Abstract
Sarcoidosis is a devastating inflammatory disease affecting many organs, especially the lungs and lymph nodes. Bone marrow-derived mesenchymal stromal cells (MSCs) can “reprogram” various types of macrophages towards an anti-inflammatory phenotype. We wanted to determine whether alveolar macrophages from sarcoidosis subjects behave similarly by mounting an anti-inflammatory response when co-cultured with MSCs. Fifteen sarcoidosis and eight control subjects underwent bronchoscopy and bronchoalveolar lavage (BAL). Unselected BAL cells (70–94% macrophages) were isolated and cultured with and without MSCs from healthy adults. Following stimulation of the cultured cells with lipopolysaccharide, the medium was removed to measure interleukin 10 and tumor necrosis factor alpha (IL-10 and TNF-α). In two additional sarcoidosis subjects, flow cytometry was used to study intracellular cytokines and surface markers associated with alveolar macrophages to confirm the results. Unselected BAL cells from sarcoidosis subjects co-cultured with MSCs showed a reduction in TNF-α (pro-inflammatory M1) and an increase in IL-10 (anti-inflammatory M2) in 9 of 11 samples studied. Control subject samples showed few, if any, differences in cytokine production. Unselected BAL cells from two additional patients analyzed by flow cytometry confirmed a switch towards an anti-inflammatory state (i.e., M1 to M2) after co-culture with MSCs. These results suggest that, similarly to other macrophages, alveolar macrophages also respond to MSC contacts by changing towards an anti-inflammatory phenotype. Based on our results, we hypothesize that mesenchymal stromal cells applied to the airways might alleviate lung inflammation and decrease steroid need in patients with sarcoidosis.
Keywords: sarcoidosis, alveolar macrophages, bone marrow stromal cells, cell therapy
1. Introduction
Bone marrow stromal cells (called MSCs) regenerate skeletal cells in the marrow niche environment and support hematopoiesis. MSCs are a very heterogeneous population of cells [1] that can differentiate to various lineages of mesenchymal tissues, including bone, muscle, and adipose tissue [2]. The observation that they can modulate the immune system resulted in extensive research on their use to treat inflammatory conditions, including T-cell-mediated diseases such as graft-versus-host disease (GVHD) [3], as well as granulomatous disorders such as Crohn’s disease [4]. MSCs decrease inflammation and promote tissue repair through cell–cell interactions including mitochondrial transfer [5], and by way of paracrine effects [6,7]. In clinical trials to date, they have demonstrated an excellent safety profile [8,9].
It is generally agreed that MSCs can reprogram activated macrophages to adopt an anti-inflammatory state (i.e., the phenotype moves from M1 towards M2) [10,11,12,13]. Cyclooxygenase-2 (COX-2) signaling contributes to driving this change and was first shown in vivo in a mouse model of sepsis [14]. Inflammatory stimuli, such as lipopolysaccharide (LPS) and TNF-α, interact with toll-like receptors in MSCs to induce nuclear factor kappa B (NF-kB), which in turn increases the production of COX-2. This enzyme increases the production of Prostaglandin E2 (PGE2) [14], which interacts with receptors on macrophages to increase IL-10 and decrease TNF-α production. MSCs from a variety of tissues seem to behave the same way [13]. Engulfment of apoptotic MSCs by macrophages was recently shown to increase the production of other immune suppressive factors, demonstrating an additional mechanism of action [15].
Sarcoidosis is a multisystem granulomatous inflammatory disease propelled primarily by dysregulated macrophage TNF-α production and increased T-helper-1 (Th1 and Th17) cell proliferation [16]. It often involves the lungs and the lymphatic system, but the etiology remains largely unknown [17,18]. Oligoclonal expansion of patient T-cells suggests that sarcoidosis may be an antigen-driven immune process [19,20] in genetically disposed hosts [21,22]. Macrophage activation plays a key role in disease pathogenesis [23], and macrophage-directed recruitment of T-helper cells is now recognized as having significant importance in the disease [24]. Currently available treatment options include glucocorticoids, cytotoxic drugs, and anti-TNF-α monoclonal antibodies, most of which have high risk/benefit profiles [25].
We hypothesized that human MSCs may have a beneficial effect if they can reprogram alveolar macrophages (AMs) of patients with sarcoidosis. To look for such an effect in vitro, we recruited biopsy-confirmed sarcoidosis subjects and control subjects to provide bronchoalveolar lavage (BAL) samples for co-culture experiments with human MSCs.
It has been shown that pro-inflammatory macrophages from a variety of sources convert to anti-inflammatory phenotype when co-cultured with MSCs. The results presented here demonstrate for the first time that AMs from patients with sarcoidosis behave similarly. The conversion is achieved by MSCs inducing IL-10 and decreasing TNF-α production of AMs. Given their known safety profile, we suggest that MSCs may offer a safe alternative or adjunctive treatment to drug therapy.
2. Methods
2.1. Clinical Procedure
Seventeen adult sarcoidosis subjects (Scadding [26] stages 0–IV), who were receiving minimal or no anti-inflammatory treatment, and eight control subjects were recruited to the NIH and consented to protocol 96-H-0100 (NCT00001532) to undergo bronchoscopy with BAL. Subjects received a medical evaluation and underwent laboratory and pulmonary function testing prior to the procedure. They received IV conscious sedation and topical anesthesia of their airways. Lavages were performed with 270–300 mL of sterile saline in aliquots of 30 mL each; the right and left upper lobes were primarily targeted. Samples were aspirated into Lukens traps and placed on ice. Approximately 10 ml of the BAL fluid return (BALF) was analyzed for viability and via cytometry to obtain white blood cell (WBC) counts. Viability was determined using Trypan Blue staining, while cell differential counts were performed after cytocentrifuge and Diff-QuikTM staining. The remaining volume was submitted for in vitro co-culture experiments with MSCs. For details of culture and co-culture conditions, as well as the ELISA protocol, please see the online data supplement.
2.2. Cell Culture
Cryopreserved clinical-grade human MSCs derived from iliac crest biopsies of healthy adult donors were obtained from the Clinical Center of the NIH, Bethesda, MD under protocol 10-CC-0053 (NCT01071577), expanded, and cryopreserved in aliquots of 1 to 4 million cells. For testing, the aliquots were removed from liquid nitrogen storage, thawed, and cultured in minimal essential medium (MEM-α) supplemented with 10% fetal bovine serum (FBS), 1% GlutaMax, and 1% PenStrep (herein named “MSC medium”). Macrophage medium consisted of RPMI 1640 supplemented with 10% FBS, 1% PenStrep and 0.00035% beta-mercaptoethanol. Freezing medium consisted of 50% MSC or macrophage medium, depending on the cell type, 40% FBS, and 10% dimethyl sulfoxide (DMSO). Cells were expanded in culture in 5% CO2 and 20% O2 at 37 °C.
Unselected BAL cells, with 70–94% AMs, were filtered upon receipt from the clinic in preparation for the co-culture assay. BAL cells were centrifuged, re-suspended in macrophage medium, counted, and cultured overnight at 37 °C in a 96-well plate with and without passage 4–6 clinical-grade MSCs. Most samples were plated in a ratio of 100,000 BAL cells to 10,000 MSCs in 200 µL of macrophage medium per well in eight replicates. One-half of the replicates were stimulated with 1 µg/mL of LPS the following morning, and supernatants were removed at 6 and 24 h for ELISAs to determine concentrations of TNF-α and IL-10, respectively.
Passage 5 clinical-grade MSCs from two healthy adult donors were combined and plated into Nunc™ 6-well UpCell™ Surface culture plates (ThermoFisher Scientific, Waltham, MA, USA) at a concentration of 3–4 × 104 cells per well in MSC medium. Cells were incubated overnight at 37 °C with 5% CO2 and 20% O2. MSC medium was removed and replaced with 3 ml of macrophage medium with and without 3–4 × 105 unselected BAL cells. Additionally, BAL cells were plated in the absence of MSCs. Plates were incubated for 16 hours as above.
2.3. Osteogenic and Adipogenic Differentiation Assay of MSCs
To demonstrate stem cell properties of MSCs, in vitro bone and adipose differentiation assays were performed as previously reported [27]. In brief, MSCs were plated in 6-well plates in complete medium and were cultured either in osteogenic or adipogenic medium for 16 days, at which time cells were fixed and stained with Alizarin Red or Oil Red, respectively. Representative microscopic pictures are shown (Figure S1).
2.4. ELISA
Pilot experiments were performed to determine the optimal ratio of MSCs to mononuclear BAL cells and time points for cytokine detection. To harvest the samples, co-culture plates were centrifuged, and the supernatants were transferred to low-absorbance plates for temporary storage at −20 °C or immediate use in an ELISA. A 1:5 dilution of the supernatant was performed to prevent the TNF-α concentrations from exceeding the concentration of the highest standard (1000 pg/mL).
ELISAs for human IL-10 and TNF-α were performed using DuoSet ELISA kits (DY217B, DY210, DY417, DY410) from R&D Systems (Minneapolis, MN, USA) according to the manufacturer’s instructions. The plates were analyzed using a Turner BioSystems (Sunnyvale, CA, USA) Modulus Microplate Reader calibrated for ELISA analysis at 450 nm using 3,3’,5,5’-Tetramethylbenzidine (TMB). ELISAs were repeated for samples that markedly overshot the high standard or had inexplicably high variance among replicates.
2.5. Flow Cytometry
Antibodies used were anti-CD68 Allophycocyanin (APC), anti-CD11c Phycoerythrin (PE), anti-CD11c Peridinin Chlorophyll Protein Complex (PerCP)-Cy5.5, anti-CD80 PE-Cy5, anti-CD163 Brilliant Violet 711, anti-CD206 APC/Fire 750, anti-TNF-α Brilliant Violet 785, and anti-IL-10 PE (BioLegend, San Diego, CA, USA).
For surface staining, cells were harvested by placing plates at 4 °C for 30 min and trituration to avoid the potential loss of select M2 cell surface markers by enzymatic degradation. Fc receptors were blocked with Human TruStain FcX (BioLegend) for 10 min at room temperature. Surface antibodies were added and incubated for 20 min at 4 °C in the dark and then washed with 5% FBS in PBS three times. Cell viability was determined using 4’,6-diamidino-2-phenylindole (DAPI). Single-stain compensation controls were prepared using UltraComp eBeads (Invitrogen, Waltham, MA, USA) according to the manufacturer’s instructions. Cell surface staining was analyzed using a BD LSRFortessa (Becton, Dickinson and Company, BD Biosciences, San Jose, CA, USA) immediately following staining. FlowJo software version 10.5.3 (FlowJo LLC, Ashland, Oregon) was used to analyze the acquired data.
For detection of cytokine expression inside the AMs, Monensin (eBioscience, Waltham, MA, USA) and Brefeldin A (eBioscience) were titrated to determine the protein transport inhibitor best suited for our cytokines and cells of interest [11]. Monensin was chosen and added at a final concentration of 2 M 4 h prior to harvesting cells to block secretion of cytokines. Cells were harvested as indicated above. Briefly, after staining with Zombie UV™ Fixable Viability Kit (BioLegend) and blocking of Fc receptor-mediated nonspecific binding, cells were stained with antibodies against surface markers for 20 min at 4 °C in the dark and washed as above. Cells were fixed for 20 min at room temperature and permeabilized using the Intracellular Fixation and Permeabilization Buffer Set (eBioscience) according to the manufacturer’s instructions. Cytokine-specific antibodies were added and incubated for 30 min at 4 °C in the dark and washed. Single-stain compensation controls were prepared as above. The Zombie UV™ single-stain compensation control was prepared using ArC™ Amine Reactive Compensation Bead Kit (Invitrogen) according to the manufacturer’s instructions. Samples were analyzed for flow cytometry as above. For all gatings, fluorescence minus one controls (FMOs) were used.
3. Results
The magnitude of any change in cytokine production was found to be positively correlated with the number of MSCs in the co-culture (Figure S2). To produce consistent, statistically significant changes in cytokine production, a ratio of 1 MSC to 10 monocyte/macrophage/BAL cells was sufficient. After the conclusion of the pilot experiments, this ratio was used throughout the study.
Seventeen subjects with biopsy-confirmed sarcoidosis and a compatible clinical history were selected to participate in the study. They were using little or no anti-inflammatory drugs at the time of the BAL harvest (Table 1). All five Scadding stages were represented in the sarcoidosis cohort: Stage 0: 3 (17.6%); Stage I: 3 (17.6%); Stage II: 1 (5.9%); Stage III: 6 (35.3%); and Stage IV: 4 (23.5%). Eight healthy adults were recruited as controls. Sarcoidosis subjects had a significantly lower mean forced vital capacity (FVC), forced expired volume in 1 second (FEV1), and diffusion adjusted for hemoglobin (DLCO adj.) (Table S1); and significantly higher serum angiotensin-converting enzyme (ACE) levels and peripheral blood monocyte numbers compared with control subjects (Tables S2 and S3). Peripheral blood lymphocytes were similar in the two cohorts (Table S3); however, sarcoidosis subjects had a significantly greater percentage of lymphocytes in their BAL (Table 2).
Table 1.
Demographics and Basic Clinical Features.
| Parameter | Controls (n = 7) |
Sarcoidosis (n = 15) |
p-Value |
|---|---|---|---|
| Mean Value | Mean Value | ||
| Total Participants | |||
| Female (number)/(%) | 4/(50%) | 11/(73.3%) | NS |
| Age (years) | 45.4 | 53.1 | NS |
| Race | |||
| Black (number)/(%) | 5/(62.5) | 8/(53.3) | NS |
| White (number)/(%) | 2/(25) | 5/(33.3) | NS |
| Asian (number)/(%) | 1/(12.5) | 0 | NS |
| Multiracial (number)/(%) | 0 | 2/(13.3) | NS |
| Height (cm), (SD) | 174, (0.91) | 168, (0.36) | NS |
| Modification of MRC Dyspnea Scale | 0 | 0.9, (1.36) | 0.027 |
| Inhaled Steroid (number/(%)), (SD) | 0 | 5/(33.3%), (0.49) | 0.019 |
| Prednisone (number / (%)), (SD) | 0 | 2/(20%), (0.35) | NS |
Abbreviations: Medical Research Council (MRC). SD: standard deviation. NS: not significant.
Table 2.
Bronchoalveolar Lavage Cell Counts.
| Parameter | Controls (n = 7) |
Sarcoidosis (n = 15) |
p-Value | ||
|---|---|---|---|---|---|
| Mean Value | SD | Mean Value | SD | ||
| BAL Cell count (× 107) | 21 | 9.7 | 22.6 | 14.8 | NS |
| BAL Lymphocytes (%) | 6.57 | 6.4 | 16.96 | 12.5 | 0.02 |
| BAL Macrophage (%) | 80.25 | 33.2 | 70.21 | 23.7 | NS |
| BALF % return (%) | 48.6 | 8.2 | 51.67 | 12.2 | NS |
| Viability of cells (%) | 80.57 | 15.7 | 84.6 | 8.0 | NS |
Abbreviation: NS: not significant.
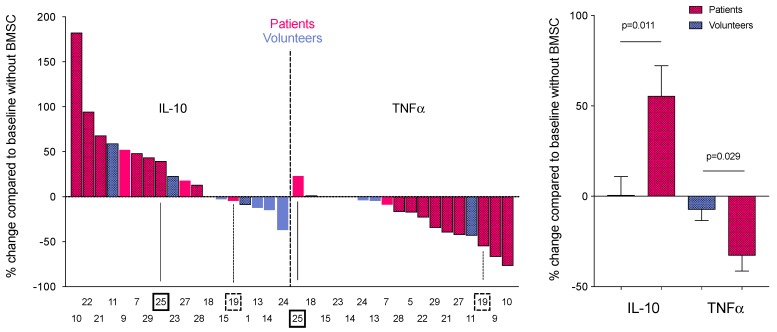
ELISA assays were performed to detect the cytokine (TNF-α and IL-10) production by the isolated BAL cells in culture with and without human MSCs. Samples of medium were collected 6 and 24 h after LPS stimulation of co-cultured MSCs and BAL cells from sarcoidosis and control subjects. ELISA measurements of these co-culture supernatants showed that BAL cells from sarcoidosis subjects significantly decreased their TNF-α production (p = 0.029) and increased their IL-10 production (p = 0.011), unlike cells from control subjects (Figure 1A,B). This result seems to reflect a shift from a pro-inflammatory M1 to a more anti-inflammatory M2 phenotype.
Figure 1.
Percent change in cytokine production of bone marrow stromal cell (MSC) and bronchoalveolar lavage (BAL) cell co-cultures stimulated with lipopolysaccharide (LPS). (A) Cytokine (IL-10—left half and TNF-α—right half) concentrations were measured from tissue culture medium of LPS-stimulated co-cultures of MSCs and BAL cells from sarcoidosis (red) and control (blue) subjects. Of the 11 cases presented, 2 sarcoidosis samples (19 and 25) diverged from the trend in one cytokine or the other (indicated by arrows and asterisks). Control subject samples showed few, if any, differences in cytokine production with no obvious trend. (B) Average percent changes in anti-inflammatory (IL-10) and the pro-inflammatory (TNF-α) cytokines in the co-culture medium are shown for both groups. The graph shows that all of the samples from the sarcoidosis subjects shift towards an anti-inflammatory state (increase in IL-10 and decrease in TNF-α), while samples from the control subjects do not (n = 9 for sarcoidosis subjects, n = 7 for control subjects, unpaired student’s t test; data are shown as mean ± SEM; p = 0.011 for IL-10 and 0.029 for TNF-α).
Cytokine production was also evaluated in co-cultures not stimulated with LPS. In these samples, IL-10 and TNF-α were either not detected or barely detectable in the culture medium. MSCs alone did not produce any measurable IL-10 or TNF-α in the presence or absence of LPS, indicating that BAL cells produced the measured cytokines.
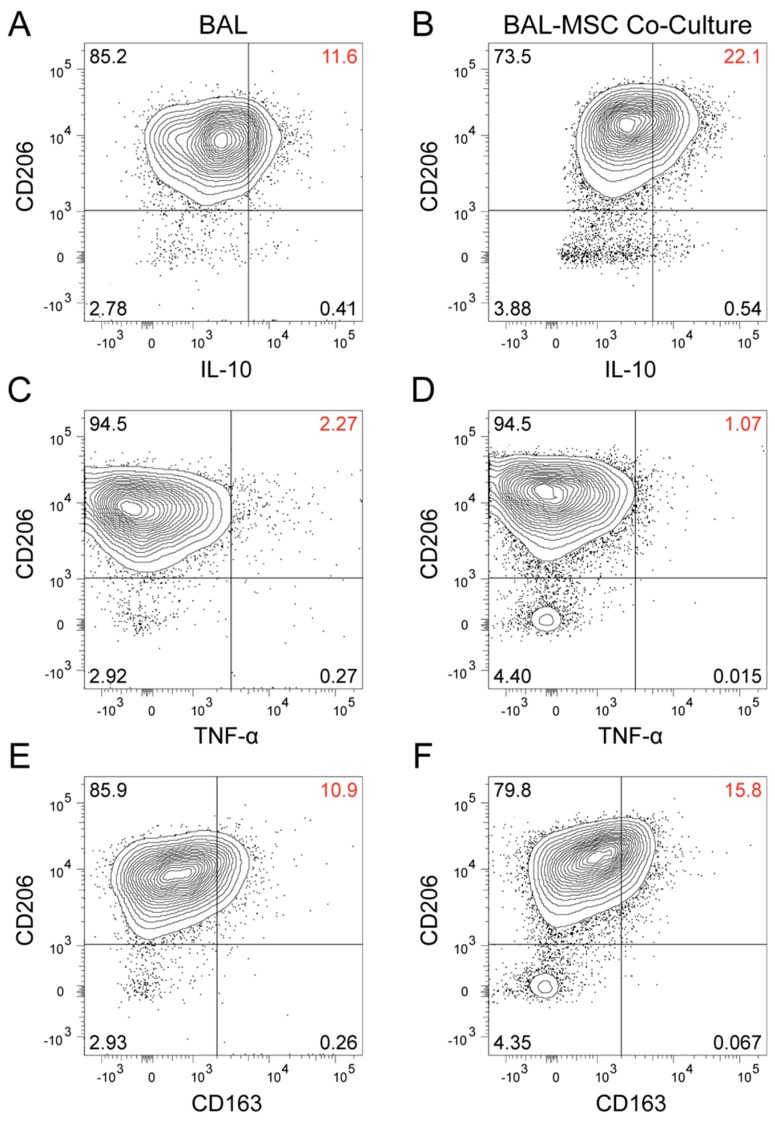
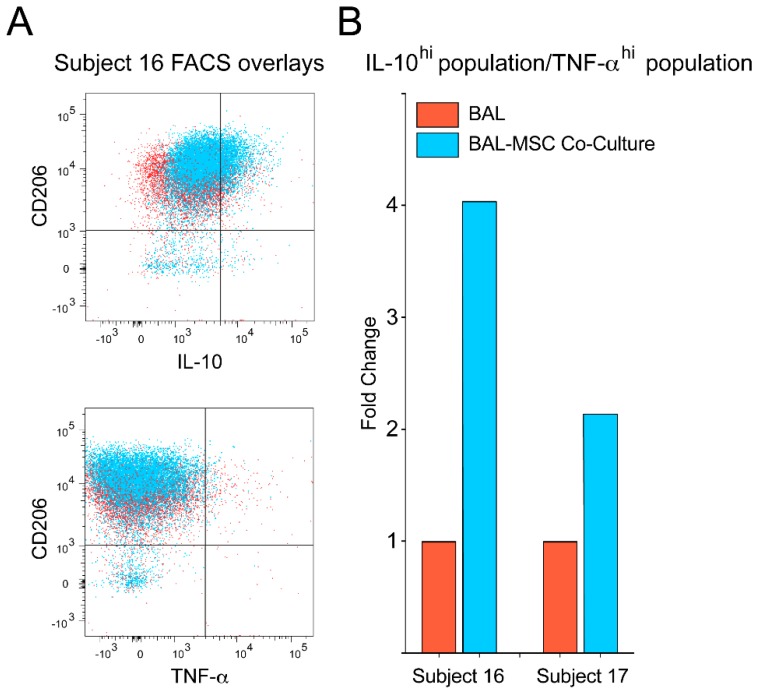
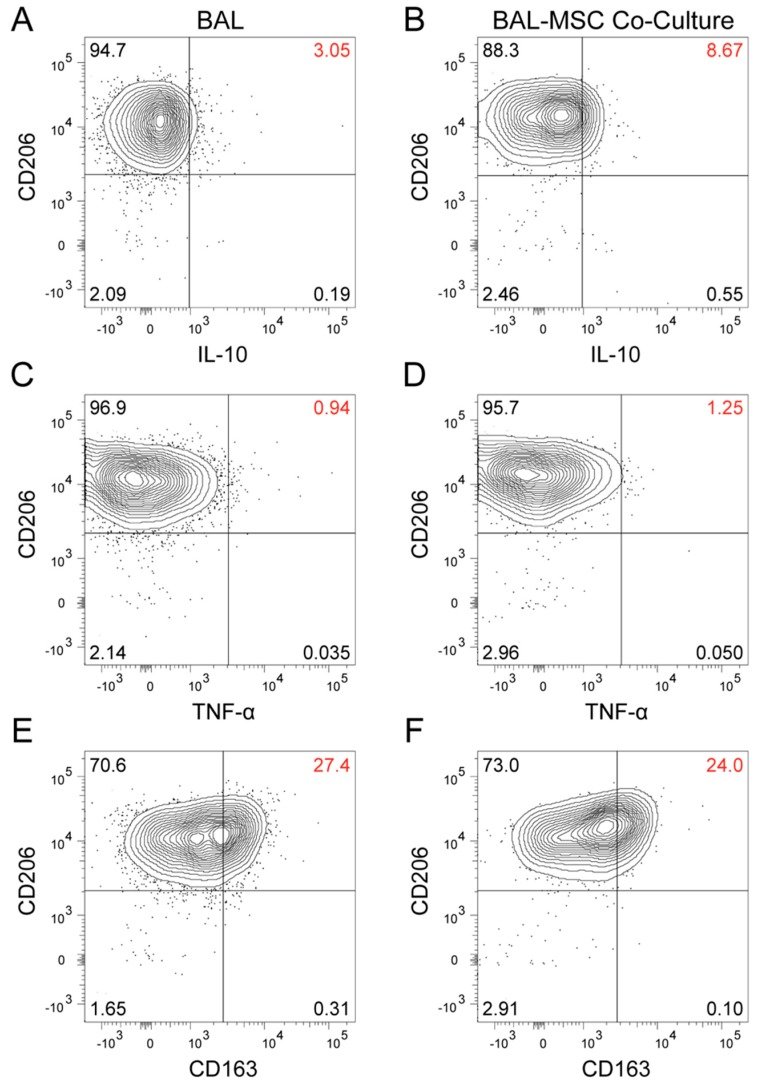
To confirm this and determine whether it was macrophages in the BAL preparations that made the cytokines we measured, we studied intracellular IL-10 and TNF-α in a population of AMs identified by flow cytometry and also examined macrophage-associated cell surface antigens. Because the detection of the cytokines was quite sensitive in the analyzed cells, it was unnecessary to add LPS to stimulate their production. BAL cells from two additional sarcoidosis subjects were studied. Subject 16 was more symptomatic and functionally impaired, and appeared to have a more active disease process (Table S4). Subject 17 had clinically inactive disease, a lower number of AMs, and a lower peripheral CD4+/CD8+ ratio (Tables S4 and S5). Sixteen hours after BAL cells were placed in culture with and without MSCs, they were harvested, processed, and analyzed by flow cytometry. We focused on CD206+ cells, which are considered to be the AMs [28,29] among the BAL cells. In AMs from subject 16, twice as many cells produced IL-10 when they were co-cultured with MSCs (22.1%) as they did when cultured without them (11.6%) (Figure 2A,B). In parallel with the increase in IL-10, there was a decrease in TNF-α (from 2.27% to 1.07%) (Figure 2C,D), resulting in a 4-fold increase in the IL-10/TNF-α ratio (Figure 3). AMs from subject 17 seemed less MSC-sensitive. While the IL-10 level more than doubled (3.05% to 8.67%) (Fig. 4A and 4B), there was a slight increase in TNF-α (0.94% to 1.25%) (Figure 4C,D), and the IL-10/TNF-α ratio only increased 2-fold (Figure 3B).
Figure 2.
Flow cytometry analysis of mononuclear cells (90% of which were macrophages) freshly prepared from a sarcoidosis subject (sarcoidosis subject 16). Bronchoalveolar lavage (BAL) cells were plated and cultured for 16 h with (B,D,F) and without (A,C,E) the presence of bone marrow stromal cells (MSCs). Surface marker CD206 was used to identify alveolar macrophages (AMs), and intracellular flow cytometry was performed to determine changes in their cytokine production. Co-cultured BAL cells increased their IL-10 (A,B) and decreased their TNF-α (C,D) production. CD163 (a surface marker thought to indicate an anti-inflammatory state) also increased (E,F). These changes suggest a shift from a pro-inflammatory towards an anti-inflammatory state of AMs following contact with MSCs.
Figure 3.
Intracellular cytokine ratios of alveolar macrophages (AMs) suggest a change from M1 (pro-inflammatory) to M2 (anti-inflammatory) status after co-culture with bone marrow stromal cells (MSCs). (A) Bronchoalveolar lavage (BAL) cells are depicted in red, BAL cells from co-cultures are pictured in blue. The co-cultured BAL cells demonstrate a clear shift towards the right, indicating an increase in the mean fluorescent intensity (MFI) of the IL-10+ population, and upwards, indicating an increase in the MFI of CD206 in these cells. Due to the lower percentage of cells producing TNF-α, a leftward shift is not as evident, although an upward shift indicating increased MFI of CD206 in these cells is present. (B) The fold change of the ratio of IL-10 to TNF-α for the BAL–MSC co-culture is shown for both subjects (sarcoidosis subjects 16 and 17). BAL cells cultured alone are depicted by the red bars (the ratio was converted to a value of 1), and co-cultured BAL cells are depicted by the blue bars (representing the fold increase of the ratio). The bar graph shows that—although not to the same extent—the BAL cells shifted towards a more anti-inflammatory state in both subjects.
Figure 4.
Flow cytometry analysis of mononuclear cells (73% of which were macrophages) freshly prepared from a sarcoidosis subject (sarcoidosis subject 17). Bronchoalveolar lavage (BAL) cells were plated and cultured for 16 hours with (B,D,F) and without (A,C,E) the presence of bone marrow stromal cells (MSCs). Surface marker CD206 was used to identify alveolar macrophages (AMs), and intracellular flow cytometry was performed to determine changes in their cytokine production. Co-cultured BAL cells almost tripled their IL-10 (A,B) while their TNF-α (C,D) production barely changed. Interestingly, CD163 (a surface marker thought to indicate an anti-inflammatory state) decreased (E,F). Overall, these changes may suggest a shift from a pro-inflammatory towards an anti-inflammatory state of AMs following contact with MSCs.
Consistent with recent publications [28,29], our data confirm that CD206 was nearly ubiquitously expressed in the AΜ population of the sarcoidosis subjects investigated (Figure 2 and Figure 4). In both subjects, the mean fluorescent intensity of CD206 increased upon co-culture with MSCs (Figure 2, Figure 3A and Figure 4). CD163, a M2 macrophage marker, increased in AMs co-cultured with MSCs in subject 16 (10.9% to 15.8%) (Figure 2E,F), but decreased in subject 17 (27.4% to 24%) (Figure 4E,F) compared with AMs cultured alone. Furthermore, there was no CD163 heterogeneity observed in the AMs (Figure 2E,F and Figure 4E,F).
At the time point studied, CD11c and CD80 (two M1 macrophage markers) in AMs co-cultured with MSCs increased from 25.3% to 40.1% and from 20.9% to 41.1%, respectively, in subject 16 (Figure S3). In subject 17, CD11c and CD80 decreased from 56.9% to 55.2% and from 49.8% to 41.9%, respectively, compared with AMs alone (Figure S4). Similar results for the surface markers were seen in fixed and unfixed cells.
4. Discussion
Our results demonstrate that alveolar macrophages from an inflammatory environment (sarcoidosis patients) respond to cues from co-cultured MSCs and change to a more anti-inflammatory phenotype. Although this effect has been reported in other tissue macrophages, the finding that AMs from patients with sarcoidosis respond to MSCs has clinical significance and suggests that a cellular therapy should be tested.
We have shown previously that the inflammatory environment has a profound effect on MSC-mediated immunomodulation. Extracellular inflammatory signals, including pro-inflammatory cytokines, and small molecules such as prostaglandins and nitric oxide are able to augment the production of MSC-derived immunomodulatory factors and ultimately lead to enhanced immunosuppression [14]. Alveolar macrophages isolated from sarcoidosis patients are in an activated state and produce significantly higher levels of inflammatory cytokines, such as TNF-α compared with control AMs [30]. When MSCs are exposed to this sarcoidosis-derived inflammatory milieu (as opposed to a homeostatic, control macrophage-produced environment) they are more likely to induce an anti-inflammatory character in AMs, which could ultimately lead to a more consistent and substantial reduction in macrophage-secreted TNF-α and an increase in IL-10 levels.
Bone marrow stromal cells have been shown to have multiple immunomodulatory properties, including reprogramming of macrophages. MSCs change macrophages functionally and phenotypically from activated, pro-inflammatory (M1) cells to anti-inflammatory (M2) cells [14]. This reprogramming is mediated by the COX-2 pathway in MSCs, which increases their PGE2 production. PGE2 then binds to the E2 and E4 receptors on the surface of macrophages to induce increased IL-10 production. In addition, MSCs can attenuate T-cell activation and induce tolerance by interfering with the maturation of dendritic cells [31]; this may be due in part to induction of Signal Transducer and Activators of Transcription-3 (STAT-3) signaling and cell-to-cell contact [32]. Given the above, we questioned if MSCs might alleviate lung inflammation in patients with sarcoidosis.
Sarcoidosis is a multi-system disease characterized by noncaseating granulomatous inflammation in a Th1/Th17 cytokine environment with elevated levels of IL-2, IL-12, IL-17, TNF- α, and IFN-γ [24]. Potential targets for therapy include TNF-α-blocking agents (monoclonal antibodies) and other agents that target pro-inflammatory pathways [33]. In a small phase I trial, four patients with chronic sarcoidosis, stages II and III, were given placental mesenchymal-like cells intravenously twice in a one-week period and followed for two years. Two of the four patients were able to discontinue prednisone and had significant improvements in their chest radiographs [34].
Due to the lack of an appropriate animal model, we tested this hypothesis by isolating primary cells from subjects’ BAL and cultured briefly in the presence and absence of healthy human MSCs. This “ex-vivo” experimental setup mimics the interaction between MSCs and AMs. We looked for changes in cytokine production of the isolated AMs as well as changes in surface marker expression [35].
While changes in AM IL-10 and TNF-α production in the presence of MSCs (Figure 1B and Figure 3B) indicated transition to an anti-inflammatory state, surface marker expression of the AMs was not typical of what is seen in a M2 shift (Suppl. Figures S2A–D and S3A–D). This might be a result of different timing of expression between surface markers and cytokines. However, recent evidence suggests that AMs exhibit a hybrid M1/M2 phenotype [28,29], implying that we do not understand the unique dynamics of surface marker expression in this specific macrophage population at the current time. Therefore, the cytokine data may provide a more reliable readout of the inflammatory state of the AM population, and we may need to reconsider the M1/M2 paradigm as a general feature of all macrophages [36].
In our cytokine ELISA assays of co-culture medium, two sarcoidosis subjects did not follow the trend of decrease in TNF-α and increase in IL-10 (like all the other patients). In one subject (19 in Figure 1), both TNF-α and IL-10 decreased in the culture medium when his/her BAL cells were co-cultured with MSCs in the presence of LPS. This patient had an active sinus infection at the time of BAL harvest that might account for the deviation. The other unusual response had a slight increase in TNF-α, but a larger increase in IL-10 production (25 in Figure 1). This patient failed to stop his/her steroid intake as all the others did. It is important to mention though, that in spite of the deviation from the other responses, even in these two patients the change induced in the AMs by the MSCs was tilted towards anti-inflammatory change, shifting the ratio towards less TNF-α and more IL-10. In a separate assay, one of the two sarcoidosis subjects whose BAL was analyzed by flow cytometry showed a similar change—a small increase in TNF-α and a larger increase in IL-10. This patient had clinically inactive disease overall. It seems that macrophage-derived pro-inflammatory signals are necessary for the MSCs to exert their effect efficiently [37].
The use of MSCs in humans with pulmonary diseases has recently been reviewed [38,39]. We hypothesized that MSC cellular therapy, as a local treatment through the airways, might decrease lung inflammation in patients with sarcoidosis. The route of delivery of MSCs may also influence treatment efficacy and can affect which molecular pathways lead to therapeutic immunomodulation. When treating an airway disease, such as sarcoidosis, MSCs can be injected either intravenously or delivered directly into the airways. In rodents, intravenously injected MSCs are entrapped in the pulmonary vasculature, and hence will be concentrated in the lungs [40]. In humans, MSCs can be found in the lungs only temporarily after intravenous infusion, which is followed by quick redistribution into the reticuloendothelial system, primarily the spleen and the liver [41]. Intravascular delivery will initiate a process called instant-blood-mediated inflammatory reaction (IBMIR), which is a complex inflammatory phenomenon that will eventually eliminate living/apoptotic MSCs from capillaries and may result in a more sustained monocyte/macrophage-mediated anti-inflammatory state [42,43]. Intrabronchial delivery of MSCs will avoid IBMIR and will result in direct interactions with alveolar inflammatory cells, including alveolar macrophages and lymphocytes. Although it is unclear if either of these delivery methods would be superior in terms of treatment efficacy, intrabronchial delivery of MSCs may lead to a more concentrated intrapulmonary effect in humans, thereby increasing treatment efficacy, needing a lower number of cells and minimizing the risks of possible systemic, IBMIR-related thrombo-inflammatory side effects [42].
Our data suggest that MSCs should be further studied as a potential therapy for pulmonary sarcoidosis due to their ability to decrease inflammation and possibly reduce the need for steroids. MSCs have been used in clinical settings for over a decade now, and although they were not always efficacious (depending on the disease and trial conditions), the consensus is that the therapy is safe [9,44,45]. A pilot study in pre-term infants suggested that intratracheal administration of MSCs was feasible and free from significant adverse events [46]. Endobronchial delivery of MSCs is being tested in patients with idiopathic pulmonary fibrosis (NCT01919827); the results are pending. Phase 1 clinical trials using MSCs from bone marrow [47], placenta [48], and adipose tissue [49] in the treatment of idiopathic pulmonary fibrosis have demonstrated a safe profile without evidence of worsening fibrosis due to the therapy. Although idiopathic pulmonary fibrosis and sarcoidosis are different diseases, these data are important when thinking about cell therapy of sarcoidosis as M2 macrophages have been suggested to increase pulmonary fibrosis [50]. Whether MSCs inhibit or promote fibrosis in the human lung is currently unknown, although there is evidence in mice of both pro and antifibrotic activity [51].
Interestingly, in mice, the ontogeny of the macrophages appears to be crucial for the development of fibrosis [52]. Therefore, targeting specific macrophage populations to alter their profibrotic profile or prevent their entrance into the lung may prove beneficial. Unfortunately, there is currently no available mouse model for sarcoidosis, so the applicability of these studies to our patient population is unknown.
A variety of strategies have been tested to improve the efficiency of MSC therapy in respiratory diseases [53]. Kardia et al. [54] used fibroblast cells from rabbits, which resemble MSCs, to show that cells grown in tissue culture can be aerosolized without showing any sign of stress (i.e., vacuolation) afterwards. Most of the fibroblasts survived and proliferated rapidly. A recent study reported that MSC viability was better via compressed nebulization compared with ultrasonic or mesh device delivery [55]. In addition to optimizing the mode of administration, other details such as dose and timing need to be investigated. One advantage of MSCs is that they can be banked, and frozen aliquots can be studied and used. While questions have been raised about a possible loss of efficacy when cells are frozen [56], in certain clinical scenarios this may not be a serious problem [57]. Because of the possibility that cryopreservation may compromise the immunomodulatory activity of MSCs however [45], we used cultured, freshly trypsinized cells in our studies. It is unclear if, and to what extent, freezing would change the effect of MSCs on alveolar macrophages. Only future clinical trials can tell if there are clinically relevant differences between these two cellular products.
Given the known safety of MSC cellular therapy and the efficacy of these cells in countering inflammation, we suggest that their use in sarcoidosis could be steroid-sparing or supplant the need for systemic steroids.
Acknowledgments
We would like to thank Ildiko Szalayova for her expert help in running some ELISA assays and Elina Stregevsky (Combined Technical Research Core, NIDCR, NIH) for her kind and professional assistance and for the support by the Intramural Research Programs of NIDCR and NHLBI, NIH and the Dean’s Research Fund of Semmelweis University.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/1/278/s1. Figure S1: In vitro differentiation of MSCs; Figure S2: Optimization of coculture cell ratios; Figure S3: Flow cytometry analysis of M1 and M2 macrophage markers in subject 16; Figure S4: Flow cytometry analysis of M1 and M2 markers in subject 17. Table S1: Pulmonary functional results; Table S2: Laboratory results; Table S3: Peripheral blood lymphocyte phenotype; Table S4: Summary of clinical data of subjects 16 and 17; Table S5: BAL counts and peripheral blood lymphocyte phenotype in subjects 16 and 17.
Author Contributions
I.M.C., C.H., M.B., and M.K. collected and isolated BAL cells. I.M.C., K.N., G.S. and L.V.-C. set up and maintained cell cultures. G.S. performed in vitro differentiation assays, L.V.-C. characterized cells using flow cytometry, I.M.C., M.B., and M.K. performed ELISA assays. I.M.C., C.H., and E.M. collected and analyzed data. I.M.C., C.H., and J.R.F. assisted with manuscript preparation. E.M. wrote the manuscript. C.H. developed intracellular flow cytometry protocol. C.H. and E.M. prepared Figures and formatted Tables. J.R.F. prepared Tables. K.N. and E.M. designed the study. C.H., J.M., and J.R.F. participated in study design. K.N. set up the cytokine assays. S.M. collected BAL fluid and subjects’ intake. M.A. performed flow cytometry optimization. J.R.F. performed all bronchoscopies and collected and evaluated patients’ data. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Intramural Research Programs of NIDCR and NHLBI, NIH and the Dean’s Research Fund of Semmelweis University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Phinney D.G. Functional heterogeneity of mesenchymal stem cells: Implications for cell therapy. J. Cell Biochem. 2012;113:2806–2812. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 2.Caplan A.I. Mesenchymal stem cells. J. Orthop. Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 3.Le Blanc K., Rasmusson I., Sundberg B., Gotherstrom C., Hassan M., Uzunel M., Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y., Ding Z., Han C., Shi H., Cui L., Lin R. Efficacy of Mesenchymal Stromal Cells for Fistula Treatment of Crohn’s Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2017;62:851–860. doi: 10.1007/s10620-017-4453-x. [DOI] [PubMed] [Google Scholar]
- 5.Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K., Rowlands D.J., Quadri S.K., Bhattacharya S., Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat. Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal S., Pittenger M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 7.Nauta A.J., Fibbe W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 8.Zhao R., Su Z., Wu J., Ji H.L. Serious adverse events of cell therapy for respiratory diseases: A systematic review and meta-analysis. Oncotarget. 2017;8:30511–30523. doi: 10.18632/oncotarget.15426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Blanc K., Davies L.C. MSCs-cells with many sides. Cytotherapy. 2018;20:273–278. doi: 10.1016/j.jcyt.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Chiossone L., Conte R., Spaggiari G.M., Serra M., Romei C., Bellora F., Becchetti F., Andaloro A., Moretta L., Bottino C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells. 2016;34:1909–1921. doi: 10.1002/stem.2369. [DOI] [PubMed] [Google Scholar]
- 11.Kim J., Hematti P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp. Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stagg J., Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Curr. Mol. Med. 2013;13:856–867. doi: 10.2174/1566524011313050016. [DOI] [PubMed] [Google Scholar]
- 13.Vasandan A.B., Jahnavi S., Shashank C., Prasad P., Kumar A., Prasanna S.J. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci. Rep. 2016;6:38308. doi: 10.1038/srep38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemeth K., Leelahavanichkul A., Yuen P.S., Mayer B., Parmelee A., Doi K., Robey P.G., Leelahavanichkul K., Koller B.H., Brown J.M., et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S., von Bonin M., Barbieri L., Halai K., Ward S., et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017:9. doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 16.Fehrenbach H., Zissel G., Goldmann T., Tschernig T., Vollmer E., Pabst R., Muller-Quernheim J. Alveolar macrophages are the main source for tumour necrosis factor-alpha in patients with sarcoidosis. Eur. Respir. J. 2003;21:421–428. doi: 10.1183/09031936.03.00083002. [DOI] [PubMed] [Google Scholar]
- 17.Iannuzzi M.C., Fontana J.R. Sarcoidosis: Clinical presentation, immunopathogenesis, and therapeutics. JAMA. 2011;305:391–399. doi: 10.1001/jama.2011.10. [DOI] [PubMed] [Google Scholar]
- 18.Patterson K.C., Chen E.S. The Pathogenesis of Pulmonary Sarcoidosis and Implications for Treatment. Chest. 2017 doi: 10.1016/j.chest.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 19.Grunewald J., Olerup O., Persson U., Ohrn M.B., Wigzell H., Eklund A. T-cell receptor variable region gene usage by CD4+ and CD8+ T cells in bronchoalveolar lavage fluid and peripheral blood of sarcoidosis patients. Proc. Natl. Acad. Sci. USA. 1994;91:4965–4969. doi: 10.1073/pnas.91.11.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katchar K., Wahlstrom J., Eklund A., Grunewald J. Highly activated T-cell receptor AV2S3(+) CD4(+) lung T-cell expansions in pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 2001;163:1540–1545. doi: 10.1164/ajrccm.163.7.2005028. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann S., Franke A., Fischer A., Jacobs G., Nothnagel M., Gaede K.I., Schurmann M., Muller-Quernheim J., Krawczak M., Rosenstiel P., et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat. Genet. 2008;40:1103–1106. doi: 10.1038/ng.198. [DOI] [PubMed] [Google Scholar]
- 22.Valentonyte R., Hampe J., Huse K., Rosenstiel P., Albrecht M., Stenzel A., Nagy M., Gaede K.I., Franke A., Haesler R., et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat. Genet. 2005;37:357–364. doi: 10.1038/ng1519. [DOI] [PubMed] [Google Scholar]
- 23.Kjellin H., Silva E., Branca R.M., Eklund A., Jakobsson P.J., Grunewald J., Lehtio J., Wheelock A.M. Alterations in the membrane-associated proteome fraction of alveolar macrophages in sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2016;33:17–28. [PubMed] [Google Scholar]
- 24.Facco M., Cabrelle A., Teramo A., Olivieri V., Gnoato M., Teolato S., Ave E., Gattazzo C., Fadini G.P., Calabrese F., et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011;66:144–150. doi: 10.1136/thx.2010.140319. [DOI] [PubMed] [Google Scholar]
- 25.James W.E., Baughman R. Treatment of sarcoidosis: Grading the evidence. Expert Rev. Clin. Pharm. 2018;11:677–687. doi: 10.1080/17512433.2018.1486706. [DOI] [PubMed] [Google Scholar]
- 26.Scadding J.G. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br. Med. J. 1961;2:1165–1172. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nemeth K., Mayer B., Sworder B.J., Kuznetsov S.A., Mezey E. A practical guide to culturing mouse and human bone marrow stromal cells. Curr. Protoc. Immunol. 2013:102. doi: 10.1002/0471142735.im22f12s102. [DOI] [PubMed] [Google Scholar]
- 28.Mitsi E., Kamng’ona R., Rylance J., Solorzano C., Jesus Reine J., Mwandumba H.C., Ferreira D.M., Jambo K.C. Human alveolar macrophages predominately express combined classical M1 and M2 surface markers in steady state. Respir. Res. 2018;19:66. doi: 10.1186/s12931-018-0777-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y.R., Hotten D.F., Malakhau Y., Volker E., Ghio A.J., Noble P.W., Kraft M., Hollingsworth J.W., Gunn M.D., Tighe R.M. Flow Cytometric Analysis of Myeloid Cells in Human Blood, Bronchoalveolar Lavage, and Lung Tissues. Am. J. Respir. Cell Mol. Biol. 2016;54:13–24. doi: 10.1165/rcmb.2015-0146OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng L., Teschler H., Guzman J., Hubner K., Striz I., Costabel U. Alveolar macrophage TNF-alpha release and BAL cell phenotypes in sarcoidosis. Am. J. Respir. Crit. Care Med. 1995;152:1061–1066. doi: 10.1164/ajrccm.152.3.7663784. [DOI] [PubMed] [Google Scholar]
- 31.Beyth S., Borovsky Z., Mevorach D., Liebergall M., Gazit Z., Aslan H., Galun E., Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 32.Gur-Wahnon D., Borovsky Z., Beyth S., Liebergall M., Rachmilewitz J. Contact-dependent induction of regulatory antigen-presenting cells by human mesenchymal stem cells is mediated via STAT3 signaling. Exp. Hematol. 2007;35:426–433. doi: 10.1016/j.exphem.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Le V., Crouser E.D. Potential immunotherapies for sarcoidosis. Expert Opin. Biol. Ther. 2018;18:399–407. doi: 10.1080/14712598.2018.1427727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baughman R.P., Culver D.A., Jankovi V., Fischkoff S., Brockway G., Lower E.E. Placenta-derived mesenchymal-like cells (PDA-001) as therapy for chronic pulmonary sarcoidosis: A phase 1 study. Sarcoidosis Vasc. Diffus. Lung Dis. 2015;32:106–114. [PubMed] [Google Scholar]
- 35.Roszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hussell T., Bell T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 37.Mezey E., Nemeth K. Mesenchymal stem cells and infectious diseases: Smarter than drugs. Immunol. Lett. 2015;168:208–214. doi: 10.1016/j.imlet.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Broekman W., Khedoe P., Schepers K., Roelofs H., Stolk J., Hiemstra P.S. Mesenchymal stromal cells: A novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax. 2018;73:565–574. doi: 10.1136/thoraxjnl-2017-210672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruk D., Heijink I.H., Slebos D.J., Timens W., Ten Hacken N.H. Mesenchymal Stromal Cells to Regenerate Emphysema: On the Horizon? Respiration. 2018 doi: 10.1159/000488149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eggenhofer E., Benseler V., Kroemer A., Popp F.C., Geissler E.K., Schlitt H.J., Baan C.C., Dahlke M.H., Hoogduijn M.J. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front. Immunol. 2012;3:297. doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gholamrezanezhad A., Mirpour S., Bagheri M., Mohamadnejad M., Alimoghaddam K., Abdolahzadeh L., Saghari M., Malekzadeh R. In vivo tracking of 111In-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl. Med. Biol. 2011;38:961–967. doi: 10.1016/j.nucmedbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Caplan H., Olson S.D., Kumar A., George M., Prabhakara K.S., Wenzel P., Bedi S., Toledano-Furman N.E., Triolo F., Kamhieh-Milz J., et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019;10:1645. doi: 10.3389/fimmu.2019.01645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moll G., Ankrum J.A., Kamhieh-Milz J., Bieback K., Ringden O., Volk H.D., Geissler S., Reinke P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol. Med. 2019;25:149–163. doi: 10.1016/j.molmed.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Thomspon M., Wolfe D., Champagne J., Mei S.H., Lalu M., Fergusson D., Winston B., Marshall J., Walley K., English S., et al. Safety of cell therapy with mesenchymal stromal cells): An updated systematic review and meta-analysis of randomized controlled trials (safecell update) Cytotherapy. 2018;20:S53–S54. doi: 10.1016/j.jcyt.2018.02.146. [DOI] [Google Scholar]
- 45.Moll G., Geissler S., Catar R., Ignatowicz L., Hoogduijn M.J., Strunk D., Bieback K., Ringden O. Cryopreserved or Fresh Mesenchymal Stromal Cells: Only a Matter of Taste or Key to Unleash the Full Clinical Potential of MSC Therapy? Adv. Exp. Med. Biol. 2016;951:77–98. doi: 10.1007/978-3-319-45457-3_7. [DOI] [PubMed] [Google Scholar]
- 46.Gao P., Zhou Y., Xian L., Li C., Xu T., Plunkett B., Huang S.K., Wan M., Cao X. Functional effects of TGF-beta1 on mesenchymal stem cell mobilization in cockroach allergen-induced asthma. J. Immunol. 2014;192:4560–4570. doi: 10.4049/jimmunol.1303461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glassberg M.K., Minkiewicz J., Toonkel R.L., Simonet E.S., Rubio G.A., DiFede D., Shafazand S., Khan A., Pujol M.V., LaRussa V.F., et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest. 2017;151:971–981. doi: 10.1016/j.chest.2016.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chambers D.C., Enever D., Ilic N., Sparks L., Whitelaw K., Ayres J., Yerkovich S.T., Khalil D., Atkinson K.M., Hopkins P.M. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19:1013–1018. doi: 10.1111/resp.12343. [DOI] [PubMed] [Google Scholar]
- 49.Tzouvelekis A., Paspaliaris V., Koliakos G., Ntolios P., Bouros E., Oikonomou A., Zissimopoulos A., Boussios N., Dardzinski B., Gritzalis D., et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J. Transl. Med. 2013;11:1–13. doi: 10.1186/1479-5876-11-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pechkovsky D.V., Prasse A., Kollert F., Engel K.M., Dentler J., Luttmann W., Friedrich K., Muller-Quernheim J., Zissel G. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin. Immunol. 2010;137:89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 51.Tzouvelekis A., Toonkel R., Karampitsakos T., Medapalli K., Ninou I., Aidinis V., Bouros D., Glassberg M.K. Mesenchymal Stem Cells for the Treatment of Idiopathic Pulmonary Fibrosis. Front. Med. 2018;5:1–8. doi: 10.3389/fmed.2018.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Misharin A.V., Morales-Nebreda L., Reyfman P.A., Cuda C.M., Walter J.M., McQuattie-Pimentel A.C., Chen C.I., Anekalla K.R., Joshi N., Williams K.J.N., et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J. Exp. Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silva L.H.A., Antunes M.A., Dos Santos C.C., Weiss D.J., Cruz F.F., Rocco P.R.M. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res. Ther. 2018;9:45. doi: 10.1186/s13287-018-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kardia E., Yusoff N.M., Zakaria Z., Yahaya B. Aerosol-based delivery of fibroblast cells for treatment of lung diseases. J. Aerosol Med. Pulm. Drug Deliv. 2014;27:30–34. doi: 10.1089/jamp.2012.1020. [DOI] [PubMed] [Google Scholar]
- 55.Aver’yanov A.V., Konoplyannikov A.G., Antonov N.S., Osipova G.L., Vasil’eva O.S., Sakharova M.G., Tatarskii A.R., Kobylyansky V.I. Survival of Mesenchymal Stem Cells in Different Methods of Nebulization. Bull. Exp. Biol. Med. 2018;164:576–578. doi: 10.1007/s10517-018-4034-9. [DOI] [PubMed] [Google Scholar]
- 56.Chinnadurai R., Copland I.B., Garcia M.A., Petersen C.T., Lewis C.N., Waller E.K., Kirk A.D., Galipeau J. Cryopreserved Mesenchymal Stromal Cells Are Susceptible to T-Cell Mediated Apoptosis Which Is Partly Rescued by IFNgamma Licensing. Stem Cells. 2016;34:2429–2442. doi: 10.1002/stem.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cruz F.F., Borg Z.D., Goodwin M., Sokocevic D., Wagner D., McKenna D.H., Rocco P.R., Weiss D.J. Freshly thawed and continuously cultured human bone marrow-derived mesenchymal stromal cells comparably ameliorate allergic airways inflammation in immunocompetent mice. Stem Cells Transl. Med. 2015;4:615–624. doi: 10.5966/sctm.2014-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.