Abstract
Inositol pyrophosphates (PP-InsPs) are an emerging class of “high-energy” intracellular signaling molecules, containing one or two diphosphate groups attached to an inositol ring, that are connected with phosphate sensing, jasmonate signaling, and inositol hexakisphosphate (InsP6) storage in plants. While information regarding this new class of signaling molecules in plants is scarce, the enzymes responsible for their synthesis have recently been elucidated. This review focuses on InsP6 synthesis and its conversion into PP-InsPs, containing seven and eight phosphate groups (InsP7 and InsP8). These steps involve two types of enzymes: the ITPKs that phosphorylate InsP6 to InsP7, and the PPIP5Ks that phosphorylate InsP7 to InsP8. This review also considers the potential roles of PP-InsPs in plant hormone and inorganic phosphate (Pi) signaling, along with an emerging role in bioenergetic homeostasis. PP-InsP synthesis and signaling are important for plant breeders to consider when developing strategies that reduce InsP6 in plants, as this will likely also reduce PP-InsPs. Thus, this review is primarily intended to bridge the gap between the basic science aspects of PP-InsP synthesis/signaling and breeding/engineering strategies to fortify foods by reducing InsP6.
Keywords: inositol, inositol phosphate, inositol pyrophosphate, inositol phosphate signaling, inositol phosphate kinases, PPIP5K, ITPK, phytate
1. Introduction
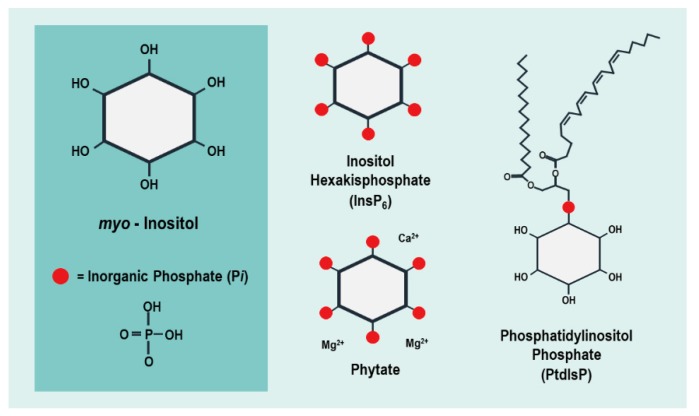
The inositol phosphate (InsP) signaling pathway has been implicated in a diverse array of cellular processes across eukaryotic organisms (for reviews, see [1,2,3]). Inositol phosphates are intracellular signaling molecules built around a simple 6-carbon myo-inositol (inositol) scaffold used in signal transduction (Figure 1). Differing phosphorylation patterns on the inositol ring convey specific cellular information, and several combinations of phosphorylation events are possible. Inositol hexakisphosphate (InsP6), also called phytate when complexed with cations, is a molecule where all hydroxyl (OH) groups on the inositol ring are phosphorylated, that serves as both a storage pool of phosphate as well as a signaling molecule in plants [4,5,6].
Figure 1.
Simplified structures of myo-inositol, InsP6, phytate, and PtdInsP. Inorganic phosphate (Pi) groups, covalently bound to the myo-inositol ring by an oxygen molecule, are represented in red. For simplification purposes, this figure does not take into account the axial and equatorial positions of the moieties.
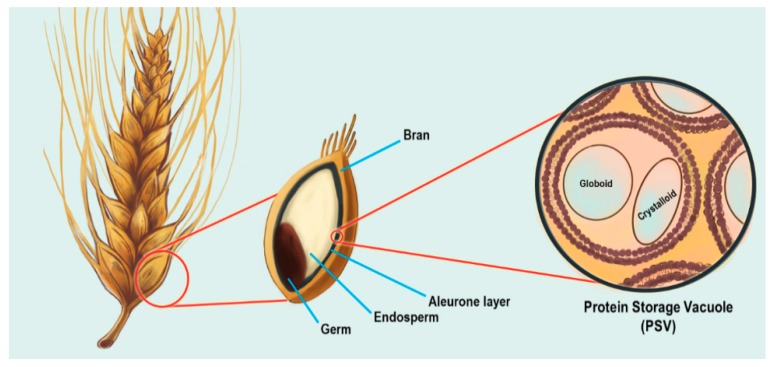
InsP6 is the major phosphorus storage sink within the plant seed, comprising up to ~1% of an Arabidopsis seed’s dry weight, and roughly 65–85% of total seed phosphorus in cereal crops [6]. InsP6 is one of the most highly electronegative molecules present in the cell, resulting in a greatly limited ability to cross cell membranes. It can be transported into the vacuole by MRP5, an ABC-transporter localized in the vacuolar membrane that has been shown to specifically transport InsP6 [4]. During seed development, the electronegative properties of InsP6 result in the chelation of positively charged metal ions such as Mg2+, Fe2+, Zn2+, Mn2+, Ca2+, and these InsP6 metal complexes accumulate within the protein storage vacuole (PSV) (Figure 2) [7,8].
Figure 2.
Diagram illustrating phytate storage in wheat granules. Phytate and other cations are stored in the protein storage vacuole (PSV) within small, crystalline storage bodies, known as globoids [9,15]. While the location of globoids varies across species, a majority of these are found within the aleurone layer of cereal crops, such as wheat and barley, as well as oilseed crops including peanuts, hemp, sunflower, and cotton [6,9,16]. Maize globoids are concentrated within the germ, whereas soybean phytate is distributed throughout the entire seed [6,17,18]. PSVs also contain crystalloid bodies, containing stored proteins and lipids [9].
While InsP6 is important for plant survivorship and yield, it is also, unfortunately, an anti-nutrient, as it can prevent useful cations and minerals from being absorbed by the animal gut [9,10,11]. Additionally, non-ruminant animals cannot digest InsP6, and that which is excreted from livestock is a major environmental concern as it leads to phosphorus pollution, eutrophication, and toxified watersheds [12,13]. Given this, plant breeders and biotechnologists have sought to limit the production of InsP6 in plants, resulting in the so-called low-phytate crops [14]. However, reducing InsP6 synthesis and accumulation in plants can come with consequences, such as a significant decline in vital plant signaling molecules derived from InsP6.
Our primary expertise and focus are on an emerging class of InsPs derived from InsP6 that contain one or two diphosphate or pyrophosphate groups (PP) attached to the inositol ring (Figure 1) [5,19]. These inositol pyrophosphates (PP-InsPs) are gaining significant attention due to their newly discovered roles in energetic metabolism as well as hormone signaling and Pi sensing (for a review, see [20]). The presence of pyrophosphate bonds in PP-InsPs has resulted in their consideration as “energetic signaling” molecules, and we note that PP-InsPs are similar in structure to ADP or ATP. PP-InsP biosynthesis is well described in non-plant eukaryotes, such as yeast and mammals, and many physiological roles have been linked to these molecules [1,2,3]. Elucidating the route of PP-InsP biosynthesis in plants has recently been accomplished and is fundamentally critical to our understanding of these molecules, which is described in the following sections. We will start with an overview of how plants synthesize the precursor to PP-InsPs, InsP6, as several of the enzymes in this pathway are key to understanding the PP-InsP pathway.
2. InsP6 Synthesis: The Lipid-Dependent Pathway
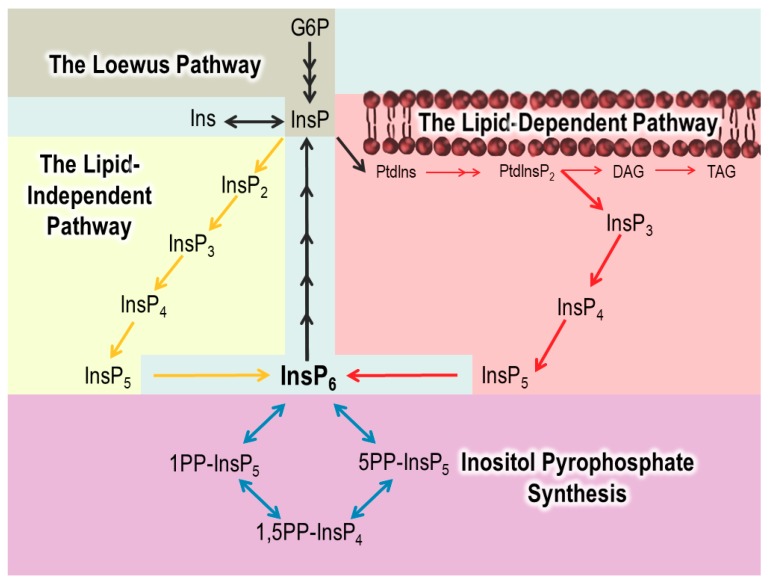
InsP6 can be synthesized by two interconnected pathways in plants. The pathways are named for their starting material: the Lipid-Dependent pathway and the Lipid-Independent pathway (Figure 3). The Lipid-Dependent pathway is present in all eukaryotic organisms [21,22,23,24,25]. The Lipid-Independent pathway for synthesizing InsP6 was originally discovered in Dictyostelium in a landmark paper by Stephens and Irvine in 1990, and followed up on in Spirodela polyrhiza [26,27]. This pathway was thought be unique to these organisms, along with land plants. However, a very recent publication by Desfougères et al. shows that the Lipid-Independent pathway is also present in mammals [28]. This work is the first to report evidence of the Lipid-Independent pathway in mammals and will be crucial for exploring the evolution of enzymes across organisms.
Figure 3.
A simplified view of the InsP synthesis and degradation pathway. InsP synthesis starts in the Loewus Pathway (tan), where InsP is synthesized from Glucose-6-Phosphate (G6P). InsPs are synthesized through the Lipid-Dependent (pink) or Lipid-Independent (yellow) pathways. PP-InsPs in plants are synthesized from InsP6 (purple). The enzymes involved in the pathway are discussed throughout the review.
The lipid component in the Lipid-Dependent pathway is phosphatidylinositol phosphate (PtdInsP), a molecule containing inositol as the head group (Figure 1). While plants synthesize a myriad of lipid-soluble PtdInsPs, phosphatidylinositol (4,5) bisphosphate (PtdIns(4,5)P2), is important for the Lipid-Dependent pathway as it is acted on by the enzyme phospholipase C (PLC) [29]. Phospholipases, by definition, hydrolyze phospholipids. The hydrolysis of PtdIns(4,5)P2 by PLC produces Ins(1,4,5)P3 and diacylglycerol (DAG), which essentially converts a phosphorylated lipid-signaling molecule (PtdIns(4,5)P2) into a water-soluble, InsP-signaling molecule (Ins(1,4,5)P3) (Figure 3) [29].
Ins(1,4,5)P3 can be subsequently phosphorylated into InsP4, and then InsP5, by a dual specific inositol polyphosphate multikinase (IPMK). The IPMK enzyme is encoded by two genes in Arabidopsis, AtIPK2α and AtIPK2β (Table 1) [30]. Both genes encode enzymes with a 6/3-kinase activity, catalyzing the conversion of Ins(1,4,5)P3 to Ins(1,4,5,6)P4 and to a final Ins(1,3,4,5,6)P5 product [31]. 5-kinase activity towards Ins(1,3,4,6)P4 and Ins(1,2,3,4,6)P5 was also reported by Stevenson-Paulik et al. Notably, these genetic studies show that the AtIPK2β gene can complement a yeast ipk2 mutant, and that Arabidopsis T-DNA loss-of-function atipk2β mutants have a 35% reduction in seed InsP6 (Table 1) [30]. atipk2α mutants are not easily studied as those that are recovered are lethal. This, along with the generally ubiquitous expression of AtIPK2α, supports the idea that AtIPK2α supplies the major IPK2 or IPMK activity in the plant cell.
Table 1.
Loss-of-function Arabidopsis mutants and impacts on InsP6 and PP-InsP levels. Arabidopsis is a simple model system that can be used to gauge the impacts of genetic changes on InsPs. The table shows the impact on InsP6 and PP-InsPs in Arabidopsis mutants for enzymes involved in InsP synthesis. Mutants for enzymes important in both the Lipid-Dependent and Lipid-Independent pathways are indicated (*).
| Pathway | Mutant | Impact on InsP6 | Impact on PP-InsPs |
|---|---|---|---|
| Lipid-Dependent Pathway | plc | Nine genes; characterized single mutants have no change in InsP6 [32] | No Change [32] |
| ipk2α * | Lethal Knock-Out [30] | Unknown | |
| ipk2β * | 35% reduction in mass seed InsP6; no change in seedling tissue as measured by radiolabeling [30] | Unknown | |
| ipk1 * | 83% reduction in mass seed InsP6; 93% reduction in seedlings as measured by radiolabeling [30] | InsP7 and InsP8 are reduced [33,34] | |
| Lipid-Independent Pathway | mik | 62–66% reduction in mass seed InsP6 [35] | Unknown |
| lpa1 | 47–57% reduction in mass seed InsP6 [35] | Unknown | |
| itpk1–4 | 46% reduction in mass seed InsP6 in Atitpk1 mutants; no changes in Atitpk2 or Atitpk3; 40–51% reduction in Atitpk4 [35] | Atitpk1 and Atitpk4 have reduced InsP7 and InsP8 [33,36] | |
| Phytate Storage | mrp5 | 73–80% reduction in mass seed InsP6 [35]; decreased InsP6 in seeds and vegetative tissue as measured by radiolabeling [4] | Elevated InsP7 and InsP8 [5] |
| PP-InsP Synthetic Pathway | vip1 | No change reported [34,37] | Increased InsP7 and Decreased InsP8 [34,37] |
| vip1/vip2 | No change reported [37] | Increased InsP7 and Decreased InsP8 [37,38] |
The last step in the Lipid-Dependent pathway of InsP6 biosynthesis is the phosphorylation of Ins(1,3,4,5,6)P5 to InsP6. This step is catalyzed by only one type of enzyme in nature, the inositol pentakisphosphate 2-kinase (IPK1), so named because all known IPK1 enzymes phosphorylate the 2-position of InsP5 [30]. While there are seven genes in Arabidopsis that are predicted to encode IPK1 enzymes, the At5g42810 gene (AtIPK1) is the only one actively expressed in plants [30]. Complementation assays reveal that AtIPK1 is able to complement a yeast ipk1 mutant, restore InsP6 levels, and rescue the mutant’s temperature-sensitive growth phenotype [39]. As loss of IPK1 function results in an 83% reduction in InsP6 in seeds (Table 1), this shows that IPK1 plays a major role in maintaining seed InsP6 levels [30].
3. InsP6 Synthesis: The Lipid-Independent Pathway
Given the importance of Pi storage in plants, it is not surprising that plants evolved a separate way to synthesize InsP6, apart from the PtdInsP pathway. In the Lipid-Independent pathway, “free” myo-inositol is acted on by a series of inositol kinases. The first is the myo-inositol kinase (MIK), which was first identified in maize as a product of the Low Phytic Acid gene [40]. Loss-of-function maize lpa3 mutants have reduced InsP6 and elevated inositol levels in the seeds [40]. Arabidopsis atmik mutants have a large reduction in total seed mass InsP6 levels (Table 1) [35]. The second step in the Lipid-Independent pathway is likely catalyzed by a gene/protein named LPA1 in rice [41]. This gene product was originally categorized as a potential 2-phosphoglycerate kinase that impacts InsP6 accumulation [41]. However, recent structural modeling indicates that InsP3 can be accommodated in the active site of this kinase, supporting a role for it as an InsP kinase [42].
The next step in this pathway should involve an inositol kinase capable of phosphorylating an InsP2 substrate. As no such kinase has been identified, this prompted speculation that InsP2’s conversion to InsP3 is catalyzed by a moonlighting function of another inositol phosphate kinase, which has yet to be identified. The last novel component of the Lipid-Independent pathway is the inositol triphosphate kinase (ITPK), which can phosphorylate specific InsP3 and InsP4 molecules [39,43]. A gene encoding ITPK1 was also identified in a maize mutant named lpa2 [44]. There are four ITPK enzymes (AtITPK1–4) in Arabidopsis [45]. It is interesting to note that only Arabidopsis atitpk1 and atitpk4 mutants have reduced seed InsP6 levels (Table 1) [33,35]. Our own work with AtITPK1 and AtITPK2 enzymes shows that these proteins are also efficient at converting InsP6 to InsP7 [38]. Laha et al. additionally used NMR to show that 5PP-InsP5 is the isoform synthesized by AtITPK1 and AtITPK2 [36]. These are key findings that highlight the catalytic flexibility of the ITPKs, as well as indicating that the Lipid-Independent pathway may have an important relationship with, and impact on, PP-InsP synthesis.
The ITPKs are thought to act in concert with the IPK2 enzymes in producing Ins(1,3,4,5,6)P5 in the Lipid-Independent pathway [27,31,43]. The final step is the conversion of Ins(1,3,4,5,6)P5 to InsP6. Both pathways utilize IPK1 to synthesize InsP6 and converge at this last step in InsP synthesis. This further highlights the importance of IPK1 in InsP6 synthesis. A very recent publication on non-plant organisms suggests that there might be some conserved functions of the Lipid-Independent pathway in other eukaryotes. Humans, for example, contain ITPK genes, and the expression of these enzymes appears to complement mutants defective in PLC, which cannot synthesize InsP6 [28,46]. Although more work needs to be done, this suggests that animals may also utilize ITPKs in a Lipid-Independent InsP6 pathway. In the same work, these authors also found that the plant ITPK1 could complement the yeast PLC mutant, suggesting that the plant ITPK may also have a very flexible substrate preference, and may act at several different steps in the Lipid-Independent pathway [28].
4. PP-InsP Synthetic Pathway
While a great majority of InsP6 is stored as phytate, a small pool can be further phosphorylated to form PP-InsPs. Our group and others have examined a class of enzymes involved in PP-InsP synthesis named the diphosphoinositol-pentakisphosphate kinases (PPIP5Ks), known as VIP or VIH in plants, and Vip1 in Chlamydomonas reinhardtii (algae) [5,34,47]. Mammalian and yeast PPIP5K enzymes phosphorylate the 1-position on InsP6 and 5PP-InsP5 to generate an InsP7 molecule, 1PP-InsP5, and an InsP8 molecule, 1,5(PP)2-InsP4, respectively [48,49,50,51,52]. Two Arabidopsis genes, AtVIP1 and AtVIP2, are orthologous to the mammalian PPIP5K genes [5,34]. AtVIP1 (also referred to as AtVIH2) and AtVIP2 (AtVIH1) are dual-domain enzymes, consisting of an ATP-grasp N-terminal kinase domain (KD) and a C-terminal histidine phosphatase domain (PD) [5]. We recently found that the KD of both AtVIP enzymes can phosphorylate 5PP-InsP5 in vitro [38]. Additionally, the Hothorn group used NMR and showed that the product of the AtVIP enzymes is indeed 1,5-PP-InsP4 [37].
A second class of enzymes, known as the inositol hexakisphosphate kinases (IP6Ks), function in non-plant organisms by phosphorylating the 5-position of InsP5 and InsP6 to generate InsP7 [53]. While the genes coding for IP6Ks are present in humans and yeast, there is no identifiable IP6K gene in the plant genome [5]. This prompted us and other groups to speculate the AtVIPs might be bifunctional enzymes, phosphorylating both InsP6 and InsP7. While our biochemical analyses of the AtVIP KDs did not rule out the possibility that these enzymes can phosphorylate InsP6, they suggested that other enzymes likely had to exist in plants to drive this reaction [38]. As the ITPKs phosphorylate the 5-position of lower InsPs, we decided to target this class of enzymes, and found that both AtITPK1 and AtITPK2 are able to phosphorylate InsP6 in vitro [36,38]. We also demonstrated that the AtITPK1 product could be further phosphorylated by the AtVIP1-KD, resulting in InsP8 [38]. Based on our findings, as well as recent work by Laha et al., we conclude that the AtITPKs are the missing enzyme in the pathway [36,38].
5. How Do PP-InsPs Function in Plants?
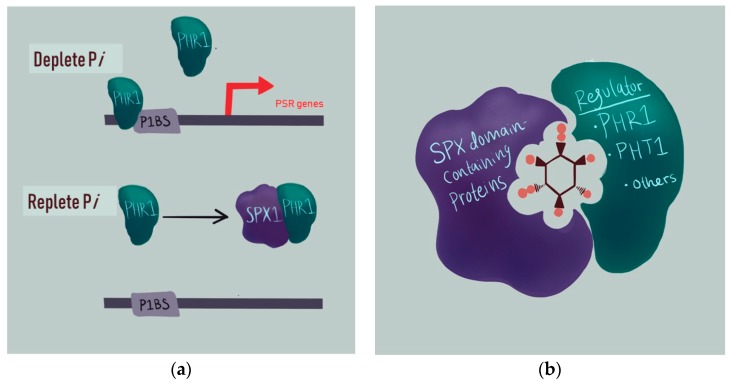
Williams et al. suggested, in a review published in 2015, that a major function of PP-InsPs in plants was as a “glue” to bring together various protein binding partners [20]. At the time of the review, it was known that InsP6 could bind to the transport inhibitor response 1 (TIR1) auxin receptor [54]. Additionally, InsP7 was hypothesized to bind to the jasmonate (JA) receptor based on structural modeling experiments [34]. Exciting data, of importance to crop breeders, details how InsP6 and InsP7 are able to complex with key proteins involved in Pi-sensing in plants [55]. Soils depleted in phosphorus lead plants to induce a suite of molecular and physiological mechanisms to enhance Pi scavenging, known as the Pi starvation response (PSR) [56,57]. The PSR is facilitated by an increase in transcription of a group of PSR genes, leading to increases in Pi transport and uptake. Upregulation of PSR gene expression is regulated by Phosphate Starvation Response Regulator 1 (PHR1), a transcription factor, along with its homologs [56,57]. PHR1 and homologous transcription factors have a high binding affinity for promoters containing PHR1 binding sequences (P1BS), which allows for the binding and up-regulation of PSR genes under low Pi conditions [56,57].
InsP6 and PP-InsPs regulate Pi sensing via facilitating complex formations between the PHR1 transcription factor and the SPX domain-containing proteins (Figure 4) [58]. PHR and SPX proteins isolated from Oryza sativa (rice), known as OsPHR2 and OsSPX4, respectively, can complex with InsP6 or InsP7 in vitro [55]. InsP8 has an even lower dissociation constant than InsP7 in the OsPHR2–OsSPX4 complex formation [59]. Together, these data support the idea that InsP8 is the main mediator of the PHR1:SPX complex formation in plants.
Figure 4.
Model depicting how PP-InsPs regulate plant Pi sensing and the Pi starvation response (PSR). (a) PSR gene regulation under deplete (top) and replete Pi conditions (bottom). SPX1 binds to PHR1 under replete Pi, preventing SPX1 from binding to promoters containing P1BS. Under deplete Pi, PHR1 is uninhibited from binding to P1BS promoters. Adapted from [60]. (b) Model depicting a complex formation between SPX1, PHR1, and InsP8. This model represents interactions between other SPX proteins and PSR regulators, such as PHR1 and its homolog, PHT1, along with others.
While InsP7 has a ~7-fold stronger binding affinity than InsP6 to OsSPX4, recent genetic analyses greatly support the idea that InsP8 is the major signaling molecule, or proxy, that conveys information on the Pi status within the plant cell [37,61]. First, atipk1, atitpk1, atitpk4 and atvip1/vip2 mutants commonly show defects in Pi sensing, such that the PSR is turned on even when grown under Pi-replete conditions [30,33,37,61]. Additionally, all of these particular mutants have decreased InsP8 levels (Table 1). In the case of atvip1/vip2 double mutants, the PSR is likely so highly upregulated that growth becomes stunted and lethality occurs in double homozygotes [37,61]. In contrast, atipk1 and atitpk1 mutants can grow fairly normally under certain conditions, and can be stimulated to further increase the PSR under Pi-replete conditions [33,62]. All of this information suggests that InsP8 functions to turn off the PSR.
We now know in greater detail that PP-InsPs likely function in binding to plant hormone receptors and transcription factor complexes involved in Pi sensing. One example where PP-InsPs potentially function as cofactors is in the case of auxin signaling [54]. Auxin is a phytohormone which regulates numerous plant developmental processes and responses to environmental stress [63,64]. Auxin modulates gene expression by binding to the auxin receptor TIR1, an F-box protein, and mediates the SCF ubiquitin–ligase-catalyzed proteolysis of AUX/IAA transcriptional repressors [65,66]. The crystallized Arabidopsis TIR1 protein complex has a tightly bound InsP6 in the leucine-rich repeat (LRR) domain of TIR1, suggesting that InsP6 is a cofactor for the auxin receptor [54].
A later study identified Ins(1,2,4,5,6)P5 in the crystallized structure of a homologous plant hormone-JA co-receptor [67]. JA is a phytohormone critical for environmental and pathogen defense signaling as well as plant physiology [68]. Similar to auxin signaling with TIR1, JA is also perceived by interactions between F-box protein, coronatine-insensitive 1 (COI1), and the JA zim domain (JAZ) transcriptional repressors [69,70,71]. JA gene regulation is modulated by JA-hormone binding to COI1 and the degradation of JAZ repressors, freeing repressed transcription factors to upregulate JA genes [67]. Sheard et al. showed that Ins(1,2,4,5,6)P5 binds to and stabilizes COI, suggesting that Ins(1,2,4,5,6)P5 is a cofactor for the JA receptor [67,72]. A study using structural modeling predicted that the PP-InsPs have a much stronger binding affinity for COI1-JAZ1 than Ins(1,2,4,5,6)P5 and InsP6, suggesting that the PP-InsPs might be the true cofactor for the JA receptor [34].
6. Consequences for Targeting InsP6 for Reduction
With our current understanding of PP-InsP synthesis, and analyses of genetic mutants in the pathway, it seems reasonable to question whether reducing InsP6 in plants will also result in reduction in PP-InsPs. Most characterized Arabidopsis mutants showing alterations in InsP6 also have impacted the intracellular levels of InsP7 and InsP8 (Table 1). Mutants with reduced PP-InsPs, such as atipk1, atitpk1, and atvip1/vip2 mutants, show an upregulation of the PSR, which could impact engineered crop performance in the field [33,37,61]. Specifically, altered Pi sensing could negatively impact plant growth and development, reduce viability, and alter root architecture [56,57]. Alterations in Pi sensing can affect other signaling systems, such as the sensing and accumulation of nitrate and other micronutrients, along with the ABA signaling pathway, often linked to stress pathways [73,74].
Given the link between InsP8 and JA signaling, decreasing PP-InsPs might result in crops that are more susceptible to pathogens and insects [34]. This impact is seen in Arabidopsis atvip1 mutants, which have reduced InsP8, and show increased susceptibility to insect herbivory as compared to WT plants [34]. Additionally, both transgenic potato plants and Arabidopsis mutants with reduced myo-inositol phosphate synthase (MIPS), the first committed step in inositol synthesis, have decreased InsP6 and are more susceptible to pathogenic viruses [75]. While the PP-InsPs were not quantified in these mutants, it is possible that the PP-InsPs were also reduced and are a causative factor in the increased susceptibility to pathogens.
7. Concluding Remarks
Ultimately, understanding the genetic mechanisms for controlling PP-InsP synthesis and function is critical for developing novel low-phytate crops that are not compromised by changes in PP-InsP signaling. A recent review nicely details existing transgenic strategies used to reduce phytate in plants [76]. A common strategy is to use tissue-specific promoters to drive the overexpression of enzymes that break down InsP6, such as Phytase, or to knock-down the expression of genes required for InsP6 synthesis. By doing so, the idea is that only specific tissues will have reduced InsP6. This strategy works well to reduce InsP6 in seeds, for example, without negatively impacting vegetative tissues. One potential drawback of this approach is that the reduction in InsP6 may also affect the precursor available for PP-InsP synthesis in these transgenic plants. It is also important to consider that plants store approximately 1% InsP7 and InsP8 in seeds [5]. We do not know much currently about whether and how PP-InsPs might regulate seed phosphate storage, however, we point out that existing data on mrp5 InsP6 transporter mutants indicate that InsP6 modulation in seed can result in increases in PP-InsPs [5]. Given the emerging role of PP-InsPs in controlling critical plant sensing and signaling pathways, the future development of strategies for phytate reduction without compromising PP-InsP synthesis and function should be considered by plant breeders.
Author Contributions
Conceptualization, C.F., G.G. and O.A.; writing—original draft preparation, C.F., G.G. and O.A.; writing—review and editing, C.F. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the NSF for funding to GG (MCB 1616038). This work is supported in part by the USDA National Institute of Food and Agriculture, Hatch project VA-136334.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Livermore T., Azevedo C., Kolozsvari B., Wilson M., Saiardi A. Phosphate, inositol and polyphosphates. Biochem. Soc. Trans. 2016;44:253–259. doi: 10.1042/BST20150215. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty A. The inositol pyrophosphate pathway in health and diseases. Biol. Rev. Camb. Philos. Soc. 2019;93:1203–1227. doi: 10.1111/brv.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shears S.B. Intimate connections: Inositol pyrophosphates at the interface of metabolic regulation and cell signaling. J. Cell Physiol. 2018;233:1897–1912. doi: 10.1002/jcp.26017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagy R., Grob H., Weder B., Green P., Klein M., Frelet-Barrand A., Schjoerring J.K., Brearley C., Martinoia E. The Arabidopsis ATP-binding cassette protein AtMRP5/AtABCC5 is a high affinity inositol hexakisphosphate transporter involved in guard cell signaling and phytate storage. J. Biol. Chem. 2009;284:33614–33622. doi: 10.1074/jbc.M109.030247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai M., Rangarajan P., Donahue J.L., Williams S.P., Land E.S., Mandal M.K., Phillippy B.Q., Perera I.Y., Raboy V., Gillaspy G.E., et al. Two inositol hexakisphosphate kinases drive inositol pyrophosphate synthesis in plants. Plant J. 2014;80:642–653. doi: 10.1111/tpj.12669. [DOI] [PubMed] [Google Scholar]
- 6.Raboy V. Seed Total Phosphate and Phytic Acid. In: Kriz A.L., Larkins B.A., editors. Molecular Genetic Approaches to Maize Improvement. Springer; Berlin, Germany: 2009. pp. 41–53. Biotechnology in Agriculture and Forestry. [Google Scholar]
- 7.Otegui M.S., Capp R., Staehelin L.A. Developing Seeds of Arabidopsis Store Different Minerals in Two Types of Vacuoles and in the Endoplasmic Reticulum. Plant Cell. 2002;14:1311–1327. doi: 10.1105/tpc.010486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S.-Y., Huang T.-K., Kuo H.-F., Chiou T.-J. Role of vacuoles in phosphorus storage and remobilization. J. Exp. Bot. 2017;68:3045–3055. doi: 10.1093/jxb/erw481. [DOI] [PubMed] [Google Scholar]
- 9.Bohn L., Meyer A.S., Rasmussen Søren K. Phytate: Impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B. 2008;9:165–191. doi: 10.1631/jzus.B0710640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raboy V. Seeds for a better future: ‘Low phytate’ grains help to overcome malnutrition and reduce pollution. Trends Plant Sci. 2001;6:458–462. doi: 10.1016/S1360-1385(01)02104-5. [DOI] [PubMed] [Google Scholar]
- 11.Cowieson A.J., Acamovic T., Bedford M.R. Phytic acid and phytase: Implications for protein utilization by poultry. Poult. Sci. 2006;85:878–885. doi: 10.1093/ps/85.5.878. [DOI] [PubMed] [Google Scholar]
- 12.Abelson P.H. A Potential Phosphate Crisis. Science. 1999;283:2015. doi: 10.1126/science.283.5410.2015. [DOI] [PubMed] [Google Scholar]
- 13.Sharpley A.N., Withers P.J.A. The environmentally-sound management of agricultural phosphorus. Fertil. Res. 1994;39:133–146. doi: 10.1007/BF00750912. [DOI] [Google Scholar]
- 14.Raboy V. Progress in Breeding Low Phytate Crops. J. Nutr. 2002;132:503S–505S. doi: 10.1093/jn/132.3.503S. [DOI] [PubMed] [Google Scholar]
- 15.Isayenkov S. Plant vacuoles: Physiological roles and mechanisms of vacuolar sorting and vesicular trafficking. Cytol. Genet. 2014;48:127–137. doi: 10.3103/S0095452714020042. [DOI] [PubMed] [Google Scholar]
- 16.Perera I., Fukushima A., Tatsuki A., Horiguchi G., Saman S., Naoki H. Expression regulation of myo-inositol 3-phosphate synthase 1 (INO1) in determination of phytic acid accumulation in rice grain. Sci. Rep. 2019;9:14866. doi: 10.1038/s41598-019-51485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Dell B.L., De Boland A.R., Koirtyohann S.R. Distribution of phytate and nutritionally important elements among the morphological components of cereal grains. J. Agric. Food Chem. 1972;20:718–723. doi: 10.1021/jf60181a021. [DOI] [Google Scholar]
- 18.Erdman J. Oilseed phytates: Nutritional implications. J. Am. Oil Chem. Soc. 1979;56:736–741. doi: 10.1007/BF02663052. [DOI] [Google Scholar]
- 19.Shears S.B. Inositol pyrophosphates: Why so many phosphates? Adv. Biol. Regul. 2015;57:203–216. doi: 10.1016/j.jbior.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams S.P., Gillaspy G.E., Perera I.Y. Biosynthesis and possible functions of inositol pyrophosphates in plants. Front. Plant Sci. 2015;6:67. doi: 10.3389/fpls.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odom A.R., Stahlberg A., Wente S.R., York J.D. A Role for Nuclear Inositol 1,4,5-Trisphosphate Kinase in Transcriptional Control. Science. 2000;287:2026–2029. doi: 10.1126/science.287.5460.2026. [DOI] [PubMed] [Google Scholar]
- 22.Perera N.M., Michell R.H., Dove S.K. Hypo-osmotic Stress Activates Plc1p-dependent Phosphatidylinositol 4,5-Bisphosphate Hydrolysis and Inositol Hexakisphosphate Accumulation in Yeast. J. Biol. Chem. 2004;279:5216–5226. doi: 10.1074/jbc.M305068200. [DOI] [PubMed] [Google Scholar]
- 23.York J., Odom A.R., Murphy R., Ives E., Wente S.R. A Phospholipase C-Dependent Inositol Polyphosphate Kinase Pathway Required for Efficient Messenger RNA Export. Science. 1999;285:96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
- 24.Fujii M., York J.D. A role for rat inositol polyphosphate kinases rIPK2 and rIPK1 in inositol pentakisphosphate and inositol hexakisphosphate production in rat-1 cells. J. Biol. Chem. 2005;280:1156–1164. doi: 10.1074/jbc.M412006200. [DOI] [PubMed] [Google Scholar]
- 25.Seeds A.M., Sandquist J.C., Spana E.P., York J.D. A molecular basis for inositol polyphosphate synthesis in Drosophila melanogaster. J. Biol. Chem. 2004;279:47222–47232. doi: 10.1074/jbc.M408295200. [DOI] [PubMed] [Google Scholar]
- 26.Stephens L.R., Irvine R.F. Stepwise phosphorylation of myo-inositol leading to myo-inositol hexakisphosphate in Dictyostelium. Nature. 1990;346:580–583. doi: 10.1038/346580a0. [DOI] [PubMed] [Google Scholar]
- 27.Brearley C.A., Hanke D.E. Metabolic evidence for the order of addition of individual phosphate esters in the myo-inositol moiety of inositol hexakisphosphate in the duckweed Spirodela polyrhiza L. Biochem. J. 1996;314:227–233. doi: 10.1042/bj3140227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desfougères Y., Wilson M.S.C., Laha D., Miller G.J., Saiardi A. ITPK1 mediates the lipid-independent synthesis of inositol phosphates controlled by metabolism. Proc. Natl. Acad. Sci. USA. 2019;116:24551–24561. doi: 10.1073/pnas.1911431116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson-Paulik J., Bastidas R., Chiou S.-T., Frye R., York J. Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc. Natl. Acad. Sci. USA. 2005;102:12612–12617. doi: 10.1073/pnas.0504172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson-Paulik J., Odom A.R., York J.D. Molecular and Biochemical Characterization of Two Plant Inositol Polyphosphate 6-/3-/5-Kinases. J. Biol. Chem. 2002;277:42711–42718. doi: 10.1074/jbc.M209112200. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Q., van Wijk R., Shahbaz M., Roels W., van Schooten B., Vermeer J.E.M., Zarza X., Guardia A., Scuffi D., García-Mata C., et al. Arabidopsis Phospholipase C3 is Involved in Lateral Root Initiation and ABA Responses in Seed Germination and Stomatal Closure. Plant Cell Physiol. 2018;59:469–486. doi: 10.1093/pcp/pcx194. [DOI] [PubMed] [Google Scholar]
- 33.Kuo H.-F., Hsu Y.-Y., Lin W.-C., Chen K.-Y., Munnik T., Brearley C. Arabidopsis inositol phosphate kinases IPK1 and ITPK1 constitute a metabolic pathway in maintaining phosphate homeostasis. Plant J. 2018;95:613–630. doi: 10.1111/tpj.13974. [DOI] [PubMed] [Google Scholar]
- 34.Laha D., Johnen P., Azevedo C., Dynowski M., Weiß M., Capolicchio S., Mao H., Iven T., Steenbergen M., Freyer M., et al. VIH2 Regulates the Synthesis of Inositol Pyrophosphate InsP8 and Jasmonate-Dependent Defenses in Arabidopsis. Plant Cell. 2015;27:1082–1097. doi: 10.1105/tpc.114.135160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S.-I., Tai T.H. Identification of genes necessary for wild-type levels of seed phytic acid in Arabidopsis thaliana using a reverse genetics approach. Mol. Genet. Genom. 2011;286:119–133. doi: 10.1007/s00438-011-0631-2. [DOI] [PubMed] [Google Scholar]
- 36.Laha D., Parvin N., Hofer A., Giehl R.F.H., Fernandez-Rebollo N., von Wirén N., Saiardi A., Jessen H.J., Schaaf G. Arabidopsis ITPK1 and ITPK2 Have an Evolutionarily Conserved Phytic Acid Kinase Activity. ACS Chem. Biol. 2019;14:2127–2133. doi: 10.1021/acschembio.9b00423. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J., Lau K., Puschmann R., Harmel R.K., Zhang Y., Pries V., Gaugler P., Broger L., Dutta A.K., Jessen H.J., et al. Two bifunctional inositol pyrophosphate kinases/phosphatases control plant phosphate homeostasis. eLife. 2019;8:e43582. doi: 10.7554/eLife.43582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adepoju O., Williams S.P., Craige B., Cridland C.A., Sharpe A.K., Brown A.M., Land E., Perera I.Y., Mena D., Sobrado P., et al. Inositol Trisphosphate Kinase and Diphosphoinositol Pentakisphosphate Kinase Enzymes Constitute the Inositol Pyrophosphate Synthesis Pathway in Plants (pre-print) bioRxiv. 2019:724914. doi: 10.1101/724914. [DOI] [Google Scholar]
- 39.Sweetman D., Johnson S., Caddick S.E.K., Hanke D.E., Brearley C.A. Characterization of an Arabidopsis inositol 1,3,4,5,6-pentakisphosphate 2-kinase (AtIPK1) Biochem. J. 2006;394:95–103. doi: 10.1042/BJ20051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi J., Wang H., Hazebroek J., Ertl D.S., Harp T. The maize low-phytic acid 3 encodes a myo-inositol kinase that plays a role in phytic acid biosynthesis in developing seeds. Plant J. 2005;42:708–719. doi: 10.1111/j.1365-313X.2005.02412.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim S.-I., Tai T.H. Genetic analysis of two OsLpa1-like genes in Arabidopsis reveals that only one is required for wild-type seed phytic acid levels. Planta. 2010;232:1241–1250. doi: 10.1007/s00425-010-1243-5. [DOI] [PubMed] [Google Scholar]
- 42.Kishor D.S., Lee C., Lee D., Venkatesh J., Seo J., Chin J.H., Jin Z., Hong S.-K., Ham J.-K., Koh H.J. Novel allelic variant of Lpa1 gene associated with a significant reduction in seed phytic acid content in rice (Oryza sativa L.) PLoS ONE. 2019;14:e0209636. doi: 10.1371/journal.pone.0209636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stiles A.R., Qian X., Shears S.B., Grabau E.A. Metabolic and signaling properties of an Itpk gene family in Glycine max. FEBS Lett. 2008;582:1853–1858. doi: 10.1016/j.febslet.2008.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi J., Wang H., Wu Y., Hazebroek J., Meeley R.B., Ertl D.S. The Maize Low-Phytic Acid Mutant lpa2 Is Caused by Mutation in an Inositol Phosphate Kinase Gene. Plant Physiol. 2003;131:507–515. doi: 10.1104/pp.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sweetman D., Stavridou I., Johnson S., Green P., Caddick S.E.K., Brearley C.A. Arabidopsis thaliana inositol 1,3,4-trisphosphate 5/6-kinase 4 (AtITPK4) is an outlier to a family of ATP-grasp fold proteins from Arabidopsis. FEBS Lett. 2007;581:4165–4171. doi: 10.1016/j.febslet.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 46.Majerus P.W., Wilson D.B., Zhang C., Nicholas P.J., Wilson M.P. Expression of inositol 1,3,4-trisphosphate 5/6-kinase (ITPK1) and its role in neural tube defects. Adv. Enzyme Regul. 2010;50:365–372. doi: 10.1016/j.advenzreg.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Couso I., Evans B.S., Li J., Liu Y., Ma F., Diamond S., Allen D.K., Umen J.G. Synergism between Inositol Polyphosphates and TOR Kinase Signaling in Nutrient Sensing, Growth Control, and Lipid Metabolism in Chlamydomonas. Plant Cell. 2016;28:2026–2042. doi: 10.1105/tpc.16.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi J., Williams J., Cho J., Falck J.R., Shears S. Purification, Sequencing, and Molecular Identification of a Mammalian PP-InsP5 Kinase That Is Activated When Cells Are Exposed to Hyperosmotic Stress. J. Biol. Chem. 2007;282:30763–30775. doi: 10.1074/jbc.M704655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fridy P.C., Otto J.C., Dollins D.E., York J.D. Cloning and characterization of two human VIP1-like inositol hexakisphosphate and diphosphoinositol pentakisphosphate kinases. J. Biol. Chem. 2007;282:30754–30762. doi: 10.1074/jbc.M704656200. [DOI] [PubMed] [Google Scholar]
- 50.Lin H., Fridy P.C., Ribeiro A.A., Choi J.H., Barma D.K., Vogel G., Falck J.R., Shears S.B., York J.D., Mayr G.W. Structural Analysis and Detection of Biological Inositol Pyrophosphates Reveal That the Family of VIP/Diphosphoinositol Pentakisphosphate Kinases Are 1/3-Kinases. J. Biol. Chem. 2009;284:1863–1872. doi: 10.1074/jbc.M805686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulugu S., Bai W., Fridy P.C., Bastidas R.J., Otto J.C., Dollins D.E., Haystead T.A., Ribeiro A.A., York J.D. A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science. 2007;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- 52.Weaver J.D., Wang H., Shears S.B. The kinetic properties of a human PPIP5K reveal that its kinase activities are protected against the consequences of a deteriorating cellular bioenergetic environment. Biosci. Rep. 2013;33:e00022. doi: 10.1042/BSR20120115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Draškovič P., Saiardi A., Bhandari R., Burton A., Ilc G., Kovačevič M., Snyder S.H., Podobnik M. Inositol Hexakisphosphate Kinase Products Contain Diphosphate and Triphosphate Groups. Chem. Biol. 2008;15:274–286. doi: 10.1016/j.chembiol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Tan X., Calderon-Villalobos L.I.A., Sharon M., Zheng C., Robinson C.V., Estelle M., Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- 55.Wild R., Gerasimaite R., Jung J.-Y., Truffault V., Pavlovic I., Schmidt A., Saiardi A., Jessen H.J., Poirier Y., Hothorn M., et al. Control of eukaryotic phosphate homeostasis by inositol polyphosphate sensor domains. Science. 2016;352:986–990. doi: 10.1126/science.aad9858. [DOI] [PubMed] [Google Scholar]
- 56.Rouached H., Arpat A.B., Poirier Y. Regulation of Phosphate Starvation Responses in Plants: Signaling Players and Cross-Talks. Mol. Plant. 2010;3:288–299. doi: 10.1093/mp/ssp120. [DOI] [PubMed] [Google Scholar]
- 57.Chien P.-S., Chiang C.-P., Leong S.J., Chiou T.-J. Sensing and Signaling of Phosphate Starvation—From Local to Long Distance. Plant Cell Physiol. 2018;59:1714–1722. doi: 10.1093/pcp/pcy148. [DOI] [PubMed] [Google Scholar]
- 58.Azevedo C., Saiardi A. Eukaryotic Phosphate Homeostasis: The Inositol Pyrophosphate Perspective. Trends Biochem. Sci. 2017;42:219–231. doi: 10.1016/j.tibs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Ried M. Inositol pyrophosphates promote the interaction of SPX domains with the coiled-coil motif of PHR transcription factors to regulate plant phosphate homeostasis (pre-print) bioRxiv. 2019 doi: 10.1101/2019.12.13.875393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puga M.I., Mateos I., Charukesi R., Wang Z., Franco-Zorrilla J.M., de Lorenzo L., Irigoyen M.L., Masiero S., Bustos R., Rodríguez J., et al. SPX1 is a phosphate-dependent inhibitor of PHOSPHATE STARVATION RESPONSE 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2014;111:14947–14952. doi: 10.1073/pnas.1404654111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong J., Ma G., Sui L., Wei M., Satheesh V., Zhang R., Ge S., Li J., Zhang T.-E., Wittwer C., et al. Inositol Pyrophosphate InsP8 Acts as an Intracellular Phosphate Signal in Arabidopsis. Mol. Plant. 2019;12:1463–1473. doi: 10.1016/j.molp.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Kuo H.-F., Chang T.-Y., Chiang S.-F., Wang W.-D., Charng Y., Chiou T.-J. Arabidopsis inositol pentakisphosphate 2-kinase, AtIPK1, is required for growth and modulates phosphate homeostasis at the transcriptional level. Plant J. 2014;80:503–515. doi: 10.1111/tpj.12650. [DOI] [PubMed] [Google Scholar]
- 63.Teale W.D., Paponov I.A., Palme K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006;7:847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- 64.Woodward A.W., Bartel B. Auxin: Regulation, Action, and Interaction. Ann. Bot. 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gray W.M., Kepinski S., Rouse D., Leyser O., Estelle M. Auxin regulates SCF TIR1-dependent degradation of AUX/IAA proteins. Nature. 2001;414:271–276. doi: 10.1038/35104500. [DOI] [PubMed] [Google Scholar]
- 66.Tan X., Zheng N. Hormone signaling through protein destruction: A lesson from plants. Am. J. Physiol. Endocrinol. Metab. 2009;296:E223–E227. doi: 10.1152/ajpendo.90807.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheard L.B., Tan X., Mao H., Withers J., Ben-Nissan G., Hinds T.R., Kobayashi Y., Hsu F.-F., Sharon M., Browse J., et al. Jasmonate perception by inositol phosphate-potentiated COI1-JAZ co-receptor. Nature. 2010;468:400–405. doi: 10.1038/nature09430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Browse J. Jasmonate Passes Muster: A Receptor and Targets for the Defense Hormone. Ann. Rev. Plant Biol. 2009;60:183–205. doi: 10.1146/annurev.arplant.043008.092007. [DOI] [PubMed] [Google Scholar]
- 69.Chini A., Fonseca S., Fernández G., Adie B., Chico J.M., Lorenzo O., García-Casado G., López-Vidriero I., Lozano F.M., Ponce M.R., et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- 70.Cui M., Du J., Yao X. The Binding Mechanism Between Inositol Phosphate (InsP) and the Jasmonate Receptor Complex: A Computational Study. Front. Plant Sci. 2018;9:963. doi: 10.3389/fpls.2018.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mosblech A., Thurow C., Gatz C., Feussner I., Heilmann I. Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana. Plant J. 2011;65:949–957. doi: 10.1111/j.1365-313X.2011.04480.x. [DOI] [PubMed] [Google Scholar]
- 72.Yu H., Wu J., Xu N., Peng M. Roles of F-box proteins in plant hormone responses. Acta Biochim. Biophys. Sin. Shanghai. 2007;39:915–922. doi: 10.1111/j.1745-7270.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- 73.Hu B., Chu C. Nitrogen-phosphorus interplay: Old story with molecular tale. New Phytol. 2019 doi: 10.1111/nph.16102. [DOI] [PubMed] [Google Scholar]
- 74.Baek D., Chun H.J., Yun D.-J., Kim M.C. Cross-talk between Phosphate Starvation and Other Environmental Stress Signaling Pathways in Plants. Mol. Cells. 2017;40:697–705. doi: 10.14348/molcells.2017.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murphy A.M., Otto B., Brearley C.A., Carr J.P., Hanke D.E. A role for inositol hexakisphosphate in the maintenance of basal resistance to plant pathogens. Plant J. 2008;56:638–652. doi: 10.1111/j.1365-313X.2008.03629.x. [DOI] [PubMed] [Google Scholar]
- 76.Reddy C.S., Kim S.-C., Kaul T. Genetically modified phytase crops role in sustainable plant and animal nutrition and ecological development: A review. 3 Biotech. 2017;7:195. doi: 10.1007/s13205-017-0797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]