Abstract
Recent evidence suggests that replacing saturated fat with unsaturated fat is beneficial for cardiovascular health. This study compared the effects of Brazil nut oil (BNO) and soybean oil (SO) supplementation for 30 days on anthropometric, blood pressure, biochemical, and oxidative parameters in patients with metabolic syndrome (MS). Thirty-one patients with MS were randomly allocated to receive 30 sachets with 10 mL each of either BNO (n = 15) or SO (n = 16) for daily supplementation. Variables were measured at the beginning of the study and after 30 days of intervention. No change in anthropometric and blood pressure variables were observed (p > 0.05). Total (p = 0.0253) and low-density lipoprotein (p = 0.0437) cholesterol increased in the SO group. High-density lipoprotein cholesterol decreased (p = 0.0087) and triglycerides increased (p = 0.0045) in the BNO group. Malondialdehyde levels decreased in the BNO group (p = 0.0296) and total antioxidant capacity improved in the SO group (p = 0.0110). Although the addition of oils without lifestyle interventions did not affect anthropometric findings or blood pressure and promoted undesirable results in the lipid profile in both groups, daily supplementation of BNO for 30 days decreased lipid peroxidation, contributing to oxidative stress reduction.
Keywords: metabolic syndrome, cardiovascular diseases, Brazil nuts, plant oils, Bertholletia
1. Introduction
Metabolic syndrome (MS) includes the combination of at least three cardiovascular risk factors, such as obesity, dyslipidemia, hypertension, and hyperglycemia or type 2 diabetes, which are associated with insulin resistance, oxidative stress, and inflammatory status [1]. MS is highly prevalent in countries with a high rate of obesity and Western eating patterns, with consequential increases in health costs [2].
There is currently no effective recommendation for the prevention of MS other than lifestyle-based interventions aimed at normalizing body weight and maintaining good cardiometabolic control, including the maintenance of adequate levels of serum lipids, glucose, and blood pressure [2].
Recent evidence suggests that some nutrients, foods, and dietary patterns have positive effects on the management of MS, although there is still no consensus on which strategy is best [3,4,5]. However, most of the guidelines suggest that diet plays a fundamental role in the prevention and treatment of MS. Food guidelines for macronutrient intake and cardiovascular health generally emphasize the consumption of foods rich in monounsaturated fatty acids (MUFAs) and a reduction in saturated fatty acid (SFA) intake [6,7,8,9].
Given the current evidence on the role of food in the prevention of nutrition-related diseases, foods with biologically active ingredients that contribute to the prevention of these diseases are increasingly sought [10,11]. The biodiversity of the Amazon provides many plants that are rich in bioactive compounds. The Brazil nut (Bertholletia excelsa), one of the most important oilseeds from this region, has an internationally known nutritional richness [12]. Bioactive substances present in Brazil nuts have already been identified [13] along with their proven beneficial anti-inflammatory [14] and endothelial effects [15]. This oleaginous plant has a complex matrix, rich in bioactive substances, such as selenium, α- and γ-tocopherols, phenolic compounds, folate, magnesium, calcium, proteins, MUFAs, and polyunsaturated fatty acids (PUFAs) [14]. The high content of MUFAs and PUFAs in foodstuffs is potentially beneficial to cardiovascular health, especially when compared to diets rich in SFAs [16].
The oil from the Brazil nut has a high nutritional value, including an unsaturated fatty acid (UFA) content of approximately 75%, constituted predominantly of oleic and linoleic acids, and phytosterols and vitamins A and E [17].
Considering the current evidence on the effects of UFA intake on cardiovascular health, this study aimed to compare the effects of Brazil nut oil and soybean oil supplementation for 30 days on anthropometric, blood pressure, biochemical, and oxidative parameters in patients with MS.
2. Materials and Methods
2.1. Participants
The participants were adults aged between 36 and 65 years, of both genders, diagnosed with MS according to the criteria of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III, 2001) [18]. All participants were recruited from the Nutrition Clinic of the João de Barros Barreto University Hospital (HUJBB), located in the city of Belém, Pará, Brazil. Individuals with thyroid function alterations, neurodegenerative, or chronic liver diseases and smokers were excluded from the study.
All participants were duly informed of the procedures used and gave their written consent. Data collection was carried out between September 2017 and October 2017. The research was approved by the Research Ethics Committee of the HUJBB (protocol No. 68270517.0.0000.0017) and registered at Brazilian Clinical Trials Registry (RBR-5d25w3).
2.2. Experimental Design
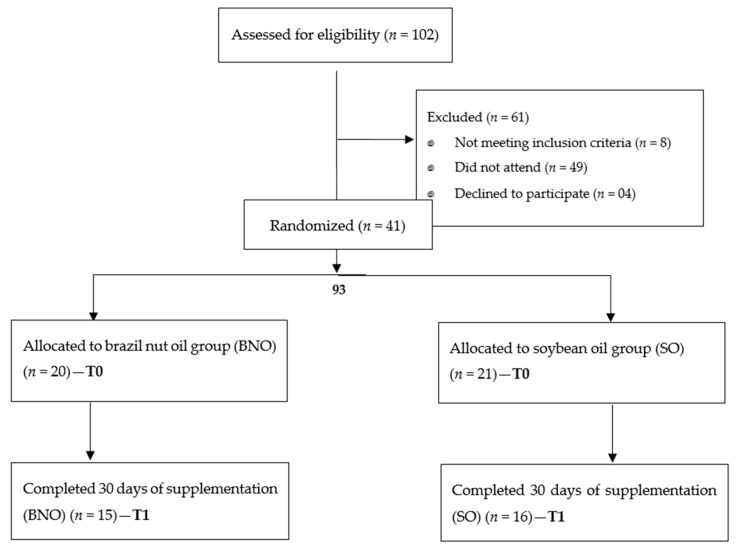
A randomized, double-blind, placebo-controlled clinical trial was conducted on 41 patients with MS. The participants were randomly separated into two groups through the distribution of unidentified sachets. This allowed the formation of the experimental group, which received extra-virgin Brazil nut oil (BNO, n = 20), and the control group, which received soybean oil (SO, n = 21). SO was chosen as the placebo, considering that it is the most used by the Brazilian population in culinary preparations. Neither the participant nor the investigators (responsible for data collection, data analysis, and oil delivery) knew to which group the research participants belonged. A flow chart representing the experimental protocol is presented in Figure 1.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of participants representing the experimental groups.
Volunteers from both groups received 300 mL of the oils, stored in identical 10 mL sachets of opaque and non-toxic material, which were previously randomly coded; only at the end of the analysis were the codes revealed. Each participant was instructed to use one sachet for lunch, without heating, once a day, for 30 consecutive days (10 mL/day).
The lifestyle habits of each subject were assessed through an interview and clinical consultation. Subjects were guided to maintain their eating habits and lifestyle, and to avoid food supplements.
All evaluations were performed at the beginning of the study (T0) and after 30 days of intervention (T1).
2.3. Oils
Brazil nut oil was obtained from an industry specialized in the production of Amazonian oils, located in Tapanã, Pará, Brazil, while soybean oil was purchased from a supermarket in Belém, Pará, Brazil.
Determination of the Fatty Acid Profile of Brazil Nut Oil
The composition of fatty acids was determined by converting fatty acids into methyl esters (FAMEs) based on the method proposed by Rodrigues et al. [19]. The fatty composition was then examined using gas chromatography (CP3380) equipped with a flame ionization detector and with a CP-Sil 88 capillary column (60 m in length, with an internal diameter of 0.25 mm and a thickness of 0.25 mm; Varian Inc., CA, USA). The operating conditions included helium as a carrier gas, with a flow rate of 0.9 mL/min, a detector (FD) at 250 °C, an injector (split ratio of 1:100) at 245 °C, and an injection volume of 1 μL. Program temperature of the column occurred over 4 min at 80 °C and a subsequent increase to 220 °C at 4 °C/min. Individual fatty acid peaks were identified by comparing the obtained retention times with the retention times of known standard fatty acid mixtures (74X, Nu-check-Prep., Inc., MN, USA), examined under the same operating conditions.
2.4. Assessments
2.4.1. Body Composition and Blood Pressure Parameters
The same trained evaluator collected the anthropometric measurements (weight, height, and waist circumference), as described by Lohman, Roche and Martorell [20] and as recommended by the World Health Organization [21]. Percent body fat (%BF) was obtained for each participant through bioimpedance.
All participants were evaluated in the morning. Body mass index (BMI) was calculated by measuring weight and height and dividing the weight in kilograms (kg) by the square of height in meters (m). Weight was measured with patients barefoot and wearing light clothing, and height was determined using a portable stadiometer.
Waist circumference (WC) was measured using an inelastic and flexible measuring tape with a scale ranging from 0 to 150 cm and resolution of 0.1 cm.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) at rest were measured with the arm positioned and supported at the level of the heart and the palm of the hand facing upwards, according to the procedures described by Veiga et al. [22]. Three measurements were taken and the results of these were used to determine the participant’s mean SBP and DBP.
2.4.2. Biochemical Parameters
Participants fasted for 12 h before their blood samples were collected by venipuncture. All samples were centrifuged at 3000 rpm for 15 min, and the plasma and serum obtained were stored at −70 °C until analysis was performed.
Fasting blood glucose, total cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides were analyzed in a biochemical self-analyzer (Dade AR®) using Dade Behring kits. Low-density lipoprotein (LDL) cholesterol was determined according to the Friedwald equation [23].
2.4.3. Oxidative Parameters
The total antioxidant capacity was determined using the Trolox equivalent antioxidant capacity (TEAC) assay. Trolox (6-hydroxy-2,5,7,8-tetramethyl-croman-2-carboxylic acid; SigmaAldrich 23881-3) is a powerful water-soluble antioxidant analog to vitamin E. We used the method proposed by Miller et al. [24] and modified by Re et al. [25], which is a colorimetric technique based on the reaction between ABTS (SigmaAldrich A1888) and potassium persulfate (K2S2O8; Sigma-Aldrich 60490), resulting in the production of ABTS+• radical cation (radical 2,2-azinobis [3-ethylbenzothiazoline-6-sulfonate], diammonium salt), green/blue chromophore [26]. The addition of antioxidants (present in the sample) to this preformed cation reduces ABTS proportionally to the antioxidant capacity, the concentration of antioxidants, and the duration of the reaction. This process was measured by spectrophotometry by observing the change in absorbance, read at 734 nm for 5 min. The results were expressed in μM/mL.
Lipid peroxidation was determined using the method originally created by Khonn and Liversedge [27] and adapted by Percário et al. [28]. It is a technique based on the reaction of malondialdehyde (MDA) with thiobarbituric acid (TBA; Sigma-Aldrich T5500) at pH 2.5 at 94 °C, forming a pink MDA–TBA complex, with absorption at 535 nm. Since the reaction is not specific for MDA, because TBA can react with sugars, amino acids, proteins, and bilirubin, the term thiobarbituric acid-reactive substances (TBARSs) is used [29]. TBARS levels were expressed as malondialdehyde (MDA) equivalent.
The technical procedure consisted of an initial preparation of monobasic potassium phosphate (KH2PO4 75 mM, Synth, 35210) in acidified water (pH 2.5). This solution was used in the preparation of TBA (10 nM). One hundred microliters of the sample was added to 500 µL of TBA 10 nM solution. It was then water bath-heated (94 °C for 60 min). After incubation, the solution was cooled to room temperature for 10 min. Then, 2.0 mL of 1-butyl alcohol was added and it was vigorously homogenized in a vortex and later subjected to centrifugation at 2500 rpm for 10 min before 1.0 mL of supernatant was collected for spectrophotometric reading at 535 nm. The MDA standard (1,1,3,3, tetrahydroxypropane—Sigma-Aldrich, T9889) was used to generate the standard curve, and the results were expressed in μM/L.
2.5. Statistical Analysis
Descriptive and inferential statistics were used to evaluate the difference between T0 and T1 in the two groups (BNO and SO). Quantitative variables were presented by central tendency and variation measures. The Shapiro–Wilk test was used to evaluate the distribution of quantitative variables. Gender was compared using Fisher’s exact test. In order to evaluate the difference between the groups, we used the Student’s t-test (for normal samples) and the Mann–Whitney U-test (when non-normality was detected in the sample). To evaluate intragroup differences (T0 × T1), either the Student’s t-test or the Wilcoxon Signed-rank test was used for paired samples for data with normal and non-normal distributions, respectively. The Alpha Error = 0.05 (5%) was previously set for null hypothesis rejection. Statistical processing was performed using the software BioEstat version 5.3 [30].
3. Results
The fatty acid profiles of Brazil nut and soybean oils are described in Table 1. Both are rich in unsaturated fatty acids (BNO: 72.89% and SO: 84.47%); oleic MUFA w9 predominates in BNO (37.58%), while linoleic PUFA w6 predominates in SO (54.6%).
Table 1.
Profile of fatty acids from Brazil nut oil and soybean oil.
| Fatty Acid | Usual Name | Brazil Nut Oil (%) | Soybean Oil * (%) |
|---|---|---|---|
| C8:0 | Caprylic Acid | 0.03 | - |
| C10:0 | Capric Acid | 0.04 | - |
| C12:0 | Lauric Acid | 1.19 | - |
| C14:0 | Myristic Acid | 0.72 | - |
| C16:0 | Palmitic Acid | 14.89 | 11.50 |
| C16:1 | Palmitoleic Acid | 0.32 | - |
| C17:0 | Margaric Acid | 0.07 | - |
| C18:0 | Stearic Acid | 10.04 | 3.62 |
| C18:1 | Oleic Acid | 37.58 | 24.0 |
| C18:2 | Linoleic Acid | 34.71 | 54.6 |
| C18:3 | α-Linolenic Acid | 0.28 | 5.87 |
| C20:0 | Arachidic Acid | 0.08 | 0.46 |
| C22:0 | Behenic Acid | 0.05 | - |
| ∑ Saturated | 27.11 | 15.58 | |
| ∑ Monounsaturated | 37.90 | 24.0 | |
| ∑ Polyunsaturated | 34.99 | 60.47 |
* Source: Zaunschirm et al. [31].
The total sample was composed of 31 individuals, divided into two groups (BNO n = 15 and SO n = 16). The distribution by gender did not show any significant difference between the groups (p > 0.5), and the two groups were mostly composed of women (BNO: 73.3% and SO: 93.7%). Similarly, age did not show any significant difference between the groups (p > 0.5), ranging from 36 to 64 years in the BNO group (mean 58.5 ± 8.4) and from 43 to 65 years in the SO group (mean 58.3 ± 5.7 years).
The anthropometric and blood pressure data are described in Table 2. There were no significant differences in BMI, %BF, WC, SBP, and DBP values between the groups at the beginning of the study (T0) and at the end of the intervention (T1). Similarly, there was no difference in BMI, %BF, WC, SBP, and DBP between the groups before and after the use of oils (T1 − T0).
Table 2.
Anthropometric and blood pressure characteristics of individuals with metabolic syndrome (MS) before and after supplementation with Brazil nut oil or soybean oil.
| BNO | SO | |||||
|---|---|---|---|---|---|---|
| Before | After | Δ | Before | After | Δ | |
| Sample size | 15 | 15 | 15 | 16 | 16 | 16 |
| BMI | 32.2 | 31.5 | −0.7 | 30.8 | 30.8 | −0.1 |
| ±4.4 | ±4.4 | 1.2 | ±4.4 | ±4.5 | 0.3 | |
| p-value (intragroup) | 0.0566 | 0.4539 | ||||
| T0 | T1 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.4125 | 0.6601 | 0.1280 | |||
| %BF | 39.9 | 40.3 | 0.4 | 43.7 | 44.3 | 0.6 |
| ±8.1 | ±8.7 | 1.4 | ±6.6 | ±6.4 | 1.4 | |
| p-value (intragroup) | 0.9089 | 0.8045 | ||||
| T0 | T0 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.0782 | 0.0904 | 0.8712 | |||
| WC | 109.1 | 108.2 | −1.3 | 102.3 | 101.3 | −1.0 |
| ±10.3 | ±8.8 | 2.7 | ±10.7 | ±9.8 | 3.6 | |
| p-value (intragroup) | 0.3224 | 0.2859 | ||||
| T0 | T1 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.1303 | 0.0939 | 0.9569 | |||
| SBP | 132.0 | 130.6 | −1.4 | 133.9 | 125.8 | −8.2 |
| ±19.8 | ±16.7 | 12.7 | ±17.3 | ±14.4 | 16.6 | |
| p-value (intragroup) | 0.5434 | 0.4456 | ||||
| T0 | T1 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.7824 | 0.4073 | 0.3705 | |||
| DBP | 78.5 | 78.2 | −0.3 | 78.8 | 76.3 | −2.5 |
| ±11.8 | ±10.4 | 4.1 | ±6.8 | ±11.0 | 10.2 | |
| p-value (intragroup) | 0.8062 | 0.3051 | ||||
| T0 | T1 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.5532 | 0.4768 | 0.5673 | |||
BNO, Brazil nut oil; SO, soybean oil; BMI, body mass index; %BF, percent body fat; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure. p-value (BNO × SO) by Student’s t-test (IMC, WC, SBP, and DBP). Data are expressed as the mean ± standard deviation.
The biochemical data are shown in Table 3. Regarding the lipid profile, the SO group showed an increase in total cholesterol at the end of the intervention (T1 − T0) (p-value = 0.0253) and in LDL cholesterol values (p-value = 0.0437). The BNO group, in turn, showed a decrease in HDL cholesterol (T1 − T0) (p-value = 0.0087) and an increase (p = 0.0045) in triglycerides. There was no difference between the glycemic values of the groups at the beginning of the study (T0) (p-value = 0.2582); after 30 days of intervention, the BNO group showed significantly higher levels of glucose (p = 0.0282) and the variation in glucose between the groups (T1 − T0) was significant (p = 0.0368), although glycemic levels intragroup did not significantly change at the end of the supplementation (T1 − T0) (p-value > 0.05).
Table 3.
Biochemical characteristics of individuals with MS before and after supplementation with Brazil nut oil or soybean oil.
| BNO | SO | |||||
|---|---|---|---|---|---|---|
| Before | After | Δ | Before | After | Δ | |
| Sample size | 15 | 15 | 16 | 16 | 16 | 16 |
| Cholesterol | 243.1 | 240.0 | −3.1 | 216.6 | 240.5 | 23.9 |
| ±59.9 | ±53.4 | ±66.2 | ±35.2 | ±38.8 | ±38.6 | |
| p-value (intragroup) | 0.8604 | 0.0253 * | ||||
| T0 | T1 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.3738 | 0.9370 | 0.2684 | |||
| LDL | 161.3 | 151.3 | −9.9 | 132.6 | 155.3 | 22.7 |
| ±58.8 | ±59.0 | ±68.6 | ±37.5 | ±38.7 | ±43.6 | |
| p-value (intragroup) | 0.5823 | 0.0437 * | ||||
| T0 | T1 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.2599 | 0.9656 | 0.2434 | |||
| HDL | 36.5 | 29.7 | −6.8 | 40.2 | 42.6 | 2.2 |
| ±8.1 | ±8.2 | ±8.7 | ±12.2 | ±14.3 | ±9.9 | |
| p-value (intragroup) | 0.0087 * | 0.3499 | ||||
| T0 | T1 | Δ (T1 = T0) | ||||
| p-value (BNO × SO) | 0.2948 | 0.1665 | 0.0121 * | |||
| Triglycerides | 226.7 | 295.3 | 68.6 | 218.3 | 213.8 | −4.5 |
| ±32.1 | ±73.8 | ±83.6 | ±36.6 | ±46.0 | ±−4.5 | |
| p-value (intragroup) | 0.0045 *b | 0.8501 | ||||
| T0 | T1 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.8451 | 0.0021 *a | 0.0066 *a | |||
| Glucose | 153.4 | 157.6 | 4.1 | 115,8 | 106.8 | −9.0 |
| ±78.1 | ±68.2 | ±43.1 | ±32 | ±26.9 | ±38.8 | |
| p-value (intragroup) | 0.7143 | 0.3678 | ||||
| T0 | T1 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.5016 | 0.0282 *c | 0.0368 * | |||
BNO, Brazil nut oil; SO, soybean oil; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides. * (Before × After): Student’s t-test for paired samples (TC, LDL, HDL). a* Comparison (BNO × SO): the Mann–Whitney U-test (TG). b* Intragroup comparison by Wilcoxon Signed-Rank test. (TG). c* The Mann–Whitney U-test for paired samples (glucose). Data are expressed as the mean ± standard deviation.
Table 4 shows the biomarkers of oxidative metabolism before and after 30 days of supplementation. After the intervention, MDA levels were significantly lower (p-value = 0.0296) in the group that received Brazil nut oil. The soybean group showed statistically significant improvement in TEAC (p-value = 0.0110), suggesting an improvement in antioxidant capacity (Figure 1).
Table 4.
Markers of the oxidative metabolism of individuals with MS before and after supplementation with Brazil nut oil or soybean oil.
| BNO | SO | |||||
|---|---|---|---|---|---|---|
| Before | After | Δ | Before | After | Δ | |
| Sample size | 14 | 14 | 14 | 15 | 15 | 15 |
| TEAC | 3.20 | 3.26 | 0.06 | 3.39 | 3.50 | 0.10 |
| ±0.80 | ±0.69 | ±1.12 | ±0.10 | ±0.10 | ±0.13 | |
| p-value (intragroup) | 0.8727 | 0.0110 * | ||||
| T0 | T1 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.3041 | 0.4767 | 0.6112 | |||
| MDA | 3.18 | 2.27 | −0.84 | 2.91 | 2.60 | −0.31 |
| ±0.93 | ±0.96 | ±1.92 | ±0.48 | ±1.42 | ±1.51 | |
| p-value (intragroup) | 0.0296 * | 0.4511 | ||||
| T0 | T1 | Δ (T1 − T0) | ||||
| p-value (BNO × SO) | 0.9658 | 0.8362 | 0.5053 | |||
| MDA/triglycerides (nmol/mg) | 1.40 | 0.77 | −0.63 | 1.33 | 1.22 | −0.12 |
| MDA/TEAC | 0.99 | 0.69 | −0.3 | 0.86 | 0.74 | −0.12 |
* Student’s t-test for paired samples. Data are expressed as the mean ± standard deviation. TEAC, Trolox equivalent antioxidant capacity; MDA, malondialdehyde; T0, beginning of the study; T1, after 30 days of intervention.
Study data are available in supplemental material (Table S1).
4. Discussion
To the best of our knowledge, this is the first study to compare the effects of 10 mL supplementation of Brazil nut and soybean oils in patients with MS.
Brazil nut and soybean oils are rich in UFAs, with values of 72.89% and 84.47% [31], respectively. Oleic acid w9 predominates in BNO (34.71%) and linoleic acid w6 predominates in SO (56.6%) [31]. These results are consistent with what has been reported in the literature [17,32,33].
The influence of the type of fatty acid ingested on CVD risk factors and on plasma concentrations of lipids and lipoprotein has been demonstrated for a long time in several experimental and population studies [34,35,36].
The results of our clinical trial showed that supplementation of 10 mL of Brazil nut and soybean oils for 30 days did not promote significant changes in anthropometric and blood pressure parameters. Similar results were found in other studies with approximate intervention periods [37,38]. Longer intervention periods are needed in order to determine whether this supplementation could have a more significant impact on these parameters [39,40,41,42].
Many mechanisms suggest that the PUFAs have effects on the modulation of pathways that could influence lipid profile. For example, PUFAs can decrease the synthesis of very low-density lipoprotein (VLDL), both due to the greater catabolism of these fatty acid in peroxisomes and its interference with nuclear receptors. Other effects include the increase in the fluidity of cell membranes of hepatocytes, interfering with the activity of LDL receptors [43] and with the amount of hepatic apoprotein B/E receptors [44]. In addition, PUFAs can promote changes in the LDL spatial configuration, providing a lower volume available for the incorporation and transport of cholesterol [45], besides decreasing triglyceridemia due to the stimulation of the hydrolysis of apo B-100 [46].
Oleic acid (MUFA), in turn, helps in reducing LDL plasma levels without oxidizing it [47], probably because it is a better substrate for acyl-coenzyme A:cholesterol acyltransferase (ACAT) in the liver, and therefore, free cholesterol is rapidly esterified, not leading to the suppression of LDL receptors [48]. Moreover, this fatty acid induces less endogenous synthesis of cholesterol than other types of fatty acid [49].
It is important to note that the intake of SFAs and total fat cannot be neglected as their high consumption can promote changes in glucose homeostasis, high blood pressure, and hypertriglyceridemia, regardless of the amount of MUFAs and PUFAs ingested [50].
In our trial, the BNO group showed an increase in triglyceride levels and a decrease in HDL levels, while the control group (SO) showed an increase in total cholesterol and LDL levels. Both groups were mostly composed of women with median ages of 58.5 ± 8.4 (BNO group) and 58.3 ± 5.7 (SO group) years; this is the age at which many women are in menopause. It is known that menopause may contribute to lipid profile alteration [51], but in our study, this effect was not evaluated. Studies evaluating the effects of oil supplementation in patients with chronic diseases present divergent data on lipid profiles; Harris, Hutchins and Fryda [38] showed negative results [38], while others found improvements in these parameters [52,53,54]. These differences among studies may be explained by the different nutritional contexts in which the studies were performed, in addition to the dosage and the period of use of the oils.
Although recommendations for the treatment of MS usually include the replacement of saturated fat and monosaccharide by unsaturated fats, it is not yet clear which metabolic parameters are improved when saturated fat is replaced by higher concentrations of MUFAs or PUFAs [42]. Some studies that replaced SFAs by UFAs also obtained inconsistent results in cardiometabolic risk factors [55,56]. Moreover, other factors may lead to increased serum lipids and blood glucose, such as a low-fiber diet, a high intake of refined carbohydrates and saturated fat, and a sedentary lifestyle [51]. According to Astrup [57], the replacement of saturated fat by polyunsaturated fat present in vegetable oils may even increase the risk of CVD death if they are not from the omega-3 series.
Supplementation of the two types of oils (SO and BNO) did not significantly change the glucose levels, which is consistent with previous studies [42,58,59].
In our study, oxidative metabolism was evaluated by measuring lipid peroxidation products, such as MDA, and the total antioxidant capacity in plasma. The analysis of the total antioxidant capacity is recommended more than the analysis of isolated antioxidants, due to their interaction in plasma or serum and because the total antioxidant capacity represents the cumulative action of all antioxidants present [26].
The decrease in MDA levels observed after the use of Brazil nut oil thus suggests a reduction in lipid peroxidation, showing a desirable effect of this oil in the treatment of MS. This can be evidenced by the reduction in the MDA/triglycerides ratio (approximately 50%) (Table 4) and allows us to conclude that, despite the increase in triglycerides, Brazil nut oil was able to decrease this parameter.
The increase in total antioxidant capacity detected in the SO group may be associated with the tocopherol contents present in this oil [31]. In contrast, it may be related to the observed increase in total cholesterol and LDL, indicating a probable defense response of the body against vascular oxidative stress [60]. This response occurs through a negative feedback loop that can activate enzymatic pathways of the antioxidant defense system in order to reduce intracellular ROS levels and thereby minimize oxidative damage [61].
The effect on oxidative stress depends on the type and total amount of macronutrients consumed [62]. These two aspects may contribute to the oxidative stress and favor the development of obesity and chronic diseases [63]. The molecular mechanisms of nutrient-mediated oxidative stress, however, are complex and still not well-known [64].
Several studies indicate that when polyphenols are present in the diet, they hinder the development of disorders related to oxidative stress, such as cancer, cardiovascular diseases, diabetes mellitus, and MS [65]. These compounds are commonly found in products of plant origin and represent the most abundant sources of antioxidants in our diet [66].
Vegetable oils are sources of bioactive compounds such as tocopherols and phenolic compounds. The average tocopherol content in Brazil nut and soybean oils is approximately 190 µg/g and 980 µg/g, respectively, while for phenolic compounds, these values are 150 mg gallic acid equivalent (GAE)/100 g for BNO and 6 mg GAE/100 g for SO [31,67,68,69,70,71]. Despite containing lower tocopherol levels and SO [31], this amount still provides antioxidant effects [68]. Moreover, its content of phenolic compounds reaffirms the antioxidant power of BNO [72], which probably determined its effect on lipid peroxidation.
Given these results, it can be observed that both oils are sources of antioxidants. Soybean oil is rich in tocopherols and Brazil nut oil in phenolic compounds. The performance of antioxidants, however, depends on several factors (e.g., the type and location of the generated free radicals and the optimal doses). In this sense, it is possible for an antioxidant to act as a protector in one system but fail to protect or even increase induced damage to other tissues [73]. Furthermore, evidence suggests that in addition to phenolic compounds, MUFAs present in Brazil nuts improve oxidative stress [74].
This study shows that the potential benefits of BNO for the treatment of MS are limited without an overall healthy lifestyle.
5. Conclusions
Although the addition of oils without lifestyle interventions did not alter the anthropometric and blood pressure parameters and promoted undesirable results in the lipid profile, daily supplementation of 10 mL of Brazil nut oil for 30 days decreased lipid peroxidation, contributing to oxidative stress reduction.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/12/1/46/s1. Table S1: Study data.
Author Contributions
Conceptualization, L.M.C.e.S., M.L.P.d.M. and L.H.M.d.S.; Data curation, L.M.C.e.S. and L.H.M.d.S.; Formal analysis, L.M.C.e.S., S.M.d.S. and B.A.Q.G.; Funding acquisition, L.M.C.e.S. and L.H.M.d.S.; Investigation, L.M.C.e.S., M.L.P.d.M. and L.H.M.d.S.; Methodology, L.M.C.e.S., M.L.P.d.M. and L.H.M.d.S.; Project administration, L.M.C.e.S. and L.H.M.d.S.; Resources, M.C.M. and L.H.M.d.S.; Supervision, M.L.P.d.M. and L.H.M.d.S.; Validation, L.M.C.e.S., M.L.P.d.M., F.V.F.R., M.C.M., S.M.d.S., B.A.Q.G. and L.H.M.d.S.; Visualization, L.M.C.e.S. and S.M.d.S.; Writing—original draft, L.M.C.e.S.; Writing—review and editing, L.M.C.e.S., M.L.P.d.M., F.V.F.R., M.C.M., S.M.d.S., B.A.Q.G. and L.H.M.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES) Foundation [$300,000].
Conflicts of Interest
The authors declare no conflict of interest. The sponsors had no role in the design, execution, interpretation, or writing of the study.
References
- 1.Mohamed S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. Trends Food Sci. Technol. 2014;35:114–128. doi: 10.1016/j.tifs.2013.11.001. [DOI] [Google Scholar]
- 2.Pérez-Martínez P., Mikhailidis D.P., Athyros V.G., Bullo M., Couture P., Covas M.I., de Koning L., Delgado-Lista J., Díaz-Lopez A., Drevon C.A., et al. Lifestyle recommendations for the prevention and management of metabolic syndrome: An international panel recommendation. Nutr. Rev. 2017;75:307–326. doi: 10.1093/nutrit/nux014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minich D.M., Bland J.S. Dietary management of the metabolic syndrome beyond macronutrients. Nutr. Rev. 2008;66:429–444. doi: 10.1111/j.1753-4887.2008.00075.x. [DOI] [PubMed] [Google Scholar]
- 4.Abete I., Astrup A., Martinez J.A., Thorsdottir I., Zulet M.A. Obesity and the metabolic syndrome: Role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr. Rev. 2010;68:214–231. doi: 10.1111/j.1753-4887.2010.00280.x. [DOI] [PubMed] [Google Scholar]
- 5.Keane D., Kelly S., Healy N.P., McArdle M.A., Holohan K., Roche H.M. Diet and metabolic syndrome: An overview. Curr. Vasc. Pharmacol. 2013;11:842–857. doi: 10.2174/15701611113116660173. [DOI] [PubMed] [Google Scholar]
- 6.Evert A.B., Boucher J.L., Cypress M., Dunbar S.A., Franz M.J., Mayer-Davis E.J., Neumiller J.J., Nwankwo R., Verdi C.L., Urbanski P., et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36:3821–3842. doi: 10.2337/dc13-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Agriculture Organization of the United Nations . Fats and Fatty Acids in Human Nutrition. Food and Agriculture Organization of the United Nations; Rome, Italy: 2010. [Google Scholar]
- 8.Aranceta J., Pérez-Rodrigo C. Recommended dietary reference intakes, nutritional goals and dietary guidelines for fat and fatty acids: A systematic review. Br. J. Nutr. 2012;107:8–22. doi: 10.1017/S0007114512001444. [DOI] [PubMed] [Google Scholar]
- 9.Santos R.D., Gagliardi A.C.M., Xavier H.T., Magnoni C.D., Cassani R., Lottenberg A.M., Casella F.A., Araújo D.B., Cesena F.Y., Alves R.J., et al. First guidelines on fat consumption and cardiovascular health [Article in Portuguese] Arq. Bras. Cardiol. 2013;100(Suppl. 3):1–40. doi: 10.1590/S0066-782X2013000900001. [DOI] [PubMed] [Google Scholar]
- 10.Siró I., Kápolna E., Kápolna B., Lugasi A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite. 2008;51:456–467. doi: 10.1016/j.appet.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 11.Alkhatib A., Tsang C., Tiss A., Bahorun T., Arefanian H., Barake R., Khadir A., Tuomilehto J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients. 2017;9:1310. doi: 10.3390/nu9121310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos O.V., Corrêa N.C.F., Soares F.A.S.M., Gioelli L.A., Costa C.E.F., Lannes S.C.S. Chemical evaluation and thermal behavior of Brazil nut oil obtained by different. Food Res. Int. 2012;47:253–258. doi: 10.1016/j.foodres.2011.06.038. [DOI] [Google Scholar]
- 13.Kocygit A., Koylu A.A., Keles H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr. Metab. Cardiovasc. Dis. 2006;16:202–209. doi: 10.1016/j.numecd.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Ros E. Nuts and novel biomarkers of cardiovascular disease. Am. J. Clin. Nutr. 2009;89:1649–1656. doi: 10.3945/ajcn.2009.26736R. [DOI] [PubMed] [Google Scholar]
- 15.Ros E., Nunez I., Perez-Heras A., Serra M., Gilabert R., Casals E., Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation. 2004;109:1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 16.Ros E., Mataix J. Fatty acid composition of nuts–implications for cardiovascular health. Br. J. Nutr. 2006;96:29–35. doi: 10.1017/BJN20061861. [DOI] [PubMed] [Google Scholar]
- 17.Yang J. Brazil nuts and associated health benefits: A review. LWT Food Sci. Technol. 2009;42:1573–1580. doi: 10.1016/j.lwt.2009.05.019. [DOI] [Google Scholar]
- 18.NCEP. Adult Treatment Panel III Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. doi: 10.1161/circ.106.25.3143. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues A.M.C., Darnetb S., Silva L.H.M. Fatty Acid Profiles and Tocopherol Contents of Buriti (Mauritia flexuosa), Patawa (Oenocarpus bataua), Tucuma (Astrocaryum vulgare), Mari (Poraqueiba paraensis) and Inaja (Maximiliana maripa) Fruits. J. Braz. Chem. Soc. 2010;21:2000–2004. doi: 10.1590/S0103-50532010001000028. [DOI] [Google Scholar]
- 20.Lohman T.G., Roche A.F., Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics; Champaign, IL, USA: 1988. [Google Scholar]
- 21.World Health Organization . Physical Status: The Use and Interpretation of Anthropometry—Report of a WHO Expert Committee. World Health Organization; Geneva, Switzerland: 1995. (WHO Technical Report Series No. 854). [PubMed] [Google Scholar]
- 22.Veiga E.V., Nogueira M.S., Cárnio E.C., Marques S., Lavrador M.A., De Moraes S.A., Souza L.A.C., Lima N.K.C., Nobre F. Assessment of the techniques of blood pressure measurement by health professionals [Article in Portuguese] Arq. Bras. Cardiol. 2003;80:89–93. doi: 10.1590/S0066-782X2003000100008. [DOI] [PubMed] [Google Scholar]
- 23.Friedwald W., Levy A.L., Frederickson D.S. Estimation of concentrations of low-density cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–4502. [PubMed] [Google Scholar]
- 24.Miller N., Rice-Evans C., Davies M., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. (Lond.) 1993;84:407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 25.Re R., Pellegrini R., Protegente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorizaton assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 26.Vasconcelos S.M.L., Goulart M.O.F., Moura J.B.F., Manfredini V., Benfato M.S., Kubota L.T. Reactive oxygen and nitrogen species, antioxidants and markers of oxidative damage in human blood: Main analytical methods for their determination [Article in Portuguese] Chem. Nova. 2007;30:1323–1338. doi: 10.1590/S0100-40422007000500046. [DOI] [Google Scholar]
- 27.Khonn H.I., Liversedge M. On a new aerobic metabolite whose production by brain is inhibited by apomorphine, methine, ergotamine, epinephrine and menadione. J. Pharmacol. Exp. Ther. 1944;83:292–300. [Google Scholar]
- 28.Percário S., Vital A., Jablonka F. Malondialdehyde dosage [Article in Portuguese] Newslab. 1994;2:46–50. [Google Scholar]
- 29.Mayne S.T. Antioxidant nutrients and chronic disease: Use of biomarkers of exposure and oxidative stress status in epidemiologic research. J. Nutr. 2003;133:933–940. doi: 10.1093/jn/133.3.933S. [DOI] [PubMed] [Google Scholar]
- 30.Ayres M., Ayres M., Jr., Ayres D.L., Santos A.A.S. BioEstat 5.3: Aplicações Estatísticas Nas Áreas das Ciências Biológicas e Médicas. 5th ed. Publicações Avulsas do Mamirauá; Belém, Brazil: 2007. p. 361. [Google Scholar]
- 31.Zaunschirm M., Pignitter M., Kienesberger J., Hernler N., Riegger C., Eggersdorfer M., Somoza V. Contribution of the ratio of tocopherol homologs to the oxidative stability of commercial vegetable oils. Molecules. 2018;23:206. doi: 10.3390/molecules23010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin C.A., Visentainer J.V., de Oliveira A.N., de Oliveira C.C., Matsushita M., de Souza N.E. Fatty acid contents of Brazilian soybean oils with emphasis on trans-fatty acids. J. Braz. Chem. Soc. 2008;19:117–122. doi: 10.1590/S0103-50532008000100017. [DOI] [Google Scholar]
- 33.Muniz M.A.P., Santos M.N.F., Costa C.E.F., Morais L., Lamarão M.L.N., Ribeiro-Costa R.M., Silva-Junior O.C. Physicochemical characterization, fatty acid composition, and thermal analysis of Bertholletia excelsa HBK oil. Pharmacogn. Mag. 2015;11:147–151. doi: 10.4103/0973-1296.149730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keys A., Kimura N., Kusukawa A., Bonte-Stewart B., Larsen N., Keys M.H. Lessons from serum cholesterol studies in Japan, Hawaii and Los Angeles. Ann. Intern. Med. 1958;48:83–94. doi: 10.7326/0003-4819-48-1-83. [DOI] [PubMed] [Google Scholar]
- 35.Connor S.L., Gustafson J.R., Artaud-Wild S.M., Flavell D.P., Classick-Kohn C.J., Hatcher L.F., Connor W.E. The cholesterol/saturated-fat index: An indication of the hypercholesterolaemic and atherogenic potential of food. Lancet. 1986;1:1229–1232. doi: 10.1016/S0140-6736(86)91384-X. [DOI] [PubMed] [Google Scholar]
- 36.Lands B. A critique of paradoxes in current advice on dietary lipids. Prog. Lipid Res. 2008;47:77–106. doi: 10.1016/j.plipres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Pilar B.C., Güllich A.P.C., Ströher D.J., Zuravski L., Mezzomo J., Coelho R.P., Faoro D., Piccoli J.D., Manfredini V. 28-days dietary supplementation with golden flaxseed improves biochemical and oxidative parameters in patients with metabolic syndrome. J. Funct. Foods. 2014;10:232–242. doi: 10.1016/j.jff.2014.06.020. [DOI] [Google Scholar]
- 38.Harris M., Hutchins A., Fryda L. The impact of virgin coconut oil and high-oleic safflower oil on body composition, lipids, and inflammatory markers in postmenopausal women. J. Med. Food. 2017;20:345–351. doi: 10.1089/jmf.2016.0114. [DOI] [PubMed] [Google Scholar]
- 39.Paschos G.K., Magkos F., Panagiotakos D.B., Votteas V., Zampelas A. Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur. J. Clin. Nutr. 2007;61:1201–1206. doi: 10.1038/sj.ejcn.1602631. [DOI] [PubMed] [Google Scholar]
- 40.Amir K., Watanabe M., Yokotsuka M., Kobayashi Y. Changes in serum fatty acids and effects of metabolic syndrome induced by intermittent flaxseed-oil supplementation. Ningen Dock. 2011;26:927–934. doi: 10.11320/ningendock.26.927. [DOI] [Google Scholar]
- 41.Hensler M., Bardova K., Jilkova Z.M., Wahli W., Meztger D., Chambon P., Kopecky J., Flachs P. The inhibition of fat cell proliferation by n-3 fatty acids in dietary obese mice. Lipids Health Dis. 2011;10:2–7. doi: 10.1186/1476-511X-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller M., Sorkin J.D., Mastella L., Sutherland A., Rhyne J., Donnelly P., Simpson K., Goldberg A.P. Poly is more effective than mono-unsaturated fat for dietary management in the metabolic syndrome: The muffin study. J. Clin. Lipidol. 2016;10:996–1003. doi: 10.1016/j.jacl.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tripodi A., Loria P., Dilengite M.A., Carulli N. Effect of fish oil and coconut oil diet on the LDL receptor activity of rat liver plasma membranes. Biochim. Biophys. Acta. 1991;1083:298–304. doi: 10.1016/0005-2760(91)90086-W. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez M.L., McNamar D.J. Dietary fat-mediated changes in hepatic apoprotein B/E receptor in the guinea pig: Effect of polyunsaturated, monounsaturated, and saturated fat. Metabolism. 1989;38:1094–1102. doi: 10.1016/0026-0495(89)90046-2. [DOI] [PubMed] [Google Scholar]
- 45.Spritz N., Mishkel M.A. Effects of dietary fats on plasma lipids and lipoproteins: A hypothesis for the lipid-lowering effect of unsaturated fatty acids. J. Clin. Investig. 1969;48:78–86. doi: 10.1172/JCI105976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan M., Cederbaum A.I., Zhang Y.L., Ginsberg H.N., Williams K.J., Fisher E.A. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J. Clin. Investig. 2004;113:1277–1287. doi: 10.1172/JCI19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reaven P., Parthasarathy S., Grasse B.J., Miller E., Almazan F., Mattson F.H., Khoo J.C., Steinberg D., Witztum J.L. Feasibility of using an oleate-rich diet to reduce the susceptibility of low-density lipoprotein to oxidative modification in humans. Am. J. Clin. Nutr. 1991;54:701–706. doi: 10.1093/ajcn/54.4.701. [DOI] [PubMed] [Google Scholar]
- 48.Grundy S.M. George Lyman Duff Memorial Lecture. Multifactorial etiology of hypercholesterolemia. Implications for prevention of coronary heart disease. Arterioscler. Thromb. 1991;11:1619–1625. doi: 10.1161/01.ATV.11.6.1619. [DOI] [PubMed] [Google Scholar]
- 49.Jones P.J.H., Lichtenstein A.H., Schaefer E.J., Namchuk G.L. Effect of dietary fat selection on plasma cholesterol synthesis in older, moderately hypercholesterolemic humans. Arterioscler. Thromb. 1994;14:542–548. doi: 10.1161/01.ATV.14.4.542. [DOI] [PubMed] [Google Scholar]
- 50.Hosseinpour-Niazi S., Mirmiran P., Fallah-Ghohroudi A., Azizi F. Combined effect of unsaturated fatty acids and saturated fatty acids on the metabolic syndrome: Tehran lipid and glucose study. J. Health Popul. Nutr. 2015;33:5. doi: 10.1186/s41043-015-0015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faludi A.A., Izar M.C.O., Saraiva J.F.K., Chacra A.P.M., Bianco H.T., Afiune Neto A., Bertolami A., Pereira A.C., Lottenberg A.M., Sposito A.C., et al. Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose [Article in Portuguese] Arq. Bras. Cardiol. 2017;109(Suppl. 1):1–76. doi: 10.5935/abc.20170121. [DOI] [PubMed] [Google Scholar]
- 52.Zibaeenezhad M.J., Farhadi P., Attar A., Mosleh A., Amirmoezi F., Azimi A. Effects of walnut oil on lipid profiles in hyperlipidemic type 2 diabetic patients: A randomized, double-blind, placebo-controlled trial. Nutr. Diabetes. 2017;7:259. doi: 10.1038/nutd.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen T., Xing G., Zhu J., Zhang S., Cai Y., Li D., Xu G., Xing E., Rao J., Shi R. Effects of 12-week supplementation of marine Omega-3 PUFA-based formulation Omega3Q10 in older adults with prehypertension and/or elevated blood cholesterol. Lipids Health Dis. 2017;16:253. doi: 10.1186/s12944-017-0617-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oliveira-de-Lira L., Santos E.M.C., de Souza R.F., Matos R.J.B., Silva M.C.D., Oliveira L.D.S., Nascimento T.G.D., Schemly P.A.L.S., Souza S.L. Supplementation-dependent effects of vegetable oils with varying fatty acid compositions on anthropometric and biochemical parameters in obese women. Nutrients. 2018;10:932. doi: 10.3390/nu10070932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hodson L., Skeaff C.M., Chisholm W.A. The effect of replacing dietary saturated fat with polyunsaturated or monounsaturated fat on plasma lipids in free-living young adults. Eur. J. Clin. Nutr. 2001;55:908–915. doi: 10.1038/sj.ejcn.1601234. [DOI] [PubMed] [Google Scholar]
- 56.Tierney A.C., McMonagle J., Shaw D.I., Gulseth H.L., Helal O., Saris W.H., Paniagua J.A., Gołąbek-Leszczyñska I., Defoort C., Williams C.M., et al. Effects of dietary fat modification on insulin sensitivity and on other risk factors of the metabolic syndrome—LIPGENE: A European randomized dietary intervention study. Int. J. Obes. (Lond.) 2011;35:800–809. doi: 10.1038/ijo.2010.209. [DOI] [PubMed] [Google Scholar]
- 57.Astrup A. Yogurt and dairy product consumption to prevent cardiometabolic diseases: Epidemiologic and experimental studies. Am. J. Clin. Nutr. 2014;99:1235–1242. doi: 10.3945/ajcn.113.073015. [DOI] [PubMed] [Google Scholar]
- 58.Vijayakumar M., Vasudevan D.M., Sundaram K.R., Krishnan S., Vaidyanathan K., Nandakumar S., Chandrasekhar R., Mathew N. A randomized study of coconut oil versus sunflower oil on cardiovascular risk factors in patients with stable coronary heart disease. Indian Heart J. 2016;68:498–506. doi: 10.1016/j.ihj.2015.10.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karupaiah T., Chuah K., Chinna K., Matsuoka R., Masuda Y., Sundram K., Michihiro S. Comparing effects of soybean oil- and palm olein-based mayonnaise consumption on the plasma lipid and lipoprotein profiles in human subjects: A double-blind randomized controlled trial with cross-over design. Lipids Health Dis. 2016;15:131. doi: 10.1186/s12944-016-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwak H.K., Yoon S. Relation of serum total antioxidant status with metabolic syndrome risk factors in Korean adults. Nutr. Res. Pract. 2007;1:335–340. doi: 10.4162/nrp.2007.1.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demirbag R., Yilmaz R., Kunt A.S., Gur M., Ulucay A., Unlu D. Relationship between plasma total antioxidant capacity and thoracic aortic intima-media thickness. Echocardiography. 2006;23:183–188. doi: 10.1111/j.1540-8175.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 62.Kitabchi E., McDaniel K.A., Wan J.Y., Tylavsky F.A., Jacovino C.A., Sands C.W., Nyenwe E.A., Stentz F.B. Effects of high-protein versus high-carbohydrate diets on markers of β-cell function, oxidative stress, lipid peroxidation, proinflammatory cytokines, and adipokines in obese, premenopausal women without diabetes: A randomized control trial. Diabetes Care. 2013;36:1919–1925. doi: 10.2337/dc12-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuzefovych L.V., Musiyenko S.I., Wilson G.L., Rachek L.I. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS ONE. 2013;8:540–559. doi: 10.1371/journal.pone.0054059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tan B.L., Norhaizan M.E., Liew W. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018;2018:9719584. doi: 10.1155/2018/9719584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scalbert A., Manach C., Morand C., Rémésy C., Jiménez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 66.Scalbert A., Williamson G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000;130:2073–2085. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 67.Lima J.R., Gonçalves L.A. Quantificação de tocoferóis em óleos de milho, soja, castanha-do-pará e castanha de caju por cromatografia líquida de alta eficiência em fase reversa. Alim. Nutr. Araraquara. 1997;8:65–73. [Google Scholar]
- 68.Silveira C.S. Ph.D. Thesis. Programa de Pós-Graduação em Ciência e Tecnologia de Alimentos, Faculdade de Agronomia, Universidade Federal de Pelotas; Pelotas Rio Grande do Sul, Brazil: 2015. Caracterização Físico-Química e Avaliação Biológica de Produtos da Castanha-do-Brasil (Bertholletia Excelsa H.B.K.) p. 146. [Google Scholar]
- 69.Funasaki M., Menezes I.S., Barroso H.S., Zanotto S.P., Carioca C.R.F. Tocopherol profile of Brazil nut oil from different geographic areas of the Amazon region. Acta Amaz. 2013;43:505–510. doi: 10.1590/S0044-59672013000400012. [DOI] [Google Scholar]
- 70.Yang J., Liu R.H., Halim L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT Food Sci. Technol. 2009;42:1–8. doi: 10.1016/j.lwt.2008.07.007. [DOI] [Google Scholar]
- 71.Abe L.T., Lajolo F.M., Genovese M.I. Comparison of phenol content and antioxidant capacity of nuts. Ciênc. Tecnol. Aliment. 2010;30:254–259. doi: 10.1590/S0101-20612010000500038. [DOI] [Google Scholar]
- 72.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 73.Singh B., Singh J.P., Kaur A., Singh N. Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review. Food Chem. 2018;261:75–86. doi: 10.1016/j.foodchem.2018.04.039. [DOI] [PubMed] [Google Scholar]
- 74.Maranhão P.A., Kraemer-Aguiar L.G., De Oliveira C.L., Kuschnir M.C.C., Vieira Y.R., Souza M.G.C., Koury J.C., Bouskela E. Brazil nuts intake improves lipid profile, oxidative stress and microvascular function in obese adolescents: A randomized controlled trial. Nutr. Metab. (Lond.) 2011;8:32. doi: 10.1186/1743-7075-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.