Abstract
Background and Aim:
The development of resistance to anthelmintic drugs has prompted research into alternative methods of controlling intestinal nematodes in ruminants. This study aimed at evaluating the in vitro and in vivo anthelmintic efficacy and toxicity of chitosan encapsulated bromelain in Small East African goats in Kenya.
Materials and Methods:
Adult mortality assay was performed using live Haemonchus contortus worms treated with encapsulated bromelain solution ranging from 0.125 mg/ml to 2 mg/ml. Percentage mortality of worms was calculated after 24 h and the lethal concentration 50% (LC50) determined. For the in vivo study, 18 healthy male indigenous goats were divided into six groups of three goats each. The encapsulated bromelain was orally administered in increasing dosages (3-30 mg kg) once daily, for 14 days. The packed cell volume (PCV), aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, creatinine, and fecal egg count (FEC) were determined on a weekly basis. At the end of the study, the goats were sacrificed and gross pathology and histopathology of main organs assessed.
Results:
Albendazole had the highest (p<0.05) anthelmintic effect on the worms. An LC50 of 0.05 mg/ml, 0.445 mg/ml, and 0.155 mg/ml was observed for albendazole, plain bromelain, and encapsulated bromelain, respectively. The PCV of treated and untreated goats did not show any significant difference (p>0.05), varied from 29.3% to 35.1%, and was within the normal range of the animal. Likewise, no significant differences (p>0.05) were observed between the AST, ALT, urea, and creatinine levels of treated and the control (non-treated) goats. No adverse clinical symptoms, toxicity of the main organs, and mortality in goats were associated with the chitosan encapsulated bromelain after administration of dose up to 30 mg/kg for 14 days. Therefore, the lethal dose 50 of encapsulated bromelain may be considered to be >30 mg/kg. On day 28 post-treatment, the encapsulated bromelain showed a higher in vivo FEC reduction (68.8%) as compared to the plain bromelain (32.4%).
Conclusion:
Our results show that bromelain encapsulated in chitosan may be safe and effective in reducing the burden of gastrointestinal tract strongyle nematodes in goats. However, there is a need for further studies to establish the dosage of the encapsulated bromelain to be administered in a single dose for the treatment of goats against gastrointestinal strongyles. In addition, species-specific studies on the efficacy of encapsulated bromelain on strongyles are necessary to evaluate its effectiveness against the entire Strongyloididae family.
Keywords: anthelmintic efficacy, bromelain, chitosan, goats, nanoencapsulation, toxicity
Introduction
Helminthosis is an important disease associated with the infection of helminths in the gastrointestinal tract of ruminants [1]. Currently, it is one of the most economically important diseases of livestock in tropics and has a significant economic impact on the well-being of farmers in developing countries [2]. The disease causes the suppression of weight gain, reduction of reproductive performances and leads to high mortality in extreme cases [3,4]. Farmers rely on the aggressive use of anthelmintics to treat and control helminthosis. While commercial anthelmintic usage has shown beneficial effects on animal health, it is also associated with problems, including consumer concern over potential synthetic drug residues in animal products [5,6], and loss of efficacy as a result of the emergence of resistance [7,8]. Given the widespread occurrence of resistance, there is a need for new alternatives involving the development of new drugs with different modes of action, with a specific focus on products arising from plants [9].
Pineapple fruits, stem, and peels are good sources of bromelain which have been proven to have anthelmintic activities [10]. The use of bromelain as anthelmintics has faced some constraints including administration challenges [11]. Studies have shown that after administration, bromelain anthelmintic activity is lowered by the low pH found in the abomasum of ruminants. Moreover, the rumen microbiota can cause deterioration of the drug efficacy, resulting in an ineffective contact between worms and drugs [11]. The study by Hunduza [12] showed that the encapsulation of bromelain with chitosan enhanced it’s in vitro activity against all the stages of Haemonchus contortus isolated from goats. It revealed that encapsulated bromelain had a higher (p>0.05) egg hatch inhibition activity (half maximal inhibitory concentration [IC50=0.249]) than extracted (IC50=0.325) and pure bromelain (IC50=0.327). The study also indicated that encapsulated bromelain had significantly higher (p<0.05) absorbance than that of plain bromelain at low pH (2.0 and 3.0) showing that chitosan nanocarriers stabilize and maintain the activity of bromelain. This implies that bromelain encapsulated in chitosan can be used to target and affect parasites found in the abomasum of ruminants which has a low pH. However, there is a need to further conduct research on the drug by evaluating its toxicity and efficacy against helminthosis in vivo in goats.
Therefore, this study aimed at evaluating the in vitro and in vivo efficacy and toxicity of a bromelain chitosan formulation in Small East African goats in Kenya.
Materials and Methods
Ethical approval
Approval for animal experiments was obtained from the Jomo Kenyatta University of Agriculture and Technology (JKUAT) Animal Ethics Committee (REF: JKU/2/4/896B). The protocols were approved by the Institutional Animal Care and Use Committee at JKUAT and conducted in compliance with Kenya’s National ethical standards to minimize animal suffering.
Study site
The study was carried out at JKUAT, Kiambu County, Kenya. The university is located at latitude 1°05 S and longitude 37°00 E. It lies at an altitude of 1525 m above sea level and receives an annual rainfall of 850 mm with temperatures ranging from 13°C to 26°C [13].
Study animals
Eighteen Small East African healthy male indigenous goats, aged between 8 and 30 months old and weighing between 13 and 21 kg, were purchased from local farmers. They were ear-tagged and kept in a goat house where they were acclimatized to the diet over the course of 14 days before commencing the study. Animals were group-housed in pens of size 2 m×2 m (three goats in each) inside the main goat house which was located within the JKUAT. Each animal was screened for the presence of strongyle eggs by fecal egg count (FEC) examination before the start of the experiment. Goats were fed on 1.5 kg of concentrate feed and 1 kg of wheat hay twice each day (at 9 a.m. and 3 p.m.). The concentrate feed comprised beet liquid molasses, maize germ, and soybean meal (Aroma Feed Suppliers, Kenya). Supplementation of essential minerals was done using feed blocks (Aroma Feed Suppliers, Kenya). Along with the experiment, albendazole (Sigma-Aldrich, USA) was administered to goats as a positive control. Commercial bromelain (Sigma-Aldrich, USA) was used as a parallel control test.
Extraction of bromelain and encapsulation in chitosan nanoparticles
Bromelain extraction was performed as described by Kahiro et al. [14]. The extracted bromelain was purified using a 10kDa dialysis membrane. The ionic gelation method was used to encapsulate bromelain into chitosan [12] where equal volume (30 ml) of extracted bromelain (4 mg/ml) was mixed with 1% sodium tripolyphosphate (STPP) and rotary mixed for 1 min. Using a syringe, 12 ml of the bromelain-STPP mixture was added to 20 ml of 1% chitosan under vigorous and continuous stirring. The resultant suspension was centrifuged at 15,000 rpm for 45 min and the obtained pellet washed with distilled water before freeze-drying. The aliquots of bromelain loaded chitosan nanocarrier pellet were frozen at −60°C and placed in the freeze-dryer (MRC equipment manufacturer, Israel). The freeze-drying was allowed to run until all the samples were completely dried. Successful conjugation of bromelain to the chitosan nanoparticles was confirmed by Fourier transform infrared spectrophotometer analysis.
In vitro adult worm mortality assay
Adult mortality assay was conducted according to the procedure by Eguale et al. [15] and Hunduza [12]. Briefly, ten actively moving worms were placed in Petri dishes and exposed to encapsulated bromelain solution ranging from 0.125 mg/ml to 2 mg/ml. Albendazole prepared in concentrations ranging from 0.125 mg/ml to 2 mg/ml was used as the positive control while phosphate-buffered saline was used as the negative control. Commercial bromelain was also prepared in concentrations ranging from 0.125 mg/ml to 2 mg/ml and was used as a parallel control test. Each test was done in triplicate. After 24 h, the number of live and dead worms was counted and the percentage mortality calculated using the formula:

Animal treatments
Treatment groups were formed after randomization based on the number of eggs per gram (EPG) of feces (EPG values), such that the mean EPG of the animals in each group was more than 500 [16]. Each group had three animals. The treatment was done orally every morning (9 a.m.) for 14 days. Groups 1, 2, and 3 received 30 mg/kg, 10 mg/kg, and 3 mg/kg of encapsulated bromelain, respectively. Group 4 received 30 mg/kg of plain bromelain. Group 5 was the positive control (Albendazole, 7.5 mg/kg body weight) and Group 6 was the negative infected non-treated control. The above dosages were chosen following the World Association for the Advancement of Veterinary Parasitology guidelines for dose determination [17] and the results of the bromelain toxicity tests obtained in the previous studies [18-20].
In vivo toxicity assessment
Clinical observations
Goats were fasted overnight before dosing. Following the period of fasting, the animals were weighed and then the test substance was administered orally using drenching guns. Observations were made and recorded systematically and continuously as per the guidelines [21]. Animals were observed individually during the entire study period. Special attention was given during the first 4 h and daily thereafter, for a total of 14 days to observe any death or changes in general behavior and other physiological activities. Observations included changes in skin and fur, eyes and mucous membranes, respiratory system, and behavior pattern. Attention was also directed to observations of salivation, diarrhea, lethargy, and sleep in the animals [21].
Temperature and bodyweight of goats were measured at 09:00 am using a digital thermometer (Kruuse Digital Thermometer; Jørgen Kruuse) and a 100 kg spring balance scale (Salter Model, Capital Scales – Pretoria, South Africa), respectively. This was done before treatment and once a week during the experiment period. Changes in the weight of individual goats were calculated and compared with that of the control animals. Changes were considered as a result of the adverse effects of drugs if the body weight loss observed was more than 10% of the initial recorded body weight [22].
Sample collection
Blood samples (3 ml) from each goat were drawn from the jugular vein in ethylenediaminetetraacetic acid test tubes using 3 ml syringes with 3/4-inch, 20-gauge needles. This was done at 09:00 am weekly.
Packed cell volume (PCV)
The PCV was determined using the microhematocrit method [23,24]. Briefly, an aliquot of blood with anticoagulant from each goat was put in micro-capillary tubes and then centrifuged at 14,000 rpm for 10 min. After centrifugation, samples were analyzed for PCV using a micro-capillary reader (Hawksley, England).
Determination of serum biochemical parameters
Aspartate aminotransferases (AST), alanine aminotransferases (ALT), urea, and creatinine were analyzed using standard diagnostic test kits on automated clinical biochemistry analyzer (Reflotron Plus System®, model: Cobas 4800 Detection Analyzer; Mumbai, India).
Gross pathology and histopathology
At the end of the experimental period, the goats were sacrificed and examined for gross pathology. Parts of the following organs were preserved in 10% buffered formalin: Liver, kidney, spleen, and heart. Thereafter, the organs were processed for histopathology and the prepared slides examined under the microscope using the procedure as described by Slaoui and Fiette [25].
Assessment of the in vivo anthelmintic efficacy
Fecal samples were collected once a week, for 4 weeks, from the rectum of the goats using fresh gloves. Aliquots of 2 g of the fecal sample from each goat were placed in a plastic bottle (Indosurgicals Pvt. Ltd., New Delhi, India) for analysis.
The fecal samples were analyzed using a modified McMaster technique [26] with a precision of 100 EPG of feces using an Olympus B 201 microscope (Optical Element Corporation, Melville, USA) at 10×. FEC reduction (FECR) percentage was calculated using the formula:
% FECR=100×[1−(T2/T1)]
T1=Mean pre-treatment FEC in treatment group
T2=Mean post-treatment FEC in treatment group [27].
Statistical analysis
All statistical analyses and graphical presentations were carried out using R (version 3.6.0) (Foundation for Statistical Computing, Vienna, Austria) and GraphPad (Version 7.02) (GraphPad Software, Inc., La Jolla, CA, USA), respectively. Tests of significance for different drug concentrations on worm mortality were conducted by analysis of variance (ANOVA) and the significance level was set at p<0.05. Regression analysis between the log concentrations and probit-transformed responses was used to determine the lethal concentration 50% (LC50). Pre- and post-treatment comparisons of FEC, PCV, weight, and temperature were done using ANOVA (p<0.05).
Results
In vitro worm mortality assay
At all concentrations, albendazole had the greatest effect on the worms with 1 mg/ml achieving 100% worm mortality. Encapsulated bromelain showed higher activity compared to the plain bromelain and achieved 100% worm mortality at 1 mg/ml. Albendazole had significantly (p<0.05) higher activity than encapsulated bromelain and plain bromelain. The LC50 observed were 0.05 mg/ml, 0.155 mg/ml, and 0.445 mg/ml for albendazole, encapsulated bromelain, and plain bromelain, respectively (Figure-1).
Figure-1.

Mean lethal concentration 50% of bromelain, encapsulated bromelain, and albendazole. Data represent means with standard deviations of three replicates analyzed using R version 3.6.0. Small letters on top of each bar compare means, according to Tukey’s honestly significant difference test (p≤0.05). Different letters indicate significantly different values. PB: Plain bromelain, EB: Encapsulated bromelain, Alb: Albendazole.
Encapsulated bromelain toxicity assessment
Clinical observations
Administration of dose up to 30 mg/kg encapsulated bromelain did not reveal any mortality in goats during the entire observation period. Therefore, lethal dose 50 (LD50) of encapsulated bromelain may be considered to be >30 mg/kg. No treatment-related clinical symptoms of toxicity were observed during the experimental period at any of the three doses of encapsulated bromelain, as well as for the plain bromelain (30 mg/kg) and albendazole (7.5 mg/kg) used as parallel controls. The skin, fur, mucous membrane, urination, water intake, and food intake of goats were found to be normal before and after treatment. Lethargy, diarrhea, inactivity, rapid breathing, excessive salivation, liquid secretion from eyes, and rapid breathing were not observed. Goat body temperatures varied from 38.1°C to 39.2°C, which is within the normal range of the animal [28]. No significant variation of temperature and body weight (p<0.05) was observed between the control non-treated goats and the encapsulated bromelain treated goats.
Necropsy macroscopic observation and histological examination
No treatment-related pathological changes of internal organs were observed in necropsy examination. Hematoxylin and eosin staining of liver showed normal hepatic architecture, hepatocytes, and hepatic sinusoids. Histological assay of the kidney from encapsulated bromelain treated groups showed normal renal architecture with a normal appearance of glomerulus and tubules. The spleen appeared normal with no histopathological alteration. Heart sections of encapsulated bromelain treated groups presented a normal myocardial architecture (Figure-2).
Figure-2.

Histological examinations of the kidney, liver, heart, and spleen of goats after 14 days of repeated-dose toxicity study. Representative photomicrographs from kidney, liver, heart, and spleen sections stained with hematoxylin and eosin (H&E), respective groups: (a) Control non-treated group, (b) encapsulated bromelain treated group (30 mg/kg), H&E 400×.
Effect of the plant extract on the PCV and serum biochemical parameters
The PCV of untreated goats varied between 29.3% and 35.1%. The mean PCV of treated goats was similar to that of the control untreated group (p>0.05). ALT and AST levels of untreated goats ranged from 14.07 to 16.17 U/L and 105.6 to 115.5 U/L, respectively, which were within the normal range [29]. No significant difference (p>0.05) was observed between the AST and ALT levels of treated and the control non-treated goats. Likewise, urea and creatinine levels of untreated goats varied from 5.6 to 6.3 mmol/L and 55.4 to 63.07 µmol/L, respectively, and were within the normal range of the animal [29]. There was no significant difference between the urea and creatinine levels of treated goats and the control non-treated goats.
In vivo anthelmintic efficacy assessment
The percentage reduction in strongyle eggs was significantly higher (p<0.05) for the goats treated with 7.5 mg/kg of albendazole than those treated with encapsulated bromelain at any of the three tested doses (30, 10, and 3 mg/kg). Similarly, a significant (p<0.05) gradual increase in percentage reduction of strongyles eggs was observed over time for goats treated with the plain and encapsulated bromelain. On 28th day post-treatment, 68.8%, 56.6%, and 5% strongyle egg reduction were recorded for goats treated with 30, 10, and 3 mg/kg of encapsulated bromelain, respectively. In addition, 32.4% and 96.5% strongyle egg reduction was noted for goats treated with 30 mg/kg of plain bromelain and 7.5 mg/kg of albendazole, respectively. In contrast, the percentage egg count for the negative control group increased by 22% (Figure-3).
Figure-3.
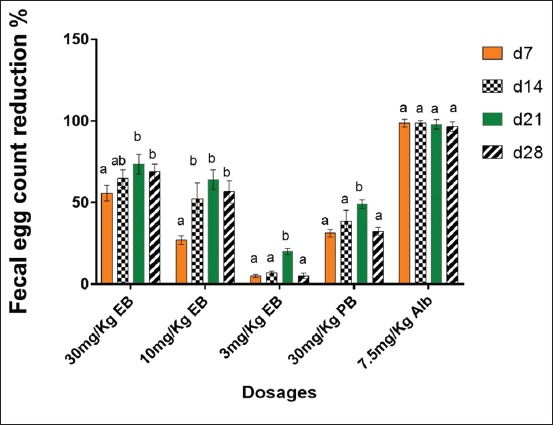
Fecal egg count reduction test. Positive values indicate a relative reduction of the number of EPG of feces and negative values a relative increase. Data represent means with standard deviations of three replicates analyzed using R version 3.6.0. Small letters on top of each bar compare means at different days post-treatment according to Turkey’s honestly significant difference test (p≤0.05). Different letters indicate significantly different values. FECR: Fecal egg count reduction, PB: Plain bromelain, EB: Encapsulated bromelain, Alb: Albendazole, EPG: Number of eggs per gram of feces, d7: Day 7 post-treatment, d14: Day 14 post-treatment, d21: Day 21 post-treatment, d28: Day 28 post-treatment.
Discussion
The current study was geared toward evaluating the in vitro and in vivo efficacy and toxicity of a bromelain chitosan formulation which can extend the release of the drug. The in vitro results demonstrated that albendazole had higher anthelmintic activity than encapsulated bromelain as well as plain bromelain. This is consistent with the findings by Hunduza [12] who also evaluated the anthelmintic activity of bromelain encapsulated in chitosan nanocarriers against H. contortus isolated from naturally infected goat and showed that albendazole had the greatest effect on the worms followed by encapsulated bromelain and extracted bromelain.
The observed albendazole and bromelain LC50s are comparable to those obtained by Cheruiyot [30] and Hunduza [12]. The small difference could be related to varying quality and source of bromelain [31]. The anthelmintic mechanism of action of pineapple cysteine proteinases is well documented [32,33]. These enzymes attack protein targets in the cuticle leading to weakening of the cuticle, blistering and rupture, and subsequent release of internal tissues. These eventually lead to the death of the worm. This property is not limited to the enzymes from pineapple and papaya but has been reported in papain homologs from figure and lattices of other plants [32-34].
Similar to the findings of this study, Dutta and Bhattacharyya [35] did not observe any toxicity after oral administration of acute and sub-acute doses of the aqueous extract of Ananas comosus (pineapple) crown leaf to rats. According to Taussig et al. [18], bromelain has very low toxicity and its LD50 has been evaluated to be >10 g/kg in mice, rates, and rabbits. Pavan et al. [19] reported that daily administration of bromelain to dogs in an increasing level up to 750 mg/kg showed no toxic effects after 6 months. Corroborating our results, Moss et al. [20] did not find any alteration in the histology of heart, kidney, or hematological parameters after administration of 1500 mg/kg bromelain per day to rats. The PCV observed in this study was lower compared to the values (32.5-43.7%) reported by Al-Bulushi et al. [36] for Sahrawi goats. Earlier reports for Jabali goats showed PCV values ranging from 37.4% to 43.7% [36]. Despite the small difference observed, the obtained PCV values were within the normal range of the animal [37]. The observation that there were no significant differences between the PCV of treated and control non-treated goats indicates that the administration of encapsulated bromelain does not affect erythrocyte production and physiology. The serum creatinine, urea, ALT, and AST levels were within the normal range of the animal and did not show any significant difference between the treated and the negative control non-treated goats indicating normal functioning kidneys and liver. The range of goat ALT level observed in this study is comparable with the finding by Tibbo et al. [38] who indicated that the ALT level of indigenous Arsi-Bale, Central Highland and Long-eared Somali goat breeds ranged from 14.0 to 20.2 U/L. The obtained values for urea were in agreement with the report of Chikwanda and Muchenje [39] and also Kaneko et al. [40] who reported urea level ranging from 3.57 to 7.14 mmol/L, which was in the normal range of the animal.
As compared to the findings of this study, Domingues et al. [41] reported a lower efficacy for bromelain in sheep infected with H. contortus. The disparity between the anthelmintic efficacy reported by Domingues et al. [41] and the findings of this study can be attributed to the differences in the administered dosages and to the fact that they used H. contortus infected sheep while the present study dealt with goats. In addition, in this study, the drug was administered for 14 days in contrast to Domingues et al. [41] single dose. In the present study, encapsulated bromelain had higher activity than plain bromelain, thus, it is clear that the encapsulation process increased the efficacy of bromelain. A similar result was reported by Hunduza [12]. Ribeiro et al. [42] further demonstrated that the encapsulation of extracted plant oils in chitosan nanoparticles leads to an increase in their efficacy against H. contortus. The observed increased activity of encapsulated bromelain as compared to plain bromelain can be attributed to the fact that encapsulation of enzymatic drugs stabilizes the protein structure, promotes an active-controlled release and stabilizes the activity presenting greater efficacy [43].
Conclusion and Recommendations
The current study demonstrates that oral administration of chitosan encapsulated bromelain up to a dosage of 30 mg/kg is not associated with any adverse clinical symptoms, toxicity of the main organs, and mortality in goats. The encapsulation of bromelain in chitosan enhances its anthelmintic properties in vitro and in vivo, and thus encapsulated bromelain can act as an important substitute to synthetic anthelmintic drugs where anthelmintic resistance has developed. However, further experiments with infected animals are essential to establish the dosage of the encapsulated bromelain to be administered in a single dose for the treatment of goats against gastrointestinal strongyles. In addition, species-specific studies on the efficacy of encapsulated bromelain on strongyles are necessary to evaluate its effectiveness against the entire Strongyloididae family.
Author’s Contributions
All authors conceived and designed the experiment. SW and JK performed the experiment. NM performed the biochemical assays. SW and JK analyzed and wrote the manuscript. All authors read and approved this manuscript.
Acknowledgments
This work was done through financial support by the Pan African University (MB 300-0002/18), Jomo Kenyatta University of Agriculture and Technology (JKUAT) and the Africa-ai-Japan Project. The authors are grateful to Matuu and Kiserian farmers for provision of goats and acknowledge the technical assistance provided by JKUAT staff, including Dr. Lewis Macharia, Grace Kimani, Perminus Kimathi, and Michael Oduor. All authors read and approved the final draft of the article.
Competing Interests
The authors declare that they have no competing interests.
Publisher’s Note
Veterinary World remains neutral with regard to jurisdictional claims in published institutional affiliation.
References
- 1.Bamaiyi P. Factors militating against the control of helminthosis in livestock in developing countries. Vet. World. 2011;5(1):42–47. [Google Scholar]
- 2.Chavan P.B, Khan L.A, Raut P.A, Maske D.K, Rahman S, Podchalwar K.S. Prevalence of nematode parasites of ruminants at Nagpur. Vet. World. 2008;1(5):140. [Google Scholar]
- 3.Waller P. From discovery to development:Current industry perspectives for the development of novel methods of helminth control in livestock. Vet. Parasitol. 2006;139(1-3):1–14. doi: 10.1016/j.vetpar.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 4.Lashari M, Tasawar Z. Prevalence of gastrointestinal parasites in sheep, Southern Punjab, Pakistan. Pak. Vet. J. 2011;31(4):295–298. [Google Scholar]
- 5.Knox D.P. Development of vaccines against gastrointestinal nematodes. Parasitology. 2000;120(7):S43–S61. doi: 10.1017/s0031182099005764. [DOI] [PubMed] [Google Scholar]
- 6.Dalton J.P, Mulcahy G. Parasite vaccines a reality? Vet Parasitol. 2001;98(1-3):149–167. doi: 10.1016/s0304-4017(01)00430-7. [DOI] [PubMed] [Google Scholar]
- 7.Wanyangu S.W, Bain R.K, Rugutt MK, Nginyi J.M, Mugambi J.M. Anthelmintic resistance amongst sheep and goats in Kenya. Prev. Vet. Med. 1996;25(3-4):285–290. [Google Scholar]
- 8.Waruiru R.M, Kogi J.K, Weda E.H, Ngotho J.W. Multiple anthelmintic resistance on a goat farm in Kenya. Vet. Parasitol. 1998;175(2-3):19. doi: 10.1016/s0304-4017(97)00195-7. [DOI] [PubMed] [Google Scholar]
- 9.Newman D.J, Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75(3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maurer H.R. Bromelain:Biochemistry, pharmacology and medical use. Cell. Mol. Life Sci. 2001;58(9):1234–1245. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buttle D, Behnke J, Bartley Y, Elsheikha H, Bartley D, Garnett M, Duce I. Oral dosing with papaya latex is an effective anthelmintic treatment for sheep infected with Haemonchus contortus. Parasit. Vectors. 2011;4(1):36. doi: 10.1186/1756-3305-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunduza A. Anthelmintic Efficacy of Bromelain Encapsulated Chitosan Nanocarrires against Haemonchus contortus. Pan African University Thesis. 2018 [Google Scholar]
- 13.Menge D.M.S, Makobe M, Monda E.O, Okemo P.O. Effects of crude extracts on some selected physiological parameters of French beans (Phaseolus vulgaris) infected with rust (Uromyces appendiculatus) Afr. J. Plant Sci. 2014;8(7):356–363. [Google Scholar]
- 14.Kahiro S.K, Kagira J.M, Maina N, Karanja S.M, Njonge F.N. Enzymatic activity of bromelain from crude extracts of crown, peels and stem of pineapples from different agro-ecological zones of Thika region, Kenya. Asian J. Biotechnol. Bioresour. Technol. 2017;1(2):1–6. [Google Scholar]
- 15.Eguale T, Tilahun G, Debella A, Feleke A, Makonnen E. In vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against Haemonchus contortus. J. Ethnopharmacol. 2007;110(3):428–433. doi: 10.1016/j.jep.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Coles G.C, Jackson F, Pomroy W.E, Prichard R.K, Son-Himmelstjerna G.V, Silvestre A. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006;136(3-4):167–185. doi: 10.1016/j.vetpar.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Wood I.B, Amaral N.K, Bairden K, Duncan J.L, Kassai T, Malone J.B, Jr, Pankavich J.A, Reinecke R.K, Slocombe O, Taylor S.M, Vercruysse J. World association for the advancement of veterinary parasitology (W.A.A.V.P.): Second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine) Vet. Parasitol. 1995;58(3):181–213. doi: 10.1016/0304-4017(95)00806-2. [DOI] [PubMed] [Google Scholar]
- 18.Taussig S.J, Yokoyama M.M, Chinen A. Bromelain:A proteolytic enzyme and its clinical application: A review. Hiroshima J. Med. Sci. 1975;24(2-3):185–193. [PubMed] [Google Scholar]
- 19.Pavan R, Jain S, Shraddha P, Kumar A. Properties and therapeutic application of bromelain:A review. Biotechnol. Res. Int 2012. 2012;1:1–6. doi: 10.1155/2012/976203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss I.N, Frazier C.V, Martin G.J. Bromelain the pharmacology of the enzyme. Arch. Int. Pharmacodyn. Ther. 1963;145(3):166–189. [PubMed] [Google Scholar]
- 21.OECD. Guideline for Testing of Chemicals Acute Oral Toxicity Acute Toxic Class Method. France: OECD; 2001. p. 14. [Google Scholar]
- 22.Raza M, Al-Shabanah O.A, El-Hadiyah T.M, Al-Majed A.A. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci. Pharm. 2002;70(2):135–145. [Google Scholar]
- 23.Githiori J.B, Hogland J, Waller P.J, Baker R.L. Evaluation of anthelmintic properties of some plants used as livestock dewormers against Haemonchus contortus infection in sheep. Parasitology. 2004;129(2):245–253. doi: 10.1017/s0031182004005566. [DOI] [PubMed] [Google Scholar]
- 24.Hansen J, Perry B. The Epidemiology, Diagnosis and Control of Helminth Parasites of Ruminants:A Handbook. Nairobi: International Livestock Research Institute; 1994. [Google Scholar]
- 25.Slaoui M, Fiette L. Histopathology procedures:From tissue sampling to histopathological evaluation. In: Gautier J.C, editor. Drug Safety Evaluation. Methods in Molecular Biology (Methods and Protocols) Vol. 691. Switzerland: Humana Press; 2011. [DOI] [PubMed] [Google Scholar]
- 26.Zajac A.Z, Conboy G.A. Veterinary Clinical Parasitology. 8th ed. Chichester: Wiley-Blackwell; 2012. pp. 8–11. [Google Scholar]
- 27.Kochapakdee S, Pandey V.S, Pralomkarn W, Choldumrongkul S, Ngampongsai W, Lawpetchara A. Anthelmintic resistance in goats in Southern Thailand. Vet. Rec. 1995;137(5):124–125. doi: 10.1136/vr.137.5.124. [DOI] [PubMed] [Google Scholar]
- 28.Swenson M.J, Reece W.O. Dukes Fisiologia dos Animais Domésticos. Guanabara Koogan S.A. Rio de Janeiro. 2006 [Google Scholar]
- 29.Jackson P.G, Cockcroft P.D. Clinical Examination of Farm Animals. Hoboken: Wiley-Blackwell; 2002. [Google Scholar]
- 30.Cheruiyot K. In vitro Ovicidal Activity of Encapsulated Ethanolic Extract of Prosopis juliflora Against Haemonchus contortus Eggs, Jomo Kenyatta University of Agriculture and Technology. Thesis. 2017 [Google Scholar]
- 31.Bresolin I, Silveira E, Tambourgi E, Mazzola P. Isolation and purification of bromelain from waste peel of pineapple for therapeutic application. Braz. Arch. Biol. Technol. 2013;56(6):971–979. [Google Scholar]
- 32.Stepek G, Lowe A.E, Buttle D.J, Duce I.R, Behnke J.M. In vitro and in vivo anthelmintic efficacy of plant cysteine proteinases against the rodent gastrointestinal nematode Trichuris muris. Parasitology. 2006;132(5):681–689. doi: 10.1017/S003118200500973X. [DOI] [PubMed] [Google Scholar]
- 33.Stepek G, Lowe A.E, Buttle D.J, Duce I.R, Behnke J.M. Anthelmintic action of plant cysteine proteinases against the rodent stomach nematode Protospirura muricola in vitro and in vivo. Parasitology. 2007a;134(1):103–112. doi: 10.1017/S0031182006001302. [DOI] [PubMed] [Google Scholar]
- 34.Stepek G, Lowe A.E, Buttle D.J, Duce I.R, Behnke J.M. The anthelmintic efficacy of plant-derived cysteine proteinases against the rodent gastrointestinal nematode Heligmosomoides polygyrus in vivo. Parasitology. 2007b;134(10):1409–1419. doi: 10.1017/S0031182007002867. [DOI] [PubMed] [Google Scholar]
- 35.Dutta S, Bhattacharyya D. Enzymatic, antimicrobial and toxicity studies of the aqueous extract of Ananas comosus (pineapple) crown leaf. J. Ethnopharmacol. 2013;150(2):451–445. doi: 10.1016/j.jep.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Al-Bulushi S, Shawaf T, Al-Hasani A. Some hematological and biochemical parameters of different goat breeds in sultanate of Oman “A preliminary study”. Vet. World. 2017;10(4):461–466. doi: 10.14202/vetworld.2017.461-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radostitis C.C, Gray D.C, Hinchcliff K.K. Blood and diseases caused by trypanosomes. In: Otto M, Clive C, Douglas C, Kenneth W, Gay C, Hinchcliff K, Russel D, editors. A Textbook: Of the Diseases of Cattle, Sheep, Pigs, Goats and Horse. 9th ed. New York: WB Saunders Company Ltd; 2000. p. 1877. [Google Scholar]
- 38.Tibbo M, Jibril Y, Woldemeskel M, Dawo F, Aragaw K, Rege J.E. Serum enzymes levels and influencing factors in three indigenous Ethiopian goat breeds. Trop. Anim. Health Prod. 2008;40(8):657–666. doi: 10.1007/s11250-008-9145-2. [DOI] [PubMed] [Google Scholar]
- 39.Chikwanda A.T, Muchenje V. Grazing system and floor type effects on blood biochemistry, growth and carcass characteristics of Nguni goats. Asian-Australas J. Anim. Sci. 2017;30(9):1253–1260. doi: 10.5713/ajas.16.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaneko J.J, Harvey J.W.M, Bruss L. Clinical Biochemistry of Domestic Animals. 6th ed. Burlington, MA: Academic Press; 2008. [Google Scholar]
- 41.Domingues L.F, Giglioti R, Feitosa K.A, Fantatto R.R, Rabelo M.D, de Sena Oliveira M.C, Bechara G.H, de Oliveira G.P, Barioni Junior W, de Souza Chagas A.C. In vitro and in vivo evaluation of the activity of pineapple (Ananas comosus) on Haemonchus contortus in Santa Inês sheep. Vet Parasitol. 2013;197(1-2):263–270. doi: 10.1016/j.vetpar.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro J.C, Ribeiro W.L.C, Camura-Vasconcelos A.L.F, Macedo I.T.F, Santos J.M.L, Paula H.C.B, Bevilaqua C.M.L. Efficacy of free and nanoencapsulated Eucalyptus citriodora essential oils on sheep gastrointestinal nematodes and toxicity for mice. Vet. Parasitol. 2014;204(2014):243–248. doi: 10.1016/j.vetpar.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 43.Bhatnagar P, Patnaik S, Srivastava A.K, Mudiam M.K.R, Shukla Y, Panda A.K, Gupta K.C. Anti-cancer activity of bromelain nanoparticles by oral administration. J. Biomed. Nanotechnol. 2014;10(12):3558–3575. doi: 10.1166/jbn.2014.1997. [DOI] [PubMed] [Google Scholar]


