Abstract
Tumor-derived extracellular vesicles (tdEVs) are attracting much attention due to their essential function in intercellular communication and their potential as cancer biomarkers. Although tdEVs are significantly more abundant in blood than other cancer biomarkers, their concentration compared to other blood components remains relatively low. Moreover, the presence of particles in blood with a similar size as that of tdEVs makes their selective and sensitive detection further challenging. Therefore, highly sensitive and specific biosensors are required for unambiguous tdEV detection in complex biological environments, especially for decentralized point-of-care analysis. Here, we report an electrochemical sensing scheme for tdEV detection, with two-level selectivity provided by a sandwich immunoassay and two-level amplification through the combination of an enzymatic assay and redox cycling on nanointerdigitated electrodes to respectively enhance the specificity and sensitivity of the assay. Analysis of prostate cancer cell line tdEV samples at various concentrations revealed an estimated limit of detection for our assay as low as 5 tdEVs/μL, as well as an excellent linear sensor response spreading over 6 orders of magnitude (10–106 tdEVs/μL), which importantly covers the clinically relevant range for tdEV detection in blood. This novel nanosensor and associated sensing scheme opens new opportunities to detect tdEVs at clinically relevant concentrations from a single blood finger prick.
Keywords: Nanoelectrodes, redox cycling, enzymatic amplification, tumor-derived extracellular vesicles, microfluidics
Liquid biopsies are highly promising for metastatic cancer disease management.1 In this noninvasive approach, a sample of blood (typically a few mL, e.g., from a finger prick) is screened for the presence of tumor biomarkers, such as circulating tumor DNA (ctDNA), miRNAs, tumor-derived extracellular vesicles (tdEVs), or circulating tumor cells (CTCs).2 It has been established that, compared to imaging techniques (magnetic resonance imaging (MRI) in conjunction with computed tomography (CT)), CTC quantification in liquid biopsies has a better prognostic value,1,3 while being significantly less demanding from a clinical point-of-view: the procedure is much more patient-friendly, cheaper and does not require any administration of toxic contrast agents. Furthermore, blood analysis can be repeated at higher frequency (e.g., a few times per month vs a few times per year for MRI), while allowing close monitoring of a patient’s response to therapy. However, the main challenge to be overcome in this approach is the extremely low relative concentration of CTCs. Moreover, for every CTC (typically <10 CTCs per mL of blood), there are millions of white blood cells and billions of red blood cells. In contrast to CTCs, tdEVs, which are constantly released by tumor cells in blood, occur at a much higher concentration (10–106 EVs per μL of blood).4 Extracellular vesicles (EVs) are nanometer-sized (30 nm to 1 μm) particles, enclosed by a phospholipid bilayer membrane and containing a great variety of biological molecular information on their cells and/or tissues of origin.5−7 EVs are shed by all cell types and found in all bodily fluids, where they play an important role in (inter)cellular communication.8,9 All EVs share the same generic EV-membrane protein repertoire, e.g., CD9, CD63, and CD81 being present on the vast majority of blood cell-derived EVs.10,11 Next to this, tdEVs exhibit membrane proteins that are specific of their cellular origin, e.g., cancer biomarkers HER2, EGFR, and epithelial cell adhesion molecule (EpCAM).1,10,12 Notably, EpCAM has been widely used for the isolation and detection of both CTCs and tdEVs,1 which are found in blood from the early stages of cancer to metastasis. The concentration of both CTCs and tdEVs increases with the progression of the tumor.13−16 The widespread tdEV concentrations naturally occurring in blood make them promising alternative cancer biomarkers. The variation between patients with low and high tdEV abundance is relatively much higher than for CTCs (a factor of 106 for EVs vs. a factor of maximally ∼103 for CTCs), and therefore statistically more unlikely to yield false-negative results.
However, before tdEVs can be considered in clinical routines and liquid biopsy analysis, reliable, unambiguous, highly sensitive and specific methods must be developed for their isolation, detection, and quantification in complex matrices such as blood. Species in the EV size range are difficult to characterize using existing analytical techniques suitable to single molecules or cells, which are, respectively, smaller and larger than EVs. Furthermore, blood comprises various other entities in the same size range as EVs and often present with much higher concentrations, such as protein aggregates, lipoproteins, cell debris and, most notably, noncancerous EVs, from which tdEVs need to be unequivocally distinguished.17 EVs are often studied using flow cytometry and/or fluorescence microscopy.18−24 Although these techniques provide unique molecular information on tdEVs, they often lack the sensitivity and/or resolution required to detect both the rarest and/or smallest tdEVs. These techniques also require substantial sample volumes. In contrast, single EVs can be detected using atomic force microscopy (AFM),25 nanoparticle tracking analysis (NTA),26 resistive pulse sensing,26 Raman spectroscopy27,28 or a combination of some of these techniques, whose throughput and level of technicality is however too low for practical medical/clinical use.
Altogether there is a clear need to be able to detect tdEVs at concentrations as low as 1–100 per μL and across an extended clinically relevant concentration range.4 However, only a few endeavors have led to the development of sensors sensitive enough to detect such low concentrations. Recently, Zhang et al.29 reported immunocapturing of tdEVs on antibody-modified herringbone in microfluidic channels followed by their detection using fluorescence microscopy, after amplification of the signal using an enzymatic reaction. Although they reported a limit of detection (LOD) of 10 EVs/μL, the signal was barely distinguishable from the background, and their approach worked over a linear detection range from 10–103 EVs/μL. Using amperometric detection of enzymatic activity after magnetic immuno-enrichment with nanocubes, Boriachek et al.30 analyzed EVs from placental cells using placental alkaline phosphatase as a marker. Their reported LOD was as low as 1 tdEV/μL. However, again, the linear range of their assay only covered a 1–104 EVs/μL concentration range. Huang et al.31 developed an electrochemical detection platform using aptamers as detection probes and a combination of hemin/G-quadruplex DNAzyme-peroxidase reaction and complex rolling circle amplification to achieve signal amplification. Although they achieved a detection limit of ∼1 tdEV/μL, they as well had a narrow linear range of detection from 1 to 103 EVs/μL, making this assay less versatile for clinical applications.
Here, we report an ultrasensitive tdEV detection assay at clinically relevant concentrations using a double amplification mechanism combining redox cycling and an enzymatic reaction, as well as a sandwich immunoassay ensuring a two-level selectivity. The assay is implemented in a lab-on-a-chip format allowing the analysis of small sample amounts, in the (low) microliter range. Uniquely, the linear dynamic range achieved with our assay spanning 6 orders of magnitude largely overlaps with the range of tdEV concentrations naturally occurring in cancer patient blood. Using tdEVs obtained from the cell culture medium, we experimentally demonstrated a LOD of 10 tdEVs/μL well above the background signal in our assay and extrapolated a theoretical LOD as low as a 5 tdEVs/μL from the established calibration curve. Compared to previously reported methods, our antifouling coating in combination with our 2-fold selective scheme awards excellent specificity for tdEV detection compared to EVs of other origins, as demonstrated here using platelet-derived EVs (giving 60 times less signal at a 102-fold higher concentration).
Sandwich Immunoassay on Nanoscale Interdigitated Electrodes
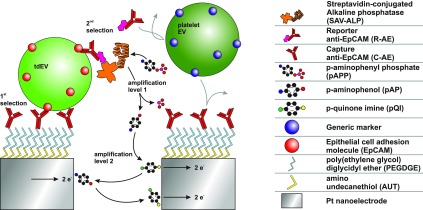
The detection principle of our assay is illustrated in Figure 1. To achieve an amplification level that is powerful enough to detect tdEVs at physiologically relevant concentrations, we use a two-level amplification strategy: (i) a first enzymatic amplification using alkaline phosphatase (ALP), releasing electrochemically active species, followed by (ii) electrical signal amplification via electrochemical redox cycling on nanoscale interdigitated electrodes (nIDEs). Given the complexity of the targeted biological sample, exquisite selectivity is required to get a signal that solely arises from the presence of tumor-derived species, i.e., with a low background signal. Here, a sandwich immunoassay with tumor-specific antibodies is implemented, providing a two-level selectivity. The assay comprises a capture anti-EpCAM (C-AE) antibody that captures tdEVs and a reporter antibody (anti-EpCAM, R-AE) conjugated to ALP through biotin–streptavidin interactions. Here, the same antibody clone is employed for the two steps of the immune-affinity assay, VU1D9, which is an anti-EpCAM clone proven to be stable and to have high affinity for EpCAM (Kd ∼ 2.7 × 10–10 M).32 Tethering of C-AE on the electrodes involves three steps. First, amine-terminated thiol (amino-undecanethiol, AUT) is self-assembled on the Pt electrodes. Second, an amine-reactive bifunctional poly(ethylene glycol) diglycidyl ether (PEGDGE) is reacted with the AUT layer to add an antifouling linker layer. Subsequently, the C-AE antibody is covalently linked under mild conditions at a slightly basic pH (8.3) and high ionic strength (2 M sodium phosphate).33 This C-AE enables the specific capture of tdEVs derived from EpCAM-expressing human prostate adenocarcinoma cell lines (LNCaP) and provides as such the first level of selectivity. After their capture, the tdEVs interact with biotinylated reporter anti-EpCAM antibodies (R-AE), to provide the second level selectivity, since noncancerous EVs (e.g., blood cell-derived) would not be recognized by either the C-AEs or the R-AEs. The use of the same antibody clones for both immuno-affinity steps can lead to the partial dissociation of the antibody/antigen complex formed between the C-AE and EVs to form a new antibody/antigen complex with R-AE, releasing thereby the EVs from the surface. Using a distinct pair of antibodies as C-AE and R-AE (e.g., VU1D9 and HO-3) would be more favorable in this context. However, considering it may be entropically unfavorable for R-AE to approach the C-AE/antigen complex and because each EV should be captured through multiple antigen/antibody interactions, it seems very unlikely that EVs are released during the second immune-affinity step. The biotin moiety of R-AE next interacts with streptavidin-conjugated alkaline phosphatase (SAV-ALP). ALP is well-known for its ability to cleave substrates containing phosphate groups. Here, the substrate (para-aminophenyl phosphate, pAPP) is chosen since its uncleaved form is electrochemically inert, while its cleaved form, para-aminophenol (pAP), is electrochemically active37. This enzymatic reaction provides the first amplification mechanism. During electrochemical measurements, one of the working electrodes of the nIDE is kept at the reduction potential (a potential well below the formal potential) of pAP, while the second working electrode is swept from a potential below to a potential above its formal potential. At the anode, pAP is oxidized into para-quinone imine (pQI), which diffuses toward the cathode. At the cathode, pQI is reduced back into pAP. The electrochemical reaction can be denoted as pAP ⇆ pQI + 2e–. Since the gaps between the electrodes of the nIDEs are small (120 nm), the pAP and pQI molecules continuously and efficiently shuttle between the two working electrodes via diffusion, producing a steady-state current, which directly scales with the tdEV concentration. This redox cycling provides the second level of amplification.
Figure 1.
Schematic illustration of tdEV sensing using a sandwich immunoassay and redox cycling on nIDEs resulting in a two-level selectivity and a two-level amplification. tdEVs are captured using C-AE tethered to electrodes (first level of selectivity). The binding of R-AE to the tdEVs completes the antibody–antigen-antibody sandwich (second-level selectivity), after which the enzyme ALP is introduced using a biotin–SAV interaction. ALP provides an enzymatic amplification of pAPP to pAP by substrate cleavage (first-level amplification), which is followed by an electrochemical signal amplification via the oxidation of pAP to pQI and subsequent redox cycling thereof between the nIDE electrodes (second-level amplification).
The nIDEs comprised two sets of interdigitated nanoelectrode arrays (100 nm width, 30 μm length, 120 nm spacing, and 70 nm height) defined by electron-beam lithography and platinum evaporation. The narrow spacing between the electrodes tremendously enhanced the redox cycling performance, while still allowing the capture of the smallest (most abundant) EVs in the void between the electrodes.34 EVs larger than 120 nm can be captured on the top surface of the electrodes and can simply contribute to the signal following the same mechanism.
The sensing area of the nIDEs was embedded in a poly(dimethylsiloxane) (PDMS) microfluidic channel (0.2 × 3 × 6 mm3) to facilitate the exchange of reagents by simple micropipetting directly through the channel inlet during surface modification and electrochemical measurements (see Supporting Information, Figure SI-1).35 A pipette tip was installed in the outlet of the microfluidic device as a collection reservoir facilitating the back and forth injection of the sample using a pipette and handling volumes larger than the microchannel itself. After the introduction of every new component, a washing step was implemented by injecting 100 μL of phosphate-buffered saline (PBS).
tdEV Detection on nIDEs
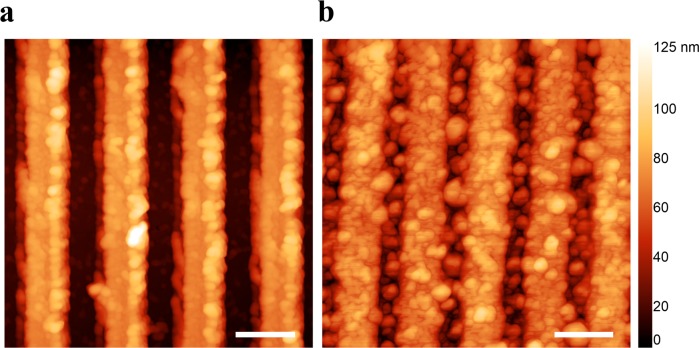
tdEVs were prepared following the protocols described in SI-2. In brief, tdEVs were isolated from LNCaP cells cultured in serum-free medium. The tdEV samples were first characterized using Nanoparticle Tracking Analysis (NTA) to estimate the tdEV concentration (i.e., 106/μL). The surface functionalization of the electrodes and tdEV capture steps were both evaluated using AFM. AFM images acquired on a functionalized unpatterned (plain) Pt surface are presented in Figure SI-2 (see Supporting Information). EV capture was next validated on patterned (nIDE) devices using AFM. A sample of LNCaP-derived tdEVs (25 μL, concentration of 106 EVs/μL, as determined by NTA), was incubated on the C-AE-functionalized electrode surface. As depicted in Figure 2, which presents AFM images before and after the capture of tdEVs on C-AE functionalized electrodes, circular objects were found on the electrodes, with an estimated diameter of 30–150 nm. These objects correspond to relatively small EVs,26 which are the least susceptible to shear forces when flushing the microchannel to remove unbound species, since the Stokes drag force linearly scales with the object size (while neglecting viscous deformation or size-dependence of the affinity). The system was not intentionally designed to exclude larger tdEVs, since EVs captured on the nIDEs were not expected to short-circuit the electrodes due to their dielectric properties. It may be that tdEVs are captured in the space between the electrodes, which is not modified with the antifouling layer and the antibodies. These objects could be captured through antigen/antibody interactions by antibodies present on the sidewalls of the electrodes. Alternatively, some tdEVs could be nonspecifically bound on the surface. The presence of the latter EVs does not influence the outcome of the measurements, which directly depends on the interactions with the second antibody (second level of selectivity).
Figure 2.
Atomic force microscope height images: (a) bare electrodes before chemical modification and (b) after modification and capture of EpCAM-positive tdEVs derived from LnCAP cell lines on nIDEs. The captured objects are 30–150 nm in diameter, which is in good agreement with small EV dimensions (scale bar: 200 nm).
After validation of the surface chemistry on plane substrates (see SI-4), the same functionalization protocol was applied on nIDEs before electrochemical measurements. 100 μL of a tdEV solution in PBS was injected into the microfluidic channel, and this solution was flushed back and forth multiple times during the incubation for 90 min. Different concentrations in EVs (initially 106 EVs per μL) were tested to study the concentration-dependent response of our nanosensor and associated sensing assay. Following this, the microchannel was flushed with a PBS solution to remove unspecifically adsorbed particles on the electrodes. Consequently, the IDEs were incubated with the biotinylated R-AE, (10 μL, 25 mg/mL in PBS, 30 min incubation) and washed with PBS. Subsequently, SAV-ALP was introduced (10 μL, 10 U solution in PBS, 30 min incubation) to interact with the biotin on the R-AE. Next, the IDEs were washed with PBS and incubated with a pAPP solution (100 μL, 10 mM in PBS, 45 min incubation), before electrochemical measurements were started. It should be noted that while the performance of ALP is optimal under alkaline conditions, a physiological pH is preferred for handling EVs. Consequently, the ALP incubation step was performed in PBS (pH 7.4) at room temperature, without active temperature control. Negative control measurements were performed using platelet-derived EVs (pdEV) at 108 pdEVs/μL, which are not EpCAM positive, and do not interact as such with anti-EpCAM antibodies (both C-AE and R-AE). Finally, to evaluate the amplification and thereby gain in sensitivity provided by the nanoscale electrodes, their performance was compared to that of microscale IDEs (μIDEs), which were 3 μm wide, 70 nm high and spaced by 3 μm. The height and total sensing area of sets of electrodes were kept the same for both devices to facilitate the performance comparison.
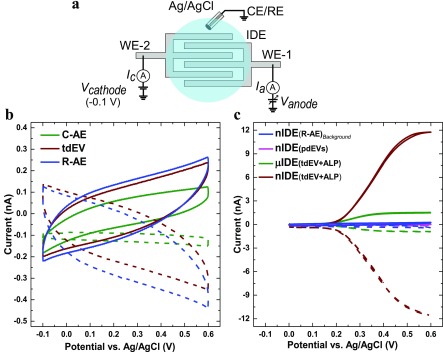
The schematic representation of the measurement setup is provided in Figure 3a; it includes two sets of independent working electrodes (WE-1 and WE-2) and an external Ag/AgCl reference electrode (RE). A fixed potential (−0.1 V) was applied to WE-2 with respect to RE. Since the RE current is very low, the stability of RE was not compromised, even when there was no additional counter electrode (CE). Hence, the CE terminal of the potentiostat was connected to RE. The voltammetric responses (scan rate of 50 mV/s) of the nIDEs were first recorded after different steps of functionalization, i.e., (1) after C-AE functionalization, (2) after tdEV capture, (3) after R-AE immobilization, and (4) after conjugation of the biotinylated R-AE to SAV-ALP and subsequent washing, as well as in the presence of pdEVs instead of tdEVs (negative control).
Figure 3.
Cyclic voltammograms of tdEVs on nIDEs: evaluation of the device specificity. (a) Schematic representation of the electrochemical measurement setup. Cyclic voltammograms (CVs) recorded (b) after various steps of functionalization of the nIDEs, after C-AE surface modification (green), after tdEV capture (brown), and after formation of a sandwich with R-AE (blue), and (c) in the presence of tdEVs on nIDEs (brown) and on μIDEs (green), or in the presence of pdEVs on nIDEs (magenta). The background signal (blue) corresponds to a device after the antibody sandwich formation. The currents Ianode (solid lines) and Icathode (dashed lines) were measured at the anode and the cathode set of electrodes of the nIDEs/μIDEs, respectively. CVs were acquired for a 1 mM pAPP solution in PBS (pH 7.4) between −0.1 V and +0.6 V vs Ag/AgCl at a scan rate of 50 mV/s.
Figure 3b presents typical cyclic voltammograms obtained after the different surface functionalization steps, yet before the introduction of ALP on the surface. In all three cases considered here, no significant change in the recorded current was observed when pAPP was added in the solution, and a maximum current of 262 pA was recorded at 0.6 V vs. Ag/AgCl after addition of the R-AE. Since pAPP is electrochemically inactive, no redox activity is expected, as observed here. Noteworthy, the recorded voltammograms are similar to the I/V characteristics of an RC series circuit, with capacitive charging and discharging upon voltage sweeping, and a hysteresis. Furthermore, a change in the maximal amplitude at 0.6 V vs. Ag/AgCl was observed after each surface functionalization step. In particular, a considerable capacitive change was found after the immobilization of the tdEVs, which can be noticed with the change in the maximal current value (at 0.6 V vs. Ag/AgCl) from 125.1 ± 6.5 pA to 245.1 ± 28.3 pA after the immobilization. Although the capacitive change after the formation of the sandwich assay with R-AE is discernible, it is relatively low compared to the preceding surface functionalization step with 106tdEVs/μL (from 245.1 ± 28.3 pA to 267.6 ± 15 pA). This behavior might be indicative of capacitive charging through the vesicles. On the sample with negative controls (with 108pdEVs/μL), as discussed below, this capacitance change was however not observed, corroborating this argument. The contrast between the signals recorded for our positive and negative control samples also indicates that the antifouling layer plays an essential role in our device, and has performed as expected.
In a following step, we compared the response of nIDEs and μIDEs, using similar conditions as before (10 μL, 106 tdEVs/μL, 1 mM pAPP in PBS (pH 7.4), scan rate of 50 mV/s), after the introduction of the SAV-ALP. Figure 3c presents characteristic sigmoidal curves of diffusion-limited redox cycling currents on closely spaced working electrodes. The limiting current of nIDEs increases with decreasing the gap size between the electrodes. Therefore, although the total sensing surface area of the nIDEs and μIDEs was the same, the 3-μm gap between the μIDEs resulted in a significantly lower limiting current (1.53 ± 0.01 nA) compared to the nIDEs, which were separated by 120 nm (11.76 ± 0.04 nA). Furthermore, the collector efficiency (ratio of cathode-to-anode limiting currents) of μIDEs was found to be only 62.3% compared to 99.8% for the nIDEs. This significant difference indicates that the redox mediator molecules cycle fewer times between the anode and the cathode for the μIDEs before diffusing into the bulk solution. Altogether, the nanoscale electrodes provided an ∼8 times larger amplification of the signal than their microscale counterparts.
Next, we investigated the specificity of our device for the capture and analysis of tdEVs (Figure 3c). tdEVs and pdEV samples were analyzed under the same conditions as before. We compared the response of (1) tdEV+R-AE (blue curve), but before the incubation with SAV-ALP on nIDEs, (2) pdEV on nIDEs (magenta curve), (3) tdEV on nIDEs (brown curve), and (4) tdEV on μIDEs (green curve), after introduction of all required reagents for the assay. The capacitive current (267.6 ± 15 pA) recorded for the tdEV+R-AE sample (106 particles/μL) was higher than for the pdEV sample (170.1 ± 13.2 pA) at 0.6 V for ∼108 particles/μL. Moreover, comparing the limiting currents, ∼60 times amplification in signal was observed compared to the control sample with pdEV for nIDE devices (magenta curve). Again, these results collectively suggest that tdEVs are specifically captured on the electrode surface, while pdEVs are not, illustrating the specificity of the nanosensor.
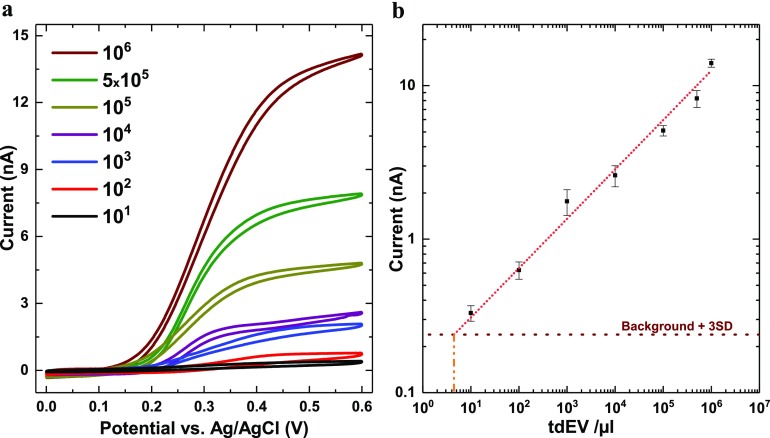
As a next step, the sensitivity and dynamic range of the assay were evaluated, through serial dilution of the initial tdEV sample on three different devices (n = 3). For each device and for each concentration, CV measurements were performed as before, with 1 mM pAPP in PBS buffer at pH 7.4 with a scan rate of 50 mV/s. This recording was repeated three times with a 5 min interval to demonstrate the stability of the measurements. The CVs recorded after varying the tdEV concentration between 10 and 106 EVs per μL are presented in Figure 4a, showing a significant influence of the tdEV concentration. From these data recorded using three independent devices, a calibration curve was established (Figure 4b) using the limiting current at 0.6 V revealing an excellent linear dynamic range spanning at least 6 orders of magnitude and successful measurements at least down to 10 tdEVs/μL, with a readout distinctively above the background signal. By extrapolating the slope of this calibration curve, the current LOD for our assay was evaluated to be ca. 5 tdEVs/μL.
Figure 4.
Sensitivity and dynamic range of the assay for the detection of tdEVs. (a) Anodic cyclic voltammograms recorded at the anode for tdEV samples with concentrations ranging from 10 to 106 tdEVs per μL. (b) Associated calibration curve (based on the limiting currents recorded at 0.6 V vs Ag/AgCl) revealing a dynamic range spanning at least 6 orders of magnitude (number of devices, n = 3). The horizontal dotted line depicts the background level plus three times the standard deviation (SD) of the redox current; from this horizontal line and the calibration curve, a theoretical LOD as low as 5 tdEVs/μL is found. (Conditions: 1 mM pAPP solution in PBS (pH 7.4); scan rate of 50 mV/s.)
In conclusion, we report a novel electrochemical biosensor and associated measurement principle for the highly selective, highly sensitive, and robust quantification of tumor-derived extracellular vesicles. For this, we used a two-level amplification of the signal and a two-level specificity. High assay sensitivity was attained through enzymatic amplification combined with redox cycling between nanoscale interdigitated electrodes. In addition, the high specificity was achieved from the presence of two independent selection steps in the sandwich immunoassay. Using the herein reported device and sensing protocol, we have reached a very high sensitivity that is clinically relevant for the detection of tdEVs, with a measured LOD as low as 10 tdEVs per μL, while extrapolation of the calibration curve suggests a projected LOD of 5 tdEVs/μL. While having an LOD in the same order of magnitude as the currently most sensitive reported systems, importantly, the detection range largely covers the concentration of tdEVs (10–106 tdEVs/μL) found in metastatic cancer patients. Further optimization of the nanosensor and assay performance is ongoing via changes in and stabilization of experimental conditions like pH, incubation time, and operating temperature. Furthermore, the herein used poly(ethylene glycol)-based antifouling layer may not be sufficient when working with samples in more complex media. In that case, the antifouling layer can be adjusted through the incorporation, for instance, of zwitterionic polymer brushes.36 Importantly, this technology can be applied to a wide range of (rare) biomarkers by simply incorporating a different recognition element (e.g., a different antibody). Furthermore, this amperometric sensing method has the potential to be developed as a portable point-of-care sensing device, which can also be useful in population-wide disease screening.
Materials and Methods
Materials
Dichloromethane and ethanol (VLSI grade) were purchased from VWR (Amsterdam, The Netherlands). Acetone (VLSI grade), Harris Uni-Core 1 mm I.D. biopsy punches, sodium phosphate, 11-amino-1-undecanethiol hydrochloride (AUT), (poly(ethylene glycol) diglycidyl ether (PEGDGE), PBS tablets, bovine serum albumin (BSA), and 4-aminophenyl phosphate monosodium salt hydrate (pAPP) were obtained from Merck (Zwijndrecht, The Netherlands). Capturing anti-EpCAM (C-AE), biotinylated reporter anti-EpCAM (R-AE), and extracellular vesicles were received as a kind gift from Immunicon corp (Huntingdon Valley, United States). Streptavidin-conjugated alkaline phosphatase (SAV-ALP) was purchased from Thermo Fisher (Eindhoven, The Netherlands). Sylgard 184 was obtained from Farnell (Utrecht, The Netherlands). Buffers were filtered through a 0.2 μm syringe filter before use (Whatman, Little Chalfont, United Kingdom).
Device Fabrication
nIDEs and μIDEs were fabricated using a combination of optical and electron-beam lithography (EBL) and metal evaporation and lift-off process. First, a 300 nm SiO2 layer was thermally grown on a 10 cm Si (100) wafer at 1000 °C. A first optical lithography step was carried out, followed by evaporation of metals (Ti, adhesion layer, and Pt) and lift-off for defining the markers for EBL. Subsequently, nIDEs were patterned using EBL. CHF3-based plasma etching was next performed to recess the adhesive metal layer into the substrate in the following step. After the etching, metal deposition (Ti, 5 nm; Pt, 70 nm) and lift-off were done to form the IDEs. Successively, the nIDEs were connected to contact pads via contact leads using a second optical lithography step followed by another metal evaporation (Ti, 5 nm; Pt, 100 nm) and lift-off. A 300 nm layer of parylene-C was evaporated as a first passivation layer. A third optical lithography step was done, and using the patterned photoresist as a mask, SiO2 (30 nm) was evaporated on top of the parylene-C layer. This oxide layer covered the entire device except for the contact pads and a rectangular window of 30 × 70 μm2 over the nIDEs. The two passivation layers (parylene and SiO2) prevented current leakage through the contact leads. The fabricated chips were then diced to 25 × 20 mm2 chips (Disco DAD321 dicing machine). Bare Pt substrates to validate and characterize the surface functionalization were prepared by sputtering a 10 nm layer of Ta as an adhesive layer followed by a 100 nm layer of Pt on Mempax glass wafers.
Surface Modification
The platinum nIDEs were first cleaned by rinsing sequentially in dichloromethane, acetone, and ethanol (bare Pt substrates were ultrasonicated in the same solvents for 7 min each). Platinum surfaces were finally cleaned in O2 plasma (Diener Pico, Diener Electronics, Ebhausen, Germany) for 30 s. After placing the PDMS device on the chip (see Supporting Information, SI-1), the microchannel was filled with a 1 mM AUT solution in ethanol to form a self-assembled thiol monolayer under static incubation at room temperature overnight. Next, the channels were washed with 1 mL of ethanol, blown dry with N2, filled with neat poly(ethylene glycol) diglycidyl ether (PEGDGE), and left overnight at 40 °C. Afterward, the channels were again rinsed with 1 mL of ethanol and blown dry with N2. This yields an antibiofouling layer, which was subsequently functionalized with antibodies. Specifically, a capturing anti-EpCAM (C-AE) solution was diluted in sodium phosphate buffer (pH 8.3) to a final concentration of 25 mg/mL and injected in the microchannel for overnight incubation. In this step, epoxide groups in the PEGDGE molecules reacted primarily with amines on the lysine residues on the antibody molecules. Prior to the EV capture, unreacted epoxide groups were blocked with a filtered 1% BSA solution in PBS for 1 h at room temperature, and the device was rinsed with 1 mL of PBS.
Characterization of the Surface Functionalization
The antibody functionalization was validated on plain Pt-coated silica substrates before being applied on devices with IDEs (see SI-4 and SI-5). AFM analysis in the air of the dried substrates confirmed the successful and selective capture of tdEVs on the C-AE functionalized Pt surface. Circular objects (10 nm ±1 nm in height and 0.1–1 μm in width) were found in all studied regions of 10 × 10 μm2, corresponding to EVs, which have collapsed while drying. In contrast, for negative control samples [(1) no addition of PEGDGE, (2) no antibody conjugation, (3) no incubation with EVs, or (4) incubation with EpCAM-negative EVs derived from the PC3 cell line], no EV was found (Figure SI-2). These conditions were subsequently used to functionalize the IDEs.
Acknowledgments
Prof. Leon Terstappen, Dr. Cees Otto, and Dr. Herman Offerhaus are acknowledged for sharing their invaluable insights. Dr. Sidharam Pujari is acknowledged for his consulting on the antifouling layer. Mr. Tom Stelwagen is also acknowledged for his help with device fabrication.
Glossary
Abbreviations
- AFM
atomic force microscopy
- ALP
alkaline phosphatase
- AUT
amino-undecanethiol
- BSA
bovine serum albumin
- C-AE
capturing anti-EpCAM
- CE
counter electrode
- CT
computed tomography
- CTC
circulating tumor cell
- ctDNA
circulating tumor DNA
- CV
cyclic voltammogram
- EBL
e-beam lithography
- EpCAM
epithelial cell adhesion molecule
- EV
extracellular vesicle
- IDE
interdigitated electrode Kd dissociation constant
- LOD
limit-of-detection
- MRI
magnetic resonance imaging
- nIDE
nanointerdigitated electrode array
- NTA
Nanoparticle Tracking Analysis
- pAP
para-aminophenol
- pAPP
para-aminophenyl phosphate
- PBS
phosphate buffered saline
- PDMS
poly(dimethylsiloxane)
- PEGDGE
poly(ethylene glycol) diglycidyl ether
- pEV
platelet-derived extracellular vesicle
- pQI
para-quinone imine
- R-AE
reporting anti-EpCAM
- RE
reference electrode
- SAV-ALP
streptavidin-conjugated alkaline phosphatase
- tdEV
tumor-derived extracellular vesicle
- WE
working electrode
- μIDE
microinterdigitated electrode array
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.nanolett.9b02741.
Description of PDMS device design, fabrication, and operation; tdEV preparation protocol from prostate cancer cell lines; EV preparation protocol from platelets; AFM and XPS results (PDF)
Author Contributions
D.G.M., P.B., S.L.G., and W.G.vdW. conceived the experiments. D.G.M. fabricated the IDEs and performed the electrochemical measurements and the data analysis. P.B. performed the surface functionalization and characterization and the microfluidics. D.G.M. and P.B. wrote the manuscript with contributions from all authors. All authors approved the content of the manuscript.
Author Contributions
∇ D.G.M. and P.B. contributed equally.
This work is part of the Perspectief Program Cancer ID (project no. 14196), which is funded by The Netherlands Organization for Scientific Research (NWO).
The authors declare no competing financial interest.
Supplementary Material
References
- Nanou A.; Coumans F. A.; van Dalum G.; Zeune L. L.; Dolling D.; Onstenk W.; Crespo M.; Fontes M. S.; Rescigno P.; Fowler G.; et al. Circulating tumor cells, tumor-derived extracellular vesicles and plasma cytokeratins in castration-resistant prostate cancer patients. Oncotarget 2018, 9 (27), 19283. 10.18632/oncotarget.25019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alix-Panabières C.; Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin. Chem. 2013, 59 (1), 110–118. 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- Budd G. T.; Cristofanilli M.; Ellis M. J.; Stopeck A.; Borden E.; Miller M. C.; Matera J.; Repollet M.; Doyle G. V.; Terstappen L. W.; et al. Circulating tumor cells versus imaging—predicting overall survival in metastatic breast cancer. Clin. Cancer Res. 2006, 12 (21), 6403–6409. 10.1158/1078-0432.CCR-05-1769. [DOI] [PubMed] [Google Scholar]
- Coumans F.; Dalum G.; Terstappen L. W. M. M. CTC Technologies and Tools. Cytometry, Part A 2018, 93 (12), 1197–1201. 10.1002/cyto.a.23684. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan R.; Soon R. H.; Zhang P.; Jiang K.; Lim C. T. Cancer diagnosis: from tumor to liquid biopsy and beyond. Lab Chip 2018, 19 (1), 11–34. 10.1039/C8LC00684A. [DOI] [PubMed] [Google Scholar]
- Poudineh M.; Sargent E. H.; Pantel K.; Kelley S. O. Profiling circulating tumour cells and other biomarkers of invasive cancers. Nature Biomedical Engineering 2018, 2 (2), 72. 10.1038/s41551-018-0190-5. [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M.; Siljander P. R.-M.; Andreu Z.; Bedina Zavec A.; Borràs F. E.; Buzas E. I.; Buzas K.; Casal E.; Cappello F.; Carvalho J. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles 2015, 4 (1), 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G.; D’Angelo G.; Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19 (4), 213. 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Mathieu M.; Martin-Jaular L.; Lavieu G.; Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21 (1), 9. 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- Reátegui E.; Vos K. E.; Lai C. P.; Zeinali M.; Atai N. A.; Aldikacti B.; Floyd F. P.; Khankhel A.; Thapar V.; Hochberg F. H.; et al. Engineered nanointerfaces for microfluidic isolation and molecular profiling of tumor-specific extracellular vesicles. Nat. Commun. 2018, 9 (1), 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliha N.; Wiencek Y.; Heider U.; Jüngst C.; Kladt N.; Krauthäuser S.; Johnston I. C.; Bosio A.; Schauss A.; Wild S. A novel multiplex bead-based platform highlights the diversity of extracellular vesicles. J. Extracell. Vesicles 2016, 5 (1), 29975. 10.3402/jev.v5.29975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav S.; Boriachek K.; Islam M. N.; Lobb R.; Möller A.; Hill M. M.; Hossain M. S. A.; Nguyen N. T.; Shiddiky M. J. An Electrochemical Method for the Detection of Disease-Specific Exosomes. ChemElectroChem 2017, 4 (4), 967–971. 10.1002/celc.201600391. [DOI] [Google Scholar]
- Julich H.; Willms A.; Lukacs-Kornek V.; Kornek M. Extracellular vesicle profiling and their use as potential disease specific biomarker. Front. Immunol. 2014, 5, 413. 10.3389/fimmu.2014.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobb R. J.; Lima L. G.; Möller A. In Exosomes: key mediators of metastasis and pre-metastatic niche formation, Seminars in cell & developmental biology; Elsevier: 2017; pp 3–10. [DOI] [PubMed] [Google Scholar]
- Galindo-Hernandez O.; Villegas-Comonfort S.; Candanedo F.; González-Vázquez M.-C.; Chavez-Ocaña S.; Jimenez-Villanueva X.; Sierra-Martinez M.; Salazar E. P. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch. Med. Res. 2013, 44 (3), 208–214. 10.1016/j.arcmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Coumans F.; Doggen C. J. M.; Attard G.; De Bono J.; Terstappen L. W. M. M. All circulating EpCAM+ CK+ CD45– objects predict overall survival in castration-resistant prostate cancer. Annals of oncology 2010, 21 (9), 1851–1857. 10.1093/annonc/mdq030. [DOI] [PubMed] [Google Scholar]
- Hattori Y.; Shimada T.; Yasui T.; Kaji N.; Baba Y. Micro-and Nanopillar Chips for Continuous Separation of Extracellular Vesicles. Anal. Chem. 2019, 6514. 10.1021/acs.analchem.8b05538. [DOI] [PubMed] [Google Scholar]
- Shpacovitch V.; Hergenroeder R. Optical and surface plasmonic approaches to characterize extracellular vesicles. A review. Anal. Chim. Acta 2018, 1005, 1–15. 10.1016/j.aca.2017.11.066. [DOI] [PubMed] [Google Scholar]
- van der Pol E.; Sturk A.; van Leeuwen T.; Nieuwland R.; Coumans F.; Mobarrez F.; Arkesteijn G.; Wauben M.; Siljander P. R.-M.; Sanchez-Lopez V.; Otero-Candelera R.; Ramon L. A.; Dolz S.; Vila V.; Mackman N.; Geddings J.; Mullier F.; Bailly N.; Han J.-Y.; Kwaan H. C.; Weiss I. M.; Buzas E. I.; Pallinger E.; Harrison P.; Kraan J.; Hedley B. D.; LazoLangner A.; Enjeti A.; Norris P. J.; Paris C.; Susen S.; Bonnefoy A.; Delorme I.; Chandler W. L.; Hau C.; Aass H. C. D.; Connor D.; Wu X.; Dragovic R.; Uotila L. M.; Lacroix R.; Robert S. Standardization of extracellular vesicle measurements by flow cytometry through vesicle diameter approximation. J. Thromb. Haemostasis 2018, 16 (6), 1236–1245. 10.1111/jth.14009. [DOI] [PubMed] [Google Scholar]
- Nolan J. P.; Duggan E.. Analysis of Individual Extracellular Vesicles by Flow Cytometry. In Flow Cytometry Protocols; Springer, 2018; pp 79–92. [DOI] [PubMed] [Google Scholar]
- Contreras-Naranjo J. C.; Wu H.-J.; Ugaz V. M. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip 2017, 17 (21), 3558–3577. 10.1039/C7LC00592J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisey C. L.; Dorayappan K. D. P.; Cohn D. E.; Selvendiran K.; Hansford D. J. Microfluidic affinity separation chip for selective capture and release of label-free ovarian cancer exosomes. Lab Chip 2018, 18 (20), 3144–3153. 10.1039/C8LC00834E. [DOI] [PubMed] [Google Scholar]
- Im H.; Lee K.; Weissleder R.; Lee H.; Castro C. M. Novel nanosensing technologies for exosome detection and profiling. Lab Chip 2017, 17 (17), 2892–2898. 10.1039/C7LC00247E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa H.; Suzuki K.; Aoki K.; Arimoto T.; Yasui T.; Kaji N.; Ishikawa T.; Ochiya T.; Baba Y. Imaging of angiogenesis of human umbilical vein endothelial cells by uptake of exosomes secreted from hepatocellular carcinoma cells. Sci. Rep. 2018, 8 (1), 6765. 10.1038/s41598-018-24563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuana Y.; Oosterkamp T.; Bahatyrova S.; Ashcroft B.; Garcia Rodriguez P.; Bertina R.; Osanto S. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J. Thromb. Haemostasis 2010, 8 (2), 315–323. 10.1111/j.1538-7836.2009.03654.x. [DOI] [PubMed] [Google Scholar]
- Van Der Pol E.; Hoekstra A.; Sturk A.; Otto C.; Van Leeuwen T.; Nieuwland R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J. Thromb. Haemostasis 2010, 8 (12), 2596–2607. 10.1111/j.1538-7836.2010.04074.x. [DOI] [PubMed] [Google Scholar]
- Lee W.; Nanou A.; Rikkert L.; Coumans F. A.; Otto C.; Terstappen L. W.; Offerhaus H. L. Label-Free Prostate Cancer Detection by Characterization of Extracellular Vesicles Using Raman Spectroscopy. Anal. Chem. 2018, 90 (19), 11290–11296. 10.1021/acs.analchem.8b01831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman P.; Enciso-Martinez A.; Rho H. S.; Pujari S. P.; Lenferink A.; Zuilhof H.; Terstappen L. W. M. M.; Otto C.; Le Gac S. Immuno-capture of extracellular vesicles for individual multi-modal characterization using AFM, SEM and Raman spectroscopy. Lab Chip 2019, 19, 2526. 10.1039/C9LC00081J. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Zhou X.; He M.; Shang Y.; Tetlow A. L.; Godwin A. K.; Zeng Y. Ultrasensitive detection of circulating exosomes with a 3D-nanopatterned microfluidic chip. Nature Biomedical Engineering 2019, 3, 438. 10.1038/s41551-019-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boriachek K.; Masud M. K.; Palma C.; Phan H.-P.; Yamauchi Y.; Hossain M. S. A.; Nguyen N.-T.; Salomon C.; Shiddiky M. J. Avoiding Pre-Isolation Step in Exosome Analysis: Direct Isolation and Sensitive Detection of Exosomes Using Gold-Loaded Nanoporous Ferric Oxide Nanozymes. Anal. Chem. 2019, 91, 3827. 10.1021/acs.analchem.8b03619. [DOI] [PubMed] [Google Scholar]
- Huang R.; He L.; Xia Y.; Xu H.; Liu C.; Xie H.; Wang S.; Peng L.; Liu Y.; Liu Y.; et al. A Sensitive Aptasensor Based on a Hemin/G-Quadruplex-Assisted Signal Amplification Strategy for Electrochemical Detection of Gastric Cancer Exosomes. Small 2019, 15 (19), 1900735. 10.1002/smll.201900735. [DOI] [PubMed] [Google Scholar]
- Schasfoort R. B.; Andree K. C.; van der Velde N.; van der Kooi A.; Stojanović I.; Terstappen L. W. Interpolation method for accurate affinity ranking of arrayed ligand–analyte interactions. Anal. Biochem. 2016, 500, 21–23. 10.1016/j.ab.2016.01.023. [DOI] [PubMed] [Google Scholar]
- Hermanson G. T.Bioconjugate Techniques, 2nd ed.; Elsevier Inc., 2008. [Google Scholar]
- Sridhar A.; van den Berg A.; Le Gac S. Non-Invasive Monitoring of Osteogenic Differentiation on Microtissue Arrays under Physiological Conditions Using Scanning Electrochemical Microscopy. Electroanalysis 2014, 26, 1881–1885. 10.1002/elan.201400229. [DOI] [Google Scholar]
- Obeid S.; Sung P.-S.; Le Roy B.; Chou M.-L.; Hsieh S.-L.; Elie-Caille C.; Burnouf T.; Boireau W. NanoBioAnalytical characterization of extracellular vesicles in 75-nm nanofiltered human plasma for transfusion: A tool to improve transfusion safety. Nanomedicine 2019, 20, 101977. 10.1016/j.nano.2019.02.026. [DOI] [PubMed] [Google Scholar]
- Rho H. S.; Yang Y.; Veltkamp H.; Gardeniers H.. Direct Delivery of Reagents from a Pipette Tip to a PDMS Microfluidic Device. In Chips and Tips; RSC Blogs, 2015. [Google Scholar]
- Baggerman J.; Smulders M. M.; Zuilhof H. Romantic Surfaces: A Systematic Overview of Stable, Biospecific, and Antifouling Zwitterionic Surfaces. Langmuir 2019, 35 (5), 1072–1084. 10.1021/acs.langmuir.8b03360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.