risks of all-cause and suicide mortality in mental disorders: A meta-review. World Psychiatry. 2014;13:153–160. doi: 10.1002/wps.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gump B.B., Matthews K.A., Eberly L.E., Chang Y.F. Depressive symptoms and mortality in men: Results from the Multiple Risk Factor Intervention Trial. Stroke. 2005;36:98–102. doi: 10.1161/01.STR.0000149626.50127.d0. [DOI] [PubMed] [Google Scholar]
- 4.Anderson R.J., Freedland K.E., Clouse R.E., Lustman P.J. The prevalence of comorbid depression in adults with diabetes: A meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 5.Kim W.K., Shin D., Song W.O. Depression and Its Comorbid Conditions More Serious in Women than in Men in the United States. J. Women’s Health. 2015;24:978–985. doi: 10.1089/jwh.2014.4919. [DOI] [PubMed] [Google Scholar]
- 6.Ancelin M.L., Farre A., Carriere I., Ritchie K., Chaudieu I., Ryan J. C-reactive protein gene variants: Independent association with late-life depression and circulating protein levels. Transl Psychiatry. 2015;5:e499. doi: 10.1038/tp.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Souza S., Thompson J.M., Slykerman R., Marlow G., Wall C., Murphy R., Ferguson L.R., Mitchell E.A., Waldie K.E. Environmental and genetic determinants of childhood depression: The roles of DAT1 and the antenatal environment. J. Affect. Disord. 2016;197:151–158. doi: 10.1016/j.jad.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q., Shelton R.C., Dwivedi Y. Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: A systematic review and meta-analysis. J. Affect. Disord. 2018;225:422–428. doi: 10.1016/j.jad.2017.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Y., Wang N., Yu B., Zhang Q., Liu L., Meng G., Wu H., Du H., Shi H., Guo X., et al. Dietary patterns are associated with depressive symptoms among Chinese adults: A case-control study with propensity score matching. Eur. J. Nutr. 2017;56:2577–2587. doi: 10.1007/s00394-016-1293-y. [DOI] [PubMed] [Google Scholar]
- 10.Liu X., Yan Y., Li F., Zhang D. Fruit and vegetable consumption and the risk of depression: A meta-analysis. Nutrition. 2016;32:296–302. doi: 10.1016/j.nut.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Li F., Liu X., Zhang D. Fish consumption and risk of depression: A meta-analysis. J. Epidemiol. Community Health. 2016;70:299–304. doi: 10.1136/jech-2015-206278. [DOI] [PubMed] [Google Scholar]
- 12.Xu H., Li S., Song X., Li Z., Zhang D. Exploration of the association between dietary fiber intake and depressive symptoms in adults. Nutrition. 2018;54:48–53. doi: 10.1016/j.nut.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Li Z., Wang W., Xin X., Song X., Zhang D. Association of total zinc, iron, copper and selenium intakes with depression in the US adults. J. Affect. Disord. 2018;228:68–74. doi: 10.1016/j.jad.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Sun C., Wang R., Li Z., Zhang D. Dietary magnesium intake and risk of depression. J. Affect. Disord. 2019;246:627–632. doi: 10.1016/j.jad.2018.12.114. [DOI] [PubMed] [Google Scholar]
- 15.Hibbeln J.R., Nieminen L.R., Blasbalg T.L., Riggs J.A., Lands W.E. Healthy intakes of n-3 and n-6 fatty acids: Estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006;83:1483s–1493s. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- 16.Lucas M., Mirzaei F., O’Reilly E.J., Pan A., Willett W.C., Kawachi I., Koenen K., Ascherio A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: A 10-y prospective follow-up study. Am. J. Clin. Nutr. 2011;93:1337–1343. doi: 10.3945/ajcn.111.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuoka Y.J., Sawada N., Mimura M., Shikimoto R., Nozaki S., Hamazaki K., Uchitomi Y., Tsugane S. Dietary fish, n-3 polyunsaturated fatty acid consumption, and depression risk in Japan: A population-based prospective cohort study. Transl. Psychiatry. 2017;7:e1242. doi: 10.1038/tp.2017.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsujiguchi H., Thi Thu Nguyen T., Goto D., Miyagi S., Kambayashi Y., Hara A., Yamada Y., Nakamura H., Shimizu Y., Hori D., et al. Relationship between the Intake of n-3 Polyunsaturated Fatty Acids and Depressive Symptoms in Elderly Japanese People: Differences According to Sex and Weight Status. Nutrients. 2019;11:775. doi: 10.3390/nu11040775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosso G., Micek A., Marventano S., Castellano S., Mistretta A., Pajak A., Galvano F. Dietary n-3 PUFA, fish consumption and depression: A systematic review and meta-analysis of observational studies. J. Affect. Disord. 2016;205:269–281. doi: 10.1016/j.jad.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Keshavarz S.A., Mostafavi S.A., Akhondzadeh S., Mohammadi M.R., Hosseini S., Eshraghian M.R., Chamari M. Omega-3 supplementation effects on body weight and depression among dieter women with co-morbidity of depression and obesity compared with the placebo: A randomized clinical trial. Clin. Nutr. ESPEN. 2018;25:37–43. doi: 10.1016/j.clnesp.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Ciappolino V., Delvecchio G., Agostoni C., Mazzocchi A., Altamura A.C., Brambilla P. The role of n-3 polyunsaturated fatty acids (n-3PUFAs) in affective disorders. J. Affect. Disord. 2017;224:32–47. doi: 10.1016/j.jad.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 22.Gu M., Li Y., Tang H., Zhang C., Li W., Zhang Y., Li Y., Zhao Y., Song C. Endogenous Omega (n)-3 Fatty Acids in Fat-1 Mice Attenuated Depression-Like Behavior, Imbalance between Microglial M1 and M2 Phenotypes, and Dysfunction of Neurotrophins Induced by Lipopolysaccharide Administration. Nutrients. 2018;10:1351. doi: 10.3390/nu10101351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beydoun M.A., Fanelli Kuczmarski M.T., Beydoun H.A., Hibbeln J.R., Evans M.K., Zonderman A.B. ω-3 fatty acid intakes are inversely related to elevated depressive symptoms among United States women. J. Nutr. 2013;143:1743–1752. doi: 10.3945/jn.113.179119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appleton K.M., Peters T.J., Hayward R.C., Heatherley S.V., McNaughton S.A., Rogers P.J., Gunnell D., Ness A.R., Kessler D. Depressed mood and n-3 polyunsaturated fatty acid intake from fish: Non-linear or confounded association? Soc. Psychiatry Psychiatr. Epidemiol. 2007;42:100–104. doi: 10.1007/s00127-006-0142-3. [DOI] [PubMed] [Google Scholar]
- 25.Astorg P., Couthouis A., Bertrais S., Arnault N., Meneton P., Guesnet P., Alessandri J.M., Galan P., Hercberg S. Association of fish and long-chain n-3 polyunsaturated fatty acid intakes with the occurrence of depressive episodes in middle-aged French men and women. Prostaglandins Leukot Essent Fat. Acids. 2008;78:171–182. doi: 10.1016/j.plefa.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Villegas A., Henriquez P., Figueiras A., Ortuno F., Lahortiga F., Martinez-Gonzalez M.A. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur. J. Nutr. 2007;46:337–346. doi: 10.1007/s00394-007-0671-x. [DOI] [PubMed] [Google Scholar]
- 27.Horikawa C., Otsuka R., Kato Y., Nishita Y., Tange C., Rogi T., Kawashima H., Shibata H., Ando F., Shimokata H. Longitudinal Association between n-3 Long-Chain Polyunsaturated Fatty Acid Intake and Depressive Symptoms: A Population-Based Cohort Study in Japan. Nutrients. 2018;10:1655. doi: 10.3390/nu10111655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai Z.G., Bo A., Wu S.J., Gai Q.Y., Chi I. ω-3 polyunsaturated fatty acids and reduction of depressive symptoms in older adults: A systematic review and meta-analysis. J. Affect. Disord. 2018;241:241–248. doi: 10.1016/j.jad.2018.07.057. [DOI] [PubMed] [Google Scholar]
- 29.Wolfe A.R., Ogbonna E.M., Lim S., Li Y., Zhang J. Dietary linoleic and oleic fatty acids in relation to severe depressed mood: 10 years follow-up of a national cohort. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2009;33:972–977. doi: 10.1016/j.pnpbp.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Berger M.E., Smesny S., Kim S.W., Davey C.G., Rice S., Sarnyai Z., Schlogelhofer M., Schafer M.R., Berk M., McGorry P.D., et al. Omega-6 to omega-3 polyunsaturated fatty acid ratio and subsequent mood disorders in young people with at-risk mental states: A 7-year longitudinal study. Transl. Psychiatry. 2017;7:e1220. doi: 10.1038/tp.2017.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beydoun M.A., Fanelli Kuczmarski M.T., Beydoun H.A., Rostant O.S., Evans M.K., Zonderman A.B. Associations of the Ratios of n-3 to n-6 Dietary Fatty Acids With Longitudinal Changes in Depressive Symptoms Among US Women. Am. J. Epidemiol. 2015;181:691–705. doi: 10.1093/aje/kwu334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahluwalia N., Dwyer J., Terry A., Moshfegh A., Johnson C. Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv. Nutr. 2016;7:121–134. doi: 10.3945/an.115.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroenke K., Spitzer R.L., Williams J.B., Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: A systematic review. Gen. Hosp. Psychiatry. 2010;32:345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Spitzer R.L., Kroenke K., Williams J.B. Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 35.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J., Sun B., Zhang D. Association of Dietary n3 and n6 Fatty Acids Intake with Hypertension: NHANES 2007–2014. Nutrients. 2019;11:1232. doi: 10.3390/nu11061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun B., Shi X., Wang T., Zhang D. Exploration of the Association between Dietary Fiber Intake and Hypertension among U.S. Adults Using 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines: NHANES 2007–2014. Nutrients. 2018;10:1091. doi: 10.3390/nu10081091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simopoulos A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011;44:203–215. doi: 10.1007/s12035-010-8162-0. [DOI] [PubMed] [Google Scholar]
- 39.Belmaker R.H., Agam G. Major depressive disorder. N. Engl. J. Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 40.He K., Liu K., Daviglus M.L., Jenny N.S., Mayer-Davis E., Jiang R., Steffen L., Siscovick D., Tsai M., Herrington D. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am. J. Cardiol. 2009;103:1238–1243. doi: 10.1016/j.amjcard.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blondeau N., Nguemeni C., Debruyne D.N., Piens M., Wu X., Pan H., Hu X., Gandin C., Lipsky R.H., Plumier J.C., et al. Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: A versatile potential therapy for stroke. Neuropsychopharmacology. 2009;34:2548–2559. doi: 10.1038/npp.2009.84. [DOI] [PubMed] [Google Scholar]
- 42.Bagga D., Wang L., Farias-Eisner R., Glaspy J.A., Reddy S.T. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl. Acad. Sci. USA. 2003;100:1751–1756. doi: 10.1073/pnas.0334211100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haag M. Essential fatty acids and the brain. Can. J. Psychiatry. 2003;48:195–203. doi: 10.1177/070674370304800308. [DOI] [PubMed] [Google Scholar]
- 44.Knuppel S., Norman K., Boeing H. Is a Single 24-hour Dietary Recall per Person Sufficient to Estimate the Population Distribution of Usual Dietary Intake? J. Nutr. 2019;149:1491–1492. doi: 10.1093/jn/nxz118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.