Abstract
Degenerative cervical myelopathy (DCM) is estimated to be the most common cause of adult spinal cord impairment. Evidence that is suggestive of a genetic basis to DCM has been increasing over the last decade. A systematic search was conducted in MEDLINE, EMBASE, Cochrane, and HuGENet databases from their origin up to 14th December 2019 to evaluate the role of single genes in DCM in its onset, clinical phenotype, and response to surgical intervention. The initial search yielded 914 articles, with 39 articles being identified as eligible after screening. We distinguish between those contributing to spinal column deterioration and those contributing to spinal cord deterioration in assessing the evidence of genetic contributions to DCM. Evidence regarding a total of 28 candidate genes was identified. Of these, 22 were found to have an effect on the radiological onset of spinal column disease, while 12 genes had an effect on clinical onset of spinal cord disease. Polymorphisms of eight genes were found to have an effect on the radiological severity of DCM, while three genes had an effect on clinical severity. Polymorphisms of six genes were found to have an effect on clinical response to surgery in spinal cord disease. There are clear genetic effects on the development of spinal pathology, the central nervous system (CNS) response to bony pathology, the severity of both bony and cord pathology, and the subsequent response to surgical intervention. Work to disentangle the mechanisms by which the genes that are reviewed here exert their effects, as well as improved quality of evidence across diverse populations is required for further investigating the genetic contribution to DCM.
Keywords: genetics, single nucleotide polymorphism, degenerative cervical myelopathy, ossification posterior longitudinal ligament, severity, surgery
1. Introduction
Degenerative cervical myelopathy (DCM) is estimated to be the most common cause of spinal cord impairment in the adult population and its incidence is expected to rise as the population continues to age [1]. The term DCM is relatively new, and it was proposed to unify degenerative pathologies with a common injury mechanism (subacute, progressive spinal cord injury) and treatment (decompressive surgery) [1]. This includes both cervical spondylosis (such as degenerative disc disease or osteophyte formation) and the ossification of the posterior longitudinal ligament (OPLL) or ligamentum flavum (OLF) [1,2,3,4]. These aetiologies were often previously separately considered, as cervical spondylotic myelopathy (CSM) and OPLL.
The trajectory of DCM between patients is heterogenous and currently unpredictable and unexplained [3]. For example, mechanical compression is an imaging hallmark of the disease. However, the location and amount of compression does not correlate with the disease symptoms [5,6,7]. In fact, the clinical phenotype can range from asymptomatic to severe disability, nearly independent from the amount of compression. Furthermore, patients’ response to surgical decompression, the mainstay of treatment, is variable: it achieves excellent improvements in some patients, whereas in others these do not occur [8]. Such variation between patients has led to increasing interest in the genetic basis of this condition. One study reported a relative risk of 5.21 for the development of DCM in first-degree relatives of patients [9].
So far, the effects of genes involved in inflammation, bone, and lipid metabolism have been linked to both the pathogenesis of DCM and the response to surgical intervention [10,11]. However, these studies have failed to disentangle their relationship to spinal degeneration and myelopathy. This is important, as the fact that symptom progression and severity of spinal cord compression correlate poorly suggests that the genetic polymorphisms that contribute to spinal column degeneration may be distinct from those that influence the development of myelopathy in response to the resulting spinal cord compression.
Moreover, reviews have focused on CSM or OPLL, as opposed to DCM. Genes that influence how the spinal cord copes with mechanical stress may be identifiable in studies that investigate the severity of myelopathy and, in particular, the response to surgery.
Therefore, the objectives of this review are to provide a synthesis of the published literature on a genetic contribution to the susceptibility to develop degenerative spinal column changes that lead to DCM, the heterogeneity in severity of the clinical manifestation of DCM, and the heterogeneity in response to surgery, in order to evaluate the genes that are specifically linked to the onset and recovery of myelopathy.
2. Methods
A systematic review was conducted in accordance with the PRISMA guidelines; a PRISMA checklist is presented in the Supplementary Data [12]. A search was conducted in MEDLINE, EMBASE, Cochrane, and HuGENet databases for all relevant papers from database origin up to 14th December 2019. The full search strategy is presented in the Supplementary Data and it was developed in conjunction with the Medical Library at the University of Cambridge School of Clinical Medicine. Reference lists of key articles were systematically examined to identify further eligible articles.
Titles and abstracts were screened for relevance and, subsequently, full text papers were screened for eligibility, according to the following inclusion criteria:
Primary clinical trial
DCM is the primary condition being addressed
Focus on genetics (specific gene identified)
Human study
English language
Full text article
Animal studies, case reports, letters, editorials, reviews, technical notes, commentaries, proposals, and corrections were excluded. In addition, articles meeting the following criteria were excluded:
Paediatric studies (patients < 18 years)
Focus on acute trauma and acute spinal cord injury
Focus on thoracic or lumbar spine
Two authors independently assessed the full-texts of potentially relevant articles (DHP and BMD), with any disagreements being resolved through discussion until agreement was reached.
Data that were extracted from the eligible articles included: study design, number of cases, number of controls, participant demographics, patient disease profile, gene studied, polymorphism/haplotype studied, and effects of polymorphisms and haplotypes on DCM susceptibility/severity/response to surgery (principal summary measures: odds ratios). The risk of bias was assessed through an evaluation of study design, methods of study population selection, matching of controls to cases, and the consideration of publication source. The MINORS methodological items were used to give structure to this process [13]. The GRADE guidelines were used to rate the quality of evidence for each candidate gene, and across genes for each of the three main questions (susceptibility, severity, response) [14].
Meta-analysis using the Cochrane Review Manager 5.3 software was used for polymorphisms, where more than one study had investigated the same polymorphism and the requisite data were available.
3. Results
After removing duplicates, a total of 914 articles were screened and 39 were eligible for inclusion (Figure 1). In total, 37 articles addressed the genetics of susceptibility to developing DCM, 13 articles addressed the genetics of heterogeneity in DCM severity (either radiological or clinical severity) and six addressed the genetics of response to surgery. A total of 28 genes were identified, with key information regarding each candidate gene presented in Tables 1–3.
Figure 1.
PRISMA flow diagram of search and screening.
3.1. What are the Genetic Effects on Susceptibility to Development of DCM?
Evidence regarding the onset of DCM/OPLL was identified for 28 genes: ACE, APOE, BID, BMP2, BMP4, BMP9, COL6A1, COL9A2, COL11A2, FGF2, FGFR1, FGFR2, HIF1A, IL1B, IL15RA, IL18RAP, leptin receptor, NPPS, OPG, OPN, RUNX2, TGFB1, TGFB3, TGFBR2, TLR5, VDBP, VDR, and VKORC1. Of these 28 genes, 22 were found to be associated with the radiological onset of spinal pathology, while 12 were associated with the clinical development of DCM (i.e., spinal cord pathology). For six genes, no significant effect of polymorphisms has been found by the studies reviewed to date: FGF2, FGFR2, IL18RAP, leptin receptor, TLR5, and VDBP. Most of the genes (19, 68%) have been investigated by only a single study. Bone morphogenetic protein genes (9, 32%) and collagen genes were the most studied gene groups (8, 29%). Table 1 presents full information for each gene.
Table 1.
Susceptibility to radiological or clinical degenerative cervical myelopathy (DCM).
| Candidate Gene | Papers Investigating | Study Population Location | No. of Patients | No. of Controls | Matching of Controls | Radiological or Clinical Onset of DCM | Proposed Mechanism | Odds Ratio (Susceptibility) | p-Value (Susceptibility) |
|---|---|---|---|---|---|---|---|---|---|
| ACE | Kim et al. (2014) [15] | South Korea | 95 OPLL | 274 | Controlled for age and sex in logistic regression models | Radiological | D/D genotype | 2.20 | 0.002 |
| APOE | Setzer et al. (2008) [16] | Germany | 60 CSM | 46 | Age, sex. Controls were patients with cervical spondylosis without CSM | Clinical | ε4 allele | 3.50 | 0.008 |
| Diptiranhan et al. (2019) [17] | India | 100 CSM | 100 | Clinical | ε2 allele vs. ε3 allele | 4.4 | 0.002 | ||
| ε2 allele vs. ε4 allele | 6.69 | 0.009 | |||||||
| BID | Chon et al. (2014) [18] | Korea | 157 OPLL | 209 | Controlled for age and sex in logistic regression models | Radiological | rs8190315 (Ser10 Gly) G allele | 2.66 | 0.005 |
| rs2072392 (Asp60Asp) C allele | 2.66 | 0.005 | |||||||
| BMP2 | Wang et al. (2008) [19] | China | 57 OPLL | 135 | Age, sex | Radiological | Ser87Ser A/G allele | 0.081 | |
| Ser37Ala G allele | <0.001 | ||||||||
| Liu et al. (2010) [20] | China | 82 (48 OPLL, 12 OLF, 22 both) | 118 | Age, sex | Radiological | rs1005464 G allele | 0.435 | ||
| Yan et al. (2013) [21] | China | 420 OPLL | 506 | Age, sex | Radiological | 109T>G G allele (Ser37Ala G allele) | <0.001 | ||
| 570A>T T allele | 0.005 | ||||||||
| Kim et al. (2014) [22] | South Korea | 110 OPLL | 211 | No. Controls were family members | Radiological | Ser87Ser A/G allele | 0.411 | ||
| Ser37Ala G allele | 0.670 | ||||||||
| BMP4 | Meng et al. (2010) [23] | China | 179 OPLL | 288 | Radiological | −5826G>A A allele | 0.495 | ||
| 6007C>T T allele | 1.57 (only males) | 0.014 | |||||||
| Ren et al. (2012)a [24] | China | 450 OPLL | 550 | Age, sex, BMI, bone mineral density, exercise level, sleeping habit, smoking status, alcohol consumption. | Radiological | rs762642 T>G G allele | 0.353 | ||
| intron 2 (54422783) G>T T allele | 0.868 | ||||||||
| rs762643 C>A A allele | 0.365 | ||||||||
| rs2855530 C>G G allele | 0.661 | ||||||||
| rs2761884 C>A A allele | 0.469 | ||||||||
| intron 5 (54419501) G>A A allele | 0.684 | ||||||||
| intron 5 (54419206) C>T T allele | 0.598 | ||||||||
| intron 5 (54419150) C>T T allele | 3.48 | <0.001 | |||||||
| rs10130587 C>G G allele | 0.926 | ||||||||
| rs35107139 T>G G allele | 0.953 | ||||||||
| rs2761880 A>G G allele | 0.221 | ||||||||
| rs74486266 T>C C allele | 0.861 | ||||||||
| rs17563 C>T T allele | 2.22 | <0.001 | |||||||
| rs76335800 A>T T allele | 1.99 | <0.001 | |||||||
| 3’-UTR (54416600) A>T T allele | 0.190 | ||||||||
| rs11335370 T>- deletion | 0.608 | ||||||||
| intron 6 (54416219) C>T T allele | 0.344 | ||||||||
| rs59702220 TT>- deletion | 0.220 | ||||||||
| Haplotype TGGGCTT | 2.54 | <0.001 | |||||||
| Wang et al. (2013) [25] | China | 499 CSM | 602 | Age, sex, BMI | Clinical | −5826G>A A allele | 0.214 | ||
| 6007C>T T allele | 0.51 | <0.001 | |||||||
| BMP9 | Ren et al. (2012)b [26] | China | 450 OPLL | 550 | Age, sex, BMI, bone mineral density, exercise level, sleeping habit, smoking status, alcohol consumption. | Radiological | rs3758496 | 0.301 | |
| rs12252199 | 0.233 | ||||||||
| rs7923671 | 0.163 | ||||||||
| rs75024165 | 1.82 | <0.001 | |||||||
| rs34379100 | 1.95 | 0.003 | |||||||
| rs9421799 | 0.69 | 0.004 | |||||||
| Haplotype CTCA | 2.37 | <0.001 | |||||||
| BMPR1A | Wang et al. (2018) [27] | China | 356 OPLL | 617 | Age, sex | Radiological | −349C>T T allele | <0.001 | |
| 4A>C C allele | <0.001 | ||||||||
| 1327C>T T allele | 0.311 | ||||||||
| 1395G>C | 0.586 | ||||||||
| COL6A1 | Tanaka et al. (2003) [28] | Japan | 342 | 298 | Age | Radiological | rs7671 G>C allele | 0.020 | |
| rs2072699 G>A allele | 0.958 | ||||||||
| intron 2 (+758) C allele | 0.019 | ||||||||
| rs760437 C>T allele | 0.435 | ||||||||
| rs754507 A>C allele | 0.062 | ||||||||
| intron 4 (+20) C allele | 0.267 | ||||||||
| intron 4 (+37) G allele | 0.010 | ||||||||
| rs2839076 G>C allele | 0.043 | ||||||||
| intron 9 (+62) C allele | 0.007 | ||||||||
| rs2277813 C>G allele | 0.057 | ||||||||
| rs2277814 G>A allele | 0.205 | ||||||||
| rs1980982 T>C allele | 0.0008 | ||||||||
| intron 15 (+39) T allele | 0.008 | ||||||||
| rs760439 G>A allele | 0.048 | ||||||||
| rs2850173 C>A allele | 0.053 | ||||||||
| rs2075893 T>C allele | 0.021 | ||||||||
| rs2742071 T>C allele | 0.219 | ||||||||
| rs2850174 T>G allele | 0.238 | ||||||||
| rs2850175 A>C allele | 0.001 | ||||||||
| rs2839077 C>T allele | 0.005 | ||||||||
| rs2276254 A>C allele | 0.00009 | ||||||||
| rs2276255 A>G allele | 0.048 | ||||||||
| rs2276256 G>C allele | 0.504 | ||||||||
| Intron 32 (-29) C allele | 0.000003 | ||||||||
| rs2236485 G>A allele | 0.0002 | ||||||||
| rs2236486 A>G allele | 0.00005 | ||||||||
| rs2236487 A>G allele | 0.00006 | ||||||||
| rs2236488 C>T allele | 0.020 | ||||||||
| rs1053312 G>A allele | 0.044 | ||||||||
| rs1053315 G>A allele | 0.040 | ||||||||
| exon 35 (+205) T allele | 0.677 | ||||||||
| rs1053320 C>T allele | 0.021 | ||||||||
| Kong et al. (2007) [29] | China | 183 (90 OPLL, 61 OLF, 32 OPLL and OLF) | 155 | Sex | Radiological | Promoter (−572) T allele | 2.94 | 0.00003 | |
| intron 32 (-29) C allele | 1.89 | 0.004 | |||||||
| Liu et al. (2010) [20] | China | 82 (48 OPLL, 12 OLF, 22 both) | 118 | Age, sex | Radiological | rs9978314 T allele | 0.7618 | ||
| rs2276255 G allele | 0.7354 | ||||||||
| Kim et al. (2014) [22] | South Korea | 110 OPLL | 211 | No. Controls were family members | Radiological | Promoter (−572) T allele | 0.282 | ||
| intron 33 (+20) G allele | 0.625 | ||||||||
| COL9A2 | Wang et al. (2012) [30] | China | 172 CSM | 176 | Age, sex, BMI | Clinical | Trp2+ allele | 1.78 | 0.048 |
| Trp3+ allele | 0.087 | ||||||||
| COL11A2 | Koga et al. (1998) [31] | Japan | 124 paired siblings, 137 OPLL patients | 212 | No | Clinical | Promoter (−182) C allele | 0.0240 | |
| intron 6 (−4) T allele | 0.0004 | ||||||||
| exon 43 (+24) G allele | 0.0210 | ||||||||
| exon 46 (+18) T allele | 0.0333 | ||||||||
| Maeda et al. (2001) [32] | Japan | 195 OPLL | 187 | No | Radiological | intron 6 (−4) T allele | 1.99 | 0.0003 | |
| exon 6 (+28) G allele | 1.84 | 0.0012 | |||||||
| Horikoshi et al. (2006) [33] | Japan | 711 OPLL | 896 | Age | Clinical | rs9277933 (IVS6-4T>A) | 0.130 | ||
| rs2071025 (IVS29+37C>T) | 0.270 | ||||||||
| FGF2 | Jun & Kim (2012) [34] | South Korea | 157 OPLL | 222 | Age, sex | Radiological | rs1476217 C allele | 0.220 | |
| rs308395 G allele | 0.580 | ||||||||
| rs3747676 T allele | 0.100 | ||||||||
| FGFR1 | Jun & Kim (2012) [34] | South Korea | 157 OPLL | 222 | Age, sex | Radiological | rs13317 C allele | 2 | 0.02 |
| FGFR2 | Jun & Kim (2012) [34] | South Korea | 157 OPLL | 222 | Age, sex | Radiological | rs755793 C allele | 0.110 | |
| rs1047100 A allele | 0.580 | ||||||||
| rs3135831 T allele | 0.590 | ||||||||
| HIF1A | Wang et al. (2014) [35] | China | 230 CSM | 284 | Age, sex, BMI | Clinical | 1772C>T T allele | 0.760 | |
| 1790G>A A allele | 1.62 | <0.001 | |||||||
| IL15RA | Kim et al. (2011) [36] | South Korea | 166 OPLL | 230 | Age, sex | Radiological | rs2296139 A allele | 1.00 | |
| rs2228059 A allele | 1.52 | 0.009 | |||||||
| Guo et al. (2014) [37] | China | 235 OPLL | 250 | Age | Clinical | rs2296139 G allele | 0.849 | ||
| rs2228059 A allele | 1.63 | <0.001 | |||||||
| IL18RAP | Diptiranhan et al. (2019) [17] | India | 100 CSM | 100 | Clinical | rs1420106 | >0.05 | ||
| rs917997 | >0.05 | ||||||||
| Leptin receptor | Tahara et al. (2005) [38] | Japan | 156 OPLL | 93 | Age | Radiological | A861G | 0.669 | |
| NPPS | Nakamura et al. (1999) [39] | Japan | 323 OPLL | 332 | Age | Clinical | IVS20–11delT | 0.0029 | |
| Koshizuka et al. (2002) [40] | Japan | 180 OPLL | 265 | Age, sex | Clinical | IVS15-14T>C | 3.01 | 0.022 | |
| Tahara et al. (2005) [38] | Japan | 156 OPLL | 93 | Age | Radiological | IVS20–11delT | 0.512 | ||
| Horikoshi et al. (2006) [33] | Japan | 711 OPLL | 896 | Age | Clinical | IVS15-14T>C | 0.320 | ||
| He et al. (2013) [41] | China | 95 OPLL | 90 | Age, sex | Radiological | A533C | 0.430 | ||
| C973T | <0.001 | ||||||||
| IVS15-14T>C | 0.026 | ||||||||
| IVS20–11delT | 0.093 | ||||||||
| OPG | Yu et al. (2018) [42] | China | 494 CSM | 515 | Clinical | 950T>C C allele | <0.01 | ||
| 1181G>C C allele | >0.05 | ||||||||
| 163A>G G allele | >0.05 | ||||||||
| OPN | Wu et al. (2014) [43] | China | 187 CSM | 233 | Age, sex, BMI | Clinical | −66T>G G allele | 1.55 | 0.002 |
| −156G/GG GG genotype | 0.651 | ||||||||
| −443C/T C allele | 0.580 | ||||||||
| RUNX2 | Liu et al. (2010) [20] | China | 82 (48 OPLL, 12 OLF, 22 both) | 118 | Age, sex | Radiological | rs967588C>T T allele | 0.1939 | |
| rs16873379 T>C C allele | 0.169 | ||||||||
| rs1406846 T>A A allele | 0.6646 | ||||||||
| rs3749863 A>C C allele | 0.8637 | ||||||||
| rs6908650 G>A A allele | 0.6362 | ||||||||
| rs1321075 C>A A allele | 0.5255 | ||||||||
| rs2677108 T>C C allele | 0.6657 | ||||||||
| rs16873437 G>T T allele | 0.6387 | ||||||||
| rs7771889 C>G G allele | 0.7854 | ||||||||
| rs12333172 C>T T allele | 0.8128 | ||||||||
| rs9296459 A>G G allele | 0.2542 | ||||||||
| Chang et al. (2017) [44] | China | 80 OPLL | 80 | Age, sex, BMI, smoking history, alcohol intake | Clinical | rs967588C>T T allele | 0.47 | 0.033 | |
| rs16873379 T>C C allele | 0.48 | 0.033 | |||||||
| rs1406846 T>A A allele | 5.67 | <0.001 | |||||||
| rs3749863 A>C C allele | 0.171 | ||||||||
| rs6908650 G>A A allele | 0.959 | ||||||||
| rs1321075 C>A A allele | 0.050 | ||||||||
| rs2677108 T>C C allele | 0.295 | ||||||||
| TGFB1 | Kamiya et al. (2001) [45] | Japan | 46 OPLL | 273 | Age, BMI | Radiological | 869T>C CC genotype | 4.5 | 0.0004 |
| Horikoshi et al. (2006) [33] | Japan | 711 OPLL | 896 | Age | Clinical | IVS2+114G>A A allele | 0.330 | ||
| Han et al. (2013) [46] | South Korea | 98 OPLL | 200 | Age, sex | Radiological | 869T>C CC genotype | 0.656 | ||
| −509C>T TT genotype | 0.931 | ||||||||
| TGFB3 | Horikoshi et al. (2006) [33] | Japan | 711 OPLL | 896 | Age | Clinical | IVS1-1284G>C CC genotype | 1.46 | 0.044 |
| TGFBR2 | Jekarl et al. (2013) [47] | South Korea | 21 OPLL | 42 | None mentioned. | Radiological | 445T>A A allele | 2.81 | 0.007 |
| 571G>A A allele | 8.73 | 0.024 | |||||||
| 1167C>T T allele | 0.888 | ||||||||
| TLR5 | Chung et al. (2011) [48] | South Korea | 166 OPLL | 231 | Age, sex | Radiological | rs2072493 G allele | 0.457 | |
| rs57441714 C allele | 0.457 | ||||||||
| rs5744168 T allele | 0.543 | ||||||||
| VDBP | Song et al. (2018) [49] | China | 318 CSM | 282 | Age, sex, BMI, smoking | Clinical | Thr420Lys | 0.973 | 0.834 |
| VDR | Kobashi et al. (2008) [50] | Japan | 63 OPLL | 126 | Age, sex | Radiological | FokI FF genotype | 2.33 | 0.0073 |
| Wang et al. (2010) [51] | China | 154 CSM | 156 | Age, sex, BMI, desk work time, smoking | Clinical | FokI T allele | >0.05 | ||
| BsmI A allele | >0.05 | ||||||||
| ApaI A allele | 2.88 | <0.001 | |||||||
| TaqI C allele | 4.67 | <0.001 | |||||||
| Liu et al. (2010) [20] | China | 82 (48 OPLL, 12 OLF, 22 both) | 118 | Age, sex | Radiological | rs11168287 G allele | 0.5933 | ||
| rs11574079 A allele | 2.68 | 0.0714 | |||||||
| rs2189480 C allele | 0.4197 | ||||||||
| rs3847987 C allele | 0.6687 | ||||||||
| rs12721370 T allele | 0.4000 | ||||||||
| Song et al. (2018) [49] | China | 318 CSM | 282 | Age, sex, BMI, smoking | Clinical | FokI FF genotype | 1.461 | 0.001 | |
| VKORC1 | Chin et al. (2013) [52] | South Korea | 98 OPLL | 200 | Age, sex, hypertension, diabetes mellitus | Radiological | −1639G>A GA genotype | 5.22 (female patients only) | 0.004 (Non-significant in male/mixed) |
3.1.1. Spinal Pathology
The majority of studies investigating the genetics of susceptibility to DCM used the radiological definition of cases. Therefore, these studies assess the development of bony spinal pathology (an initial stage in overall DCM development).
Kim et al. (2014) investigated the ACE gene, finding the deletion/deletion genotype of the intron 16 polymorphism (rs4646994) to be associated with an increased risk of developing radiological OPLL (AOR 2.20, p = 0.002) [15]. Similarly, two SNPs of the BID gene (rs8190315, rs2072392) were associated with the development of OPLL (OR 2.66, p = 0.005 for both) [18].
Four studies have investigated the role of variants in BMP2. Wang et al. (2008) found no significant effect of the Ser87Ser SNP, but found the Ser37Ala SNP was associated with an increased risk of OPLL development (p < 0.001) [19]. Interestingly, however, patients with the GG genotype of Ser87Ser had significantly greater number of ossified vertebrae, which suggested the A allele restricts ectopic ossification in OPLL. Meanwhile, the Ser37Ala SNP had no significant effect on the number of ossified vertebrae.
Yan et al. (2013) also found the Ser37Ala SNP to be associated with increased risk (p < 0.001) [21], although a more recent study that compared OPLL patients to their family members found no effect of either the Ser87Ser or Ser37Ala SNPs on risk of OPLL (p = 0.411, p = 0.670, respectively) [22]. Additionally, the 570A>T SNP in the BMP2 gene was not found to be significantly associated with risk of OPLL [21]. Liu et al. (2010) used a patient cohort that included OPLL, OLF, and OPLL + OLF patients, but found no effect of the rs1005464 intronic SNP on the susceptibility of radiological DCM development [20].
In the BMP4 gene, the 6007C>T SNP was found to be associated with an increased risk of developing radiological OPLL in male patients (OR 1.57, p = 0.014), although the effect is lost when males and females are considered together (p = 0.493) [23]. In the same SNP, the CT and TT genotypes were associated with a greater number of ossified vertebrae (p = 0.043) [23], as was a haplotype (TGGGCTT) containing seven SNPs (p = 0.002). Ren et al. (2012a) identified three SNPs that significantly increase the risk of OPLL: rs54419150 (OR 3.48, p < 0.001), rs17563 (OR 2.22, p < 0.001), and rs76335800 (OR 1.99, p < 0.001). Linkage disequilibrium studies also identified the haplotype block TGGGCTT containing these three SNPs to be significantly associated with the occurrence of OPLL (OR 2.54, p < 0.001) [24].
In the BMP9 gene, two SNPs and a haplotype containing four SNPs were found to be associated with an increased risk of OPLL development: rs75024165 (OR 1.82, p < 0.001), rs34379100 (OR 1.95, p = 0.003), and haplotype CTCA (OR 2.37, p < 0.001). The haplotype was also associated with development of a greater number of ossified vertebrae (p = 0.001). A further SNP (rs9421799) was found to be protective (OR 0.69, p = 0.004), while three SNPs had no significant effect [26].
Wang et al. (2018) investigated the BMPR1A gene, finding two SNPs (-349C>T, 4A>C) that were associated with an increased risk of OPLL development (p < 0.001 both), and two (1327C>T, 1395G>C) with no significant effect [27]. Furthermore, patients with the C allele of the 4A>C SNP were more likely to have a greater number of ossified vertebrae on lateral cervical radiograph (p < 0.001).
The COL6A1 gene has been the subject of four studies. Tanaka et al. (2003) investigated 32 SNPs in the COL6A1 gene, of which 21 were significantly associated with OPLL (see Table 1) [28]. Further work by Kong et al. (2007) was consistent with these findings, with intron 32 (-29) C allele conferring a greater risk of OPLL (OR 1.89, p = 0.004) [29]. However, Liu et al. (2010) reported no significant effect of the rs2276255 SNP on the risk of OPLL or OLF development [20], in contrast to Tanaka et al.’s finding of a weak significant effect (p = 0.048). Further contradiction in the COL6A1 gene is seen in Kong et al.’s (2007) finding that the promoter (−572) SNP T allele was associated with a 2.94 times greater risk of OPLL (p = 0.0003), while Kim et al. (2014) found no significant effect (p = 0.282) [22]. Liu et al. (2010) found no effect of one additional SNP (rs9978314) on the risk of OPLL or OLF development [20].
In the COL11A2 gene, the intron 6 (−4) polymorphism was associated with a greater risk of OPLL development in two studies (OR 1.99, p = 0.0003; p = 0.0004) [31,32]. Similarly, the exon 6 (+28) polymorphism was associated with an odds ratio of 1.84 of developing OPLL (p = 0.0012) [32].
Jun & Kim (2012) investigated the FGF2, FGFR1, and FGFR2 genes in 157 OPLL patients and 222 age- and sex-matched controls [34]. Three SNPs of the FGF2 gene showed no significant effect on the likelihood of OPLL development, as did three SNPs of the FGFR2 gene. However, the rs13317 SNP in the FGFR1 gene was associated with an increased risk (OR 2.0, p = 0.02).
Kim et al. (2011) investigated two SNPs of the IL15RA (IL15Rα) gene [36]. The A allele of rs2228059 conferred a 1.52 times risk of radiological OPLL (p = 0.009), while the rs2296139 SNP had no significant effect.
The A861G polymorphism of the leptin receptor gene had no effect on the likelihood of OPLL development in a study of 156 OPLL patients and 93 age-matched controls [38].
In the NPPS gene, two studies both found no significant effect of the IVS20-11delT SNP on the likelihood of radiological OPLL (p = 0.512, p = 0.093) [38,41]. However, patients that were homozygous for the T deletion of the IVS20-11delT polymorphism had fewer ossified vertebrae and less thick ossification of their cervical vertebrae (p < 0.001 for both) [41].
The IVS15-14T>C and C973T SNPs were associated with an increased risk of radiological OPLL (p = 0.026, p < 0.001) [41]. Furthermore, patients with the T allele of the IVS15-14T>C SNP also had both a greater number of ossified vertebrae and greater thickness of ossification of their vertebrae (p < 0.001, p = 0.017, respectively). For the C973T SNP, the T allele was associated with increased thickness of ossified vertebrae (p = 0.007), but it had no effect on number of ossified vertebrae (p = 0.248). There was no effect of the A533C polymorphism on the likelihood of radiological OPLL development, or number of ossified vertebrae, or thickness of ossified vertebrae (p = 0.430, p = 0.363, p = 0.947) [41].
In a case-control study of OPLL, OLF, and OPLL+OLF patients, 11 SNPs of the RUNX2 gene had no significant association with radiological development of OPLL/OLF [20]. However, patients with the C allele of the rs16873379 SNP had a greater number of ossified vertebrae (p = 0.001), as did patients with the A allele of the rs1406846 SNP (p = 0.020), and patients with the C allele of the rs2677108 SNP (p = 0.044).
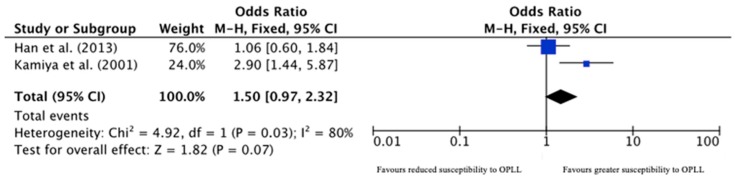
In the TGFB1 (TGFβ1) gene, the CC genotype of the 869T>C polymorphism was found to be associated with an increased risk of radiological OPLL development in one study (OR 4.5, p = 0.0004) [45], but it had no such association in a recent study that involved almost double the number of cases (p = 0.656) [46]. On meta-analysis, there was no significant effect of the 869T>C polymorphism on the susceptibility to OPLL development (OR 1.50, 95% CI 0.97–2.32, p = 0.07; Figure 2). The 509C>T was found to have no association with radiological OPLL development [46].
Figure 2.
Forest plot for TGFB1 869T>C polymorphism.
Jekarl et al. (2013) investigated three SNPs of the TGFBR2 (TGFβR2) gene, finding that two were associated with increased likelihood of OPLL development. The 445T>A polymorphism conferred a 2.81 times increased risk (p = 0.007), while the 571G>A polymorphism was associated with 8.73 times risk (p = 0.024) [47].
The TLR5 gene has been investigated by one study, which found no association of three SNPs with the likelihood of OPLL development [48].
In the VDR gene, Kobashi et al. (2008) found the FokI polymorphism to be associated with 2.33 times increased risk of OPLL development (p = 0.0073) [50]. Similarly, Liu et al. (2010) found an association between the rs11574079 polymorphism and OPLL/OLF risk (OR 2.68, p = 0.0714) [20].
The VKORC1 gene was investigated in 98 OPLL patients and 200 control subjects, with the −1639G> A polymorphism having a significant effect in female patients (OR 5.22, p = 0.004), but not when both sexes were considered together (p > 0.05) [52].
In the NPPS gene, He et al. (2013) examined the effect of four SNPs on the progression of OPLL ossification on lateral radiograph. The AA genotype of the A533C SNP and the homozygous T deletion genotype of the IVS20-11delT SNP were both associated with better responses to surgical intervention (OR 3.11, p = 0.029; OR 3.35, p = 0.007). The other two polymorphisms were not associated with any difference in response to surgery (good response defined as <2 mm increase in ossified mass of the posterior longitudinal ligament) [41].
3.1.2. Spinal Cord Pathology
Multiple studies used clinical signs and symptoms of DCM alongside positive radiological findings. Such combination interrogates the development of cord pathology, rather than simply the development of spinal pathology.
In the APOE gene, the ε4 allele was found to be associated with an increased risk of myelopathy in a case-control study, where the controls had cervical spondylosis without myelopathy (OR 3.50, p = 0.008) [16]. However, a study in an Indian population found the ε2 allele to be associated with increased risk of myelopathy when compared to both the ε3 and ε4 alleles (OR 4.4, p = 0.002; OR 6.69, p = 0.009) [17].
In the BMP4 gene, Wang et al. (2013) found the 6007C>T SNP to be protective for the development of clinical signs and symptoms of CSM (OR 0.51, p < 0.001) [25]. This is in contradiction to the evidence described above, in which this SNP was shown to be associated with an increased risk of radiological OPLL development [23,24].
The Trp2(+) allele of the COL9A2 gene was associated with an increased risk of CSM development (OR 1.78, p = 0.048), a risk that was worsened by heavy smoking (OR 5.56, p < 0.001), while the Trp3 allele had no significant effect [30].
Koga et al. (1998) identified three polymorphisms of the COL11A2 gene associated with DCM development: promoter (−182), exon 43 (+24) and exon 46 (+18) [31]. Horikoshi and colleagues investigated two additional SNPs of the COL11A2 gene, but found no significant effect for either [33].
In the HIF1A (HIF-1α) gene, Wang et al. (2014) found no effect of the 1772C>T SNP, while the 1790G>A polymorphism was associated with an increased risk of CSM development (OR 1.62, p < 0.001) [35].
In the IL15RA gene, Guo et al. (2014) found a significant effect of the A allele of the rs2228059 SNP on DCM development (OR 1.63, p < 0.001) [37]. However, there was no effect of the rs2296139 SNP on the likelihood of developing symptomatic DCM. This is in commonality with the above findings of Kim et al. (2011) who showed rs2296139 had no effect on likelihood of developing radiological OPLL while the rs2228059 SNP did [36].
In the IL18RAP gene, Diptiranhan et al. (2019) found no significant effect of either the rs1420106 or rs917997 SNPs on the development of myelopathy (p > 0.05) [17].
Three studies have looked at the NPPS gene in relation to clinical onset of spinal cprd disease [33,39,40]. Nakamura et al. (1999) found the IVS20-11delT polymorphism to be associated with an increased risk of development of DCM (p = 0.0029) [39]. There is conflicting evidence of the effect of the IVS15-14T>C polymorphism: one study found it to be associated with a 3.01 times risk of myelopathy development (p = 0.022) [40], while another found no significant effect (p = 0.320) [33].
Yu et al. (2018) found no significant effect of the 1181G>C and 163A>G polymorphisms in the osteoprotegerin (OPG) gene, but found the C allele of the 950T>C SNP to be associated with a greater risk of myelopathy (p < 0.01) [42].
Wu et al. (2014) studied three SNPs of the osteopontin (OPN) gene [43]. Two showed no significant effect, but the G allele of the -66T>G SNP was associated with an odds ratio of 1.55 of clinical onset of DCM (p = 0.002).
In the RUNX2 gene, Chang et al. (2017) found the SNPs rs967588 and rs16873379 to be protective for DCM development (OR 0.47, p = 0.033; OR 0.48, p = 0.033) [44]. The rs1406846 SNP was, on the other hand, strongly associated with DCM development (OR 5.67, p < 0.001). Four further SNPs had no significant effect.
Horikoshi et al. (2006) studied the TGFB1 (TGFβ1) and TGFB3 (TGFβ3) genes [33]. There was no significant effect of the IVS2+114G>A SNP of TGFB1, while the CC genotype IVS1-1284G>C SNP of TGFB3 was associated with an increased risk of DCM development (OR 1.46, p = 0.044).
Song et al. (2018) found no significant effect of the Thr20Lys polymorphism of the VDBP gene (OR 0.973, p = 0.834) [49].
In the VDR gene, Wang et al. (2010) found no significant effect of FokI polymorphism on CSM risk [51]. The BsmI polymorphism also had no effect on CSM risk, but the ApaI and TaqI polymorphisms conferred a 2.88 times and 4.67 times increased CSM risk (both p < 0.001). In opposition to Wang et al.’s findings, Song et al. (2018) found the ff genotype of the FokI polymorphism to be associated with a 1.985 times greater risk of myelopathy (p = 0.003) [49].
3.2. What Are the Genetic Effects on Clinical Severity of DCM?
Seven studies investigated the genetic effects on the clinical severity of DCM, while 11 investigated radiological severity (four studies investigated both). Polymorphisms of 8 genes affected radiological severity, while three genes affected clinical severity. Table 2 presents the full results.
Table 2.
Radiological or clinical severity of DCM.
| Candidate Gene | Papers Investigating | Study Population Location | No of Patients | Method of Severity Assessment | Proposed Mechanism | Outcome |
|---|---|---|---|---|---|---|
| BDNF | Abode-Iyamah et al. (2016) [53] | USA | 10 CSM | Short Form 36 Survey | Val66Met mutation | Met allele subjects had worse scores for physical functioning and social functioning (p < 0.05). Met allele subjects had worse ‘physical health summary’ scores (p = 0.02). |
| BMP2 | Wang et al. (2008) [19] | China | 57 OPLL | Number of ossified vertebrae on lateral cervical radiograph (1–7) | Ser87Ser GG genotype | Patients with GG genotype had significantly greater number of ossified vertebrae (p < 0.001) |
| Ser37Ala GG genotype | No significant difference in number of ossified vertebrae (p = 0.113) | |||||
| BMP4 | Meng et al. (2010) [23] | China | 179 OPLL | Number of ossified vertebrae on lateral cervical radiograph/CT/MRI (1–7) | −5826G>A A allele | No significant difference in number of ossified vertebrae (p = 0.324) |
| 6007C>T T allele | Patients with T allele had significantly greater number of ossified vertebrae (p = 0.043) | |||||
| Ren et al. (2012)a [24] | China | 450 OPLL | Number of ossified vertebrae on lateral cervical radiograph/CT (1–7) | Haplotype TGGGCTT | Patients with the TGGGCTT haplotype had significantly greater number of ossified vertebrae (p = 0.002) | |
| BMP9 | Ren et al. (2012)b [26] | China | 450 OPLL | Number of ossified vertebrae on lateral cervical radiograph/CT (1–7) | Haplotype CTCA | Patients with the CTCA haplotype had significantly greater number of ossified vertebrae (p = 0.001) |
| BMPR1A | Wang et al. (2018) [27] | China | 356 OPLL | Number of ossified vertebrae on lateral cervical radiograph (1–7) | 4A>C C allele | Patients with C allele had significantly greater number of ossified vertebrae (p < 0.001) |
| HIF1A | Wang et al. (2014) [35] | China | 230 CSM | mJOA score | 1772C>T T allele | No significant difference in mJOA score (p > 0.05) |
| 1790G>A A allele | Patients with A allele had significantly worse mJOA scores (p < 0.001) | |||||
| NPPS | He et al. (2013) [41] | China | 95 OPLL | Number of ossified vertebrae on lateral cervical radiograph (1–7) | A533C | No significant difference in number of ossified vertebrae (p = 0.363) |
| C973T | No significant difference in number of ossified vertebrae (p = 0.248) | |||||
| IVS15-14T>C | Patients with T allele had significantly greater number of ossified vertebrae (p < 0.001) | |||||
| IVS20–11delT | Patients homozygous for the T deletion had significantly fewer ossified vertebrae (p < 0.001) | |||||
| Ossified thickness of cervical vertebrae on lateral radiograph | A533C | No significant difference in ossified thickness of cervical vertebrae (p = 0.947) | ||||
| C973T | Patients with T allele had significantly thicker ossification of cervical vertebrae (p = 0.007) | |||||
| IVS15-14T>C | Patients with T alelle had significantly thicker ossification of cervical vertebrae (p = 0.017) | |||||
| IVS20–11delT | Patients homozygous for the T deletion had significantly less thick ossification of cervical vertebrae (p < 0.001) | |||||
| OPG | Yu et al. (2018) [42] | China | 494 CSM | mJOA score and number of ossified vertebrae | 950T>C | TT genotype associated with higher mJOA scores and fewer ossified cervical vertebrae (p < 0.05). |
| OPN | Wu et al. (2014) [43] | China | 187 CSM | mJOA score | −66T>G G allele | No significant difference in mJOA score (p > 0.05) |
| −156G/GG GG genotype | No significant difference in mJOA score (p > 0.05) | |||||
| −443C/T C allele | No significant difference in mJOA score (p > 0.05) | |||||
| RUNX2 | Chang et al. (2017) [44] | China | 80 OPLL | Number of ossified vertebrae on CT/MRI (1–7) | rs967588C>T T allele | No significant difference in number of ossified vertebrae (p = 0.784) |
| rs16873379 T>C C allele | Patients with C allele had significantly greater number of ossified vertebrae (p = 0.001) | |||||
| rs3749863 A>C C allele | No significant difference in number of ossified vertebrae (p = 0.129) | |||||
| rs6908650 G>A A allele | No significant difference in number of ossified vertebrae (p = 0.813) | |||||
| rs1321075 C>A A allele | No significant difference in number of ossified vertebrae (p = 0.610) | |||||
| rs1406846 T>A A allele | Patients with A allele had significantly greater number of ossified vertebrae (p = 0.020) | |||||
| rs2677108 T>C C allele | Patients with C allele had significantly greater number of ossified vertebrae (p = 0.044) | |||||
| VDBP | Song et al. (2018) [49] | China | 318 CSM | mJOA score | Thr420Lys | No significant difference in mJOA score (p = 0.546) |
| Number of ossified vertebrae | Thr420Lys | No significant difference in number of ossified vertebrae (p = 0.117) | ||||
| VDR | Wang et al. (2010) [51] | China | 154 CSM | Number of segmental lesions on MRI | FokI T allele | No significant difference in mean number of segmental lesions (p > 0.05) |
| BsmI A allele | No significant difference in mean number of segmental lesions (p > 0.05) | |||||
| ApaI A allele | No significant difference in mean number of segmental lesions (p > 0.05) | |||||
| TaqI C allele | No significant difference in mean number of segmental lesions (p > 0.05) | |||||
| mJOA score | FokI T allele | No significant difference in mJOA score (p > 0.05) | ||||
| BsmI A allele | No significant difference in mJOA score (p > 0.05) | |||||
| ApaI A allele | No significant difference in mJOA score (p > 0.05) | |||||
| TaqI C allele | No significant difference in mJOA score (p > 0.05) | |||||
| Song et al. (2018) [49] | China | 318 CSM | mJOA score | FokI ff genotype | No significant difference in mJOA score (p = 0.358) | |
| Number of ossified vertebrae | FokI ff genotype | No significant difference in number of ossified vertebrae (p = 0.575) |
CSM patients with the Val66Met polymorphism of the BDNF gene had more severe disease, as assessed by functional survey: worse SF-36 scores for physical functioning and physical health summary than their counterparts without the polymorphism (p < 0.05) [53].
Wang et al. (2014) studied the effect of two polymorphisms of the HIF1A gene on CSM: 1772C>T and 1790G>A [35]. While the former conferred no significant difference in mJOA score, in the latter patients with the A allele had significantly worse mJOA scores than their G allele counterparts (p < 0.001).
Yu et al. (2018) found the TT genotype of the 950T>C polymorphism in the OPG gene to be associated with higher mJOA scores and fewer ossified vertebrae (p < 0.05); the TT genotype appears to be protective [42].
Wu et al. (2014) investigated four polymorphisms of the OPN gene in 187 CSM patients, finding no significant difference of all four polymorphisms on the mJOA score [43].
There was no effect of the Thr420Lys polymorphism of the VDBP gene on mJOA score or the number of ossified segments in 318 CSM patients [49]. Similarly, four polymorphisms of the VDR gene (FokI, BsmI, ApaI, TaqI) were found to have no significant effect on mJOA score in two studies [49,51].
3.3. What Are the Genetic Effects on Response to Surgery in DCM?
The polymorphisms of five genes were associated with clinical response to surgery in DCM: APOE, BMP4, HIF1A, OPN, and RUNX2. The NPPS gene was studied for radiological response to surgery. Table 3 presents the results.
Table 3.
Response to surgery in DCM.
| Candidate Gene | Papers Investigating | Study Population Location | Number of Patients | Surgery Type | Mean Follow-Up | Method of Assessment of Response to Surgery | Improvement Defined As | Proposed Mechanism | Odds Ratio of No Improvement | Odds Ratio of Improvement | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| APOE | Setzer et al. (2009) [54] | Germany | 60 CSM | ACDF | 18.8 months | mJOA score | mJOA score +1 | ε4 allele | 3.3 (8.6 in multivariate model) | - | 0.002 (0.004 multivariate model) |
| BMP4 | Wang et al. (2013) [25] | China | 499 CSM | Anterior cervical corpectomy and fusion | 12 months | mJOA score | >50% improvement in mJOA score | −5826G>A A allele | - | - | 0.053 |
| 6007C>T T allele | - | 1.53 | 0.002 | ||||||||
| HIF1A | Wang et al. (2014) [35] | China | 230 CSM | Anterior cervical corpectomy and fusion | 24 months | mJOA score | >50% improvement in mJOA score | 1790G>A A allele | - | 1.55 | 0.024 |
| NPPS | He et al. (2013) [41] | China | 95 OPLL | 3.1 years | Progression of OPLL ossification on lateral radiograph | <2 mm increase in ossified mass of PLL | A533C AA genotype | - | 3.11 | 0.029 | |
| C973T | - | - | 0.935 | ||||||||
| IVS15-14T>C | - | - | 0.836 | ||||||||
| IVS20–11delT homozygous T deletion | - | 3.35 | 0.007 | ||||||||
| OPN | Wu et al. (2014) [43] | China | 187 CSM | Anterior cervical corpectomy and fusion | 24 months | mJOA score | >50% improvement in mJOA score | −66T>G GG genotype | 3.62 | - | 0.007 |
| RUNX2 | Chang et al. (2017) [44] | China | 80 OPLL | Laminoplasty | 12 months | mJOA score | % improvement in mJOA score | rs967588C>T T allele | - | - | >0.05 |
| rs16873379 T>C C allele | - | - | <0.05 | ||||||||
| rs3749863 A>C C allele | - | - | >0.05 | ||||||||
| rs6908650 G>A A allele | - | - | <0.05 | ||||||||
| rs1321075 C>A A allele | - | - | >0.05 | ||||||||
| rs1406846 T>A A allele | - | - | <0.05 | ||||||||
| rs2677108 T>C C allele | - | - | <0.05 |
In the APOE gene, the ε4 allele was associated with an increased risk of poor response to ACDF surgery. In a multivariate model, it was associated with an 8.6 times risk of worsening or no change in mJOA score (p = 0.004) [54].
The 6007C>T polymorphism of the BMP4 gene was associated with greater likelihood of post-surgical improvement of mJOA score (OR 1.53, p = 0.002), but the -5826G>A polymorphism had no significant effect (p = 0.053) [25].
In the HIF1A gene, the 1790G>A polymorphism was also associated with a greater likelihood of post-surgical improvement of the mJOA score (OR 1.55, p = 0.024) [35].
In the OPN gene, the GG genotype of the −66T>G SNP was found to be associated with worse response to surgical intervention, as assessed by mJOA score (OR 3.62, p = 0.007) [43]. Good surgical response was defined as >50% improvement in mJOA score.
Seven polymorphisms of the RUNX2 gene were investigated for their effect on pre- vs. post-surgical mJOA score. The patients with the CC genotype of the rs16873379 SNP improved less (52.4%) than patients with TT genotype (61.7%), an effect that is mirrored by patients with the AA genotype of the rs1406846 SNP and patients with the CC genotype of the rs2677108 SNP. Patients with the AA genotype of the rs6908650 SNP improved more (66.8%) than their counterparts with the GG genotype (57.4%). The three other polymorphisms had no significant effect on mJOA score improvement [44].
In the NPPS gene, the AA genotype of the A533C polymorphism was associated with a 3.11 times greater likelihood of radiological improvement after surgical intervention. Similarly the IVS20-11delT homozygous T deletion was associated with a 3.35 greater likelihood of improvement. For both polymorphisms, improvement was defined as an increase of <2 mm in the ossified mass of the posterior longitudinal ligament over a mean follow-up length of 3.1 years [41].
4. Discussion
The aim of this study was to critically appraise the current evidence on the genetic contribution to DCM, with specific focus on distinguishing spinal column disease from spinal cord disease. Studies were identified evaluating the susceptibility, severity, and responsiveness to surgery in DCM. Studies on spinal column disease focused on the radiological outcomes of OPLL. Evidence was identified for a number of genes, including many in the TGFβ superfamily and many known to be associated with bone development.
By further focusing on studies evaluating relationships with clinical function, versus radiological measures, a shortlist of genes that were related to spinal column disease or ‘myelopathy’ and not ‘spondylosis’ was identified: specifically, 12 genes that were associated with susceptibility, three genes with clinical severity, and five genes with response to surgical intervention. Table 4 presents a summary of the evidence for genetic effects on ‘myelopathy’, including GRADE rating for each gene. Across the three focuses of this review (susceptibility, severity, response to surgery), the GRADE rating of quality of evidence is baseline low, as all studies are observational. For all three, the quality of evidence is upgraded due to the large effects across genes, but downgraded due to inconsistency between studies.
Table 4.
Summary of candidate genes affecting myelopathy (i.e., clinical onset/severity/response to surgery rather than radiological). Colour coded for evidence level (green: unconflicted evidence, amber: conflicting evidence, red: no evidence or not yet investigated). GRADE rating of quality of evidence given for each candidate gene—baseline quality low (all observational studies); gene-specific upgrade/downgrade comments in parentheses.
| Candidate Gene | Papers Investigating | Susceptibility to Myelopathy | Severity of Myelopathy | Post-Operative Response | GRADE Rating |
|---|---|---|---|---|---|
| APOE | Setzer et al. (2008) [16] Setzer et al. (2009) [54] |
ε4 allele: OR 3.50, p = 0.008 | ε4 allele: OR of no improvement 3.3 (8.6 in multivariate model), p = 0.002 (p = 0.004) | Low (small sample size, inconsistency across ethnicities) |
|
| Diptiranhan et al. (2019) | ε2 allele: OR 6.69, p = 0.009 | ||||
| BDNF | Abode-Iyamah et al. (2016) [53] | Val66Met: Met allele subjects had worse scores for physical functioning (p < 0.05), social functioning (p < 0.05 and ‘physical health summary’ (p = 0.02) on SF-36 survey. | Low (single study, very small sample size) |
||
| BMP4 | Wang et al. (2013) [25] | 6007C>T T allele: OR 0.51, p < 0.001 | 6007C>T T allele: OR of improvement 1.53, p = 0.002 | Low (inconsistency across studies, inconsistency between CSM and OPLL studies) |
|
| COL9A2 | Wang et al. (2012) [30] | Trp2+ allele: OR 1.78, p = 0.048 | Low (single study, small sample size) |
||
| COL11A2 | Koga et al. (1998) [31] | Promoter (−182) C allele (p = 0.0240); Intron 6 (−4) T allele (p = 0.0004); Exon 43 (+24) G allele (p = 0.0210); Exon 46 (+18) T allele (p = 0.0333) |
Low | ||
| HIF1A | Wang et al. (2014) [35] | 1790G>A A allele: OR 1.62, p < 0.001 | 1790G>A A allele associated with worse mJOA scores (p < 0.001) | 1790G>A A allele: OR of improvement 1.55, p = 0.024 | Low (single study) |
| IL15RA | Guo et al. (2014) [37] | rs2228059 A allele: OR 1.63, p < 0.001 | Low | ||
| NPPS | Nakamura et al. (1999) [39] | IVS20-11delT: p = 0.0029 | Low (inconsistency across studies) |
||
| Koshizuka et al. (2002) [40] | IVS15-14T>C: OR 3.01, p = 0.022 NB. Horikoshi et al. (2006) find p = 0.320. |
||||
| OPG | Yu et al. (2018) [42] | 950T>C C allele: p < 0.01 | 950T>C TT genotype associated with higher mJOA scores and fewer ossified vertebrae (p < 0.05) | Low (single study) |
|
| OPN | Wu et al. (2014) [43] | −66T>G G allele: OR 1.55, p = 0.002 | No significant difference in mJOA score (p > 0.05). | -66T>G GG genotype: OR of no improvement 3.62, p = 0.007 | Low (single study) |
| RUNX2 | Chang et al. (2017) [44] | rs967588C>T T allele: OR 0.47, p = 0.033; rs16873379T>C C allele: OR 0.48, p = 0.033; rs1406846T>A A allele: OR 5.67, p < 0.001 |
rs16873379T>C C allele: p < 0.05; rs6908650G>A A allele: p < 0.05; rs1406846T>A A allele: p < 0.05; rs2677108T>C C allele: p < 0.05 |
Low | |
| TGFB3 | Horikoshi et al. (2006) [33] | IVS1-1284G>C CC genotype: OR 1.46, p = 0.044 | Low (single study) |
||
| VDR | Wang et al. (2010) [51] |
ApaI A allele: OR 2.88, p < 0.001; TaqI C allele: OR 4.67, p < 0.001 |
No significant difference in mJOA score (p > 0.05). | Low (inconsistency across studies) |
|
| Song et al. (2018) [49] | FokI ff genotype: OR 1.985, p = 0.003 | No significant difference in mJOA score or number of ossified vertebrae (p > 0.05) |
4.1. Spinal Column Disease: Focus on OPLL
The greatest focus of research to date has been on the bone morphogenetic proteins, a group of multifunctional growth factors that fall within the TGFβ superfamily and are involved in cartilage development and the induction of bone formation [55]. Four genes within this family of growth factors have been associated with both altered susceptibilities to bony spinal pathology and altered susceptibility to the development of myelopathy: BMP2, BMP4, BMP9, and BMPR1A. The 4A>C SNP in the BMPR1A gene is associated with a significantly greater likelihood of radiological OPLL and a significantly greater number of ossified vertebrae [27]. Similarly, the CTCA haplotype of the BMP9 gene is associated with a significantly increased risk of developing OPLL (OR 2.37), as well as a greater number of ossified vertebrae [26]. In the BMP4 gene, a haplotype of 7 SNPs is associated with both greater susceptibilities to OPLL and worse disease [24]. Moreover, the 6007C>T SNP in the BMP4 gene is associated with not only greater likelihood of developing bony pathology and greater severity of radiological disease, but also a greater likelihood of post-operative improvement of the mJOA score [23,25].
The dual role of 6007C>T SNP in the BMP4 gene merits further discussion. The T allele of the polymorphism was found to be protective for spinal cord disease [25] (AOR 0.51) and it was associated with better outcomes in mJOA score after surgery (AOR 1.53 of being in the ‘improvement’ group). Conversely, Meng et al. found the same T allele to be associated with a greater likelihood of radiological OPLL (OR 1.57) [23]. The contrasting effect of the same allele suggests the effect of the BMP4 gene is not limited to spinal pathology and the development of bony compression, but it may also influence the spinal cord response to such compression. It is unclear whether this effect is due to an intrinsic effect of BMP4 on CNS resilience or regeneration, or a treatment artifact that faster compression elicited by the 6007C>T polymorphism giving more severe bony pathology results in faster decompression and better post-operative outcomes. Nonetheless, it is clear that bone morphogenetic protein genes may have extensive influences in the pathogenesis and symptoms of DCM.
Alongside the BMP genes, several other genes should be highlighted. In the NPPS gene, the C973T polymorphism significantly affected both the susceptibility of OPLL development and the thickness of ossified vertebrae, but notably did not affect the number of ossified vertebrae.
NPPS gene polymorphisms were implicated in post-surgical improvements of spinal column disease affecting the thickness of ossified vertebrae (C973T), while others (IVS15-14T>C) affect the number of ossified vertebrae and others affect both (IVS20-11delT) [41].
Evaluation of the network of genes that were found to be associated with the development of spinal column pathology shows that, while each gene has an independent effect on susceptibility to pathology, there is clear connectedness within and across gene families (Figure 3).
Figure 3.
STRING Evidence Network for genes associated with spinal column disease.
4.2. Spinal Cord Disease
The ε4 allele of the APOE gene, an allele that is well known for its associations with both cardiovascular disease and Alzheimer’s disease, was associated with both a significantly increased likelihood of DCM development (OR 3.50) [16] and a significantly greater likelihood of failing to gain post-operative improvement (AOR 8.60 no improvement) [54]. However, this effect might not be universal across ethnicities; a study in an Indian population found the ε2 allele to be associated with development of myelopathy (OR 6.69) [17].
The 1790G>A polymorphism of the HIF1A gene displayed the opposite effect: it was associated with significantly greater likelihood of DCM development (OR 1.62), and worse disease but a greater likelihood of post-surgical improvement (OR 1.55) [35].
Reductions in Hif1α expression have been shown to be associated with the neuroprotective benefits of hyperbaric oxygen in spinal cord injury mouse models [56]. It is possible that such a mechanism is also the mediator of the HIF1A polymorphism’s effect on susceptibility, severity, and post-operative response in DCM.
The APOE gene and its product, the apolipoprotein E transporter, are well-known to be involved remyelination, with defective clearance of myelin debris by the transporter limiting the potential for remyelination [57]. In the case of both HIF1A and APOE, their effects appear to be directly exerted on the cord’s response to bony pathology, rather than via the bony pathology itself.
There appears to be delineation between genetic factors contributing to the development of bony pathology in the cervical spine, and those contributing to the CNS response to such insult. That an SNP of brain-derived neurotrophic factor (BDNF) is associated with the severity of disability (i.e., CNS response to insult) gives further weight to such a distinction [53].
As with genes that are associated with spinal pathology, the genes studied with relation to spinal cord disease have independent, but connected, effects (Figure 4).
Figure 4.
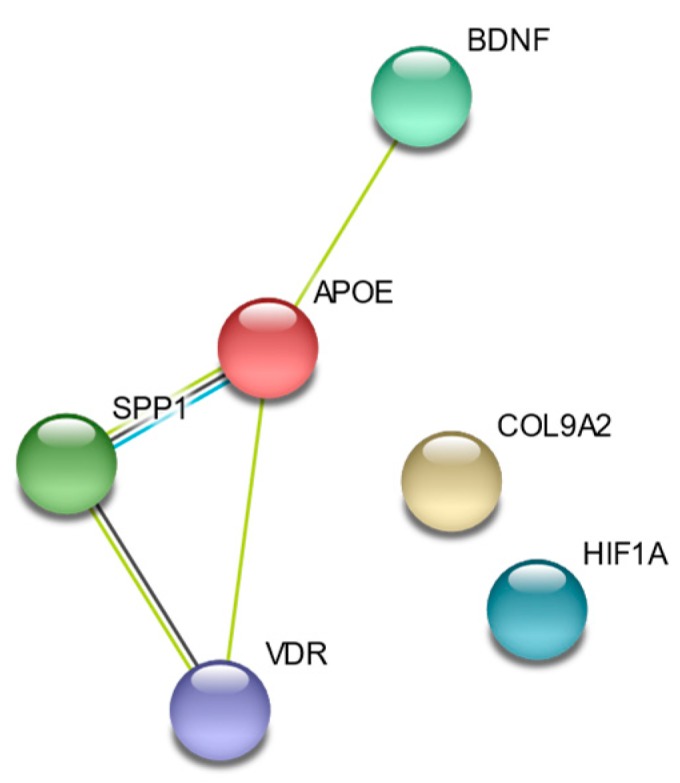
STRING Evidence Network diagram for genes associated with spinal cord disease.
4.3. Conflicting Evidence
The frequency of conflicting evidence is one striking aspect of much of the work reviewed here. The best example of this is perhaps seen in the RUNX2 gene; the rs1406846 SNP A allele is associated with 5.67 times greater likelihood of developing DCM in one study [44], but it has no significant effect in a further study using a similar number of participants from the same country [20]. Similarly the 869T>C SNP in the TGFB1 gene was associated with an odds ratio of 4.50 in one study [45], but a larger, more recent study found no significant effect of the same allele [46], with the result of meta-analysis showing no significant effect. Further examples of conflicting evidence include the IVS20-11delT polymorphism of the NPPS gene, in which one study found a significant effect on DCM susceptibility [39], but two others found no significant effect [38,41], while in the IVS15-14T>C polymorphism, two studies found an effect on susceptibility [40,41], with a further study showing no significant effect [33]. Such inconsistency might reflect the relatively small sample sizes of much of the work described here, and it indicates the need for large, well powered genetic investigations.
4.4. Limitations of Current Work
Limitations of the current work on the genetics of DCM are multiple. Firstly, many of the studies that were reviewed in this article scored poorly on the MINORS methodological items assessment [13]. None published information regarding prospective calculation of study size, few reported whether the cases and controls were demographically matched, and some did not report how participants were recruited (e.g., consecutively). As mentioned above, the sample sizes remain in the hundreds rather than thousands, which limits the degree to which their conclusions can be considered valid. Moreover, in reporting the results, many omit odds ratios, instead of reporting only p-values, which limits the degree to which such results can be interpreted.
Many of the studies reviewed here focused exclusively on Japanese, Chinese, or South Korean participants, and specifically OPLL. Interestingly, in the APOE gene ethnicity appears to result in conflicting genetic effects, with the ε2 allele associated with myelopathy in Indian populations and the ε4 allele associated with myelopathy in Chinese populations [16,17]. It is widely acknowledged that there is a greater prevalence of OPLL within Asian populations, and this might explain their disproportionate representation in the literature [1]. However, without further work across ethnicities, it remains speculation as to whether the conclusions from these studies are globally relevant and across the spectrum of DCM pathologies.
There is significant diversity in the assessment of disease severity between studies. One study used the SF-36 quality of life survey [53], three used the mJOA score [35,43,51] (a clinical score commonly used in DCM research [58,59,60,61]), while others used radiographic measures [19,23,24,26,27,41,44,51]. A similar situation is found within the literature while considering response to surgery, with one study using a cut-off for ‘improvement’ as +1 point on mJOA score [54], some using >50% increase in mJOA score [25,35,43], one using a t-test of % improvement on mJOA between homozygous groups [44], and one paper while using a radiographic definition of disease progression [41]. Such heterogeneity of outcome measures limits the degree to which the effects of genes on severity of DCM and response to surgery can be compared. The removal of surrogate outcome measures and more consistent use of a single form of outcome measure would permit more readily comparable conclusions to be drawn across different studies. We are currently undertaking RECODE DCM, an international consensus process to standardize the reporting of data elements in DCM research, and this would clearly hold benefit here (www.recode-dcm.com) [62]. For the reasons that are outlined above, the GRADE ratings of quality of evidence for each candidate gene were ‘low’ across all genes.
4.5. Future Directions
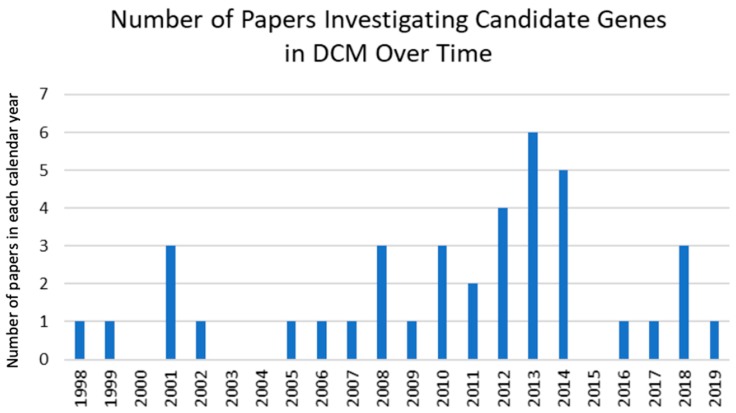
It is clear that interest in this field is building, with increasing numbers of studies focusing on genetic effects in DCM (Figure 5). However, more than half the that are genes reviewed here have been investigated by only a single study, often with small sample sizes, which suggests more intensive work in larger populations is required to further describe the genetic basis of DCM. Furthermore, all of the studies included in this review focused on individual candidate genes. While some considered the effects of haplotypes consisting of several SNPs within a single gene [24,26,29], no work has yet combined SNPs across different genes. Such combinations may exhibit effect sizes of greater magnitude than those in the current body of literature, with potential for such genetic profiles permitting greater personalization of treatment strategies. Future work should also seek to characterize the mechanism by which the genes that were reviewed here exert their effects in the pathobiology of DCM.
Figure 5.
Bar graph of number of papers investigating candidate genes in DCM in each calendar year.
5. Conclusions
While a number of limitations of the current work do exist, there is clear evidence of genetic effects of single nucleotide polymorphisms and haplotypes in DCM. Some of the genes exert their influence on the development of bony pathology, while others have effects on the spinal cord itself. Further investigation of the genetic basis of DCM requires larger study sizes, using more consistent measures of disease severity and response to surgery. The current evidence base is insufficient for translation to clinical practice for use in prognostication and management, but the potential for genetic profiles to be used in this way may well be realized once greater characterization of the genetic basis of DCM is achieved.
Acknowledgments
The authors are grateful to the Medical Library at the University of Cambridge for their assistance in the design of the search strategy for this article.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/1/282/s1, Data S1: search strategy used for MEDLINE and EMBASE databases. Data S2: search strategy used for Cochrane and HuGENet databases. Data S3: PRSIMA checklist.
Author Contributions
Conceptualization, B.M.D. and M.R.N.K.; methodology, B.M.D. and D.H.P.; formal analysis B.M.D. and D.H.P.; writing—original draft preparation, D.H.P.; writing—review and editing, B.M.D., D.H.P., O.D.M., A.R.B. and M.R.N.K.; supervision, M.R.N.K. All authors have read and agreed to the published version of the manuscript.
Funding
Research in the senior author’s laboratory is supported by a core support grant from the Wellcome Trust and MRC to the Wellcome Trust-Medical Research Council Cambridge Stem Cell Institute. MRNK is supported by a NIHR Clinician Scientist Award.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Nouri A., Tetreault L., Singh A., Karadimas S.K., Fehlings M.G. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine. 2015;40:E675–E693. doi: 10.1097/BRS.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 2.Davies B.M., Mowforth O.D., Smith E.K., Kotter M.R. Degenerative cervical myelopathy. BMJ. 2018;360:k186. doi: 10.1136/bmj.k186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tetreault L., Goldstein C.L., Arnold P., Harrop J., Hilibrand A., Nouri A., Fehlings M.G. Degenerative Cervical Myelopathy: A Spectrum of Related Disorders Affecting the Aging Spine. Neurosurgery. 2015;77:S51–S67. doi: 10.1227/NEU.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 4.Kalsi-Ryan S., Karadimas S.K., Fehlings M.G. Cervical Spondylotic Myelopathy: The Clinical Phenomenon and the Current Pathobiology of an Increasingly Prevalent and Devastating Disorder. Neuroscientist. 2013;19:409–421. doi: 10.1177/1073858412467377. [DOI] [PubMed] [Google Scholar]
- 5.Nouri A., Tetreault L., Côté P., Zamorano J.J., Dalzell K., Fehlings M.G. Does Magnetic Resonance Imaging Improve the Predictive Performance of a Validated Clinical Prediction Rule Developed to Evaluate Surgical Outcome in Patients with Degenerative Cervical Myelopathy? Spine. 2015;40:1092–1100. doi: 10.1097/BRS.0000000000000919. [DOI] [PubMed] [Google Scholar]
- 6.Witiw C.D., Mathieu F., Nouri A., Fehlings M.G. Clinico-Radiographic Discordance: An Evidence-Based Commentary on the Management of Degenerative Cervical Spinal Cord Compression in the Absence of Symptoms or With Only Mild Symptoms of Myelopathy. Glob. Spine J. 2018;8:527–534. doi: 10.1177/2192568217745519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tempest-Mitchell J., Hilton B., Davies B.M., Nouri A., Hutchinson P.J., Scoffings D.J., Mannion R.J., Trivedi R., Timofeev I., Crawford J.R., et al. A comparison of radiological descriptions of spinal cord compression with quantitative measures, and their role in non-specialist clinical management. PLoS ONE. 2019;14:e021938. doi: 10.1371/journal.pone.0219380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon S.T., Raich A., Hashimoto R.E., Riew K.D., Shaffrey C.I., Rhee J.M., Tetreault L.A., Skelly A.C., Fehlings M.G. Predictive factors affecting outcome after cervical laminoplasty. Spine. 2013;38:S232–S252. doi: 10.1097/BRS.0b013e3182a7eb55. [DOI] [PubMed] [Google Scholar]
- 9.Patel A.A., Spiker W.R., Daubs M., Brodke D.S., Cannon-Albright L.A. Evidence of an Inherited Predisposition for Cervical Spondylotic Myelopathy. Spine. 2012;37:26–29. doi: 10.1097/BRS.0b013e3182102ede. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson J.R., Patel A.A., Brodt E.D., Dettori J.R., Brodke D.S., Fehlings M.G. Genetics and Heritability of Cervical Spondylotic Myelopathy and Ossification of the Posterior Longitudinal Ligament: Results of a Systematic Review. Spine. 2013;38:S123–S146. doi: 10.1097/BRS.0b013e3182a7f478. [DOI] [PubMed] [Google Scholar]
- 11.Wang G., Cao Y., Wu T., Duan C., Wu J., Hu J., Lu H. Genetic factors of cervical spondylotic myelopathy-a systemic review. J. Clin. Neurosci. 2017;44:89–94. doi: 10.1016/j.jocn.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (MINORS): Development and validation of a new instrument. ANZ J. Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 14.Balshem H., Helfand M., Schünemann H.J., Oxman A.D., Kunz R., Brozek J., Vist G.E., Falck-Ytter Y., Meerpohl J., Norris S., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Kim D.H., Yun D.H., Kim H.-S., Min S.K., Yoo S.D., Lee K.H., Kim K.-T., Jo D.J., Kim S.K., Chung J.-H., et al. The Insertion/Deletion Polymorphism of Angiotensin I Converting Enzyme Gene is Associated with Ossification of the Posterior Longitudinal Ligament in the Korean Population. Ann. Rehabil. Med. 2014;38:1–5. doi: 10.5535/arm.2014.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setzer M., Hermann E., Seifert V., Marquardt G. Apolipoprotein E gene polymorphism and the risk of cervical myelopathy in patients with chronic spinal cord compression. Spine. 2008;33:497–502. doi: 10.1097/BRS.0b013e3181657cf7. [DOI] [PubMed] [Google Scholar]
- 17.Diptiranjan S., Harshitha S.M., Sibin M.K., Arati S., Chetan G.K., Bhat D.I. Role of APOE and IL18RAP gene polymorphisms in cervical spondylotic myelopathy in Indian population. J. Clin. Neurosci. 2019;66:83–86. doi: 10.1016/j.jocn.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 18.Chon J., Hong J.-H., Kim J., Han Y.J., Lee B.W., Kim S.-C., Kim D.H., Yoo S.D., Kim H.-S., Yun D.H. Association between BH3 interacting domain death agonist (BID) gene polymorphism and ossification of the posterior longitudinal ligament in Korean population. Mol. Biol. Rep. 2014;41:895–899. doi: 10.1007/s11033-013-2933-4. [DOI] [PubMed] [Google Scholar]
- 19.Wang H., Liu D., Yang Z., Tian B., Li J., Meng X., Wang Z., Yang H., Lin X. Association of bone morphogenetic protein-2 gene polymorphisms with susceptibility to ossification of the posterior longitudinal ligament of the spine and its severity in Chinese patients. Eur. Spine J. 2008;17:956–964. doi: 10.1007/s00586-008-0651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Zhao Y., Chen Y., Shi G., Yuan W. RUNX2 Polymorphisms Associated with OPLL and OLF in the Han Population. Clin. Orthop. Relat. Res. 2010;468:3333–3341. doi: 10.1007/s11999-010-1511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan L., Chang Z., Liu Y., Li Y.-B., He B.-R., Hao D.-J. A single nucleotide polymorphism in the human bone morphogenetic protein-2 gene (109T>G) affects the Smad signaling pathway and the predisposition to ossification of the posterior longitudinal ligament of the spine. Chin. Med. J. 2013;126:1112–1118. [PubMed] [Google Scholar]
- 22.Kim K.H., Kuh S.U., Park J.Y., Lee S.J., Park H.S., Chin D.K., Kim K.S., Cho Y.E. Association between BMP-2 and COL6A1 gene polymorphisms with susceptibility to ossification of the posterior longitudinal ligament of the cervical spine in Korean patients and family members. Genet. Mol. Res. 2014;13:2240–2247. doi: 10.4238/2014.March.31.4. [DOI] [PubMed] [Google Scholar]
- 23.Meng X., Wang H., Yang H., Hai Y., Tian B., Lin X. T allele at site 6007 of bone morphogenetic protein-4 gene increases genetic susceptibility to ossification of the posterior longitudinal ligament in male Chinese Han population. Chin. Med. J. 2010;123:2537–2542. [PubMed] [Google Scholar]
- 24.Ren Y., Feng J., Liu Z., Wan H., Li J., Lin X. A new haplotype in BMP4 implicated in ossification of the posterior longitudinal ligament (OPLL) in a Chinese population. J. Orthop. Res. 2012;30:748–756. doi: 10.1002/jor.21586. [DOI] [PubMed] [Google Scholar]
- 25.Wang D., Liu W., Cao Y., Yang L., Liu B., Yao G., Bi Z. BMP-4 polymorphisms in the susceptibility of cervical spondylotic myelopathy and its outcome after anterior cervical corpectomy and fusion. Cell. Physiol. Biochem. 2013;32:210–217. doi: 10.1159/000350137. [DOI] [PubMed] [Google Scholar]
- 26.Ren Y., Liu Z., Feng J., Wan H., Li J., Wang H., Lin X. Association of a BMP9 Haplotype with Ossification of the Posterior Longitudinal Ligament (OPLL) in a Chinese Population. PLoS ONE. 2012;7:e40587. doi: 10.1371/journal.pone.0040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H., Jin W., Li H. Genetic polymorphisms in bone morphogenetic protein receptor type IA gene predisposes individuals to ossification of the posterior longitudinal ligament of the cervical spine via the smad signaling pathway. BMC Musculoskelet. Disord. 2018;19:61. doi: 10.1186/s12891-018-1966-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T., Ikari K., Furushima K., Okada A., Tanaka H., Furukawa K.-I., Yoshida K., Ikeda T., Ikegawa S., Hunt S.C., et al. Genomewide Linkage and Linkage Disequilibrium Analyses Identify COL6A1, on Chromosome 21, as the Locus for Ossification of the Posterior Longitudinal Ligament of the Spine. Am. J. Hum. Genet. 2003;73:812–822. doi: 10.1086/378593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong Q., Ma X., Li F., Guo Z., Qi Q., Li W., Yuan H., Wang Z., Chen Z. COL6A1 Polymorphisms Associated with Ossification of the Ligamentum Flavum and Ossification of the Posterior Longitudinal Ligament. Spine. 2007;32:2834–2838. doi: 10.1097/BRS.0b013e31815b761c. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z.C., Shi J.G., Chen X.S., Xu G.H., Li L.J., Jia L.S. The role of smoking status and collagen IX polymorphisms in the susceptibility to cervical spondylotic myelopathy. Genet. Mol. Res. 2012;11:1238–1244. doi: 10.4238/2012.May.9.2. [DOI] [PubMed] [Google Scholar]
- 31.Koga H., Sakou T., Taketomi E., Hayashi K., Numasawa T., Harata S., Yone K., Matsunaga S., Otterud B., Inoue I., et al. Genetic mapping of ossification of the posterior longitudinal ligament of the spine. Am. J. Hum. Genet. 1998;62:1460–1467. doi: 10.1086/301868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maeda S., Ishidou Y., Koga H., Taketomi E., Ikari K., Komiya S., Takeda J., Sakou T., Inoue I. Functional Impact of Human Collagen α2(XI) Gene Polymorphism in Pathogenesis of Ossification of the Posterior Longitudinal Ligament of the Spine. J. Bone Miner. Res. 2001;16:948–957. doi: 10.1359/jbmr.2001.16.5.948. [DOI] [PubMed] [Google Scholar]
- 33.Horikoshi T., Maeda K., Kawaguchi Y., Chiba K., Mori K., Koshizuka Y., Hirabayashi S., Sugimori K., Matsumoto M., Kawaguchi H., et al. A large-scale genetic association study of ossification of the posterior longitudinal ligament of the spine. Hum. Genet. 2006;119:611–616. doi: 10.1007/s00439-006-0170-9. [DOI] [PubMed] [Google Scholar]
- 34.Jun J.-K., Kim S.-M. Association Study of Fibroblast Growth Factor 2 and Fibroblast Growth Factor Receptors Gene Polymorphism in Korean Ossification of the Posterior Longitudinal Ligament Patients. J. Korean Neurosurg. Soc. 2012;52:7–13. doi: 10.3340/jkns.2012.52.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z.-C., Hou X.-W., Shao J., Ji Y.-J., Li L., Zhou Q., Yu S.-M., Mao Y.-L., Zhang H.-J., Zhang P.-C., et al. HIF-1α polymorphism in the susceptibility of cervical spondylotic myelopathy and its outcome after anterior cervical corpectomy and fusion treatment. PLoS ONE. 2014;9:e110862. doi: 10.1371/journal.pone.0110862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim D.H., Jeong Y.S., Chon J., Yoo S.D., Kim H.-S., Kang S.W., Chung J.-H., Kim K.-T., Yun D.H. Association between interleukin 15 receptor, alpha (IL15RA) polymorphism and Korean patients with ossification of the posterior longitudinal ligament. Cytokine. 2011;55:343–346. doi: 10.1016/j.cyto.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Guo Q., Lv S.-Z., Wu S.-W., Tian X., Li Z.-Y. Association between single nucleotide polymorphism of IL15RA gene with susceptibility to ossification of the posterior longitudinal ligament of the spine. J. Orthop. Surg. Res. 2014;9:103. doi: 10.1186/s13018-014-0103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tahara M., Aiba A., Yamazaki M., Ikeda Y., Goto S., Moriya H., Okawa A. The extent of ossification of posterior longitudinal ligament of the spine associated with nucleotide pyrophosphatase gene and leptin receptor gene polymorphisms. Spine. 2005;30:877–880. doi: 10.1097/01.brs.0000160686.18321.ad. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura I., Ikegawa S., Okawa A., Okuda S., Koshizuka Y., Kawaguchi H., Nakamura K., Koyama T., Goto S., Toguchida J., et al. Association of the human NPPS gene with ossification of the posterior longitudinal ligament of the spine (OPLL) Hum. Genet. 1999;104:492–497. doi: 10.1007/s004390050993. [DOI] [PubMed] [Google Scholar]
- 40.Koshizuka Y., Kawaguchi H., Ogata N., Ikeda T., Mabuchi A., Seichi A., Nakamura Y., Nakamura K., Ikegawa S. Nucleotide Pyrophosphatase Gene Polymorphism Associated with Ossification of the Posterior Longitudinal Ligament of the Spine. J. Bone Miner. Res. 2002;17:138–144. doi: 10.1359/jbmr.2002.17.1.138. [DOI] [PubMed] [Google Scholar]
- 41.He Z., Zhu H., Ding L., Xiao H., Chen D., Xue F. Association of NPP1 polymorphism with postoperative progression of ossification of the posterior longitudinal ligament in Chinese patients. Genet. Mol. Res. 2013;12:4648–4655. doi: 10.4238/2013.October.18.3. [DOI] [PubMed] [Google Scholar]
- 42.Yu H.-M., Chen X.-L., Wei W., Yao X.-D., Sun J.-Q., Su X.-T., Lin S.-F. Effect of osteoprotegerin gene polymorphisms on the risk of cervical spondylotic myelopathy in a Chinese population. Clin. Neurol. Neurosurg. 2018;175:149–154. doi: 10.1016/j.clineuro.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Wu J., Wu D., Guo K., Yuan F., Ran B. OPN Polymorphism is Associated with the Susceptibility to Cervical Spondylotic Myelopathy and its Outcome After Anterior Cervical Corpectomy and Fusion. CPB. 2014;34:565–574. doi: 10.1159/000363023. [DOI] [PubMed] [Google Scholar]
- 44.Chang F., Li L., Gao G., Ding S., Yang J., Zhang T., Zuo G. Role of Runx2 polymorphisms in risk and prognosis of ossification of posterior longitudinal ligament. J. Clin. Lab. Anal. 2017;31:e22068. doi: 10.1002/jcla.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamiya M., Harada A., Mizuno M., Iwata H., Yamada Y. Association between a polymorphism of the transforming growth factor-beta1 gene and genetic susceptibility to ossification of the posterior longitudinal ligament in Japanese patients. Spine. 2001;26:1264–1266. doi: 10.1097/00007632-200106010-00017. [DOI] [PubMed] [Google Scholar]
- 46.Han I.B., Ropper A.E., Jeon Y.J., Park H.S., Shin D.A., Teng Y.D., Kuh S.-U., Kim N.-K. Association of transforming growth factor-beta 1 gene polymorphism with genetic susceptibility to ossification of the posterior longitudinal ligament in Korean patients. Genet. Mol. Res. 2013;12:4807–4816. doi: 10.4238/2013.February.28.26. [DOI] [PubMed] [Google Scholar]
- 47.Jekarl D.W., Paek C.-M., An Y.J., Kim Y.J., Kim M., Kim Y., Lee J., Sung C.H. TGFBR2 gene polymorphism is associated with ossification of the posterior longitudinal ligament. J. Clin. Neurosci. 2013;20:453–456. doi: 10.1016/j.jocn.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 48.Chung W.-S., Nam D.-H., Jo D.-J., Lee J.-H. Association of Toll-Like Receptor 5 Gene Polymorphism with Susceptibility to Ossification of the Posterior Longitudinal Ligament of the Spine in Korean Population. J. Korean Neurosurg. Soc. 2011;49:8–12. doi: 10.3340/jkns.2011.49.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song D., Wu Y., Tian D. Association of VDR-FokI and VDBP-Thr420Lys polymorphisms with cervical spondylotic myelopathy: A case-control study in the population of China. J. Clin. Lab. Anal. 2018;33:e22669. doi: 10.1002/jcla.22669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobashi G., Ohta K., Washio M., Okamoto K., Sasaki S., Yokoyama T., Miyake Y., Sakamoto N., Hata A., Tamashiro H., et al. FokI Variant of Vitamin D Receptor Gene and Factors Related to Atherosclerosis Associated With Ossification of the Posterior Longitudinal Ligament of the Spine: A Multi-Hospital Case-Control Study. Spine. 2008;33:E553–E558. doi: 10.1097/BRS.0b013e31817e9de2. [DOI] [PubMed] [Google Scholar]
- 51.Wang Z.C., Chen X.S., Wang D.W., Shi J.G., Jia L.S., Xu G.H., Huang J.H., Fan L. The genetic association of Vitamin D receptor polymorphisms and cervical spondylotic myelopathy in Chinese subjects. Clin. Chim. Acta. 2010;411:794–797. doi: 10.1016/j.cca.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 52.Chin D.-K., Han I.-B., Ropper A.E., Jeon Y.-J., Kim D.-H., Kim Y.-S., Park Y., Teng Y.D., Kim N.-K., Kuh S.-U. Association of VKORC1-1639G> A polymorphism with susceptibility to ossification of the posterior longitudinal ligament of the spine: A Korean study. Acta Neurochir. 2013;155:1937–1942. doi: 10.1007/s00701-013-1747-4. [DOI] [PubMed] [Google Scholar]
- 53.Abode-Iyamah K.O., Stoner K.E., Grossbach A.J., Viljoen S.V., McHenry C.L., Petrie M.A., Dahdaleh N.S., Grosland N.M., Shields R.K., Howard M.A., et al. Effects of brain derived neurotrophic factor Val66Met polymorphism in patients with cervical spondylotic myelopathy. J. Clin. Neurosci. 2016;24:117–121. doi: 10.1016/j.jocn.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 54.Setzer M., Vrionis F.D., Hermann E.J., Seifert V., Marquardt G. Effect of apolipoprotein E genotype on the outcome after anterior cervical decompression and fusion in patients with cervical spondylotic myelopathy. J. Neurosurg. Spine. 2009;11:659–666. doi: 10.3171/2009.7.SPINE08667. [DOI] [PubMed] [Google Scholar]
- 55.Chen D., Zhao M., Mundy G.R. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Y., Liu X., Qu S., Yang J., Wang Z., Gao C., Su Q. Hyperbaric oxygen intervention on expression of hypoxia-inducible factor-1α and vascular endothelial growth factor in spinal cord injury models in rats. Chin. Med. J. 2013;126:3897–3903. [PubMed] [Google Scholar]
- 57.Cantuti-Castelvetri L., Fitzner D., Bosch-Queralt M., Weil M.-T., Su M., Sen P., Ruhwedel T., Mitkovski M., Trendelenburg G., Lütjohann D., et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. 2018;359:684–688. doi: 10.1126/science.aan4183. [DOI] [PubMed] [Google Scholar]
- 58.Kalsi-Ryan S., Singh A.R., Massicotte E.M.M., Arnold P.M.M., Brodke D.S.M., Norvell D.C., Hermsmeyer J.T., Fehlings M.G. Ancillary Outcome Measures for Assessment of Individuals with Cervical Spondylotic Myelopathy. Spine. 2013;38:S111–S122. doi: 10.1097/BRS.0b013e3182a7f499. [DOI] [PubMed] [Google Scholar]
- 59.Davies B.M., McHugh M., Elgheriani A., Kolias A.G., Tetreault L.A., Hutchinson P.J.A., Fehlings M.G., Kotter M.R.N. Reported Outcome Measures in Degenerative Cervical Myelopathy: A Systematic Review. PLoS ONE. 2016;11:e0157263. doi: 10.1371/journal.pone.0157263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davies B.M., McHugh M., Elgheriani A., Kolias A.G., Tetreault L., Hutchinson P.J.A., Fehlings M.G., Kotter M.R.N. The reporting of study and population characteristics in degenerative cervical myelopathy: A systematic review. PLoS ONE. 2017;12:e0172564. doi: 10.1371/journal.pone.0172564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kopjar B., Tetreault L., Kalsi-Ryan S., Fehlings M. Psychometric properties of the modified japanese Orthopaedic association scale in patients with cervical spondylotic myelopathy. Spine. 2015;40:E23–E28. doi: 10.1097/BRS.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 62.Davies B.M., Khan D.Z., Mowforth O.D., McNair A.G.K., Gronlund T., Kolias A.G., Tetreault L., Starkey M.L., Sadler I., Sarewitz E., et al. RE-CODE DCM (REsearch Objectives and Common Data Elements for Degenerative Cervical Myelopathy): A Consensus Process to Improve Research Efficiency in DCM, Through Establishment of a Standardized Dataset for Clinical Research and the Definition of the Research Priorities. Glob. Spine J. 2019;9:65S–76S. doi: 10.1177/2192568219832855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.