Abstract
Background: Acute pulmonary embolism (PE) is characterized hemodynamically by abrupt obstruction in trans-pulmonary blood flow. The echocardiographic Pulmonary to Left Atrial ratio (ePLAR, tricuspid regurgitation Vmax/mitral E/e’) has been validated as a non-invasive surrogate for trans-pulmonary gradient (TPG) that accurately differentiates pre-capillary from post-capillary chronic pulmonary hypertension. This study assessed ePLAR as an incremental echocardiographic assessment tool compared with traditional measures of right ventricular pressure and function. Methods: In total, 110 (57.4 ± 17.6 years) patients with confirmed sub-massive pulmonary emboli with contemporaneous echocardiograms (0.3 ± 0.9 days) were compared with 110 age-matched controls (AMC). Results: Tricuspid velocities were higher than AMC (2.6 ± 0.6 m/s vs. 2.4 ± 0.3 m/s, p < 0.05), although still consistent with “normal” right ventricular systolic pressures (34.2 ± 13.5 mmHg vs. 25 ± 5.3 mmHg, p < 0.05) with lower mitral E/e’ values (8.2 ± 3.8 vs. 10.8 ± 5.1, p < 0.05). ePLAR values were higher than AMC (0.36 ± 0.14 m/s vs. 0.26 ± 0.10, p < 0.05) suggesting significantly elevated TPG. Detection of abnormal echocardiographic findings increased from 29% (TRVmax ≥ 2.9 m/s) and 32% (reduced tricuspid annular plane systolic excursion) to 70% with ePLAR ≥ 0.3 m/s. Conclusions: Raised ePLAR values in acute sub-massive pulmonary embolism suggest elevated trans-pulmonary gradients even in the absence of acutely increased pulmonary artery pressures. ePLAR dramatically increases the sensitivity of echocardiography for detection of hemodynamic perturbations in sub-massive pulmonary embolism patients, which may offer clinical utility in diagnosis and management.
Keywords: pulmonary embolus, echocardiography, ePLAR
1. Background
Clinically, presentations of acute pulmonary embolism typically include acute progressive shortness of breath with associated pleuritic chest pain, with or without cough/hemoptysis. Physiologic derangements manifest as tachycardia, syncope/presyncope, and in severe cases may result in systemic hypotension, hemodynamic instability, or shock. Physiologically, this phenomenon occurs due to a sudden impairment of blood flow from the right heart through the lungs to the left heart with a consequent reduction in systemic blood flow. Echocardiographically, the abrupt increase in trans-pulmonary gradient leads to acute right ventricular dysfunction (often with the classic distribution of McConnell’s sign), with or without increased right ventricular systolic pressure. Obstructed trans-pulmonary flow leads to a decrease in left atrial filling pressure, with consequently reduced left ventricular filling that may result systemic hypo-perfusion and/or cardiogenic shock.
Acute pulmonary embolism (PE) presentations carry a mortality rate as a high as 25% in the first 30 days [1]. As such, prompt and thorough evaluation and management is crucial in minimizing the potential morbidity and mortality associated [2]. The “gold standard” diagnosis of acute pulmonary embolism is via computed tomography pulmonary angiogram (CTPA), to anatomically assess clot burden and distribution [3]. Ventilation perfusion scintigraphy (V/Q scan) has comparative utility, via assessment of the burden of mismatched perfusion defects, as a diagnostic and prognostic measure [4]. The hemodynamic functional effect of PE however is typically assessed by echocardiography [5]. Specifically, patients are assessed for evidence of right ventricular (RV) dysfunction/enlargement and/or significant pulmonary hypertension, manifest as elevated right ventricular systolic pressures [6]. Based on European Society of Cardiology Guidelines 2014 [7], immediate thrombolysis is both justified and recommended in the setting of significant RV dysfunction and associated cardiogenic shock or hemodynamic instability. This includes instances where CTPA may not be feasible prior to echocardiographic evaluation.
In this setting, significant right ventricular dysfunction may be evidenced by reduced right ventricular function, e.g., McConnell’s sign (RV free wall akinesia with apical wall hypercontractility) [8], reduced TAPSE (tricuspid annular plane systolic excursion) [9], reduced tricuspid annular Doppler Tissue Imaging S’-velocity (RV S’) [10], and, more recently, reduced RV free wall longitudinal strain [11,12]. All of these are functional assessments, which suggest right ventricular dysfunction in the setting of acutely increased afterload. Importantly, however, the absence of RV dysfunction on initial echocardiography does not definitively exclude significant embolic burden in hemodynamically, normotensive stable patients [7]. Furthermore, these measurements of RV dysfunction may be confounded by RV pathology (for example with RV wall infarct, or pre-existing pulmonary hypertension) [13]. Conversely, in hemodynamically unstable patients with suspected high risk PE, the absence of echocardiographic evidence of RV dysfunction or overload can effectively exclude PE [5].
The pathophysiologic underpinning of the hemodynamic perturbation of acute PE is pre-capillary obstruction to trans-pulmonary flow. The novel parameter ePLAR (the echocardiographic Pulmonary to Left Atrial Ratio) has been validated as a non-invasive surrogate to trans-pulmonary gradient [14]. ePLAR, which assesses the relationship between right ventricular systolic pressure and left atrial pressure via the formula,
| ePLAR (m/s) = TRVmax (m/s)/mitral E/e’ | (1) |
has been shown to correlate well with invasively-derived trans-pulmonary gradient (TPG). ePLAR non-invasively differentiates between pre-capillary and post-capillary pulmonary hypertension in patients being investigated for consideration of specific pulmonary vasodilator therapies. Higher ePLAR values suggest higher trans-pulmonary gradients and pre-capillary pulmonary hypertension. Lower ePLAR values suggest higher left atrial pressures with minimal increase in trans-pulmonary gradient and post-capillary pulmonary hypertension (see Figure 1 [14]). It has also been shown that with age, as left heart filling pressures naturally rise, ePLAR declines linearly. Thus, consideration of normal vs. abnormal values in a given patient must take into consideration age.
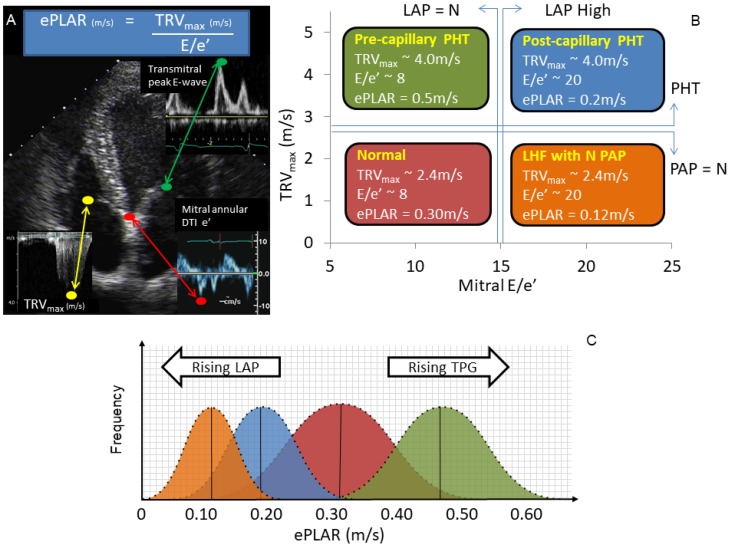
Figure 1.
ePLAR (echocardiographic Pulmonary to Left Atrial ratio = tricuspid regurgitation Vmax/mitral E/e’), explanation and example data. (A) The ePLAR comprises three simple measurements: peak tricuspid regurgitation continuous wave velocity (TRVmax) (m/s) divided by the trans-mitral peak pulsed wave Doppler E wave (cm/s) Peak Doppler Tissue Imaging mitral septal annular e’ wave (E/e’) (cm/s). (B) The four nominal patient subsets clinically encountered are demonstrated with predicted bell curves displayed. (C) Normal cases (red) will have normal pulmonary artery pressure (PAP) and left atrial pressure (LAP), normal TRVmax (e.g., 2.4 m/s), normal E/e’ (e.g., 8), and a predicted ePLAR of approximately 0.30 m/s. Patients with left heart failure (LHF) but with normal pulmonary arterial pressures (PAPmean < 25 mmHg) will have normal TRVmax (e.g., 2.4 m/s) with a high E/e’ (e.g., 20), yielding an ePLAR of approximately 0.12 m/s (orange). Patients with post-capillary pulmonary hypertension secondary to LHF will have a high TRVmax (e.g., 4.0 m/s) and a high E/e’ (e.g., 20), yielding an ePLAR of approximately 0.2 m/s (blue). Patients with pre-capillary pulmonary hypertension will have a high TRVmax (e.g., 4.0 m/s) with a normal E/e’ (e.g., 8), yielding the highest of ePLAR values-approximately 0.50 m/s in this example (green). (C) ePLAR will be higher than normal in patients with pre-capillary physiology (rising TPG) and lower than normal in patients with post-capillary physiology (rising LAP). Figure reproduced with permission [14].
It is hypothesized that ePLAR (as a measure of trans-pulmonary gradient) may have a higher diagnostic yield than other previously utilized echocardiographic markers of elevated pulmonary pressures (as assessed by TRVmax/RVSP) and RV dysfunction (reduced TAPSE or RV S’) in detecting hemodynamic perturbations in patients with acute sub-massive pulmonary embolism. Abnormally high ePLAR levels would likely be comprised of a small (if any) increase in TRVmax with significantly reduced mitral E/e’ (consistent with reduced left atrial filling pressures). Increased ePLAR in these patients will suggest increased TPG even in the absence of actual elevated right heart pressures or right ventricular dysfunction.
2. Methods
2.1. Patient Inclusion
Consecutive patients referred to three Major Australian Hospital echocardiography laboratories with pulmonary emboli confirmed by CT pulmonary angiography (CTPA) or ventilation/perfusion (V/Q) scintigraphy were studied. Patients with massive embolic load associated with shock requiring resuscitation were not included in this study. All remaining patients were shown to have sub-massive embolic burden (bilateral or saddle). All patients were studied at the time of diagnosis (< ±72 h) with comprehensive echocardiography. Human Research and Ethics Committee approval for review of data was obtained from each institution. A further cohort of age-matched, paired “normal subjects” was generated with permission from the echocardiography database of the referring institutions. These subjects were defined as normal via the criteria: normal left ventricular size and function (systolic and diastolic), normal right ventricular size and function, normal intracardiac valves, and no clinical history of pulmonary embolism, lung disease, or pulmonary arterial hypertension.
2.2. Echocardiographic Methods
All patients referred for transthoracic echocardiography with the diagnosis of suspected pulmonary embolism were studied with comprehensive 2D, Doppler and strain analysis (where available). Left heart function, size, and valvular function was assessed as per standard protocols. Left heart diastolic filling was assessed using pulsed wave Doppler at the mitral tips according to the ASE guidelines [15] Mitral annular Doppler Tissue Imaging (DTI) velocities were assessed in the annulus (septal and lateral). Right heart function was assessed using tricuspid annular plane systolic excursion (TAPSE) measured from color DTI M-mode. Tricuspid annular systolic DTI peak velocity (RV S’) was obtained by placing a cursor slightly distal to the lateral annulus in the four-chamber view. All measurements were averaged over three beats in sinus rhythm and five beats in atrial fibrillation.
2.3. Statistical Methods
Linear measurements were assessed using normalized ratios and student t-tests. Parametric variables were evaluated using the Chi squared student test. Significance was defined by p < 0.05. Graphical patient data were compared with a previously generated “standardized population” of 1000 subjects as reference for ePLAR and TRVmax values, stratified by decade (see Figure 2) [14]. Comparisons of the patients with their age-matched controls was used to generate sensitivity and specificity for each echocardiographic parameter in predicting the presence of pulmonary embolism. Receiver operator curves (ROC) curves were generated using GraphPad Prism 8, via the Hanley method, to compare the predictive power of tested parameters for pulmonary embolism.
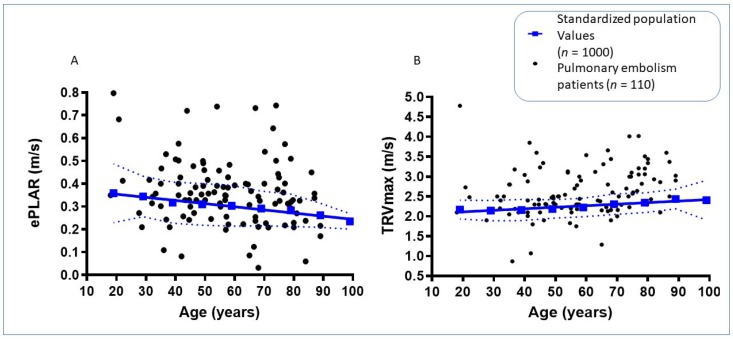
Figure 2.
(A) Age stratified echocardiographic ePLAR values (calculated as TRVmax/mitral E/e’) for CTPA or V/Q scan confirmed acute pulmonary embolism patients (n = 110) vs. ePLAR of previously documented “standardized population” [14]. (B) Age stratified echocardiographically obtained TRVmax values for patients with confirmed PE vs. “standardized normal population”.
3. Results
There were 223 patients referred for acute echocardiography with the diagnosis of possible/probable/proven sub-massive pulmonary embolism. Of these, 88 patients were subsequently shown to either not have sub-massive pulmonary emboli (32 patients with small/lobar pulmonary emboli and 56 with negative CTPA/VQ scans for PE) or had a delay of >72 h from CTPA/VQ to echocardiography. A further 25 patients were excluded with incomplete datasets. The remaining 110 patients (64 male, aged 57.4 ± 17.6 years) were shown to have bilateral pulmonary emboli (n = 97) or saddle emboli (n = 13) by CTPA/VQ scan. Echocardiography was performed at 0.3 ± 0.9 days from CTPA/VQ (range −3 to +3 days). Demographics of the patients are shown in Table 1, with statistical comparison to the aged matched control cohort.
Table 1.
Demographics of pulmonary embolism patients compared with age-matched control cohort.
| PE Patients | Age-Matched Controls | Significance (p < 0.05) | |
|---|---|---|---|
| n | 110 | 110 | - |
| Age (years) | 57.4 ± 17.6 | 58.1 ± 17.8 | 0.76 |
| Male (%) | 58 | 60 | - |
| BSA (m2) | 2.1 ± 0.3 | 1.98 ± 0.27 | 0.01 |
| Systolic blood pressure (mmHg) | 124.9 ± 15.7 | 121.5 ± 20.6 | 0.42 |
| Diastolic blood pressure (mmHg) | 74.3 ± 8.5 | 74.2 ± 7.4 | 0.93 |
| TRVmax (m/s) | 2.61 ± 0.61 | 2.36 ± 0.28 | 0.0001 |
| ePLAR (m/s) | 0.36 ± 0.14 | 0.26 ± 0.10 | <0.0001 |
| RVSP (mmHg) | 34.18 ± 13.49 | 25 ± 5.3 | <0.0001 |
| TAPSE (mm) | 21.08 ± 6.31 | 20.60 ± 5.91 | 0.60 |
| RV S’ (cm/s) | 13.49 ± 3.58 | 12.6 ± 3.3 | <0.0001 |
| Mitral E/e’ | 8.2 ± 3.8 | 10.8 ± 5.1 | 0.01 |
PE, pulmonary embolus; BSA, body surface area; TRV, Tricuspid regurgitation maximum continuous-wave Doppler velocity; ePLAR, echocardiographic Pulmonary to Left Atrial Ratio (ePLAR = tricuspid regurgitation Vmax/mitral E/e’; RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion; RV S’, right ventricular peak Doppler Tissue Imaging systolic velocity.
Based on recent guideline definitions of pulmonary hypertension [12], a TRVmax > 2.9 m/s was defined as indicative of elevated right ventricular systolic pressure. Based on the previous validation of ePLAR [14], values > 0.28 m/s were considered to be indicative of elevated TPG. Normal values for TAPSE were considered excursions distances ≥17 mm. Normal values for tricuspid annular Doppler Tissue Imaging systolic velocity (RV S’) were considered ≥9.5 cm/s [16].
Using these cut-off values, patients were separated analyzed in dichotomous assessments of ePLAR vs. TRVmax (Table 2 and Figure 3A), ePLAR vs. TAPSE (Table 3 and Figure 3B), and ePLAR vs. RV S’ (Table 4 and Figure 3C). For each respective analysis, four groups were defined: Group 1 (abnormal TRVmax or TAPSE or RV S’, elevated ePLAR ≥ 0.28 m/s), Group 2 (normal TRVmax or TAPSE or RVS’, elevated Eplar ≥ 0.28 m/s), Group 3 (abnormal TRVmax or TAPSE or RVS’, normal ePLAR < 0.28 m/s), and Group 4 (normal TRVmax or TAPSE or RVS’, normal ePLAR < 0.28 m/s).
Table 2.
Demographic and echocardiographic evaluation by Group ePLAR vs. TRVmax.
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| N | 27 | 48 | 5 | 30 |
| Age (years) | 62.95 ± 17.54 | 54.89 ± 16.03 | 77.77 ± 9.47 | 53.02 ± 18.31 |
| Male (%) | 63 | 60 | 20 | 57 |
| BSA (m2) | 2.14 ± 0.34 | 2.10 ± 0.27 | 1.97 ± 0.3 | 2.08 ± 0.42 |
| Baseline HR (bpm) | 76.8 ± 20.7 | 75.2 ± 11.84 | 66.0 ± 9.2 | 67.74 ± 18.31 |
| Time to echo (days) | 0.22 ± 1.09 | 0.23 ± 0.66 | 0.60 ± 0.89 | 0.53 ± 1.04 |
| TRVmax (m/s) | 3.38 ± 0.42 | 2.42 ± 0.26 | 3.19 ± 0.19 | 2.11 ± 0.44 |
| ePLAR (m/s) | 0.48 ± 0.08 | 0.42 ± 0.13 | 0.20 ± 0.08 | 0.20 ± 0.07 |
| TAPSE (mm) | 18.17 ± 5.46 | 24.23 ± 6.57 | 18.25 ± 3.87 | 19.79 ± 6.43 |
| S’(cm/s) | 12.68 ± 3.50 | 14.44 ± 3.95 | 12.00 ± 3.87 | 13.17 ± 2.85 |
Group 1 (Abnormal TRVmax, elevated ePLAR ≥ 0.28 m/s), Group 2 (normal TRVmax, elevated ePLAR ≥ 0.28 m/s), Group 3 (Abnormal TRVmax, normal ePLAR < 0.28 m/s), and Group 4 (normal TRVmax, normal ePLAR < 0.28 m/s).
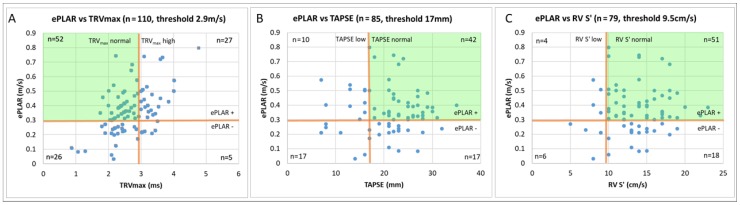
Figure 3.
Graphical display of the positive and negative diagnostic yield of ePLAR (ePLAR+ defined as ePLAR > 0.3 m/s, ePLAR-defined as ePLAR ≤ 0.3 m/s) versus TRVmax ((A) TRVmax normal defined as TRVmax ≤ 2.9 m/s, high TRVmax > 2.9 m/s), TAPSE ((B) TAPSE normal defined as TAPSE ≥ 17 mm, low TAPSE < 17 mm), and RV S’ ((C) RV S’ normal defined as RV S’ ≥ 9.5 cm/s, low RV S’ < 9.5 cm/s). Incremental diagnostic yield of ePLAR (green shaded quadrants) represented as patients with true positive ePLAR and false negative relative comparison parameter.
Table 3.
Demographic and echocardiographic evaluation by Group ePLAR vs. TAPSE.
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| N | 5 | 47 | 6 | 18 |
| Age (years) | 72.64 ± 7.81 | 54.59 ± 18.28 | 59.50 ± 19.67 | 60.82 ± 19.41 |
| Male (%) | 20 | 55 | 67 | 44 |
| BSA (m2) | 2.08 ± 0.3 | 2.09 ± 0.35 | 1.97 ± 0.31 | 1.98 ± 0.34 |
| Baseline HR (bpm) | 68.0 ± 9.1 | 73.1 ± 10.26 | 66.0 ± 8.0 | 62.8 ± 8.18 |
| Time to echo (days) | 0.20 ± 2.17 | 0.32 ± 0.81 | 0.00 ± 0.3 | 1.06 ± 1.21 |
| TRVmax (m/s) | 3.20 ± 0.69 | 2.72 ± 0.59 | 2.39 ± 0.48 | 2.22 ± 0.72 |
| ePLAR (m/s) | 0.47 ± 0.14 | 0.40 ± 0.10 | 0.22 ± 0.01 | 0.21 ± 0.08 |
| TAPSE (mm) | 10.80 ± 3.03 | 23.30 ± 4.66 | 9.17 ± 2.79 | 22.11 ± 4.07 |
| S’ (cm/s) | 9.80 ± 1.79 | 14.48 ± 3.43 | 8.00 ± 1.73 | 13.67 ± 2.91 |
Group 1 (Abnormal TAPSE, elevated ePLAR ≥ 0.28 m/s), Group 2 (normal TAPSE, elevated ePLAR ≥ 0.28 m/s), Group 3 (Abnormal TAPSE, normal ePLAR < 0.28 m/s), and Group 4 (normal TAPSE, normal ePLAR < 0.28 m/s).
Table 4.
Demographic and echocardiographic evaluation by Group ePLAR vs. RV S’.
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| N | 4 | 51 | 6 | 18 |
| Age (years) | 62.75 ± 17.86 | 54.76 ± 18.26 | 64.17 ± 20.83 | 61.32 ± 20.03 |
| Male (%) | 25 | 53 | 67 | 44 |
| BSA (m2) | 2.14 ± 0.32 | 2.07 ± 0.33 | 1.97 ± 0.3 | 1.98 ± 0.34 |
| Baseline HR (bpm) | 76.8 ± 20.7 | 75.2 ± 14.93 | 66.0 ± 9.1 | 71.3 ± 8.18 |
| Time to echo (days) | −0.25 ± 1.89 | 0.33 ± 0.86 | 0.33 ± 0.82 | 0.94 ± 1.21 |
| TRVmax (m/s) | 3.26 ± 0.54 | 2.72 ± 0.59 | 2.60 ± 0.63 | 2.19 ± 0.68 |
| ePLAR (m/s) | 0.46 ± 0.10 | 0.42 ± 0.13 | 0.19 ± 0.08 | 0.20 ± 0.07 |
| TAPSE (mm) | 13.0 ± 6.68 | 22.8 ± 5.17 | 10.7 ± 4.13 | 22.4 ± 3.99 |
| S’ (cm/s) | 8.5 ± 0.58 | 14.3 ± 3.32 | 7.8 ± 1.60 | 14.1 ± 2.40 |
Group 1 (Abnormal RV S’, elevated ePLAR ≥ 0.28 m/s), Group 2 (normal RV S’, elevated ePLAR ≥ 0.28 m/s), Group 3 (Abnormal RV S’, normal ePLAR < 0.28 m/s), and Group 4 (normal RV S’, normal ePLAR < 0.28 m/s).
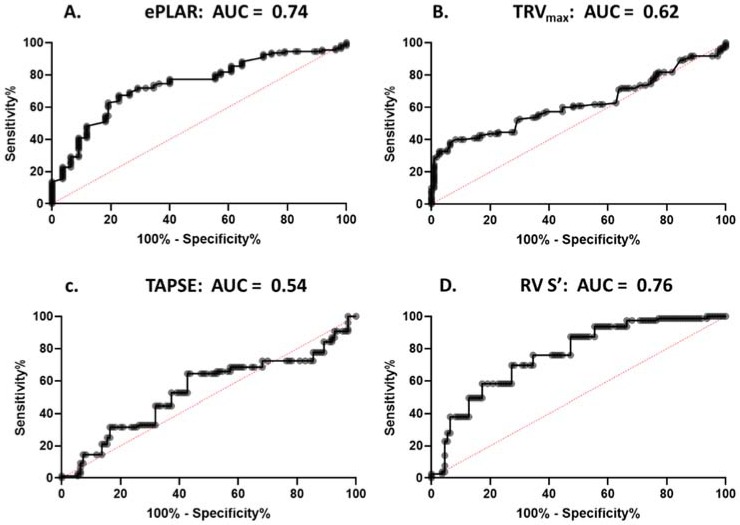
Using the standardized cut–off values listed above, sensitivity and specificity data were calculated for the prediction of pulmonary embolism, in comparison to the age matched control cohort. As shown in Table 5, ePLAR performed most strongly with sensitivity 72% (confidence interval (CI) 62–80%), specificity 66% (CI 57–75%), positive predictive value 68% (CI 62–74%), and negative predictive value 70% (CI 63–75%). TRVmax demonstrated sensitivity 29% (CI 21–39%), specificity 98% (CI 94–100%), positive predictive value 94% (CI 80–98%), and negative predictive value 58% (CI 57–70%). Right ventricular function as assessed by TAPSE demonstrated sensitivity 22% (CI 14–33%), specificity 85% (CI 77–91%), positive predictive value 52% (CI 36–66%), and negative predictive value 61% (CI 58–65%). Right ventricular function as assessed by RV S’ demonstrated sensitivity 13% (CI 6–22%), specificity 85% (CI 76–91%), positive predictive value 37% (CI 22–55%), and negative predictive value 57% (CI 55–60%). Comparison of the predictive power of each parameter was assessed using ROC curves (see Figure 4). ePLAR performed strongly (AUC 0.74, p < 0.05) compared with TRVmax (AUC 0.62, p < 0.05), TAPSE (AUC 0.54, p = 0.33), and RV S’ (AUC 0.76, p < 0.05).
Table 5.
Sensitivity and specificity of each echocardiographic parameter in the detection of acute pulmonary embolism compared to age-matched controls.
| Percentage (95% CI) | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
|---|---|---|---|---|
| ePLAR | 72% (62–80%) | 66% (57–75%) | 68% (62–74%) | 70% (63–75%) |
| TRVmax | 29% (21–39%) | 98% (94–100%) | 94% (80–98%) | 58% (57–70%) |
| TAPSE | 22% (14–33%) | 85% (77–91%) | 52% (36–66%) | 61% (58–65%) |
| RV S’ | 13% (6–22%) | 85% (76–91%) | 37% (22–55%) | 57% (55–60%) |
Figure 4.
Receiver operator curves assessing predict power of each parameter (ePLAR, TRVmax, TAPSE, and RV S’) in predicting pulmonary embolism, when compared to age-matched controls.
4. Discussion
Sub-massive acute pulmonary embolism is associated with significant morbidity and mortality, in both the acute and chronic setting. It has been well documented that early treatment with anticoagulation, and in severe cases thrombolysis, is critical in the care of these patients [2]. Anatomical assessment of clot location and burden is well achieved with CTPA and nuclear medicine V/Q scan in most cases. Echocardiography is unlikely to ever replace these tests for definitive diagnosis of PE [5,17].
However, echocardiography has two major roles in this disease spectrum [7]. Firstly, in some situations, these anatomic tests are logistically not feasible in the emergent setting, and echocardiography is used to assess for “supportive” or “surrogate” evidence to confirm or refute the clinical suspicion of PE [18]. Indeed, while clearly not the optimal test for the definitive diagnosis of PE, echocardiography is frequently requested (often before CTPA or V/Q scans can logistically be obtained) to guide emergent therapy. Clinicians have long recognized the constellation of visual cues (McConnell’s sign with preserved apical RV function [8] and reduced free wall function), in the setting of minimally elevated TRVmax/RVSP values, and underfilled left-sided chambers. Quantitative measures of RV function help reinforce these subjective impressions of the constellation of findings of significant PE [10].
In this study, patients with proven acute PE had mean TAPSE values of 21.1 mm and RV S’ of 13.5 cm/s, with 59/110 and 69/110 false negative results, respectively. As a binary diagnostic tool for RV dysfunction, TRVmax performed poorly, with only 32 out of 110 patients having appropriately elevated TRVmax ≥ 2.9 m/s. Clinically, all three parameters had very low sensitivity for the detection of acute PE (TRVmax 29%, TAPSE 22%, RV S’ 13%), though high specificity. ePLAR on the other hand was found to have much higher sensitivity (72%) with modest specificity (66%), 31/110 results being falsely normal. Although RV S’ was found to have a greater ROC AUC than ePLAR, ePLAR was found to have the highest negative predictive value of all parameters, and much higher positive predictive value than RV S’. Clinically, these data suggest that ePLAR, as a marker of impaired trans-pulmonary flow, is much more likely to register as abnormal than measures of right heart pressure (RVSP/TRVmax), or right heart function (TAPSE and RV S’), with a negative predictive value superior to these other parameters.
Secondly, and more logically, echocardiography in the setting of suspected or proven acute PE plays a major role in risk stratification and the assessment of hemodynamic distress of the right-sided circulation. Current Guidelines (both European and American) for thrombolysis or catheter-based intervention for PE recommend echocardiographic assessment of RV dysfunction [7,19], and subsequent treatment in the setting of RV dysfunction + hemodynamic instability, with or without CTPA or V/Q scan confirmation of PE [7]. Prognostic advantage has been demonstrated when these criteria lead to lysis/intervention [20]. However, in this study, only 32% had reduced TAPSE while 70% had abnormal ePLAR. It is hypothesized that ePLAR is a subtler indicator of hemodynamic disturbance. Future studies may well show that using this marker of impaired transpulmonary flow may better predict response to therapy.
Finally, this study offers mechanistic insights into the hemodynamics of acute PE, and, specifically, the physiologic underpinning of systemic hypotension and shock [21]. In this cohort, the mitral E/e’ (as a marker of left atrial pressure) was substantially lower than age-matched controls (8.2 ± 3.8 vs. 10.8 ± 5.1, p = 0.013). Even though, as a group, these pulmonary embolism patients were not pulmonary hypertensive (TRVmax 2.61 ± 0.61 m/s, RVSP 34.18 ± 13.49 mmHg), ePLAR was markedly elevated, suggesting for the first time non-invasively, elevated TPG levels. Interestingly, for each of the three echocardiographic parameters assessed dichotomously with ePLAR (Table 2, Table 3 and Table 4), patients in Group 2 (ePLAR true positive, TRVmax/TAPSE/RV S’ false negative) were significant younger than patients in Group 1 or 3 (TRVmax/TAPSE/RV S’ true positive). This may suggest an element of age related physiologic compensation or pulmonary vasculature reserve in the setting of an acute obstruction to trans-pulmonary flow [22]. Consequently, it is hypothesized that ePLAR may offer mechanistic understanding into normalization of trans-pulmonary flow in acute PE following therapy [23]. Follow up echocardiographic analysis may also provide insight into the long-term physiologic impacts of acute PE, in particular in the setting of pre-existing cardiac systolic or diastolic dysfunction.
5. Limitations
It should be noted that this a retrospective study, over several years, of a very large cohort of patients presenting with suspected or confirmed pulmonary embolus. Clinical practice varies from case to case, and across time and institutions. The subset of patients with complete datasets, adequate for calculation of all required parameters for this study, is reflected in the size of the final cohort. The age-matched control group was selected randomly from a very large database of normal individuals. The groups are reasonably well matched, but this is never a perfect process. The delay between echo and anatomic imaging study was set at a maximum of ±3 days. It could be well argued that an echocardiogram performed three days after diagnosis and onset of anticoagulation for PE may well have some dilution of the hemodynamic effects of the condition.
6. Conclusions
The majority of patients with sub-massive pulmonary embolism, in the absence of shock, demonstrated normal estimated right ventricular systolic pressure and normal right ventricular function. Despite this, these patients exhibited a significant increase in the echocardiographic Pulmonary to Left Atrial Ratio (ePLAR), suggesting increased trans-pulmonary gradient with pre-capillary obstruction to flow. This new parameter may well offer a more sensitive echocardiographic sign of abnormal hemodynamic disturbance in these patients. Future studies should focus on the value of ePLAR in acute patient triage, guidance of therapy, and prediction of prognosis in the setting of acute pulmonary embolism.
Abbreviations
| PE | Pulmonary Embolus |
| RV | Right ventricle |
| TAPSE | Tricuspid annular plane systolic excursion |
| RV S’ | Right ventricular DTI systolic velocity (measured at the tricuspid annulus) |
| PAP | Pulmonary artery pressure |
| LAP | Left atrial pressure |
| TPG | Trans-pulmonary gradient |
| RVSP | Right ventricular systolic pressure |
| TRVmax | Tricuspid regurgitation maximum continuous-wave Doppler velocity |
| ePLAR | echocardiographic Pulmonary to Left Atrial Ratio |
| CTPA | Computed tomography pulmonary angiogram |
| DTI | Doppler Tissue Imaging |
| Mitral E/e’ | Pulsed wave Doppler peak trans-mitral e-wave velocity/DTI mitral annular e’-wave velocity |
Author Contributions
Conceptualization, I.G.S. and G.M.S.; Data curation, B.T.F., I.G.S., Y.C. and W.M.S.; Formal analysis, I.G.S., and G.M.S.; Funding acquisition, B.T.F., and G.M.S.; Investigation, I.G.S., Y.C, J.H., A.Z.R., D.W., D.G.P. and W.M.S.; Methodology, I.G.S., and G.M.S.; Software, I.G.S. and W.M.S.; Validation, B.T.F., I.G.S. and W.M.S.; Writing—original draft, I.G.S. and G.M.S.; Writing—review and editing, I.G.S., W.M.S. and G.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mookadam F., Jiamsripong P., Goel R., Warsame T.A., Emani U.R., Khandheria B.K. Critical appraisal on the utility of echocardiography in the management of acute pulmonary embolism. Cardiol. Rev. 2010;18:29–37. doi: 10.1097/CRD.0b013e3181c09443. [DOI] [PubMed] [Google Scholar]
- 2.Steering Committee Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: Rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am. Heart J. 2012;163:33–38 e1. doi: 10.1016/j.ahj.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Stein P.D., Fowler S.E., Goodman L.R., Gottschalk A., Hales C.A., Hull R.D., Leeper K.V., Jr., Popovich J., Jr., Quinn D.A., Sos T.A., et al. Multidetector computed tomography for acute pulmonary embolism. N. Engl. J. Med. 2006;354:2317–2327. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 4.Anderson D.R., Kahn S.R., Rodger M.A., Kovacs M.J., Morris T., Hirsch A., Lang E., Stiell I., Kovacs G., Dreyer J., et al. Computed tomographic pulmonary angiography vs. ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: A randomized controlled trial. JAMA. 2007;298:2743–2753. doi: 10.1001/jama.298.23.2743. [DOI] [PubMed] [Google Scholar]
- 5.Pruszczyk P., Goliszek S., Lichodziejewska B., Kostrubiec M., Ciurzyński M., Kurnicka K., Dzikowska-Diduch O., Palczewski P., Wyzgal A. Prognostic value of echocardiography in normotensive patients with acute pulmonary embolism. JACC Cardiovasc. Imaging. 2014;7:553–560. doi: 10.1016/j.jcmg.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Hsiao S.H., Chang S.M., Lee C.Y., Yang S.H., Lin S.K., Chiou K.R. Usefulness of tissue doppler parameters for identifying pulmonary embolism in patients with signs of pulmonary hypertension. Am. J. Cardiol. 2006;98:685–690. doi: 10.1016/j.amjcard.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 7.Konstantinides S.V., Torbicki A., Agnelli G., Danchin N., Fitzmaurice D., Galie N., Gibbs J.S., Huisman M.V., Humbert M., Kucher N., et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014;35:3033–3073. doi: 10.5603/KP.2014.0211. [DOI] [PubMed] [Google Scholar]
- 8.McConnell M.V., Solomon S.D., Rayan M.E., Come P.C., Goldhaber S.Z., Lee R.T. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am. J. Cardiol. 1996;78:469–473. doi: 10.1016/S0002-9149(96)00339-6. [DOI] [PubMed] [Google Scholar]
- 9.Kucher N., Rossi E., De Rosa M., Goldhaber S.Z. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch. Intern. Med. 2005;165:1777–1781. doi: 10.1001/archinte.165.15.1777. [DOI] [PubMed] [Google Scholar]
- 10.Hsiao S.H., Lee C.Y., Chang S.M., Yang S.H., Lin S.K., Huang W.C. Pulmonary embolism and right heart function: Insights from myocardial Doppler tissue imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2006;19:822–828. doi: 10.1016/j.echo.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.H., Park J.H. Strain Analysis of the right ventricle using two-dimensional echocardiography. J. Cardiovasc. Imaging. 2018;26:111–124. doi: 10.4250/jcvi.2018.26.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll B.J., Heidinger B.H., Dabreo D.C., Matos J.D., Mohebali D., Feldman S.A., McCormick I., Litmanovich D., Manning W.J. Multimodality assessment of right ventricular strain in patients with acute pulmonary embolism. Am. J. Cardiol. 2018;122:175–181. doi: 10.1016/j.amjcard.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez O., Trinquart L., Planquette B., Couturaud F., Verschuren F., Caille V., Meneveau N., Pacouret G., Roy P.M., Righini M., et al. Echocardiography and pulmonary embolism severity index have independent prognostic roles in pulmonary embolism. Eur. Respir. J. 2013;42:681–688. doi: 10.1183/09031936.00097512. [DOI] [PubMed] [Google Scholar]
- 14.Scalia G.M., Scalia I.G., Kierle R., Beaumont R., Cross D.B., Feenstra J., Burstow D.J., Fitzgerald B.T., Platts D.G. ePLAR—The echocardiographic pulmonary to left atrial ratio—A novel non-invasive parameter to differentiate pre-capillary and post-capillary pulmonary hypertension. Int. J. Cardiol. 2016;212:379–386. doi: 10.1016/j.ijcard.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell C., Rahko P.S., Blauwet L.A., Canaday B., Finstuen J.A., Foster M.C., Horton K., Ogunyankin K.O., Palma R.A., Velazquez E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American society of echocardiography. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., Flachskampf F.A., Foster E., Goldstein S.A., Kuznetsova T., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Jimenez D., Aujesky D., Moores L., Gomez V., Marti D., Briongos S., Monreal M., Barrios V., Konstantinides S., Yusen R.D. Combinations of prognostic tools for identification of high-risk normotensive patients with acute symptomatic pulmonary embolism. Thorax. 2011;66:75–81. doi: 10.1136/thx.2010.150656. [DOI] [PubMed] [Google Scholar]
- 18.Bova C., Greco F., Misuraca G., Serafini O., Crocco F., Greco A., Noto A. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am. J. Emerg. Med. 2003;21:180–183. doi: 10.1016/S0735-6757(02)42257-7. [DOI] [PubMed] [Google Scholar]
- 19.Kearon C., Akl E.A., Comerota A.J., Prandoni P., Bounameaux H., Goldhaber S.Z., Nelson M.E., Wells P.S., Gould M.K., Dentali F., et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel B., Shah M., Garg L., Agarwal M., Martinez M., Dusaj R. Trends in the use of echocardiography in pulmonary embolism. Medicine. 2018;97:e12104. doi: 10.1097/MD.0000000000012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldie A.M., Eliadis P.E., Fraser J.F., Little S.G., Scalia I.G., Scalia G.M. Acute life-threatening reversible pulmonary vasoconstrictive reaction to bleomycin chemotherapy demonstrating the clinical application of ePLAR—Echocardiographic Pulmonary to Left Atrial Ratio. Int. J. Cardiol. 2016;215:438–440. doi: 10.1016/j.ijcard.2016.04.141. [DOI] [PubMed] [Google Scholar]
- 22.Tran M., Kwon A., Holt D., Kierle R., Fitzgerald B., Scalia I., Scalia W., Holt G., Scalia G. Echocardiographic Pulmonary to Left Atrial Ratio (ePLAR): A comparison study between ironman athletes, age matched controls and a general community cohort. J. Clin. Med. 2019;8:1756. doi: 10.3390/jcm8101756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scalia I.G., Riha A.Z., Kwon A., Newbigin K., Scalia G.M. Dramatic normalization of the echocardiographic pulmonary-to-left atrial ratio with thrombolysis in a case of life-threatening submassive pulmonary emboli. Case. 2017;1:124–127. doi: 10.1016/j.case.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]