Abstract
AceDoPC® is a structured glycerophospholipid that targets the brain with docosahexaenoic acid (DHA) and is neuroprotective in the experimental ischemic stroke. AceDoPC® is a stabilized form of the physiological 2-DHA-LysoPC with an acetyl group at the sn1 position; preventing the migration of DHA from the sn2 to sn1 position. In this study we aimed to know the bioavailability of 13C-labeled DHA after oral intake of a single dose of 13C-AceDoPC®, in comparison with 13C-DHA in triglycerides (TAG), using gas chromatography/combustion/isotope ratio mass spectrometry (GC/C/IRMS) to assess the 13C enrichment of DHA-containing lipids. 13C-DHA enrichment in plasma phospholipids was significantly higher after intake of AceDoPC® compared with TAG-DHA, peaking after 24 h in both cases. In red cells, 13C-DHA enrichment in choline phospholipids was comparable from both sources of DHA, with a maximum after 72 h, whereas the 13C-DHA enrichment in ethanolamine phospholipids was higher from AceDoPC® compared to TAG-DHA, and continued to increase after 144 h. Overall, our study indicates that DHA from AceDoPC® is more efficient than from TAG-DHA for a sustained accumulation in red cell ethanolamine phospholipids, which has been associated with increased brain accretion.
Keywords: plasma phospholipids, brain, gas chromatography combustion isotope ratio mass spectrometry
1. Introduction
Docosahexaenoic acid (DHA)/22:6n-3 is the long-chain polyunsaturated n-3 fatty acid (n-3 PUFA) which specifically accumulates into the brain where it plays a crucial role for the development and function [1]. Indeed, DHA is required for brain development from fetus to adult, and for cognition and visual acuity [2]. It is involved in brain development such as synaptogenesis, neurogenesis, neuronal differentiation [3], and maintenance of membrane fluidity [4]. In addition, brain DHA content is altered in neurodegenerative diseases, particularly in Alzheimer’s disease [5,6]. DHA synthesis from oral intake of its essential precursor α-linolenic acid (18:3n-3) is only 1% in men and 1–3% in women [7]. Due to this low level of synthesis, dietary pre-formed DHA is the preferred source of DHA to improve brain DHA accretion.
It is known that DHA comes from blood plasma through crossing the blood-brain barrier (BBB), both as a non-esterified fatty acid and as esterified in lyso-phosphatidylcholine (lysoPC). However, several studies have shown that, although a slower uptake of DHA was observed from DHA-containing lysoPC, a stronger DHA accumulation into the brain was found on a long term [8,9], whereas the brain uptake of DHA is faster but more limited from non-esterified DHA [10,11]. Indeed, DHA esterified at the sn2 position of lysoPC more efficiently crosses a re-constituted BBB than non-esterified DHA [12]. This has been corroborated by more recent studies showing that BBB expresses the specific binding protein Mfsd2a to facilitate this transfer [13], and that an oral intake of DHA-containing lysoPC is more efficient than non-esterified DHA to increase the brain DHA content and the memory [9]. It is noteworthy that the brain uptake of other unsaturated fatty acids, especially arachidonic acid, which is the second most abundant after DHA, also is most efficient when esterified in lysoPC [14].
However, DHA esterified at the sn2 position of lysoPC, supposed to be its physiological position within phosphoglycerides, rapidly migrates to the sn1 position [15]. We then structured such a lysoPC to prevent this migration, by acetylating the sn1 position [16]. The resulting structured phospholipid: 1-acetyl, 2-docosahexaenoyl-glycerophosphocholine has been named AceDoPC®. This compound affords more neuroprotection in a post-ischemic stroke model than does non-esterified DHA [17], and DHA incorporation into brain tissues is greater with AceDoPC than with equimolar concentrations of non-esterified DHA or of PC DHA [18]. On the other hand, additional findings showed that AceDoPC® inhibits lipopolysaccharide-induced neuro-inflammation in microglial cells through interleukin-6 signaling [19], and activates neurogenesis, but not astrogenesis, from nerve stem cells [20]. In light of this potential, it was of interest to investigate the bioavailability of DHA in humans from oral intake of AceDoPC® compared to a usual source such as TAG-DHA.
2. Materials and Methods
2.1. Human Volunteers
Three healthy men between 60 to 70 years old, with no cognitive defects, were selected. Their body mass index was between 20 to 30 Kg/m2. In whole blood of fasting subjects, glycaemia was less than 7 mmol/L, total cholesterol was less or equal to 7 mmol/L, triglycerides level was less than 1.7 mmol/L, and hemoglobin was more than 130 g/L. All subjects gave their informed consent for inclusion before participating in the study.
2.2. Experimental Procedure
The research was conducted at the clinical research center from Rhône-Alpes Research Center for Human Nutrition (CRNH-RA) under the Clinical Trial Number NCT02168738. The Sponsor was the Hospices Civils de Lyon, and the agreement was obtained from the legal authorities, after approvement by the local ethics committee. This was a double-blind randomized study with a crossover use of two sources: 13C-AceDoPC and TAG-13C-DHA. Each source contained 50 mg of 13C-DHA that was ingested after 12 h of fasting, as an alcoholic solution on a piece of bread. The blood sampling (36 mL in total) started before the DHA ingestion (T0), and after 1 (T1), 3 (T3), 6 (T6), 24 (d1), 72 (d3), and 144 (d6) hours, of the oral intake. A wash-out of 120 days between each source was applied to avoid or minimize 13C labeling of red blood cells from the first intake.
2.3. Synthesis of Labeled DHA Sources
2.3.1. 13C-AceDoPC Synthesis
The synthesis of labeled AceDoPC with 13C-DHA was similar to that of [14C]-AceDoPC as previously described [18], using a DHA ester form, U13C-DHA methyl ester (provided by Dr. Anthony Windust’s lab) uniformly labeled with carbon 13. The purity of the synthesized product was checked by High Performance Liquid Chromatography (HPLC), and 13C Nuclear Magnetic Resonance (NMR). Finally, DHA was analyzed by Gas Chromatography (GC) and the 13C enrichment was checked by GC/mass spectrometry (MS), showing uniformly labeled DHA.
2.3.2. Production of 13C-DHA-Containing Triacylglycerol
13C-TAG was produced by growing a microalgae (Crypthecodinium cohnii) strain (ATCC-30772 American Type Culture Collection) in a defined medium by Nestlé industry (Tours, France). All the products came from Sigma or Riedel-deHaën. The microalgae were developed during several days in a specific medium with salt and water in dark conditions. Microalgae were transferred in a starting medium, at pH 6.5 and 27 °C, containing 1-13C-acetate. The biomass was collected after 20 days of microalgae growing.
13C-DHA-TAG was harvested from the cells by centrifugation. The biomass was then freeze-dried, and lipids were extracted with hexane/isopropanol/water 5:5:1 (v/v/v). Lipids were separated by HPLC, using a normal phase silica column (21 × 250 mm; 10 µm). The solvent system used was a gradient consisting of hexane/2-propanol (55:4, v/v) (solvent A) to hexane/isopropanol/water (60/120/20, v/v/v) (solvent B) with a flow rate of 30 mL/min. Neutral lipids were eluted and collected between 0 and 8 min. The fatty acid composition of TAG was obtained by GC showing that DHA represented 20% of total fatty acids in TAG. Analysis of proton spectrum of 13C-TAG showed high purity of the samples according to NMR Bruker 400 MHz experiment (10 mg of samples prepared in 0.75 mL CDCL3). The GC/MS analysis confirmed eleven 13C within one molecule of DHA. The non-toxicity for human administration was checked by pharmacists from Hospices Civils de Lyon.
2.4. Blood Red Cells and Plasma Preparation
Blood samples were collected in tubes containing citrate-dextrose and the red cells and platelet-poor-plasma were obtained as described previously [21]. Plastic tubes containing whole blood were centrifuged at 200× g for 15 min at 4 °C to obtain platelet-rich plasma (PRP) and the red blood cells pellet. This PRP was acidified to pH 6.4 with citric acid to prevent platelet activation and centrifuged at 900× g for 12 min at 4 °C to pellet platelets and get the platelet poor plasma (PPP) in the supernatant. This PPP was then centrifuged at 2000× g for 10 min at 4 °C to eliminate the remaining platelets, and the plasma supernatant was added with 5 × 10−5 M butyl hydroxyl-toluene (BHT) as an antioxidant, and frozen at −80 °C until purification of lipids. Blood red cells/erythrocytes, from the first 200× g pellet, were diluted in Tyrode-HEPES buffer and centrifuged at 100× g for 10 min at 4 °C to remove contaminating white cells. After removing the supernatant, red blood cells were diluted with 9% NaCl and centrifuged at 2000× g for 10 min at 4 °C. This was repeated once and Tyrode-HEPES was added to red cells, in presence of 5 × 10−5 M BHT as an antioxidant, and stored at −80 °C.
2.5. Extraction and Separation of Lipids from Red Cells and Plasma
Total lipids were extracted from red cells and plasma according to Bligh and Dyer. Internal standards (1,2-diheptadecanoyl-glycerophosphocholine and 1,2-diheptadecanoyl-glyceroethanolamine) were added to samples before lipid extraction for quantification. Lipid classes were separated by thin-layer chromatography (TLC) with first diethylether/methanol (90:10, v/v) as the mobile phase to elute nonpolar lipids. Then, total phospholipids were extracted from the origin, or phosphatidylethanolamine (PE) and phosphatidylcholine (PC) were separated with chloroform/methanol/water (63:27:4, v/v/v). Total phospholipids or PC and PE were scraped off the plate and treated for 90 min with boron trifluoride in methanol/toluene (50:50, v/v) to obtain fatty acid methyl esters.
2.6. Quantification of 13C by Gas Chromatography Combustion-Isotope Ratio Mass Spectrometry (GC-C-IRMS)
The 13C enrichment was measured by GC-C-IRMS (gas chromatography–combustion isotope ratio mass spectrometry) (Isoprime; Elementar UK Ltd, Cheade SK8 6PT, UK.) [22]. The sample was injected into a gas chromatograph (model GC6890, Agilent Technologies, Palo Alto, CA, United States) equipped with a fused-silica column (SP2380, 30 m × 0.25 mm × 0.20 µm film thickness, Supelco). Helium was used as the carrier gas. Injection (1 µL) was performed in split less mode at 250 °C, with a split less time of 1min. DHA was separated at constant flow (1.2 mL min–1) with the following oven program: (a) 100 °C for 1 min; (b) increase at a rate of 25 °C min–1 to 175 °C; (c) hold at 175 °C for 5.3 min; (d) increase at a rate of 4 °C min−1 to 250 °C. The GC effluent was diverted to a flame ionization detector until the elution of the target peak. The effluent from the gas chromatograph was then switched into a copper oxide furnace maintained at 850 °C which produced CO2 and water from the sample. Water and CO2 passed through Nafion tubing, where water was removed, while CO2 was transferred to the IRMS instrument via an open-split interface. Before and after the CO2 peak arising from sample combustion, a reference CO2 gas calibrated against PDB was sequentially injected in the IRMS instrument for 30 s, where PDB is the Pee Dee Belemnite international standard ((13C/12C)PDB = 0.0112372). The different isotopomers were collected onto three different collectors at mass-to-charge ratio (m/z) 44 (main ion: 12C16O16O), 45 (13C16O16O, 12C16O17O), and 46 (12C17O17O, 12C16O18O, 13C16O17O). Ions at m/z 44, 45, and 46 were continuously recorded until the return of the 44 signal to the baseline value. Isotopomers at m/z 44 and 45 were measured, leading to the 13C/12C ratio. The 13CO2/12CO2 ratios of samples were expressed as δ13C ‰ relative to PDB:
To take into account the difference of labelling of the DHA between 13C-AceDoPC (22 13C atoms) and 13C-TAG (11 13C atoms) results were finally expressed in mole percent excess (MPE) calculated from the Atom%:
| At% = [100 × (13C/12C) PDB × (0.001 × δ 13Csample + 1)]/[1 + (13C/12C) PDB × (0.001 × δ 13Csample + 1) |
2.7. Measures of Quality Control
A quality control standard including DHA compound (Mix 37 from Supelco) was injected before and after each batch of analyses. Less than 4.8% variation was observed for 13C-DHA enrichment during the study period.
2.8. Statistical Analysis
Results are means ± SEM values of n = 3. Data with different signs are significantly different at p < 0.05, according to the Student t test.
3. Results
3.1. 13C-DHA Incorporation into Plasma Phospholipids
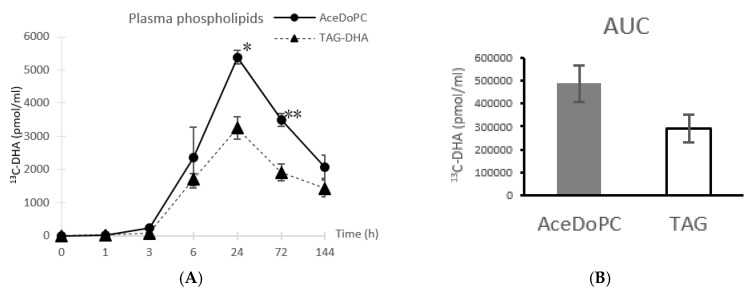
After separation of total phospholipids fraction by TLC, FAMEs were analyzed by GC-C-IRMS as described in Materials & Methods. The 13 C enrichment in DHA was calculated and reported as the amount of 13C-DHA content in the analyzed samples (Figure 1). An increase from both DHA sources (13C-AceDoPC and 13C-DHA-TAG) started after 3 h of DHA intake, but was substantial after 6 h, and peaked after 24 h. However, the 13 C-DHA peak was 2-fold higher from AceDoPC (5386 pmol/mL) compared to TAG-DHA (3247 pmol/mL). After peaking, the 13C enrichment in DHA decreased at 72 h from both sources but remained significantly different. This decrease continued until 144 h, but no more significance between the two sources could be seen. The statistical significance could then be observed after 24 h and 72 h.
Figure 1.
13C-DHA in plasma phospholipids from AceDoPC compared to TAG-DHA after 50 mg DHA intake in both esterified forms, at different times post-intake (A). Results are expressed in pmol of 13C-DHA per mL of plasma, presented as means ± SEM from three values. (B) represents area under curves (AUC) from Figure 1A. Stars indicate significant differences within each time, according to the student t test.
3.2. 13C-DHA Incorporation into Red Cells PC and PE
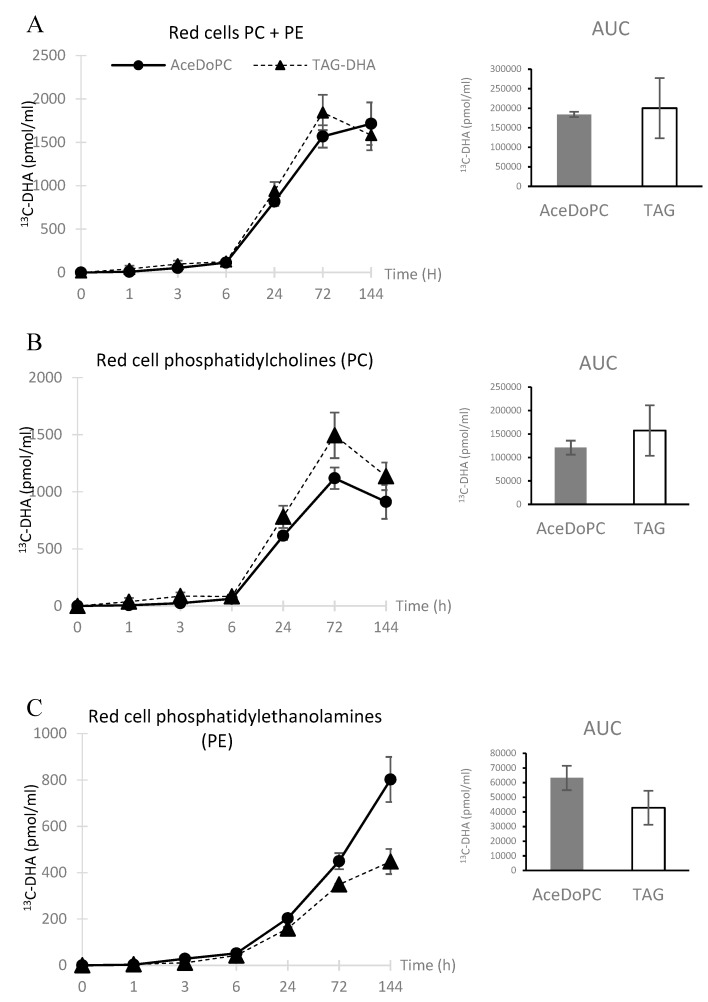
13C-DHA appeared in red blood cells (phosphatidylcholines + phosphatidylethanolamines: PC + PE) after 6 h, and increased linearly till 72 h, then plateauing at 144 h, although 13C-DHA tended to decrease from TAG-DHA while tending to increase from AceDoPC. (Figure 2A). The 13C-DHA appearance into PC exhibited a peak at 72 h and decreased at 144 h (Figure 2B). No significant differences were found for these kinetics for both sources. When studying the accumulation of 13C-DHA into PE, it tended to plateauing at 144 h from 13C-DHA-TAG whereas it sharply increased at this latter time from 13C-AceDoPC, with around twice as much of 13C-DHA from AceDoPC (Figure 2C). This strongly suggests that the transfer of DHA over time, which is expected from PC to PE, was more important from AceDoPC than from TAG-DHA.
Figure 2.
13C-DHA in red cell phospholipids after intake of 13C-DHA esterified in either AceDoPC or TAG. Fifty milligrams of 13C-DHA source were ingested, and blood samples collected at the different times shown in figures. PE & PC were separated as described in Materials & Methods. Results are expressed in pmol of 13C-DHA per mL of blood, presented as ± SEM from three values. (A) relates to 13C-DHA incorporation into red cell PC+PE. (B,C) relate to separate phospholipids PC and PE, respectively.
Overall, Figure 2 shows a transfer over time of DHA from PC to PE, which was predominant when the oral source was AceDoPC compared to TAG-DHA.
4. Discussion
It is usually considered that TAG are hydrolyzed at the intestinal level, followed by the absorption of unesterified fatty acids and the remaining 2-acyl-glycerol. Therefore, the DHA bioavailability from TAG-DHA will be affected by its position within the TAG. Considering AceDoPC, DHA being exclusively at the sn2 position, we might expect its release by phospholipase A2, as it occurs from classical phospholipids, except if AceDoPC sufficiently mimicks a lysoPC to be absorbed without hydrolysis. 13C-DHA bioavailability from TAG-DHA and AceDoPC should then be different. The results obtained above suggest a higher bioavailability of 13 C-DHA from the latter form, with an almost double amount of 13C-DHA in plasma phospholipids.
The kinetics of 13C-DHA accumulation in red cell phospholipids compared to plasma clearly show a transfer from the latter to the former. In a previous study with 13 C-DHA-TAG only, we found there was still increased accumulation in red cell phospholipids after 3 days [23]. Also, in another study looking at the 13C-DHA distribution from a single oral intake of phosphatidylcholine (13C-DHA-PC) in three human volunteers [24], the last time analysis was after 3 days which did not allow to discriminate between PC and PE accumulation kinetics in red cells. In the current study, the last blood withdrawing was done after 6 days, which reveals a plateau in DHA accumulation in total red cell phospholipids and a different kinetics between PC and PE. Interestingly, 13C-DHA in PC peaked after 3 days and started to decrease at 6 days while it still continued to accumulate into PE. Such a difference in the incorporation of 13C-DHA within erythrocyte PE is in agreement with the preferential accumulation of DHA into brain PE after dietary intake [25]. Although 13C-DHA from TAG-DHA started to plateau after 3 days, it continued to rise from AceDoPC. This suggests that AceDoPC would better promote the brain DHA accretion, as erythrocyte DHA is considered as a marker of brain DHA [26]. This agrees with the observation that AceDoPC, injected i.v. to rats, is efficient to bring DHA to the brain [18], if we speculate that part of this transporter crosses the intestine. Alternatively, it may be considered that AceDoPC might acetylate intestinal targets in an aspirin-like activity [27], then releasing lysoPC-DHA for easily crossing the intestine like other lysoPC from PC-DHA hydrolysis. The recent paper from Sugasini et al. is in favor of this easy crossing [9].
5. Conclusions
In summary, oral intake of AceDoPC, even as a single moderate dose (50 mg) would facilitate DHA accumulation in human red cell PE, which is a marker of brain DHA accretion, likely because of a similar structure to lysoPC-DHA. Showing human brain enrichment itself with DHA from dietary AceDoPC would require other approaches, such as brain imaging after intake of appropriately labeled AceDoPC.
Acknowledgments
The Authors thank, Nestlé for providing 13C-DHA-TAG, and Polaris for funding the PhD thesis salary of MH. We also wish to thank the volunteers, all the nursing and medical staff of the Rhône-Alpes Research Center for Human Nutrition, and the clinical research department of Hospices Civils de Lyon for trials sponsoring and monitoring.
Author Contributions
Conceptualization: M.P., N.B.-H., M.L. (Martine Laville), M.L. (Michel Lagarde). Methodology: M.H., H.N., M.P., M.B., N.B.-H., L.M., V.S., M.L. (Martine Laville), M.L. (Michel Lagarde). Formal analysis: M.H., H.N., M.P., M.B., N.B.-H., L.M., V.S. Resources: M.P., A.W., N.F., S.L.-P. Writing—original draft preparation: H.N., M.L. (Michel Lagarde). Writing—review and editing: M.H., H.N., M.B., N.B.-H., L.M., V.S., M.L. (Martine Laville), M.L., S.L.-P., M.L. (Michel Lagarde). Supervision: M.L. (Michel Lagarde). Project administration: M.L. (Martine Laville), M.L. (Michel Lagarde). Funding acquisition: M.L. (Martine Laville), M.L. (Michel Lagarde). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the French National Agency for Research (ANR) for the grant “Neuroprotect” (2008–2012).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Crawford M.A., Doyle W., Leaf A., Leighfield M., Ghebremeskel K., Phylactos A. Nutrition and neurodevelopmental disorders. Nutr. Health. 1993;9:81–97. doi: 10.1177/026010609300900205. [DOI] [PubMed] [Google Scholar]
- 2.Uauy R., Hoffman D.R. Essential fat requirements of preterm infants. Am. J. Clin. Nutr. 2000;71:245S–250S. doi: 10.1093/ajcn/71.1.245S. [DOI] [PubMed] [Google Scholar]
- 3.Gharami K., Das M., Das S. Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem. Int. 2015;89:51–62. doi: 10.1016/j.neuint.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Pinot M., Vanni S., Pagnotta S., Lacas-Gervais S., Payet L.A., Ferreira T., Gautier R., Goud B., Antonny B., Barelli H. Lipid cell biology. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science. 2014;345:693–697. doi: 10.1126/science.1255288. [DOI] [PubMed] [Google Scholar]
- 5.Cunnane S.C., Schneider J.A., Tangney C., Tremblay Mercier J., Fortier M., Bennett D.A., Morris M.C. Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 2012;29:691–697. doi: 10.3233/JAD-2012-110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkouch M., Hachem M., Elgot A., Lo Van A., Picq M., Guichardant M., Lagarde M., Bernoud-Hubac N. The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J. Nutr. Biochem. 2016;38:1–11. doi: 10.1016/j.jnutbio.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Burdge G. α-Linolenic acid metabolism in men and women: Nutritional and biological implications. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:137–144. doi: 10.1097/00075197-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Thies F., Pillon C., Moliere P., Lagarde M., Lecerf J. Preferential incorporation of sn-2 lysoPC DHA over unesterified DHA in the young rat brain. Am. J. Physiol. 1994;267:R1273–R1279. doi: 10.1152/ajpregu.1994.267.5.R1273. [DOI] [PubMed] [Google Scholar]
- 9.Sugasini D., Thomas R., Yalagala P.C.R., Tai L.M., Subbaiah P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017;7:11263. doi: 10.1038/s41598-017-11766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chouinard-Watkins R., Chen C.T., Metherel A.H., Lacombe R.J.S., Thies F., Masoodi M., Bazinet R.P. Phospholipid class-specific brain enrichment in response to lysophosphatidylcholine docosahexaenoic acid infusion. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:1092–1098. doi: 10.1016/j.bbalip.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Bazinet R.P., Bernoud-Hubac N., Lagarde M. How the plasma lysophospholipid and unesterified fatty acid pools supply the brain with docosahexaenoic acid. Prostaglandins Leukot. Essent. Fatty Acids. 2019;142:1–3. doi: 10.1016/j.plefa.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Bernoud N., Fenart L., Molière P., Dehouck M.-P., Lagarde M., Cecchelli R., Jecerf J. Preferential Transfer of 2-Docosahexaenoyl-1-Lysophosphatidylcholine Through an In Vitro Blood-Brain Barrier Over Unesterified Docosahexaenoic Acid. J. Neurochem. 1999;72:338–345. doi: 10.1046/j.1471-4159.1999.0720338.x. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen L.N., Ma D., Shui G., Wong P., Cazenave-Gassiot A., Zhang X., Silver D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 14.Thies F., Delachambre M.C., Bentejac M., Lagarde M., Lecerf J. Unsaturated fatty acids esterified in 2-acyl-l-lysophosphatidylcholine bound to albumin are more efficiently taken up by the young rat brain than the unesterified form. J. Neurochem. 1992;59:1110–1116. doi: 10.1111/j.1471-4159.1992.tb08353.x. [DOI] [PubMed] [Google Scholar]
- 15.Croset M., Brossard N., Polette A., Lagarde M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 2000;345:61–67. doi: 10.1042/bj3450061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polette A., Deshayes C., Chantegrel B., Croset M., Armstrong J.M., Lagarde M. Synthesis of acetyl,docosahexaenoyl-glycerophosphocholine and its characterization using nuclear magnetic resonance. Lipids. 1999;34:1333–1337. doi: 10.1007/s11745-999-0486-1. [DOI] [PubMed] [Google Scholar]
- 17.Chauveau F., Cho T.H., Perez M., Guichardant M., Riou A., Aguettaz P., Picq M., Lagarde M., Berthezène Y., Nighoghossian N., et al. Brain-targeting form of docosahexaenoic acid for experimental stroke treatment: MRI evaluation and anti-oxidant impact. Curr. Neurovasc. Res. 2011;8:95–102. doi: 10.2174/156720211795495349. [DOI] [PubMed] [Google Scholar]
- 18.Hachem M., Géloën A., Van A.L., Foumaux B., Fenart L., Gosselet F., Da Silva P., Breton G., Lagarde M., Picq M., et al. Efficient Docosahexaenoic Acid Uptake by the Brain from a Structured Phospholipid. Mol. Neurobiol. 2016;53:3205–3215. doi: 10.1007/s12035-015-9228-9. [DOI] [PubMed] [Google Scholar]
- 19.Fourrier C., Remus-Borel J., Greenhalgh A.D., Guichardant M., Bernoud-Hubac N., Lagarde M., Joffre C., Layé S. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia In Vitro. J. Neuroinflamm. 2017;14:170. doi: 10.1186/s12974-017-0939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo Van A., Sakayori N., Hachem M., Belkouch M., Picq M., Fourmaux B., Lagarde M., Osumi N., Bernoud-Hubac N. Targeting the Brain with a Neuroprotective Omega-3 Fatty Acid to Enhance Neurogenesis in Hypoxic Condition in Culture. Mol. Neurobiol. 2019;56:986–999. doi: 10.1007/s12035-018-1139-0. [DOI] [PubMed] [Google Scholar]
- 21.Lagarde M., Bryon P.A., Guichardant M., Dechavanne M. A simple and efficient method for platelet isolation from their plasma. Thromb. Res. 1980;17:581–588. doi: 10.1016/0049-3848(80)90098-5. [DOI] [PubMed] [Google Scholar]
- 22.Gabert L., Vors C., Louche-Pélissier C., Sauvinet V., Lambert-Porcheron S., Drai J., Michalski M.C. 13C tracer recovery in human stools after digestion of a fat-rich meal labelled with [1, 1, 1-13C3]tripalmitin and [1, 1, 1-13C3]triolein. Rapid Commun. Mass Spectrom. RCM. 2011;25:2697–2703. doi: 10.1002/rcm.5067. [DOI] [PubMed] [Google Scholar]
- 23.Brossard N., Croset M., Normand S., Pousin J., Lecerf J., Laville M., Tayot J.L., Lagarde M. Human plasma albumin transports [13C]docosahexaenoic acid in two lipid forms to blood cells. J. Lipid Res. 1997;38:1571–1582. [PubMed] [Google Scholar]
- 24.Lemaitre-Delaunay D., Pachiaudi C., Laville M., Pousin J., Armstrong M., Lagarde M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [13C]DHA in phosphatidylcholine. J. Lipid Res. 1999;40:1867–1874. [PubMed] [Google Scholar]
- 25.Connor W.E., Neuringer M., Lin D.S. Dietary effects on brain fatty acid composition: The reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. J. Lipid Res. 1990;31:237–247. [PubMed] [Google Scholar]
- 26.Létondor A., Buaud B., Vaysse C., Fonseca L., Herrouin C., Servat B., Layé S., Pallet V., Alfos S. Erythrocyte DHA level as a biomarker of DHA status in specific brain regions of n-3 long-chain PUFA-supplemented aged rats. Br. J. Nutr. 2014;112:1805–1818. doi: 10.1017/S0007114514002529. [DOI] [PubMed] [Google Scholar]
- 27.Acetyl,2acyl-Glycerophosphocholines for the Treatment of Inflammation or of Intestinal Cancer. WO 2017/006047 Al. Patent. 2017 Jan 12