Abstract
Background:
Cystic echinococcosis (CE), a zoonotic disease that affects animal and human health, is of increasing economic importance due to high morbidity rates and high economic losses in the livestock industry.
Aim:
The present study was conducted to purify the antigen from hydatid cyst fluid (HCF) with high diagnostic efficacy of camel hydatidosis using indirect enzyme-linked immunosorbent assay (ELISA).
Materials and Methods:
The HCF antigen was purified using Sephacryl S-300 column chromatography. Characterization of fractions was performed using reducing and non-reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis. Further, antibodies against Echinococcus granulosus cysts in camel serum were detected using indirect ELISA.
Results:
The purification process resulted in three fractions of antigens: FI, FII, and FIII. Indirect ELISA showed that higher diagnostic efficacy was observed in FI than in FII and FIII. Indirect ELISA, in which FI was utilized, showed 88% sensitivity and 91.7% specificity. Non-reducing SDS-PAGE showed that FI had two bands of molecular weights 120 and 60 kDa. Western blot analysis of FI demonstrated that 60, 38, and 22 kDa were antigenic bands when reacted with naturally infected camel sera with E. granulosus cysts. Using indirect ELISA, F1 recorded an infection percentage of 81.7% in randomly collected camel serum samples.
Conclusion:
FI is a promising antigen for accurate diagnosis of camel CE using indirect ELISA.
Keywords: camel hydatidosis, cystic echinococcosis, Echinococcus granulosus, gel filtration chromatography, hydatid cyst fluid, indirect enzyme-linked immunosorbent assay, sodium dodecyl sulfate–polyacrylamide gel electrophoresis, Western blot
Introduction
Cystic echinococcosis (CE), a zoonotic disease caused by the larval stage of the Echinococcus granulosus, is an animal and human health problem of increasing economic and public health importance [1]. CE in animals is asymptomatic and its diagnosis is made at necropsy [2,3], which depends on the analysis of morphological features using light or electron microscopy [4,5]. The serological diagnosis of CE improves the quality of the management and treatment of the disease. Enzyme-linked immunosorbent assay (ELISA) has been useful for the diagnosis of CE in domestic animals and humans [6]. It is generally cheap, quick, and requires fewer trained and specialized personnel [7-9].
Commercially available immunodiagnostic kits often depend on crude antigen preparations of E. granulosus hydatid cyst fluid (HCF); hence, it lacks satisfactory specificity and sensitivity [10]. Unsatisfactory performances may be due to the poor quality of antigen preparations. To avoid this problem, novel tests using purified antigens have been utilized in previous studies [11,12]. Purification of HCF antigens is essential to remove cross-reactivity and increase the sensitivity of techniques for the detection of low levels of antibodies [13]. El Deeb et al. [14] reported that the detected antibody of E. granulosus HCF in sheep using different antigens showed that purified HCF antigen was the most effective antigen compared with excretory/secretory and somatic antigens of protoscolex. The response of HCF antigens depends on the host and the location of the parasitic cysts [10]. Furthermore, the specificity and diagnostic efficacy of purified HCF antigen were higher than those of protoscolex antigen in serological studies on CE among camels in Egypt [15].
Antigen B and antigen 5, the most immunogenic antigen among HCF antigens, play an important role in the life cycle of the cestode [16]. However, interestingly, antigen 5 is immunoreactive in all stages of CE pathology compared with antigen B, which reveals a reduced antibody capturing activity in all CE stages [17]. Moreover, antigen 5 is one of the most immunogenic proteins present in HCF. Pagnozzi et al. [18] obtained an HCF fraction highly enriched in native antigen 5 through fast protein liquid chromatography on a Superdex-200 column with strong reactivity in both ELISA and Western blotting assays. The enriched antigen 5 fraction obtained from concentrated aliquots of sheep HCF was used for the accurate diagnosis of human CE using ELISA [19].
This study aimed to adopt a simple and inexpensive HCF purification method to obtain purified antigen of high diagnostic potency for camel CE.
Materials and Methods
Ethical approval
All experimental procedures were performed according to the institutional guidelines of the National Research Centre’s Animal Research Committee under protocol number 18/198.
Antigen preparation
The HCF antigen was prepared from hydatid cysts removed from the lungs of slaughtered camels in an abattoir in Cairo in accordance with Swarna and Parija [20]. Briefly, HCF was aseptically aspirated in a sterile tube. HCF was further centrifuged at 6000×g for 45 min at 4°C and then dialyzed against phosphate buffer saline with pH 7.2. Finally, HCF was centrifuged at 6000×g for 30 min. The supernatant was collected and its protein content was estimated using the Lowry et al. method [21].
Preparation of hyperimmune rabbit serum
Antibodies against E. granulosus HCF antigen were produced in two healthy male New Zealand rabbits free of parasitic infections, weighing 1.5 kg, and about 2 months of age. Two rabbits were subcutaneously immunized with 40 μg/kg of crude HCF antigen emulsified in Freund’s complete adjuvant according to Guobadia and Fagbemi [22]. On day 14, another dose of antigen was injected in Freund’s incomplete adjuvant according to Fagbemi et al. [23]. The second and third booster doses were administered on days 21 and 28, respectively. After 4 days, the last blood samples were collected from the ear vein and serum samples were separated.
Collection of serum samples
In total, 25 positive camel serum samples were collected from infected slaughtered camels with lung cysts during several visits to a local abattoir. Further, 12 blood samples were collected as negative samples from healthy camels free of cysts as confirmed by veterinary inspection. ELISA was used to diagnose 93 camel serum samples randomly collected from a local abattoir in Cairo. Sera were separated from all blood samples and kept at −20°C until use.
Purification of HCF antigen by gel filtration
The concentrated solution containing the HCF antigen was applied onto a Sephacryl S-300 gel filtration column chromatography (142 cm in length and 1.75 cm in diameter). The column was equilibrated and fractions were eluted with 0.02 mol/l phosphate buffer saline (pH 7.2) containing 0.03 mol/l phenylmethylsulfonyl fluoride and 0.02% NaN3 at a flow rate of 30 ml/h. The eluted fractions were collected in 175 tubes of 2 ml each. Furthermore, the proteins were monitored by measuring the absorbance at 280 nm using a spectrophotometer (Shimadzu).
ELISA
Diagnostic value of crude antigen and isolated fractions was compared using indirect ELISA with hyperimmune rabbit sera and naturally infected camel sera according to Santiago and Hillyer [24]. The most potent fraction was adopted to diagnose E. granulosus cysts in randomly collected camel sera. Checkerboard titration was used to determine the antigen concentrations and dilution of sera as well as protein A horseradish peroxidase (Sigma Chem. Co., St. Louis, USA). The cutoff values were measured as mean values +3SD [25].
Characterization of fractions
Non-reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 12% polyacrylamide gel [26] stained with silver stain [27], photographed, and analyzed using Molecular Imager Gel Doc™ XR+ with Image Lab Software (Bio-Rad, California, USA). Molecular weights of bands observed were calculated using molecular weight of standard proteins which were electrophoresed on the same gel.
Immunoblot
After another electrophoresis, under reducing condition in 10% SDS-PAGE, eluted fractions, crude HCF antigens, and Prestained Protein Ladder (Vivantis Technologies) were blotted onto nitrocellulose membrane as described by Towbin et al. [28] in a blotting system. After washing and blocking, the membrane was incubated with diluted naturally infected camel sera (1:200). Protein A horseradish peroxidase conjugate was used at a dilution of 1:2000. In addition, bands were observed by adding 4-chloro-1-naphthol (Sigma), and the membrane was photographed by Molecular Imager Gel Doc™ XR+ with Image Lab Software.
Statistical analysis
Sensitivity and specificity were calculated according to Gonzalez-Sapienza et al. [29], and the diagnostic parameters used were as follows: True-positive values (Tp), sera from camel naturally infected with E. granulosus as confirmed by parasitological examination; false-negative values (Fn), sera from camel infected with CE showing negative readings; false-positive values (Fp), sera from non-infected camels showing a positive result; and true-negative values (Tn), sera from healthy camels free of cysts as confirmed by veterinary inspection showing negative readings.
Results
Isolated fractions
The purification process resulted in the isolation of three fractions of antigens: FI, FII, and FIII (Figure-1). The protein content of the fractions FI, FII, and FIII was 54.6, 38.7, and 69.6 mg/ml, respectively.
Figure-1.
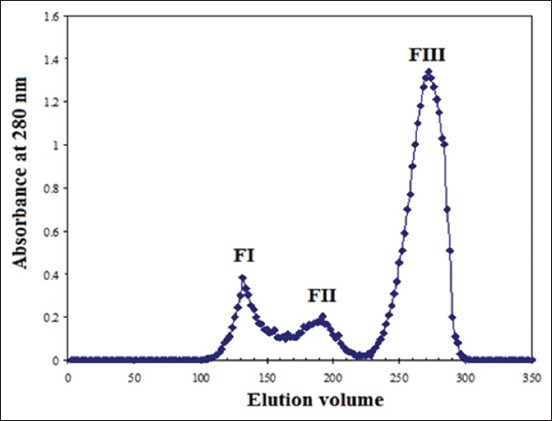
Purification profile of hydatid cyst fluid antigen on Sephacryl S 300 column chromatography.
Electrophoretic profile of the isolated fractions
FI migrates in two bands; a non-complex band with a molecular weight of 120 kDa and a complex band with a molecular weight of 60 kDa under non-reducing conditions in 12% SDS-PAGE. Conversely, FII revealed a complex band with molecular weight of 72 kDa and FIII revealed a complex band molecular weight of 74 kDa (Figure-2).
Figure-2.
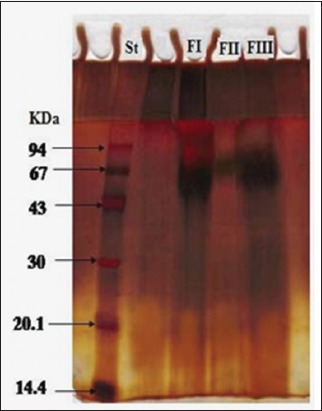
Electrophoretic profile of the three fractions of camel HCF antigen FI, FII, FIII, and Low Molecular weight standards (lane St).
Diagnostic potency of the three purified fractions
Serum samples from naturally infected camels with E. granulosus and hyperimmune rabbit sera showed a strong reaction against these three fractions. Higher potency in the detection of antibodies against CE was observed in purified FI than in FII and FIII, as shown in Figure-3.
Figure-3.
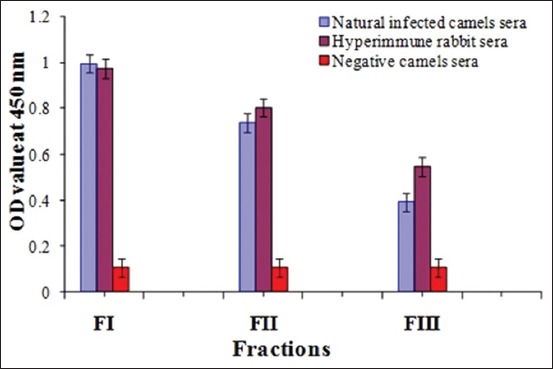
Comparative diagnostic potency of FI, FII and FIII.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of FI using indirect ELISA
Using ELISA, sensitivity of purified FI antigen against positive naturally infected camel serum samples was found to be 88%, and specificity was 91.7%. FI had PPV of 95.6% and NPV of 78.6%.
Diagnosis of camel CE
Indirect ELISA was adopted to diagnose E. granulosus cysts in camel using purified FI as an antigen. FI antigen showed that 81.7% of randomly selected camel serum samples were infected with CE, as shown in Figure-4.
Figure-4.
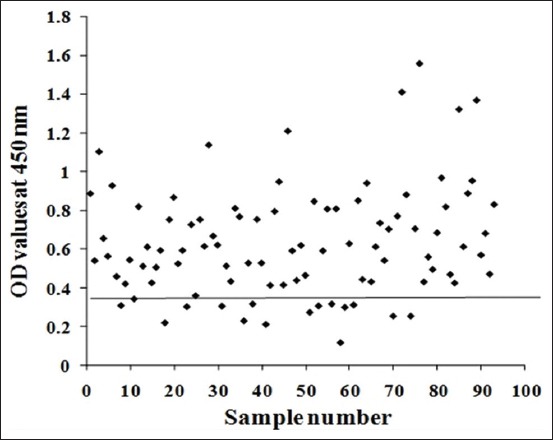
Diagnostic potency of FI in collected camel random serum samples.
Antigenic bands
The antigenic bands in crude HCF antigen were 120, 74, 60, 57, 38, 22, and 8 kDa (Figure-5 Lane B). The antigenic bands of FI antigen were identified as 60, 38, and 22 kDa using immunoblot under reducing condition, in which infected camel serum samples were utilized (Figure-5 Lane A).
Figure-5.
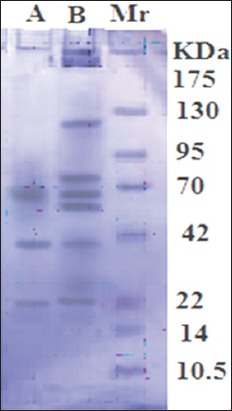
Antigenic bands under reducing condition recognized by naturally infected camel serum in crude HCF antigen (Lane B) and purified FI (Lane A). Standard’s molecular weight (Lane Mr).
Discussion
In the present study, Sephacryl S-300 column chromatography was used to purify the HCF antigen collected from camel lung cysts. Three fractions, FI, FII, and FIII, were isolated. Indirect ELISA showed that FI was the most potent diagnostic antigen. This antigen contained two bands, a non-complex band with a high molecular weight of 120 kDa and a complex band with a molecular weight of 60 kDa, as confirmed using SDS-PAGE under non-reducing conditions. The previous studies recorded antigen 5 and antigen B as the most potent diagnostic antigens of camel E. granulosus cysts [30,31]. Antigen 5 has a high molecular weight of 400 kDa and dissociates into complex components of 60-70 kDa under reducing conditions [1,11]. This behavior is similar to that of FI, which showed a band with a high molecular weight of 120 kDa that dissociated into three antigenic bands at molecular weights of 60, 38, and 22 kDa under reducing conditions by immunoblotting with positive CE camel sera.
The appearance of these bands was possibly due to the dissociation of the high molecular band into a small band of 60 kDa and two smaller bands of 38 and 22 kDa under reducing condition. This result may be in agreement with that of a previous study by Monteiro et al. [32], which suggests that native antigen 5 complex has a large molecule of 600 kDa equivalent to an oligomeric structure of about 10 units. Correspondingly, antigen 5 is an oligomeric thermolabile glycoprotein that migrates as 57 and 67 kDa bands in SDS-PAGE under non-reducing conditions and as 38 and 22 kDa bands under reducing conditions [33]. Pagnozzi et al. [18] found that antigen 5 purified by fast protein liquid chromatography, analyzed by SDS-PAGE under non-reducing and reducing conditions, and examined by immunoblotting with positive CE patient sera yields reduced antigen 5 with 38 kDa band of light reactivity and non-reduced antigen 5 with 60 kDa band, which has the strongest reactivity. The 22 kDa antigenic band of antigen 5 results due to the presence of heparin sulfate proteoglycans binding sites [34,35]. These observations led to the comparison of FI with antigen 5 where both had high-molecular-weight complex dissociating into smaller entities of diagnostic potency of camel CE. In the current study, indirect ELISA was used for serodiagnosis of camel E. granulosus cysts recording 88% sensitivity and 91.7% specificity. These results matched those of a previous study by Pagnozzi et al. [19], which reported that antigen 5 ELISAs revealed different sensitivity (88.3%) without significant differences in specificity (92.5%).
The origin of FI from camel cyst is an additional advantage of FI. Based on molecular identification studies of animal and human isolates, camel cysts are more potent in causing infection in humans [36,37]. The Egyptian G6 strain nucleotide sequence was the predominant genotype in Egypt, and its involvement has been shown by several studies [38-40].
Conclusion
The diagnostic antigen of camel origin can be successfully utilized for the diagnosis of E. granulosus cysts by ELISA, which may help control the infection and minimize transmission to humans. In addition, the antigen of camel origin could successfully be utilized in the diagnosis of human infection. A local HCF antigen FI isolated by an easy, one-step, low-cost purification method proved to exhibit high diagnostic potency for camel CE.
Authors’ Contributions
All authors participated in the study design. NIT collected lung hydatid cysts and blood samples from slaughtered camels, prepared crude antigen, performed a hyperimmune rabbit serum and carried out Western blot. MSH performed a purification of HCF antigen by Sephacryl S-300 gel filtration column chromatography. MSH and EEE carried out SDS-PAGE under non-reducing conditions. NIT, EEE and EHA shared in ELISA for the detection of CE-specific antibodies. NIT and EHA analyzed the data. NIT and EHA participated in writing the manuscript. All authors revised and approved the final manuscript.
Acknowledgments
National Research Centre, Egypt is greatly appreciated for funding this work, Agreement No. 11020201.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Deplazes P, Rinaldi L, Alvarez Rojas C.A, Torgerson P.R, Harandi M.F, Romig T, Antolova D, Schurerxx J.M, Lahmarjjjj S, Cringolix G, Magambo J, Thompson R.C.A, Jenkinsx E.J. Global distribution of alveolar and cystic echinococcosis. Adv. Parasitol. 2017;95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Kern P, da Silva A.M, Akhan O, Müllhaupt B, Vizcaychipi K.A, Budke C, Vuitton D.A. The echinococcosis:Diagnosis, clinical management and burden of disease. Adv. Parasitol. 2017;96:259–369. doi: 10.1016/bs.apar.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Craig P, Mastin A, Kesteren F, Boufana B.B. Echinococcus granulosus:Epidemiology and state-of-the-art of diagnostics in animals. Vet. Parasitol. 2015;213(3-4):132–148. doi: 10.1016/j.vetpar.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Arbabi M, Pirestani M, Delavari M, Hooshyar H, Abdoli A, Sarvi S. Molecular and morphological characterizations of Echinococcus granulosus from human and animal isolates in Kashan, Markazi province, Iran. Iran J. Parasitol. 2017;12(2):177–187. [PMC free article] [PubMed] [Google Scholar]
- 5.La-Rocca S, Farias J, Chalar C, Kun A.E, Fernandez V. Echinococcus granulosus: Insights into the protoscolex F-actin cytoskeleton. Acta Trop. 2019;199:1051222. doi: 10.1016/j.actatropica.2019.105122. [DOI] [PubMed] [Google Scholar]
- 6.Bauomi I.R, El-Amir A.M, Fahmy A.M, Zalat R.S, Diab T.M. Evaluation of purified 27.5. kDa protoscolex antigen-based ELISA for the detection of circulating antigens and antibodies in sheep and human hydatidosis. J. Helminthol. 2015;89(5):577–583. doi: 10.1017/S0022149X14000479. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Wen H, Li J, Lin R, McManus D.P. Immunology and immunodiagnosis of cystic echinococcosis:An update. Clin. Dev. Immunol 2012. 2012;10 doi: 10.1155/2012/101895. Article ID 101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunita T, Khurana S, Malla N, Dubey M.L. Immunodiagnosis of cystic echinococcosis by antigen detection in serum, urine, and saliva samples. Trop. Parasitol. 2011;1(1):33–38. doi: 10.4103/2229-5070.72107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bashiri S, Mansoor F.N, Valadkhani Z. Expansion of a highly sensitive and specific ELISA test for diagnosis of hydatidosis using recombinant EgB8/2 protein. Iran. J. Basic. Med. Sci. 2019;22(2):134–139. doi: 10.22038/ijbms.2018.29024.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sbihi Y, Rmiqui A, Rodriguez-Cabezas M.N, Orduña A, Rodriguez-Torres A, Osuna A. Comparative sensitivity of six serological tests and diagnostic value of ELISA using purified antigen in hydatidosis. J. Clin. Lab. Anal. 2001;15(1):14–18. doi: 10.1002/1098-2825(2001)15:1<14::AID-JCLA3>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golassa L, Abebe T, Hailu A. Evaluation of crude hydatid cyst fluid antigens for the serological diagnosis of hydatidosis in cattle. J. Helminthol. 2011;85(1):100–108. doi: 10.1017/S0022149X10000349. [DOI] [PubMed] [Google Scholar]
- 12.Toaleb N.I, Derbala A.A, Abdel-Rahman E.H. Comparative diagnostic evaluation of crude and isolated fractions of Echinococcus granulosus in dogs. Glob. Vet. 2011;7(6):587–592. [Google Scholar]
- 13.El Shanawany E.E, Toaleb N.I, Rahman E.H.A. Hydatid cyst germinal layer purified glycoproteins for diagnosis of camel cystic echinococcosis. Int. J. Vet. Sci. 2019;8(2):101–105. [Google Scholar]
- 14.El Deeb S, Aly I, Mahna N, Faried A, Zalat R, Younis M. Purification and characterization of Echinococcus granulosus cathepsin-B protein and evaluation of its role as a diagnostic marker. Glob. Vet. 2017;18(2):137–145. [Google Scholar]
- 15.Mousa W.M, Mahdy O.A, Abdel-Wahab A.M, El-Gameel O.A. Epidemiological and serological studies on cystic echinococcosis among camels in Egypt. J. Parasitol. Photon. 2015;105(3729-2384):212–218. [Google Scholar]
- 16.Díaz A, Casaravilla C, Barrios A.A, Ferreira A.M. Parasite molecules and host responses in cystic echinococcosis. Parasite Immunol. 2016;38(3):193–205. doi: 10.1111/pim.12282. [DOI] [PubMed] [Google Scholar]
- 17.Ahn C.S, Han X, Bae Y.A, Ma X, Kim J.T, Cai H, Yang H.J, Kang I, Wang H, Kong Y. Alteration of immunoproteome profile of Echinococcus granulosus hydatid fluid with progression of cystic echinococcosis. Parasit. Vectors. 2015;8:10. doi: 10.1186/s13071-014-0610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagnozzi D, Biosa G, Addis M.F, Mastrandrea S, Masala G, Uzzau S. An easy and efficient method for native and immunoreactive Echinococcus granulosus antigen 5 enrichment from hydatid cyst fluid. PLoS One. 2014;9(8):1–12. doi: 10.1371/journal.pone.0104962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagnozzi D, Addis M.F, Biosa G, Roggio A.M, Tedde V, Mariconti M, Tamarozzi F, Meroni V, Masu G, Masala G, Brunetti E, Uzzau S. Diagnostic accuracy of antigen 5-based ELISAs for human cystic echinococcosis. PLoS. Negl. Trop. Dis. 2016;10(3):1–14. doi: 10.1371/journal.pntd.0004585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swarna S, Parija S.C. Rev. Inst. Med. Trop. 4. Vol. 50. São. Paulo: 2008. Dot-ELISA for evaluation of hydatid cyst wall, protoscoleces and hydatid cyst fluid antigens in the serodiagnosis of cystic echinococcosis; pp. 233–236. [DOI] [PubMed] [Google Scholar]
- 21.Lowry O.H, Rosebrough N.J, Farr A.L, Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 22.Guobadia E.E, Fagbemi B.O. The isolation of Fasciola gigantica-specific antigens and their use in the serodiagnosis of fascioliasis in sheep by the detection of circulating antigens. Vet. Parasitol. 1997;68(3):269–282. doi: 10.1016/s0304-4017(96)01065-5. [DOI] [PubMed] [Google Scholar]
- 23.Fagbemi B.O, Obarisiaghon I.O, Mbuh J.V. Detection of circulating antigen in sera of Fasciola gigantica infected cattle with antibodies reactive with a Fasciola-specific 88 kDa antigen. Vet. Parasitol. 1995;58(3):235–246. doi: 10.1016/0304-4017(94)00718-r. [DOI] [PubMed] [Google Scholar]
- 24.Santiago N, Hillyer G.V. Antibody profiles by EIIB and ELISA of cattle and sheep infected with Fasciola hepatica. J. Parasitol. 1988;74(5):810–818. [PubMed] [Google Scholar]
- 25.Jin Y, Anvarov K, Khajibaev A, Hong S, Hong S. Serodiagnosis of echinococcosis by ELISA using cystic fluid from Uzbekistan sheep. Korean J. Parasitol. 2013;51(3):313–317. doi: 10.3347/kjp.2013.51.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli U.K. Cleavage of structural protein during assembly of the head of bacteriophage T. Nature. 1970;27(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Wray W, Boulikas T, Wray V.P, Hancok R. Silver staining of proteins in polyacrylamide gel. Anal. Biol. Chem. 1981;118(4):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- 28.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets:Procedure and some applications. Proc. Nat. Acad. Sci. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Sapienza G, Lorenzo C, Nieto A. Improved immunodiagnosis of cystic hydatid disease by using a synthetic peptide with higher diagnostic value than that of its parent protein Echinococcus granulosus antigen B. J. Clin. Microbiol. 2000;38(11):3979–3983. doi: 10.1128/jcm.38.11.3979-3983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortona E, Rigano R, Margutti P, Notargiacomo S, Ioppolo S, Vaccari S, Barca S, Buttari B, Profumo E, Teggi A, Siracusano A. Native and recombinant antigens in the immunodiagnosis of human cystic echinococcosis. Parasite Immunol. 2000;22(11):553–559. doi: 10.1046/j.1365-3024.2000.00336.x. [DOI] [PubMed] [Google Scholar]
- 31.Pagnozzi D, Addis M.F, Biosa G, Roggio A.M, Tedde V, Mariconti M, Tamarozzi F, Meroni V, Masu G, Masala G, Brunetti E, Uzzau S. Diagnostic accuracy of antigen 5-based ELISAs for human cystic echinococcosis. PLoS. Negl. Trop. Dis. 2016;10(3):e0004585. doi: 10.1371/journal.pntd.0004585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteiro K.M, Cardoso M.B, Follmer C, da Silveira N.P, Vargas D.M, Kitajima E.W, Zaha A, Ferreira H.B. Echinococcus granulosus antigen B structure:Subunit composition and oligomeric states. PLoS. Negl. Trop. Dis. 2012;6(3):e1551. doi: 10.1371/journal.pntd.0001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felice G.D, Pini C, Afferni C, Vicari G. Purification and partial characterization of the major antigen of Echinococcus granulosus (antigen 5) with monoclonal antibodies. Mol. Biochem. Parasitol. 1986;20(2):133–142. doi: 10.1016/0166-6851(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo C, Salinas G, Brugnini A, Wernstedt C, Hellman U, Lez-Sapienza G.G. Echinococcus granulosus antigen 5 is closely related to proteases on the trypsin family. Biochem. J. 2003;369(1):191–198. doi: 10.1042/BJ20021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmena D, Benito A, Eraso E. Antigens for the immunodiagnosis of Echinococcus granulosus infection:An update. Acta. Trop. 2006;98(1):74–86. doi: 10.1016/j.actatropica.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Azab M.E, Bishara S.A, Helmy H, Oteifa N.M, El-Hosein L.M, Ramzy R.M, Ahmed M.A. Molecular characterization of Egyptian human and animal E. granulosus isolates by RAPD-PCR technique. J. Egypt. Soc. Parasitol. 2004;34(1):83–96. [PubMed] [Google Scholar]
- 37.Shahnazi M, Hejazi H, Salehi M, Andalib A.R. Molecular characterization of human and animal Echinococcus granulosus isolates in Isfahan Iran. Acta Trop. 2011;117(1):47–50. doi: 10.1016/j.actatropica.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Aaty H.E.A, Abdel-Hameed D.M, Alam-Eldin Y.H, El-Shennawy S.F, Aminou H.A, Makled S.S, Darweesh S.K. Molecular genotyping of Echinococcus granulosus in animal and human isolates from Egypt. Act. Trop. 2012;121(2):125–128. doi: 10.1016/j.actatropica.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Omar M, Sultan K, Haridy M, Omran A. Prevalence of cystic Echinococcosis in slaughtered ruminants in different abattoirs upper Egypt. Am. J. Anim. Vet. Sci. 2013;8(3):117–121. [Google Scholar]
- 40.Khalifa N.O, Khater H.F, Nassief M.Z. Genetic fingerprint of unilocular hydatidosis in Egyptian camels and humans using nested PCR. Pak. Vet. J. 2014;34(4):522–526. [Google Scholar]


