Abstract
For floral induction in adult citrus, low temperature is one of the most important environmental factors. FLOWERING LOCUS C (FLC) plays a very important role in low-temperature-induced Arabidopsis flowering by repressed FLC expression under exposure to prolonged low-temperature conditions. However, little is known about the FLC regulation mechanism in perennial woody plants such as citrus. In this study, the functions of citrus FLC homolog (PtFLC) were investigated by ectopic expression in Arabidopsis. Transcription factor of homeodomain leucine zipper I (HD-ZIP I) as an upstream regulator of PtFLC was identified by yeast one-hybrid screen to regulate its transcription. The HD-ZIP I transcription factor was highly homologous to Arabidopsis ATHB13 and thus was named PtHB13. Ectopically expressed PtHB13 inhibited flowering in transgenic Arabidopsis. Furthermore, the expression of PtFLC and PtHB13 showed a seasonal change during the floral induction period and was also affected by low temperature. Thus, we propose that PtHB13 binds to PtFLC promoter to regulate its activity during the citrus floral induction process.
Keywords: citrus, flowering, FLC, FT, low temperature
1. Introduction
Floral induction and flowering are among the most crucial events in the plant life cycle because they must occur at in an appropriate time to ensure seed survival and subsequent germination [1,2]. It has been well known that several factors affect floral induction and flowering of plants, including autonomous and environmental factors [2]. So far, five flowering pathways (photoperiod, gibberellin, vernalization, age, and endogenous) have been identified in model plants [3,4]. Meanwhile, many floral induction, development and blooming related genes have been characterized in model plants. Among these genes, FLOWERING LOCUS T (FT), FLOWERING LOCUS C (FLC), SUPRESSOR OF OVEREXPRESSION OF CONSTANS 1 (SOC1), APETALA1 (AP1), and LEAFY (LFY) play pivotal roles by integrating signals from different flowering pathways [3,4]. In recent years, some putative homologs of these genes have been isolated and characterized in perennial woody plants [4,5]. However, the relatively poor mechanism for the regulation of these genes were related to woody plants compared with model plants.
In citrus, several factors besides internal developmental cues affect floral induction and flowering including temperature, water stress, defoliation, and fruit production [6,7,8]. In adult citrus trees, water stress and low temperature are the two major environmental factors for floral induction and flowering [9,10,11]. Low temperatures in winter inducing flowering the next spring has been reported in sweet orange, lime, and Satsuma mandarin [9,10,11]. Low-temperature treatment is known to increase floral intensity and transcript abundance of citrus FT (CiFT) in buds and leaves and expression of citrus LFY (CiLFY) in buds before flower morphological development in Satsuma mandarin [7,12]. Similar results were reported for sweet orange, low-temperature treatment to promote sweet orange flowering is also achieved by increasing the transcript abundance of CiFT in leaves and floral meristem gene CiLFY and citrus AP1 (CiAP1) in buds [7,9]. Recent reports showed that under low ambient temperatures, exogenous abscisic acid (ABA) affected the transcript abundance of CiFT in the shoots of Satsuma mandarin during the floral induction stage. Meanwhile, endogenous ABA accumulated [13]. The expression of CiFT was increased by exposure to low floral-inductive temperatures [9], which is consistent with the function of FT in Arabidopsis [14]. In contrast, the expression of AP1 and LFY increased only toward the end of floral inductive temperature treatment in sweet orange [15], which is also consistent with their function in model plants as floral meristem identity genes [16]. These studies show that CiFT is correlated with low-temperature-induced flowering, indicating that it is crucial for regulating citrus flowering.
Flowering in response to exposure to cold temperature for extended periods is termed vernalization in annual plants [17]. In Arabidopsis, FLC is involved in the vernalization induction of flowering [17,18]. Without exposure to low temperature, FLC represses flowering by repressing flowering activators such as SOC1 and FT [18,19]. In perennial herbaceous plants, PERPETUAL FLOWERING1 (FLC homolog) is transiently repressed by low temperature to allow Arabis alpina to flower in the subsequent season, but then undergoes upregulation by warm temperature to limit flowering to only the spring season [20]. In citrus, FLC homolog (PtFLC) is also isolated from trifoliate orange (Citrus trifoliata). It is regulated by alternative splicing and results in five alternative splicing transcripts. The alternative splicing pattern of PtFLC is altered through developmental stages and further influenced by seasonal low temperature [21]. The results suggested that PtFLC is also correlated with low-temperature-induced flowering. However, it remains largely unknown what kind of regulation mechanism of PtFLC participates in low-temperature-induced flowering.
In this study, overexpression of some alternative splicing transcripts of PtFLC altered flowering time in transgenic Arabidopsis. A homeodomain leucine zipper protein (PtHB13) was found and confirmed to be directly regulated PtFLC by yeast one-hybrid analysis. Overexpression of PtHB13 induced late flowering in transgenic Arabidopsis. To further investigate the molecular mechanism underlying flowering in low temperature, the expression patterns of PtFLC, PtHB13, PtFT, and PtLFY were further investigated during the entire growth period and low-temperature treatment. On the basis of these results, we discuss the role of PtFLC and PtHB13 during the citrus floral induction process.
2. Results
2.1. Transcriptional Activity and Functional Analysis of PtFLC
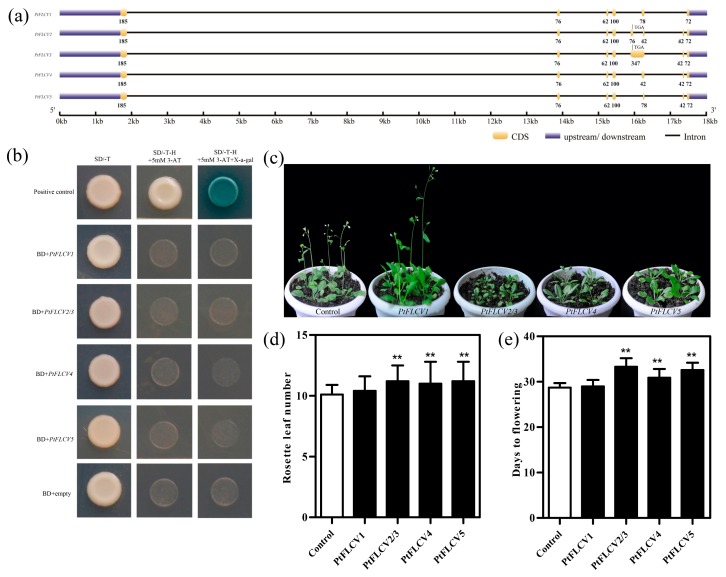
In a previous study, we identified that PtFLC has five types of alternative splicing events, including intron retention and exon skipping [21]. These alternative splicing transcripts were conceptually translated and showed four unique peptide sequences, PtFLCV1: 190aa, PtFLCV2/3: 158aa, PtFLCV4: 192aa, PtFLCV5: 204aa, with PtFLCV2 and PtFLCV3 having the same coding sequence because of early termination of translation due to intron retention (Figure 1a). Since FLC is a MADS-box transcription factor, we suspected that PtFLC may act a master regulator of flowering by influencing the expression of numerous genes. Therefore, to investigate PtFLC transcriptional activity, these alternative splicing transcripts were fused in-frame with the GAL4-binding domain (GAL4-BD) in pGBKT7, and then transformed into yeast (Figure 1b). Yeast cells transformed with positive control (pCL vector) exhibited good growth and displayed α-galactosidase activity on SD/-Trp/-His selection medium. By contrast, yeast cells carrying pBD-PtFLCV1/2/3/4/5 and the negative control plasmid pGBKT7 were unable to grow on selection medium (Figure 1b). These results indicate that PtFLC may be a repressor and suppress downstream targets.
Figure 1.
Transcriptional activity and functional analysis of citrus FLOWERING LOCUS C homolog (PtFLC). (a) Structural comparison of five alternatively spliced PtFLC transcripts, rectangular boxes indicate exons and lines indicate introns. (b) Transcriptional activation analysis of five alternatively spliced PtFLC transcripts in yeast cells. Yeast cells carrying pBD-PtFLCV1, pBD-PtFLCV2/3, pBD-PtFLCV4, pBD-PtFLCV5, pGBKT7 empty vector (as a negative control), or the positive control (pCL) were streaked on SD/-Trp/-His medium supplemented with 5-bromo-4-chloro-3-indolyl-α-galactoside (X-α-Gal) and 3-amino-1,2,4-triazole (3-AT). (c) Phenotype analysis of PtFLC transgenic Arabidopsis. (d) Numbers of leaves and days to flowering of transgenic plants from five PtFLC transcripts at time of flowering stage under long day. Asterisks indicate significant differences (Student’s t-test): ** P < 0.01.
To investigate its effects on flowering, five alternative splicing transcripts of PtFLC were introduced into wild-type Arabidopsis. In the T1 generation, more than 12 transgenic lines were obtained for each transcript. According to RT-PCR results, these transcripts of PtFLC were all detected in the corresponding transgenic plants, but no transcript was detected in the control plants. These transgenic plants were classed into two classes, I and II, based on days to flowering (Figure 1c). The class I plants (PtFLCV2/3, PtFLCV4, and PtFLCV5) flowered later than the control plants. The average day to flowering was extended in the transgenic plants, and ranged from 32.6 to 33.3 days, while that of the control plants was 28.7 days (Figure 1d). These results indicate that PtFLCV2/3, PtFLCV4, and PtFLCV5 have an effect on flowering time similar to FLC in Arabidopsis [22]. H (Poncirus trifoliata L. Raf. owever, the class II plants (PtFLCV1) did not affect the timing of flowering in transgenic Arabidopsis (Figure 1d). Similar results were also found in another perennial plant, Taihangia rupestris [23]. On the other hand, overexpression of PtFLCV2/3, PtFLCV4, and PtFLCV5 in Arabidopsis is very slight compared to the delayed flowering observed for 35S-driven expression of Arabidopsis FLC or Brassica napus FLC [23,24]. Therefore, we speculated that these alternative splicing transcripts may have functional redundancy. Another possible explanation is that alterations coding region sequences of these alternative splicing transcripts might result in their functional changes during the alternative splicing process.
2.2. Promoter Activity and Spatial Expression of PtFLC
To further investigate the expression of PtFLC, a 1.6 Kb promoter fragment from the translation start site (TSS) of PtFLC (proPtFLC) was amplified from trifoliate orange and analyzed by using PLACE software [25]. Several common elements were found, such as a TATA box, CAAT box, and the putative transcriptional start site. Meanwhile, some putative cis-acting regulatory elements involved in plant hormone-response, stress-response, light-response, and circadian control were also predicted (Figure 2a and Supplementary Table S1), implying that proPtFLC may responsed to various environmental factors. To precisely define the spatial expression pattern of proPtFLC, proPtFLC was fused to the β-glucuronidase (GUS) gene and transformed into wild-type Arabidopsis. More than 20 transgenic lines were created, which mostly showed similar GUS expression patterns. At the juvenile stage, stronger GUS staining could be detected in the whole plants (Figure 2b). Along with the growth of seedlings, although the GUS signal was present in whole plants at the adult stage, the expression was weakened compared with the juvenile stage (Figure 2b). Further analysis of GUS expression in different tissues of transgenic plants showed that GUS staining was found in roots, leaves, stems, flowers, silique pods, and mature seeds (Figure 2b). These results agree with the real-time PCR results of PtFLC expression in different citrus tissues (Figure 2c). To investigate whether the promoter activity could be induced by low temperature, seedlings were grown at 4 °C and 15 °C for 1 week in long-day conditions, and then transferred to under long-day conditions at 25 °C (Figure 2d,e). The data showed that GUS activity was decreased after low-temperature treatment. It seems that proPtFLC was inhibited by low-temperature treatment.
Figure 2.
Promoter activity and spatial expression of PtFLC. (a) Schematic diagram of predicted cis-elements in proPtFLC. (b) Histochemical β-glucuronidase (GUS) staining of proPtFLC in different tissues of transgenic Arabidopsis under long-day conditions. (c) Relative expression of PtFLC in different tissues of trifoliate orange. R, roots; S, stems; L, leaves; FL, flowers at anthesis; FR, whole fruits at 30 days after flowering. (d) Analysis of GUS activity from proPtFLC in transgenic Arabidopsis by different temperature treatments (4, 15, and 25 °C) under long-day conditions. (e) Relative GUS intensity from proPtFLC in transgenic Arabidopsis by different temperature treatments (4, 15, and 25 °C) under long-day conditions. Asterisks indicate significant differences (Student’s t-test): ** P < 0.01.
2.3. PtHB13 Directly Binds to proPtFLC
To identify the transcription factors that regulate PtFLC, a yeast one-hybrid assay was performed by using proPtFLC as bait. We obtained 50 positive clones from the screening, only 2 genes (Ciclev10032279m and Ciclev10032279m) were found after putative positive clones were re-streaked on high-stringency medium supplemented with 100 mM Aureobasidin A (AbA). Ciclev10032279m containing homeodomain belonging to the HD-ZIP I transcription factor family was identified according to the citrus genome database [26]. Ciclev10012080m is not a transcription factor and has not been selected for further study. Results of the comparison between cDNA and genomic DNA sequences revealed that the citrus HD-ZIP I transcription factor gene consisted of three exons and two introns, which were located on chromosome 4. Alignment and phylogenetic analysis showed that this gene had high similarity (63%) to Arabidopsis HB13, and thus was named PtHB13 (Supplementary Figure S1a). It is composed of an 882 bp open reading frame (ORF) encoding a 293-amino-acids putative protein. The PtHB13 protein includes a homeobox domain and a homeobox-associated leucine zipper in the middle (Figure 3a), consistent with previous reports on the HD-ZIP I protein [27]. To determine the expression pattern of PtHB13, its transcript was examined in different tissues. PtHB13 was highly expressed in leaves, roots, and stems, and slightly in flowers and fruit (Supplementary Figure S1b).
Figure 3.
PtHB13 directly binds to the promoter of PtFLC. (a) Sequence analysis of PtHB13 protein with its homolog protein. PtHB13 from Citrus trifoliate, MDP0000423596 from Malus domestica, Potri.010G093400.1 from Populus trichocarpa, Ciclev10032279m from Citrus clementina, AT1G69780.1 from Arabidopsis, Zm00008a000472 T01 from Zea mays, and Os03g07450.1 from Oryza sativa. Heavy red line indicates conserved domain of these proteins. (b) Schematic diagrams of proPtFLC and constructs of yeast one-hybrid assay. proFLC indicates the promoter fragment containing a bZIP910 site, while proFLCm is mutated form of proFLC. (c) Schematic diagrams of effector and reporter vectors for dual luciferase reporter assay. (d) Growth of yeast cells co-transformed with prey and bait on selective medium. proFLC1 contains a bZIP910, while proFLC contains a mutated form of bZIP910 site; (e) Transient expression assay of promoter activity using tobacco cells co-transformed with effector and reporters. Error bars represent ± SE (n = 3); asterisks indicate significant differences (Student’s t-test): ** P < 0.01.
A number of cis-elements were found in proPtFLC (Supplementary Table S1), including a bZIP910 element (ATGCCGTT, 741 to 749 bp upstream of the PtFLC start codon) that has been shown to be recognized by HD-ZIP I transcription factor [28]. To further confirm that PtHB13 binds to proPtFLC, yeast one-hybrid assay was performed using PtHB13 as prey, and a 125 bp fragment containing either original or mutated bZIP910 (CGATAAC) cis-element as bait (Figure 3b). The results showed that only the yeast cells co-transformed with the prey and bait containing non-mutated bZIP910 grew normally, suggesting that PtHB13 could bind to the bZIP910 in proPtFLC (Figure 3c). We subsequently further investigated whether PtHB13 activated or suppressed proPtFLC in vivo by performing dual luciferase (LUC) assay on tobacco leaves. In this study, PtHB13 was used as an effector, and two constructed with pFLC containing the original bZIP910 and proFLCm containing the mutated one were used as reporters (Figure 3d). The results show that co-transformation of the effector and the original reporter significantly elevated the promoter activity of PtFLC, whereas the activity resumed to the control level if the bZIP910 element was mutated (Figure 3e), indicating that PtHB13 activated proPtFLC by binding to the bZIP910 site.
2.4. Functional Analysis of PtHB13 in Transgenic Arabidopsis
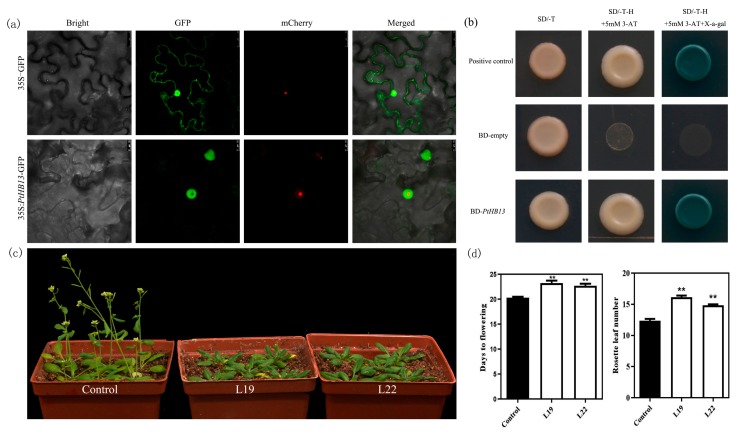
To further investigate the subcellular localization of PtHB13, the ORF of PtHB13 was fused with green fluorescent protein (GFP) gene under control of 35S promoter. Then a transient expression assay of tobacco leaves was performed (Figure 4a). The results revealed that the GFP signal was observed throughout cells with empty 35S::GFP control. By contrast, the 35S::PHB13-GFP fusion protein was only localized in the nucleus (Figure 4a). These results suggest that PtHB13 acts as a transcription factor and may be involved in transcription regulation. To investigate whether PtHB13 has transcriptional activity, PtHB13 was fused to GAL4-BD in pGBKT7 and transformed into the yeast cells (Figure 4b). The result shows that all yeast cells showed normal growth on SD/-Trp medium, whereas only BD-PtHB13 and positive control vectors survived when they were cultured on SD/-Trp/-His medium with 5 mM 3-AT add (Figure 4b). These results indicate that PtHB13 may possibly be an activator.
Figure 4.
Functional analysis of PtHB13 in Arabidopsis. (a) PtHB13-GFP localization in tobacco cells; (b) Transcriptional activation analysis of PtHB13. Yeast cells carrying pBD-PtHB13 vector, pGBKT7, or positive control (pCL) were streaked on SD/-Trp medium or SD/-Trp/-His plates supplemented with x-α-gal. (c) Phenotypes of PtHB13 transgenic Arabidopsis and control. (d) Number of days and leaves to flowering for two PtHB13 transgenic lines. Asterisks indicate significant differences (Student’s t-test): ** P < 0.01.
To evaluate the function of PtHB13 during the citrus flowering process, PtHB13 was genetically transformed into Arabidopsis (Figure 4c). A total of 23 transgenic lines were obtained, and all of them transgenic lines flowered later than the control plants. Three transgenic lines were randomly selected in the third generation for further phenotypic observation (Figure 4c). The results show that Three 35S::PtHB13 transgenic lines flowered significantly later than the control based on days to flowering and number of leaves. The average days to flowering ranged from 21.9 to 22.4 in the transgenic plants, and for the control plants the average was 20.4 days (Figure 4d). The average number of leaves at flowering of the transgenic plants ranged from 15.30 to 16.13, and in the control plants it was 12.4 (Figure 4e). These results indicate that PtHB13 may act a floral repressor in citrus flowering.
2.5. PtFLC and PtHB13 Are Regulated by Low-Temperature Changes in Citrus
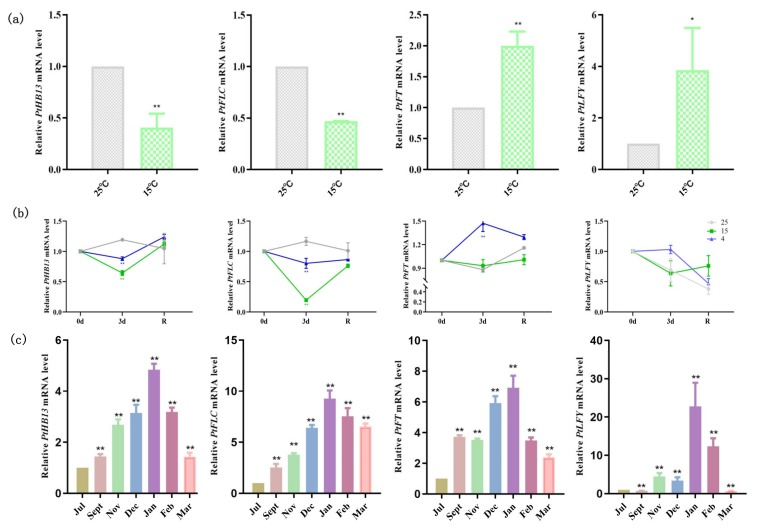
To investigate whether PtFLC and PtHB13 change with low temperature, citrus callus was treated at 25 °C and 15 °C for 1 day under long-day conditions (Figure 5a). The expression levels of PtFLC, PtHB13, PtFT, and PtLYF were measured by real-time PCR. The results show that PtFLC and PtHB13 were inhibited by 15 °C after treatment (Figure 5a). In contrast, the transcription levels of PtFT and PtLFY were induced compared with 25 °C (Figure 5a). These results suggest that low ambient temperature inhibits the expression level of PtFLC and PtHB13 and induces the expression of PtFT and PtLFY. In this study, 4-month-old seedlings of trifoliate orange also underwent the low-temperature treatment (Figure 5b). However, flowering did not occur after treatment because of their juvenility. The expression changes of these genes were also investigated with leaves during the treatment process. At three days after treatment, the expression of mRNA for PtHB13 and PtFLC was much lower in the leaves of seedlings than before treatment (Figure 5b). However, the mRNA level of the two genes increased after transfer to 25 °C. Interestingly, the expression pattern of PtFT presented an opposite expression pattern to that of PtHB13 and PtFLC (Figure 5b).
Figure 5.
PtFLC, PtHB13, PtFT, and PtLFY are regulated by temperature changes in citrus. (a) Relative expression levels of PtFLC, PtHB13, PtFT, and PtLFY in citrus callus at 25 °C and 15 °C for 1 day. (b) Changes in transcript levels of PtFLC, PtHB13, PtFT, and PtLFY under low-temperature conditions. (c) Relative expression levels of PtFLC, PtHB13, PtFT, and PtLFY in citrus trees buds from July to March. Asterisks indicate significant differences (Student’s t-test): ** P < 0.01.
To understand whether the expression of PtHB13 and PtFLC is closely related to seasonal periodicity of flowering in citrus, their expression patterns were investigated in adult trifoliate orange buds (Figure 5c). The expression pattern of PtHB13 and PtFLC fluctuated according to seasonal shifts and flower morphological changes in the adult trifoliate orange buds. Their transcript abundance was low in July, increased slowly after September, and peaked in November; as spring approached, transcript abundance decreased rapidly after accumulating during winter (Figure 5c). It is worth noting that the expression pattern of PtFT was similar to that of PtFLC. Furthermore, PtLFY also increased in December, January, and February but increased later than other genes because the fact that these floral meristem identity genes may be regulated by PtFT.
3. Discussion
Vernalization describes the ability to promote flowering after prolonged exposure to low temperature [1]. Prolonged exposure to low temperature accelerates flowering through the vernalization pathway, which silences the floral repressor FLC in vernalization-sensitive plants [17,29]. For woody plants, FLC has not been characterized except in citrus [21]. Epigenetic modifications and alternative splicing events are mechanisms that regulate gene expression prior to transcription [16,21]. In a previous study, five alternative splicing transcripts of PtFLC were found in deciduous citrus trifoliate orange [21]. In evergreen citrus, the effect of fruits on the expression of FLC was found to involved in alternate bearing of citrus clementina [8,30]. Furthermore, many aspects of homologous FLC post-translational regulation have been reported in citrus by epigenetic modification and alternative splicing [21,30]. However, it is not known how FLC is transcriptionally activated or inhibited by transcription factors.
FLC is a typical MADS-box gene that acts as a central repressor of floral transition in Arabidopsis [22]. In recent years, many of the components and environmental factors that influence the chromatin state of FLC, whether active or inactive, have been reported in model plants [18,19,31]. However, it is unknown which transcription factor drives the transcription of FLC. To identify upstream transcriptional regulators of PtFLC, an HD-ZIP I transcription factor binding to the PtFLC promoter was found by yeast one-hybrid analysis (Figure 3). It shares similarity with the Arabidopsis homeobox-leucine zipper protein AtHB13 (63%), which is involved in responding to various stresses and plays an important role during plant growth and development [27,32]. For example, AtHB13 is upregulated in Arabidopsis by drought, low temperature, and salinity, similar to its homologue HaHB1 in sunflower [27]. Under normal and mild stress conditions, overexpressing AtHB13 or HaHB1 achieves an improved yield associated with higher chlorophyll content in Arabidopsis [33,34]. Recently, AtHB13 was shown to negatively affect stem elongation in Arabidopsis [35]. In this study, we describe the mechanism by which PtHB13 directly activates transcription of PtFLC and negatively regulates floral transition by phenotypic description and biochemical analysis (Figure 4). These results indicate that PtHB13 functions as an important regulator in citrus during the low-temperature floral induction process.
In Arabidopsis, FLC is an important floral regulator by suppressing FT expression in the vernalization and autonomous pathways [36]. Extended low temperature causes a modification of the chromatin structure around the FLC promoter, and chromatin remodeling, co-transcriptional RNA processing, and polycomb silencing inhibit the transcription of FLC in Arabidopsis [37]. Unlike the case in Arabidopsis, the expression of PtFLC in adult citrus showed upregulation in autumn and winter (September to January of the following year) in adult citrus buds, followed by a decrease in the spring and summer, indicating that cold accumulation did not repress FLC expression in field conditions (Figure 5). A similar result was also described for pear [31], indicating that PtFLC may not act as a key regulator in regulating flowering transition in adult citrus by chromatin remodeling as reported for Arabidopsis [38]. One possible explanation for this observation is that the total expression level of PtFLC was dispersed because of the alternative splicing of PtFLC, which exerts its function in certain alternative splicing transcript forms at a particular development stage. Compared with PtFLC, PtHB13 presents the same expression pattern (Figure 5). These results further indicate that the expression of PtHB13 activates the expression of PtFLC by binding to its promoter, and that both PtFLC and PtHB13 play crucial roles in floral induction by low temperature. In deciduous citrus, such as trifoliate orange, growth of spring shoots ceases in April by self-pruning, and floral induction occurs in late spring and early summer [39,40]. The expression of PtFT is related to seasonal periodicity of flowering in adult trifoliate orange buds, consistent with previous reports [40]. Flower development starts soon after induction and the trees then enter a winter rest period because of low temperature. The next year, flower development resumes in early spring and the trees bloom in April [39]. In this study, PtLFY expression increased during early summer, when period evocation of flower organs was been initiated, suggesting that PtLFY is associated with flower bud development just before blooming.
4. Materials and Methods
4.1. Plant Materials
To investigate the expression of flowering-related genes, adult trifoliate orange trees were planted in the field at the National Citrus Breeding Center of Huazhong Agricultural University, Wuhan, China. In citrus, there are three important shoots during the year, spring shoots, summer shoots, and autumn shoots, and the spring shoots are the most important for citrus growth and flowering. In this study, the terminal bud and following five buds (the major node positions for flower formation) from spring shoots were sampled at important periods of flower induction (January, February, March, July, September, November, and December). To investigate whether PtFLC and PtHB13 were affected by low temperature, sweet orange (Citrus sinensis) embryogenic callus and 4-month-old potted seedlings of trifoliate orange (Citrus trifoliata) were treated by different low temperatures in this study. In the experiments of low-temperature treatment, the seedlings of trifoliate orange were transferred to three artificial incubators set at 4 °C, 15 °C (flower-inductive low temperature), and 25 °C for 3 days, and then transferred to long-day conditions (16 h light/8 h dark) at 25 °C. At least three mature and healthy leaves per tree were collected from low-temperature treatment and control plants at 0, 3, and 6 days. For temperature treatment, sweet orange callus was transferred to two artificial climate chambers set to 15 °C and 25 °C under long-day conditions with 60% air humidity. Callus was collected at 1 day under the above temperature treatment. All samples were immediately frozen in liquid nitrogen after collection and stored at −80 °C until used.
4.2. Transcriptional Activity Assay
For the yeast assay, the ORFs of PtFLC and PtHB13 without a termination codon were cloned into the pGBKT7 vector (Clontech, Palo Alto, CA, USA) to generate pBD-PtFLC and pBD-PtHB13 constructs. The pCL and the pGBKT7 vectors were used as a positive and negative controls, respectively. The yeast AH109 strain was transformed with pBD-PtFLC and pBD-PtHB13 constructs. The yeast transcriptional activity assay was performed as described previously [41]. Each experiment was independently repeated three times in this study.
4.3. Histochemical Assay of GUS Activity
To generate the proPtFLC:GUS construct, the PtFLC promoter (1600 bp fragment upstream of the TSS) was isolated from trifoliate orange leaves based on the reference citrus genome [26], and cloned into DX2181 vector containing a GUS gene. The proPtFLC:GUS construct was transformed into Arabidopsis by the floral dipping method [42]. Histochemical staining of proPtFLC:GUS was performed in Arabidopsis based on a previously reported method [43]. Three biologic repeats were performed in this study.
4.4. Subcellular Localization Analysis
To investigate the subcellular localization of PtHB13, the ORF of PtHB13 without the stop codon was inserted into the PBI121 vector containing the GFP gene under control of the CaMV 35S promoter to form a 35S:PtHB13-GFP construct. The control vector (35S:GFP) and fusion construct (35S:PtHB13-GFP) were transformed into Agrobacterium tumefaciens GV3101. Then the fusion construct and control were transformed into tobacco leaves, as described previously [44].
4.5. Arabidopsis Transformation
The full-length CDS of PtHB13 and different alternative splicing transcripts of PtFLC were amplified and inserted into the pBI121vector driven by the CaMV 35S promoter. The constructs were transformed into Agrobacterium tumefaciens GV3101 by the heat shock method. For Arabidopsis transformation, the floral dipping method was used [42]. Seeds carrying different constructs were selected on medium containing 50 mg/ml kanamycin under long-day conditions at 25 °C. The transgenic plants from different constructs were also confirmed in the T1 generation by PCR amplification. To investigate the flowering time of transgenic plants from different constructs, the number of leaves and days to flowering were counted when plants bore a 1 cm long inflorescence. For different temperature treatments of proPtFLC:GUS, seedlings of transgenic plants were transferred to artificial climate chambers set at 4, 15, and 25 °C and incubated for 1 week.
4.6. Yeast One-Hybrid Assay
The PtFLC promoter fragment (from -13 to -1002) was inserted into the pAbAi vector with the ClonExpress One Step Cloning Kit (Vazyme Biotech Co.,Ltd, Nanjing, China). After a self-activation test, the bait vector and AD library were co-transformed into the yeast Y1HGold strain. Yeast one-hybrid assay was performed using the Matchmaker Gold Yeast One-Hybrid Library Screening System (Clontech, Mountain View, CA, USA) according to the user manual (protocol #PT4087-1). The potential cis-elements (bZIP910) of PtFLC promoter were predicted by PLACE software [25].
4.7. Dual Luciferase Reporter Assay
To generate an effector construct, the full-length ORF of PtHB13 was fused into the pGreenII 62-SK vector using the ClonExpressTM II One Step Cloning Kit (Vazyme Biotech Co.,Ltd., Nanjing, China), while the original and mutated PtFLC promoter fragments were cloned into pGreenII 0800-LUC to generate reporters. For transient gene expression analysis, the effector and reporter constructs were co-transformed into tobacco leaf cells. Transformation and detection of LUC activity were performed as previously described [44]. The transformed tobacco leaf cells were detected by using the Dual-Luciferase® Reporter Assay System (Promega (Beijing) Biotech Co., Ltd., Beijing, China).
4.8. Real-Time PCR
The RNeasy Plant Mini kit (Qiagen, Hilden, Germany) was used to isolate citrus total RNA, and approximately 1 μg purified total RNA was used to synthesize cDNA by using the PrimeScript RT First Strand cDNA Synthesis Kit (TaKaRa, Otsu, Japan). The synthesized cDNA was diluted 1:10 as a template for real-time PCR. Primer Express software (PE Applied Biosystems, Foster City, CA, USA) was used to design real-time primers to avoid conserved regions. The GC content and length of the primers were 45–55% and about 21 bp, respectively. The PCR product sizes were 160–200 bp. These primers were tested to ensure amplification of single discrete band with no primer-dimers. Real-time PCR analysis was conducted using the ABI PRISM 7000 system (Applied Biosystems). A melting curve analysis was performed for each sample to verify the specificity of the reactions. Each reaction was performed with a 20 µL including 10.0 µL SYBR Green PCR Master Mix (TaKaRa, Otsu, Japan), 1.0 µL of cDNA template, 0.5 µL of sense and antisense primers (10 pmol L−1), and 8 µL of ddH2O with the following PCR parameters: 95 °C for 10 min, and 40 cycles of 95 °C for 15 s and 60 °C for 60 s. The relative expression levels of the target genes were calculated using the 2−ΔΔCt method by normalizing with citrus Actin according to a previously reported method [45]. Three biological repeats were assayed in this study.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/1/114/s1. Table S1. Cis-elements in proPtFLC predicted by PLACE analysis. Positions are relative to transcriptional initiation site. Orientation of the motifs is indicated (+, forward; -, reverse). Table S2. Primers used in this study. Figure S1. (a) Phylogenetic analysis of PtHB13. MEGA7 software was used to create phylogenetic trees with the following parameters: neighbor-joining method, jones-taylors-thornton model, pairwise deletion, and 1000 bootstrap replicates [46]. PtHB13 from Citrus trifoliate; BraHB13 (Brara.G02910.1) from Brassica rapa; MdHB13 (MDP0000423596) from Malus domestica; CcHB13 (Ciclev10032279m) from Citrus clementina; AtHB13 (AT1G69780) from Arabidopsis; EgHB13 (Eucgr.G02520.1) from Eucalyptus grandis, AtrHB13 (evm_27.model.AmTr) from Amborella trichopoda; MaHB13 (GSMUA_Achr7T11600_001) from Musa acuminate; TaHB13 (Traes_4DL_88ABAD6C0) from Triticum aestivum; OsHB13 (Os03g07450) from Oryza sativa; PhHB13 (PhHAL.9G597100) from Panicum hallii; ZmHB13 (Zm00008a000472) from Zea mays; VviHB13 (GSVIVT01020033001) from Vitis vinifera; FveHB13 (mrna28322.1) from Fragaria vesca; SlHB13 (Solyc05g007180.2) from Solanum lycopersicum; MeHB13 (Manes.05G098900) from Manihot esculenta; TcHB13 (Thecc1EG011764t1) from Theobroma cacao; SpHB13 (SapurV1A.0200s0040) from Salix purpurea; GmHB13 (Glyma.01G044600) from Glycine max; MtHB13 (Medtr8g468210) from Medicago truncatula; LusHB13 (Lus10037200 ) from Linum usitatissimum. (b) Relative expression of PtHB13 in various tissues of trifoliate orange: roots (R), stems (S), leaves (L), flowers at anthesis (FL) and fruits at 30 days after flowering (FR).
Author Contributions
Conceptualization, Y.-J.M.; Methodology, Y.-J.M. and P.-T.L.; Validation, L.-M.S. and R.-F.Z.; Formal analysis, H.Z.; Data curation, X.-Y.A.; Writing—review and editing, J.-Z.Z. and C.-G.H.; Supervision, J.-Z.Z.; Project administration, J.-Z.Z.; Funding acquisition, C.-G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported financially by the National Major Research and Development Plan (2018YFD1000302), the National Natural Science Foundation of China (grant nos. 31972356, 31772252, 31601743, 31672110, and 31872045), and the Fundamental Research Funds for the Central Universities (2662018JC044).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Young H., Shogo I., Takato I. Flowering time regulation: Photoperiod- and temperature-sensing in leaves. Trends Plant Sci. 2013;18:575–583. doi: 10.1016/j.tplants.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaudinier A., Blackman B.K. Evolutionary processes from the perspective of flowering time diversity. New Phytol. 2019 doi: 10.1111/nph.16205. [DOI] [PubMed] [Google Scholar]
- 3.Blümel M., Dally N., Jung C. Flowering time regulation in crops-what did we learn from Arabidopsis? Curr. Opin. Biotechnol. 2015;32:121–129. doi: 10.1016/j.copbio.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 4.Khan M.R.G., Ai X.-Y., Zhang J.-Z. Genetic regulation of flowering time in annual and perennial plants. Wiley Interdiscip. Rev. RNA. 2014;5:347–359. doi: 10.1002/wrna.1215. [DOI] [PubMed] [Google Scholar]
- 5.Wilkie J.D., Sedgley M., Olesen T. Regulation of floral initiation in horticultural trees. J. Exp. Bot. 2008;59:3215–3228. doi: 10.1093/jxb/ern188. [DOI] [PubMed] [Google Scholar]
- 6.Chaikiattiyos S., Menzel C.M., Rasmussen T.S. Floral induction in tropical fruit trees: Effects of temperature and water supply. J. Hortic. Sci. 1994;69:397–415. doi: 10.1080/14620316.1994.11516469. [DOI] [Google Scholar]
- 7.Nishikawa F. Regulation of floral induction in citrus. J. Jpn. Soc. Hortic. Sci. 2013;82:283–292. doi: 10.2503/jjshs1.82.283. [DOI] [Google Scholar]
- 8.Muñozfambuena N., Mesejo C., Carmen Gonzálezmas M., Primomillo E., Agustí M., Iglesias D. Fruit regulates seasonal expression of flowering genes in alternate-bearing ‘Moncada’ mandarin. Ann. Bot. 2011;108:511–519. doi: 10.1093/aob/mcr164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chica E.J., Albrigo L.G. Changes in CsFT transcript abundance at the onset of low-temperature floral induction in sweet orange. J. Am. Soc. Hortic. 2013;138:184–189. doi: 10.21273/JASHS.138.3.184. [DOI] [Google Scholar]
- 10.Tang L., Lovatt C.J. Effects of low temperature and gibberellic acid on floral gene expression and floral determinacy in ‘Washington’ navel orange (Citrus sinensis L. Osbeck) Sci. Hortic. 2019;243:92–100. doi: 10.1016/j.scienta.2018.08.008. [DOI] [Google Scholar]
- 11.Chica E.J., Albrigo L.G. Expression of flower promoting genes in sweet orange during floral inductive water deficits. J. Am. Soc. Hortic. 2013;138:88–94. doi: 10.21273/JASHS.138.2.88. [DOI] [Google Scholar]
- 12.Nishikawa F., Endo T., Shimada T., Fujii H., Shimizu T., Omura M., Ikoma Y. Increased CiFT abundance in the stem correlates with floral induction by low temperature in Satsuma mandarin (Citrus unshiu Marc.) J. Exp. Bot. 2007;58:3915–3927. doi: 10.1093/jxb/erm246. [DOI] [PubMed] [Google Scholar]
- 13.Endo T., Shimada T., Nakata Y., Fujii H., Matsumoto H., Nakajima N., Ikoma Y., Omura M. Abscisic acid affects expression of citrus FT homologs upon floral induction by low temperature in Satsuma mandarin (Citrus unshiu Marc.) Tree Physiol. 2017;38:755–771. doi: 10.1093/treephys/tpx145. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi Y., Kaya H., Goto K., Iwabuchi M., Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- 15.Pillitteri L.J., Lovatt C.J., Walling L.L. Isolation and characterization of LEAFY and APETALA1 homologues from Citrus sinensis L. Osbeck ‘Washington’. J. Am. Soc. Hortic. Sci. 2004;129:846–856. doi: 10.21273/JASHS.129.6.0846. [DOI] [Google Scholar]
- 16.Becker A., Theißen G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenetics Evol. 2003;29:464–489. doi: 10.1016/S1055-7903(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 17.Whittaker C., Dean C. The FLC locus: A platform for discoveries in epigenetics and adaptation. Annu. Rev. Cell Dev. Biol. 2017;33:555–575. doi: 10.1146/annurev-cellbio-100616-060546. [DOI] [PubMed] [Google Scholar]
- 18.Sheldon C.C., Rouse D.T., Finnegan E.J., Peacock W.J., Dennis E.S. The molecular basis of vernalization: The central role of FLOWERING LOCUS C (FLC) Proc. Natl. Acad. Sci. USA. 2000;97:3753–3758. doi: 10.1073/pnas.97.7.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auge G.A., Penfield S., Donohue K. Pleiotropy in developmental regulation by flowering-pathway genes: Is it an evolutionary constraint? New Phytol. 2019;224:55–70. doi: 10.1111/nph.15901. [DOI] [PubMed] [Google Scholar]
- 20.Wang R., Farrona S., Vincent C., Joecker A., Schoof H., Turck F., Alonso-Blanco C., Coupland G., Albani M.C. PEP1 regulates perennial flowering in Arabis alpina. Nature. 2009;459:423–427. doi: 10.1038/nature07988. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J.-Z., Li Z.-M., Mei L., Yao J.-L., Hu C.-G. PtFLC homolog from trifoliate orange (Poncirus trifoliata) is regulated by alternative splicing and experiences seasonal fluctuation in expression level. Planta. 2009;229:847–859. doi: 10.1007/s00425-008-0885-z. [DOI] [PubMed] [Google Scholar]
- 22.Michaels S.D., Amasino R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du X., Xiao Q., Zhao R., Wu F., Xu Q., Chong K., Meng Z. TrMADS3, a new MADS-box gene, from a perennial species Taihangia rupestris (Rosaceae) is upregulated by cold and experiences seasonal fluctuation in expression level. Dev. Genes Evol. 2008;218:281–292. doi: 10.1007/s00427-008-0218-z. [DOI] [PubMed] [Google Scholar]
- 24.Tadege M., Sheldon C.C., Helliwell C.A., Stoutjesdijk P., Dennis E.S., Peacock W.J. Control of flowering time by FLC orthologues in Brassica napus. Plant J. 2001;28:545–553. doi: 10.1046/j.1365-313X.2001.01182.x. [DOI] [PubMed] [Google Scholar]
- 25.Higo K., Ugawa Y., Iwamoto M., Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G.A., Terol J., Ibanez V., Lopez-Garcia A., Perez-Roman E., Borreda C., Domingo C., Tadeo F.R., Carbonell-Caballero J., Alonso R., et al. Genomics of the origin and evolution of Citrus. Nature. 2018;554:311–316. doi: 10.1038/nature25447. [DOI] [PubMed] [Google Scholar]
- 27.Ebrahimian-Motlagh S., Ribone P.A., Thirumalaikumar V.P., Allu A.D., Chan R.L., Mueller-Roeber B., Balazadeh S. JUNGBRUNNEN1 confers drought tolerance downstream of the HD-Zip I transcription factor AtHB13. Front. Plant Sci. 2017;8:2118. doi: 10.3389/fpls.2017.02118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathelier A., Zhao X., Zhang A.W., Parcy F., Worsley-Hunt R., Arenillas D.J., Buchman S., Chen C.-Y., Chou A., Ienasescu H., et al. JASPAR 2014: An extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res. 2013;42:D142–D147. doi: 10.1093/nar/gkt997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michaels S.D., Amasino R.M. Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell. 2001;13:935–941. doi: 10.1105/tpc.13.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agusti M., Mesejo C., Munoz-Fambuena N., Vera-Sirera F., de Lucas M., Martinez-Fuentes A., Reig C., Iglesias D.J., Primo-Millo E., Blazquez M.A. Fruit-dependent epigenetic regulation of flowering in Citrus. New Phytol. 2019 doi: 10.1111/nph.16044. [DOI] [PubMed] [Google Scholar]
- 31.Niu Q., Li J., Cai D., Qian M., Jia H., Bai S., Hussain S., Liu G., Teng Y., Zheng X. Dormancy-associated MADS-box genes and microRNAs jointly control dormancy transition in pear (Pyrus pyrifolia white pear group) flower bud. J. Exp. Bot. 2016;67:239–257. doi: 10.1093/jxb/erv454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris J.C., Hrmova M., Lopato S., Langridge P. Modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. New Phytol. 2011;190:823–837. doi: 10.1111/j.1469-8137.2011.03733.x. [DOI] [PubMed] [Google Scholar]
- 33.Cabello J.V., Chan R.L. The homologous homeodomain-leucine zipper transcription factors HaHB1 and AtHB13 confer tolerance to drought and salinity stresses via the induction of proteins that stabilize membranes. Plant Biotechnol. J. 2012;10:815–825. doi: 10.1111/j.1467-7652.2012.00701.x. [DOI] [PubMed] [Google Scholar]
- 34.Silva A.T., Ribone P.A., Chan R.L., Ligterink W., Hilhorst H.W.M. A predictive coexpression network identifies novel genes controlling the seed-to-seedling phase transition in Arabidopsis thaliana. Plant Physiol. 2016;170:2218–2231. doi: 10.1104/pp.15.01704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ribone P.A., Capella M., Chan R.L. Functional characterization of the homeodomain leucine zipper I transcription factor AtHB13 reveals a crucial role in Arabidopsis development. J. Exp. Bot. 2015;66:5929–5943. doi: 10.1093/jxb/erv302. [DOI] [PubMed] [Google Scholar]
- 36.Samach A., Onouchi H., Gold S.E., Ditta G.S., Schwarz-Sommer Z., Yanofsky M.F., Coupland G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- 37.Kwak J.S., Son G.H., Song J.T., Seo H.S. Post-translational modifications of FLOWERING LOCUS C modulate its activity. J. Exp. Bot. 2016;68:383–389. doi: 10.1093/jxb/erw431. [DOI] [PubMed] [Google Scholar]
- 38.Schilling S., Pan S., Kennedy A., Melzer R. MADS-box genes and crop domestication: The jack of all traits. J. Exp. Bot. 2018;69:1447–1469. doi: 10.1093/jxb/erx479. [DOI] [PubMed] [Google Scholar]
- 39.Li Z.-M., Zhang J.-Z., Mei L., Deng X.-X., Hu C.-G., Yao J.-L. PtSVP, an SVP homolog from trifoliate orange (Poncirus trifoliata L. Raf.), shows seasonal periodicity of meristem determination and affects flower development in transgenic Arabidopsis and tobacco plants. Plant Mol. Biol. 2010;74:129–142. doi: 10.1007/s11103-010-9660-1. [DOI] [PubMed] [Google Scholar]
- 40.Nishikawa F., Endo T., Shimada T., Fujii H., Shimizu T., Omura M. Differences in seasonal expression of flowering genes between deciduous trifoliate orange and evergreen Satsuma mandarin. Tree Physiol. 2009;29:921–926. doi: 10.1093/treephys/tpp021. [DOI] [PubMed] [Google Scholar]
- 41.Zheng X., Zhao Y., Shan D., Shi K., Wang L., Li Q., Wang N., Zhou J., Yao J., Xue Y. MdWRKY9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase MdDWF4 expression. New Phytol. 2018;217:1086–1098. doi: 10.1111/nph.14891. [DOI] [PubMed] [Google Scholar]
- 42.Clough S.J., Bent A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 2010;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 43.Sun L.M., Zhang J.Z., Mei L., Hu C.G. Molecular cloning, promoter analysis and functional characterization of APETALA 1-like gene from precocious trifoliate orange (Poncirus trifoliata L. Raf.) Sci. Hortic. 2014;178:95–105. doi: 10.1016/j.scienta.2014.08.015. [DOI] [Google Scholar]
- 44.Wang M., Dai W., Du J., Ming R., Liu J.H. ERF109 of trifoliate orange (Poncirus trifoliata (L.) Raf.) contributes to cold tolerance by directly regulating expression of Prx1 involved in antioxidative process. Plant Biotechnol. J. 2018;17:1316–1332. doi: 10.1111/pbi.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.