Abstract
There are around 140 species in the genus Callicarpa, with 23 species occurring in Vietnam. The Vietnamese Callicarpa species have been poorly studied. In this work, the leaf essential oils of C. bodinieri, C. candicans, C. formosana, C. longifolia, C. nudiflora, C. petelotii, C. rubella, and C. sinuata, have been obtained from plants growing in central Vietnam. The chemical compositions of the essential oils were determined using gas chromatography – mass spectrometry. Mosquito larvicidal activities of the essential oils were carried out against Aedes aegypti. All of the Callicarpa leaf essential oils showed larvicidal activity, but two samples of C. candicans were particularly active with 48-h LC50 values of 2.1 and 3.8 μg/mL. Callicarpa candicans essential oil should be considered as a potential alternative mosquito control agent.
Keywords: Lamiaceae, Callicarpa candicans, Callicarpa rubella, Aedes aegypti, atractylone, β-bisabolene, germacrone
1. Introduction
There are around 140 species of Callicarpa L. distributed in tropical and subtropical locations [1]. The genus has been placed in either the Verbenaceae or the Lamiaceae, but is currently placed in Lamiaceae [2,3]. Members of the genus have been used as fish poisons and in herbal medicine [1,2]. In this work, we present the essential oil compositions of several Callicarpa species growing wild in central Vietnam. In addition, some of the essential oils were screened for mosquito larvicidal activity.
Callicarpa bodinieri H. Lév. is native to western and central China [3], Vietnam, Laos, Cambodia, and Thailand [4]. The plant is used in traditional Chinese medicine to treat hematemesis (oral decoction of the leaves) and to treat wounds and bruises (fresh leaves externally) [5]. Flavonoids, sterols, triterpenoids [2], and diterpenoids [6] have been characterized in the leaves of C. bodinieri.
Callicarpa candicans (Burm. f.) Hochr. is native to southeast Asia, including China (Quangdong, Hainan), Burma, Cambodia, India, Laos, the Philippines, Thailand, and Vietnam [3]. The plant has been used as a fish poison in the Philippines [7], India [8], and Thailand [9]. In Vietnamese traditional medicine, the plant is used to prepare a tonic, to treat diseases of the liver and stomach, and externally to treat skin problems, pimples and ulcerations [10]. In Thailand, the stem bark of C. candicans is used to treat skin inflammation and swelling [11], while in the Philippines, the plant is taken to treat abdominal troubles [7], and sore throat and tonsillitis in the Mariana Islands [12]. In Vietnam, C. candicans is used as a tonic for postpartum care in women, to treat liver and abdominal pain, and as a diuretic [13]. Diterpenoids, triterpenoids, and flavonoids have been isolated from C. candicans [9,10].
Callicarpa formosana Rolfe is found in southeastern China (including Taiwan) [14], Japan, the Philippines [3], and Vietnam [15,16]. In China, C. formosana is used to treat scrofula (mycobacterial cervical lymphadenitis), and goiter [5], to stop bleeding [17], and to treat pyogenic infections [18]. Several sesquiterpenoids, diterpenoids, triterpenoids, iridoids, and flavonoids have been isolated and characterized from C. formosana [14].
Callicarpa longifolia Lam. Ranges from southern China through Malesia to Australia and from India through southeast Asia, including Vietnam [3,4,15,16,19,20,21]. Leaves of C. longifolia are used in China to treat wounds [2], while in Vietnam the plant is used to treat fever, diarrhea, abdominal pain, and as a tonic for postpartum women [13]. Kaurane diterpenoids and several flavonoids have been isolated from the leaves of C. longifolia [2,5].
Callicarpa nudiflora Hook. & Arn. is distributed from southern China through Southeast Asia as well as Burma, India, and Sri Lanka [3]. In Chinese traditional medicine, C. nudiflora is used for gastrointestinal bleeding, tuberculosis, upper respiratory tract infection, pneumonia, and bronchitis [5]. In Vietnam, the plant has been used traditionally for treating stomach bleeding and hepatitis [13]. The phytochemistry of C. nudiflora has been extensively studied. Terpenoids, including iridoids, diterpenoids, triterpenoids, as well as numerous flavonoids and phenylpropanoids have been isolated and identified in the plant [5].
Callicarpa petelotii Dop is endemic to Vietnam and recorded in the provinces of Lạng Sơn, Vĩnh Phúc, Hòa Bình, and Nghệ An [15,16]. There are no reports in the literature regarding ethnobotanical uses of the plant nor are there any phytochemical analyses reported.
Callicarpa rubella Lindl. ranges from southeastern China south through Burma, Thailand, Laos, and Vietnam [3]. In Vietnam, the fresh leaves are applied externally to treat scabies [13] or chewed to treat gum disease [22].
Callicarpa sinuata A.L. Budantzev & Phuong is endemic to Vietnam. It has been recorded in Quảng Bình province, (Vĩnh Linh: Do Linh), Sơn Trà peninsula (Đà Nãng City), and Gia Lai province [15,16]. There are no reports in the literature on the ethnobotany or phytochemistry of this species.
Mosquito-borne diseases have been a chronic menace to humans throughout history. Aedes aegypti (L.) (Diptera: Culicidae) is an important insect vector of arboviruses such as dengue [23], yellow fever [24], chikungunya [25], and Zika [26]. Dengue fever is widespread in Vietnam and epidemics are becoming more frequent [27]. Furthermore, chikungunya and Zika infections have recently been reported in Vietnam [28]. Culex quinquefasciatus Say (Diptera: Culicidae) is a vector of lymphatic filariasis [29] as well as several arboviruses such as West Nile virus and St. Louis encephalitis virus [30] and possibly Zika virus [31].
Insecticide resistance in Aedes and Culex mosquitoes has been growing throughout the world and may lead to an increase in the frequency of mosquito-borne diseases [32,33,34,35,36]. In addition to insecticide resistance, there is a chronic problem of the environmental impacts of synthetic insecticides [37,38], and there is a need for new and complementary methods for controlling insect vectors. Essential oils have shown promise as renewable and environmentally-safe alternatives to the use of synthetic insecticides [39,40,41,42,43]. As part of our continuing research on essential oils of aromatic plants from Vietnam and our search for natural mosquito control agents, we have collected and analyzed the essential oils from several species of Callicarpa growing wild in central Vietnam, and, depending on availability, the essential oils were screened for larvicidal activity against Ae. aegypti, and/or Cx. quinquefasciatus. The volatile components of C. candicans, C. longifolia, C. petelotii, and C. sinuata are reported for the first time. As far as we are aware, none of the Callicarpa essential oils presented in this work has been previously investigated in terms of mosquito larvicidal activity.
2. Results and Discussion
2.1. Essential Oil Compositions
2.1.1. Callicarpa bodinieri
The leaf essential oil of C. bodinieri was obtained from Ngoc Linh Nature Reserve, Quang Nam province. The essential oil composition is presented in Table 1. The major components in C. bodinieri leaf essential oil were caryophyllene oxide (9.8%), β-selinene (8.9%), limonene (8.0%), and α-copaene (5.4%). A total of 106 compounds were identified in the essential oil accounting for 96.2% of the composition with sesquiterpene hydrocarbons (34.2%) and oxygenated sesquiterpenoids (37.8%) making up the bulk of the composition. The volatiles, obtained by head-space solid-phase micro extraction, of C. bodinieri from China have been reported [44]. The main volatile compounds were eremophila-1(10),11-diene (30.1%), cadina-3,9-diene (15. 2%), and longifolene (5.7%), and therefore, very different from the composition of the leaf essential oil from Vietnam.
Table 1.
Chemical composition of Callicarpa bodinieri leaf essential oil from Ngoc Linh Nature Reserve, Vietnam.
| RI a | RI b | Compound | % |
|---|---|---|---|
| 923 | 924 | α-Thujene | tr c |
| 930 | 932 | α-Pinene | 1.5 |
| 945 | 945 | α-Fenchene | tr |
| 947 | 946 | Camphene | 0.1 |
| 970 | 969 | Sabinene | tr |
| 975 | 974 | β-Pinene | 1.7 |
| 977 | 974 | 1-Octen-3-ol | tr |
| 983 | 979 | Octan-3-one | tr |
| 984 | 984 | p-Menth-3-ene | tr |
| 986 | 988 | Myrcene | 0.5 |
| 1002 | 998 | Octanal | 0.3 |
| 1022 | 1024 | p-Cymene | 0.2 |
| 1027 | 1024 | Limonene | 8.0 |
| 1030 | 1026 | 1,8-Cineole | 0.1 |
| 1068 | 1063 | 1-Octanol | 0.2 |
| 1089 | 1082 | m-Cymenene | 0.1 |
| 1098 | 1095 | Linalool | 0.3 |
| 1103 | 1100 | Nonanal | 2.3 |
| 1120 | 1119 | trans-p-Mentha-2,8-dien-1-ol | 0.1 |
| 1125 | 1122 | α-Campholenal | 0.1 |
| 1131 | 1132 | cis-Limonene oxide | 0.2 |
| 1135 | 1133 | cis-p-Mentha-2,8-dien-1-ol | 0.1 |
| 1135 | 1137 | trans-Limonene oxide | 0.1 |
| 1137 | 1135 | Nopinone | tr |
| 1139 | 1135 | trans-Pinocarveol | 0.1 |
| 1183 | 1178 | Naphthalene | 0.5 |
| 1186 | 1187 | trans-p-Mentha-1(7),8-dien-2-ol | 0.2 |
| 1194 | 1195 | Myrtenal | 0.3 |
| 1204 | 1201 | Decanal | 0.3 |
| 1217 | 1215 | trans-Carveol | 0.2 |
| 1242 | 1239 | Carvone | 0.2 |
| 1260 | 1260 | Dec-(2E)-enal | 0.1 |
| 1265 | 1267 | Nonanoic acid | 0.4 |
| 1281 | 1287 | Bornyl acetate | 0.1 |
| 1286 | 1287 | Dihydroedulan IA | 0.3 |
| 1291 | 1294 | Dihydroedulan IIA | 0.3 |
| 1295 | 1298 | (Z)-Theaspirane | 0.2 |
| 1300 | 1300 | Tridecane | 0.1 |
| 1305 | 1305 | Undecanal | 0.1 |
| 1308 | 1310 | (Z)-Patchenol | tr |
| 1312 | 1314 | (E)-Theaspirane | 0.1 |
| 1344 | 1345 | α-Cubebene | 0.1 |
| 1349 | 1671 | 1-Tetradecanol | 0.1 |
| 1366 | 1373 | α-Ylangene | 0.4 |
| 1373 | 1374 | α-Copaene | 5.4 |
| 1381 | 1387 | β-Bourbonene | 0.1 |
| 1386 | 1389 | β-Elemene | 0.9 |
| 1415 | 1419 | β-Ylangene | 0.4 |
| 1416 | 1417 | β-Caryophyllene | 1.0 |
| 1424 | 1430 | γ-Maaliene | 0.3 |
| 1427 | 1431 | β-Gurjunene (= Calarene) | 2.3 |
| 1436 | 1439 | Aromadendrene | 2.3 |
| 1442 | 1447 | Selina-5,11-diene | 0.2 |
| 1449 | 1455 | Valerena-4,7(11)-diene | 0.8 |
| 1452 | 1452 | α-Humulene | 0.6 |
| 1457 | 1458 | allo-Aromadendrene | 1.8 |
| 1470 | 1475 | Selina-4,11-diene | 0.2 |
| 1472 | 1478 | γ-Muurolene | 2.7 |
| 1476 | 1483 | α-Amorphene | 0.2 |
| 1478 | 1479 | ar-Curcumene | 0.1 |
| 1486 | 1489 | β-Selinene | 8.9 |
| 1489 | 1495 | γ-Amorphene | 0.1 |
| 1493 | 1498 | α-Selinene | 0.9 |
| 1495 | 1500 | α-Muurolene | 0.6 |
| 1504 | 1505 | β-Bisabolene | 0.2 |
| 1510 | 1513 | γ-Cadinene | 1.0 |
| 1512 | 1514 | Cubebol | 0.1 |
| 1515 | 1522 | δ-Cadinene | 0.2 |
| 1517 | 1521 | trans-Calamenene | 0.5 |
| 1518 | 1528 | cis-Calamenene | 0.7 |
| 1538 | 1544 | α-Calacorene | 0.6 |
| 1558 | 1561 | (E)-Nerolidol | 0.6 |
| 1559 | 1564 | β-Calacorene | 0.8 |
| 1567 | 1566 | Maaliol | 0.5 |
| 1567 | 1567 | Palustrol | 0.6 |
| 1574 | 1577 | Spathulenol | 2.3 |
| 1579 | 1582 | Caryophyllene oxide | 9.8 |
| 1583 | 1590 | Globulol | 3.8 |
| 1585 | 1590 | β-Copaen-4α-ol | 1.3 |
| 1590 | 1594 | Salvial-4(14)-en-1-one | 0.6 |
| 1591 | 1592 | Viridiflorol | 2.2 |
| 1594 | 1595 | Cubeban-11-ol | 0.7 |
| 1599 | 1598 | Dehydroxy-iso-calamendiol | 0.4 |
| 1601 | 1602 | Ledol | 0.7 |
| 1604 | 1600 | Rosifoliol | 0.5 |
| 1607 | 1608 | Humulene epoxide II | 2.6 |
| 1612 | 1618 | 1,10-di-epi-Cubenol | 0.5 |
| 1623 | 1630 | Muurola-4,10(14)-dien-1β-ol | 1.7 |
| 1625 | 1627 | 1-epi-Cubenol | 0.6 |
| 1631 | 1642 | Caryophylla-4(12),8(13)-dien-5α-ol | 0.5 |
| 1634 | 1644 | Caryophylla-4(12),8(13)-dien-5β-ol | 0.4 |
| 1639 | 1638 | τ-Cadinol | 0.5 |
| 1641 | 1640 | τ-Muurolol | 0.6 |
| 1644 | 1644 | α-Muurolol (= δ-Cadinol) | 0.5 |
| 1653 | 1652 | α-Cadinol | 0.9 |
| 1655 | 1651 | Pogostol | 2.5 |
| 1660 | 1668 | ar-Turmerone | 0.5 |
| 1662 | 1668 | trans-Calamenen-10-ol | 0.3 |
| 1668 | 1668 | 14-Hydroxy-9-epi-(E)-caryophyllene | 0.4 |
| 1670 | 1675 | Cadalene | 0.6 |
| 1682 | 1685 | Germacra-4(15),5,10(14)-trien-1α-ol | 0.3 |
| 1806 | 1816 | Callicarpenal | 0.4 |
| 1837 | 1841 | Phytone | 1.5 |
| 1955 | 1958 | Palmitic acid | 0.7 |
| 2103 | 2109 | (E)-Phytol | 1.8 |
| 2700 | 2700 | Heptacosane | 0.6 |
| Monoterpene hydrocarbons | 12.1 | ||
| Oxygenated monoterpenoids | 2.1 | ||
| Sesquiterpene hydrocarbons | 34.2 | ||
| Oxygenated sesquiterpenoids | 37.8 | ||
| Diterpenoids | 3.3 | ||
| Others | 6.6 | ||
| Total identified | 96.2 |
a RI = Retention Index determined with respect to a homologous series of n-alkanes on a ZB-5 column. b Retention indices from the databases. c tr = trace (<0.05%).
2.1.2. Callicarpa candicans
The leaf essential oils of C. candicans have been obtained from three different locations in Central Vietnam, Nghia Dan district (Nghe An province), Dai Loc district (Quang Nam province), and Hoa Vang district (Da Nang city). The C. candicans leaf essential oils were dominated by sesquiterpene hydrocarbons and oxygenated sesquiterpenes. (E)-Caryophyllene (19.0%, 7.1%, and 15.3%), β-selinene (6.2%, 5.7%, and 4.5%), caryophyllene oxide (2.9%, 13.4%, and 3.4%), and atractylone (37.7%, 4.2%, and 42.4%), respectively, for the samples from Nghia Dan, Dai Loc, and Hoa Vang, were the major components (Table 2). The stem bark essential oil, collected from Hoa Vang, was also rich in (E)-caryophyllene (7.8%), β-selinene (7.9%), caryophyllene oxide (11.1%), and atractylone (6.2%) (Table 3). As far as we are aware, there have been no previous reports on C. candicans essential oils.
Table 2.
Chemical compositions of Callicarpa candicans leaf essential oils from Vietnam.
| RI a | RI b | Compound | % | ||
|---|---|---|---|---|---|
| Nghia Dan | Dai Loc | Hoa Vang | |||
| 873 | 863 | 2,3-Dimethyl-cyclohexa-1,3-diene | --- | tr c | --- |
| 930 | 932 | α-Pinene | --- | tr | --- |
| 975 | 974 | β-Pinene | --- | 0.1 | --- |
| 977 | 974 | 1-Octen-3-ol | 0.3 | 0.1 | 0.1 |
| 983 | 979 | 3-Octanone | 0.2 | tr | 0.1 |
| 996 | 988 | 3-Octanol | --- | tr | tr |
| 1024 | 1024 | p-Cymene | --- | 0.4 | tr |
| 1027 | 1024 | Limonene | --- | 0.1 | --- |
| 1030 | 1026 | 1,8-Cineole | --- | tr | --- |
| 1068 | 1067 | cis-Linalool oxide (furanoid) | --- | tr | --- |
| 1083 | 1086 | Terpinolene | --- | tr | --- |
| 1084 | 1084 | trans-Linalool oxide (furanoid) | --- | tr | --- |
| 1089 | 1087 | 2-Nonanone | 0.1 | 0.1 | 0.1 |
| 1099 | 1095 | Linalool | 0.6 | 1.4 | 0.4 |
| 1182 | 1178 | Naphthalene | --- | 0.3 | --- |
| 1191 | 1190 | Methyl salicylate | --- | 0.1 | 0.1 |
| 1287 | 1287 | Dihydroedulan IA | 0.1 | 0.2 | 0.1 |
| 1291 | 1293 | 2-Undecanone | 0.2 | 0.1 | 0.3 |
| 1295 | 1310 | (Z)-Theaspirane | --- | tr | --- |
| 1312 | 1314 | (E)-Theaspirane | --- | tr | --- |
| 1335 | 1335 | δ-Elemene | 0.2 | 1.1 | 0.1 |
| 1335 | 1330 | (Z)-Jasmone | --- | 0.1 | --- |
| 1344 | 1346 | α-Terpinyl acetate | --- | 0.1 | --- |
| 1366 | 1373 | α-Ylangene | --- | 0.1 | --- |
| 1376 | 1374 | α-Copaene | 0.1 | 0.1 | --- |
| 1376 | 1383 | (E)-β-Damascenone | --- | tr | --- |
| 1381 | 1383 | cis-β-Elemene | --- | --- | 0.1 |
| 1388 | 1390 | trans-β-Elemene | 0.9 | 1.5 | 1.7 |
| 1401 | 1408 | (Z)-Caryophyllene | --- | 0.1 | --- |
| 1410 | 1411 | Thymohydroquinone dimethyl ether | --- | 0.1 | --- |
| 1419 | 1417 | (E)-Caryophyllene | 19.0 | 7.1 | 15.3 |
| 1428 | 1434 | γ-Elemene | 3.2 | 0.5 | 2.3 |
| 1437 | 1439 | Aromadendrene | 0.2 | 0.2 | 0.1 |
| 1451 | 1454 | (E)-β-Farnesene | --- | --- | 0.1 |
| 1455 | 1452 | α-Humulene | 2.4 | 1.2 | 1.9 |
| 1459 | 1458 | allo-Aromadendrene | 0.2 | 0.1 | 0.1 |
| 1470 | 1475 | Selina-4,11-diene | --- | 0.2 | --- |
| 1474 | 1476 | β-Chamigrene | 0.1 | 0.1 | 0.1 |
| 1475 | 1478 | γ-Muurolene | 0.3 | 0.1 | --- |
| 1478 | 1483 | α-Amorphene | 0.3 | 0.3 | 0.2 |
| 1480 | 1487 | (E)-β-Ionone | --- | 0.7 | --- |
| 1481 | 1484 | Germacrene D | 0.9 | --- | 0.5 |
| 1484 | 1487 | Aristolochene | 0.1 | --- | --- |
| 1488 | 1489 | β-Selinene | 6.2 | 5.7 | 4.5 |
| 1493 | 1499 | Curzerene | 2.2 | 0.8 | 5.3 |
| 1495 | 1498 | α-Selinene | --- | 1.0 | 1.7 |
| 1496 | 1500 | Bicyclogermacrene | 3.0 | --- | --- |
| 1498 | 1500 | α-Muurolene | 0.2 | --- | --- |
| 1504 | 1505 | (E,E)-α-Farnesene | 0.7 | --- | 0.4 |
| 1513 | 1513 | γ-Cadinene | 0.3 | --- | --- |
| 1518 | 1522 | δ-Cadinene | 0.4 | --- | 0.1 |
| 1519 | 1520 | 7-epi-α-Selinene | 0.1 | --- | 0.1 |
| 1533 | 1528 | Zonarene | 0.1 | --- | --- |
| 1534 | 1540 | Selina-4(15),7(11)-diene | 1.5 | 1.8 | 0.9 |
| 1541 | 1545 | Selina-3,7(11)-diene | 0.5 | 0.9 | 0.2 |
| 1546 | 1548 | α-Elemol | --- | 0.4 | 0.1 |
| 1556 | 1559 | Germacrene B | 6.1 | 0.1 | 5.1 |
| 1557 | 1561 | (E)-Nerolidol | --- | 0.3 | --- |
| 1576 | 1577 | Spathulenol | 0.7 | 2.1 | 1.0 |
| 1581 | 1582 | Caryophyllene oxide | 2.9 | 13.4 | 3.4 |
| 1583 | 1590 | Globulol | --- | 0.1 | --- |
| 1593 | 1594 | Salvial-4(14)-en-1-one | 0.1 | --- | --- |
| 1598 | 1601 | trans-β-Elemenone | 0.1 | --- | --- |
| 1609 | 1608 | Humulene epoxide II | 0.3 | 1.6 | 0.4 |
| 1627 | 1629 | iso-Spathulenol | 0.1 | 0.3 | 0.1 |
| 1630 | 1642 | Caryophylla-4(12),8(13)-dien-5α-ol | --- | 0.4 | --- |
| 1636 | 1644 | Caryophylla-4(12),8(13)-dien-5β-ol | 1.2 | 1.1 | 1.3 |
| 1643 | 1644 | α-Muurolol (= Torreyol) | 0.1 | --- | --- |
| 1647 | 1642 | Selina-3,11-dien-6α-ol | --- | --- | tr |
| 1655 | 1649 | β-Eudesmol | 1.9 | 3.6 | 1.9 |
| 1662 | 1657 | Atractylone | 37.7 | 4.2 | 42.4 |
| 1666 | 1666 | Intermedeol | 0.1 | --- | --- |
| 1668 | 1668 | 14-Hydroxy-9-epi-(E)-caryophyllene | 0.2 | 2.5 | 0.4 |
| 1693 | 1693 | Germacrone | 0.3 | --- | 0.2 |
| 1696 | 1700 | Eudesm-7(11)-en-4-ol | --- | --- | tr |
| 1711 | --- | Unidentified d | 0.2 | 1.1 | 0.4 |
| 1713 | 1713 | Longifolol | --- | --- | 0.1 |
| 1736 | 1734 | 1(10),11-Eremophiladien-9-one | 0.5 | --- | --- |
| 1739 | 1746 | 8α,11-Elemodiol | --- | --- | 0.1 |
| 1768 | --- | Unidentified e | --- | 1.0 | 0.3 |
| 1799 | 1796 | (E)-Isovalencenol | --- | --- | 0.1 |
| 1858 | --- | Unidentified f | 0.6 | 10.9 | 1.8 |
| 1919 | --- | Unidentified g | --- | 1.0 | --- |
| 1936 | --- | Unidentified h | --- | 1.0 | --- |
| 1994 | 1994 | Manoyl oxide | 0.9 | 3.3 | --- |
| 1998 | 1997 | Kaur-15-ene | 0.1 | --- | --- |
| 2005 | --- | Unidentified i | 0.5 | 3.6 | 0.9 |
| 2055 | --- | Unidentified j | 0.3 | 6.6 | 0.8 |
| 2091 | --- | Unidentified k | --- | 8.5 | 1.0 |
| 2105 | 2109 | (E)-Phytol | --- | --- | 0.8 |
| Monoterpene hydrocarbons | 0.0 | 0.6 | 0.0 | ||
| Oxygenated monoterpenoids | 0.6 | 1.6 | 0.4 | ||
| Sesquiterpene hydrocarbons | 49.0 | 22.6 | 40.7 | ||
| Oxygenated sesquiterpenoids | 46.0 | 29.9 | 51.5 | ||
| Diterpenoids | 1.0 | 3.3 | 0.8 | ||
| Others | 0.8 | 1.5 | 0.8 | ||
| Total Identified | 97.3 | 59.7 | 94.2 | ||
a RI = Retention Index determined with respect to a homologous series of n-alkanes on a ZB-5 column. b Retention indices from the databases. c tr = trace (<0.05%). d MS: 220(41%), 205(20%), 202(30%), 187(30%), 162(92%), 158(33%), 149(63%), 147(61%), 121(79%), 119(84%), 107(79%), 105(73%), 97(49%), 93(61%), 91(71%), 79(48%), 77(38%), 67(53%), 55(49%), 43(100%), 41(62%). e MS: 220(64%), 202(9%), 177(100%), 159(77%), 135(49%), 123(74%), 107(83%), 93(64%), 81(58%), 67(53%), 55(55%), 43(55%), 41(65%). f MS: 233(16%), 232(100%), 204(19%), 189(21%), 161(29%), 148(31%), 147(38%), 135(32%), 134(33%), 133(44%), 121(34%), 108(48%), 105(37%), 93(53%), 91(51%), 79(44%), 77(33%), 67(22%), 55(24%), 53(23%), 41(34%). g MS: 236(12%), 222(4%), 203(4%), 193(25%), 175(25%), 161(9%), 149(22%), 147(48%), 133(14%), 121(13%), 119(13%), 107(19%), 105(26%), 93(24%), 91(27%), 79(22%), 67(17%), 55(15%), 43(100%), 41(22%). h MS: 290(23%), 165(6%), 151(100%), 138(12%), 123(25%), 109(24%), 95(17%), 81(26%), 69(35%), 55(23%), 43(14%), 41(20%). i MS: 230(70%), 215(100%), 201(44%), 187(30%), 174(33%), 160(31%), 159(34%), 145(27%), 131(27%), 117(20%), 115(18%), 105(27%), 91(50%), 79(28%), 77(32%), 53(31%), 41(27%). j MS: 233(17%), 232(100%), 217(29%), 204(15%), 189(12%), 187(12%), 176(13%), 161(19%), 148(16%), 147(23%), 133(26%), 122(51%), 121(45%), 107(52%), 105(42%), 93(63%), 91(59%), 79(57%), 77(39%), 67(29%), 55(27%), 53(36%), 41(45%). k MS: 248(4%), 230(8%), 220(16%), 205(16%), 191(15%), 175(18%), 159(14%), 147(100%), 133(16%), 121(42%), 119(33%), 107(28%), 105(40%), 93(35%), 91(45%), 79(41%), 67(24%), 55(21%), 53(24%), 41(35%).
Table 3.
Chemical composition of Callicarpa candicans stem bark essential oil from Hoa Vang, Vietnam.
| RI a | RI b | Compound | % |
|---|---|---|---|
| 978 | 974 | 1-Octen-3-ol | 1.4 |
| 996 | 988 | 3-Octanol | 0.4 |
| 1099 | 1095 | Linalool | 0.6 |
| 1191 | 1190 | Methyl salicylate | 0.9 |
| 1335 | 1336 | Bicycloelemene | 1.6 |
| 1350 | 1356 | Eugenol | 0.4 |
| 1389 | 1389 | β-Elemene | 0.6 |
| 1419 | 1417 | β-Caryophyllene | 7.8 |
| 1429 | 1434 | γ-Elemene | 1.1 |
| 1455 | 1452 | α-Humulene | 1.4 |
| 1478 | 1483 | α-Amorphene | 0.2 |
| 1483 | 1476 | β-Chamigrene | 1.5 |
| 1487 | 1496 | Indipone | 0.8 |
| 1489 | 1489 | β-Selinene | 7.9 |
| 1493 | 1498 | Curzerene | 0.6 |
| 1496 | 1498 | α-Selinene | 2.0 |
| 1519 | 1520 | 7-epi-α-Selinene | 0.6 |
| 1537 | 1528 | Zonarene | 2.6 |
| 1541 | 1545 | Selina-3,7(11)-diene | 1.1 |
| 1559 | 1559 | Germacrene B | 1.6 |
| 1560 | 1561 | (E)-Nerolidol | 0.5 |
| 1576 | 1577 | Spathulenol | 1.5 |
| 1582 | 1582 | Caryophyllene oxide | 11.1 |
| 1609 | 1608 | Humulene epoxide II | 1.3 |
| 1617 | --- | Unidentified c | 1.2 |
| 1636 | 1644 | Caryophylla-4(12),8(13)-dien-5β-ol | 1.2 |
| 1647 | 1642 | Selina-3,11-dien-6α-ol | 0.4 |
| 1654 | 1652 | α-Eudesmol | 5.3 |
| 1659 | 1657 | Atractylone | 6.2 |
| 1670 | 1668 | 14-Hydroxy-9-epi-(E)-caryophyllene | 1.4 |
| 1692 | 1693 | Germacrone | 0.3 |
| 1711 | --- | Unidentified d | 1.2 |
| 1730 | 1728 | iso-Longifolol | 0.8 |
| 1735 | 1734 | 1(10),11-Eremophiladien-9-one | 1.2 |
| 1770 | --- | Unidentified e | 1.1 |
| 1858 | --- | Unidentified f | 4.1 |
| 1985 | --- | Unidentified g | 2.2 |
| 1985 | 1987 | 1-Eicosene | 2.2 |
| 1993 | 1994 | Manoyl oxide | 6.2 |
| 1997 | 1997 | Kaur-15-ene | 0.4 |
| 2006 | --- | Unidentified h | 2.9 |
| 2054 | --- | Unidentified i | 2.9 |
| 2089 | --- | Unidentified j | 5.1 |
| 2106 | 2109 | (E)-Phytol | 0.3 |
| Monoterpene hydrocarbons | 0.0 | ||
| Oxygenated monoterpenoids | 0.6 | ||
| Sesquiterpene hydrocarbons | 30.7 | ||
| Oxygenated sesquiterpenoids | 31.8 | ||
| Diterpenoids | 6.9 | ||
| Others | 3.1 | ||
| Total Identified | 73.1 |
a RI = Retention Index determined with respect to a homologous series of n-alkanes on a ZB-5 column. b Retention indices from the databases. c MS: 207(45%), 204(53%), 189(54%), 161(39%), 147(32%), 137(30%), 135(68%), 133(31%), 123(41%), 109(47%), 95(53%), 93(50%), 81(100%), 71(60%), 67(43%), 55(45%), 43(97%), 41(37%). d MS: 220(49%), 205(29%), 202(48%), 187(40%), 162(100%), 159(41%), 149(53%), 147(58%), 131(37%), 121(77%), 119(85%), 107(68%), 105(68%), 97(47%), 93(57%), 91(62%), 79(40%), 77(31%), 67(43%), 55(46%), 43(79%), 41(48%). e MS: 220(46%), 202(9%), 187(10%), 177(100%), 159(50%), 138(27%), 135(37%), 123(58%), 107(57%), 95(36%), 93(40%), 91(37%), 81(35%), 79(29%), 67(19%), 55(29%), 43(30%), 41(29%). f MS: 233(17%), 232(100%), 217(8%), 204(22%), 189(24%), 176(17%), 161(26%), 148(26%), 147(27%), 135(27%), 134(30%), 133(38%), 122(26%), 121(30%), 108(40%), 105(30%), 93(47%), 91(41%), 79(35%), 77(25%), 67(17%), 55(19%), 53(17%), 41(24%). g MS: 236(2%), 221(3%), 218(5%), 203(7%), 182(24%), 179(27%), 162(19%), 161(28%), 143(45%), 234(30%), 125(60%), 123(64%), 121(43%), 109(55%), 107(39%), 97(53%), 95(63%), 93(47%), 81(70%), 79(37%), 71(40%), 69(69%), 67(40%), 55(100%), 43(87%), 41(70%). h MS: 230(70%), 215(100%), 201(49%), 187(31%), 174(33%), 160(25%), 159(27%), 145(21%), 131(27%), 117(15%), 105(19%), 91(36%), 79(21%), 77(23%), 55(14%), 53(23%), 41(18%). i MS: 233(18%), 232(100%), 217(31%), 204(18%), 190(14%), 187(14%), 176(13%), 161(15%), 148(14%), 147(21%), 133(21%), 122(49%), 121(38%), 107(44%), 105(34%), 93(55%), 91(46%), 79(43%), 77(28%), 67(23%), 55(22%), 53(29%), 41(30%). j MS: 342(1%), 248(4%), 230(27%), 220(21%), 215(35%), 205(20%), 203(19%), 191(18%), 175(24%), 159(22%), 147(100%), 133(20%), 131(19%), 121(45%), 119(36%), 105(48%), 91(50%), 79(43%), 77(30%), 67(26%), 55(25%), 53(29%), 43(17%), 41(35%).
2.1.3. Callicarpa formosana
The leaf essential oil of C. formosana from Vietnam was dominated by caryophyllene oxide (38.9%), β-bisabolene (18.6%), and (E)-caryophyllene (6.5%) (Table 4). The composition of the essential oil from Vietnam is notably different from that collected in Guangdong, China, which was composed largely of spathulenol (20.2%), (E)-caryophyllene (17.2%), germacrene D (8.1%), and β-eudesmol (5.5%) [45].
Table 4.
Chemical composition of Callicarpa formosana leaf essential oil from Ngoc Linh Nature Reserve, Vietnam.
| RI a | RI b | Compound | % |
|---|---|---|---|
| 930 | 932 | α-Pinene | 0.6 |
| 975 | 974 | β-Pinene | 0.3 |
| 1022 | 1024 | p-Cymene | 0.1 |
| 1026 | 1024 | Limonene | 0.1 |
| 1097 | 1095 | Linalool | 0.1 |
| 1103 | 1100 | Nonanal | 0.1 |
| 1182 | 1187 | (3Z)-Hexenyl butyrate | 0.1 |
| 1188 | 1195 | Hexyl butyrate | 0.1 |
| 1192 | 1197 | (2E)-Hexenyl butyrate | 0.1 |
| 1227 | 1221 | (3Z)-Hexenyl 2-methylbutyrate | 0.1 |
| 1232 | 1236 | Hexyl 2-methylbutyrate | tr c |
| 1234 | 1235 | (2E)-Hexenyl 2-methylbutyrate | 0.1 |
| 1285 | 1287 | Dihydroedulan IA | 0.2 |
| 1290 | 1294 | Dihydroedulan IIA | 0.1 |
| 1295 | 1298 | (Z)-Theaspirane | 0.3 |
| 1311 | 1314 | (E)-Theaspirane | 0.3 |
| 1372 | 1374 | α-Copaene | 0.1 |
| 1385 | 1389 | β-Elemene | 0.2 |
| 1398 | 1402 | α-Funebrene | 0.1 |
| 1400 | 1408 | (Z)-Caryophyllene | 0.1 |
| 1412 | 1410 | α-Cedrene | tr |
| 1416 | 1417 | (E)-Caryophyllene | 6.5 |
| 1426 | 1430 | β-Copaene | tr |
| 1430 | 1428 | Dictamnol | tr |
| 1443 | 1453 | Geranyl acetone | 0.1 |
| 1448 | 1454 | (E)-β-Farnesene | 0.2 |
| 1452 | 1452 | α-Humulene | 0.6 |
| 1471 | 1478 | γ-Muurolene | 0.1 |
| 1477 | 1479 | ar-Curcumene | 0.6 |
| 1484 | 1491 | Eremophilene | 0.1 |
| 1485 | 1489 | β-Selinene | 0.1 |
| 1491 | 1498 | α-Selinene | 0.2 |
| 1494 | 1500 | α-Muurolene | 0.1 |
| 1503 | 1505 | β-Bisabolene | 18.6 |
| 1509 | 1511 | Sesquicineole | 0.3 |
| 1517 | 1521 | trans-Calamenene | 0.2 |
| 1519 | 1521 | β-Sesquiphellandrene | 0.1 |
| 1539 | 1542 | cis-Sesquisabinene hydrate | 0.2 |
| 1547 | --- | Unidentified d | 2.1 |
| 1550 | 1555 | cis-7-epi-Sesquisabinene hydrate | 0.3 |
| 1556 | 1561 | (E)-Nerolidol | 0.5 |
| 1579 | 1582 | Caryophyllene oxide | 38.9 |
| 1603 | --- | Unidentified e | 1.8 |
| 1606 | 1608 | Humulene epoxide II | 1.5 |
| 1616 | --- | Unidentified f | 1.1 |
| 1624 | 1627 | 1-epi-Cubenol | 0.2 |
| 1629 | 1642 | Caryophylla-4(12),8(13)-dien-5α-ol | 0.6 |
| 1633 | 1644 | Caryophylla-4(12),8(13)-dien-5β-ol | 1.1 |
| 1643 | 1644 | α-Muurolol (= δ-Cadinol) | 1.0 |
| 1652 | 1656 | 14-Hydroxy-9-epi-(Z)-caryophyllene | 0.5 |
| 1654 | 1651 | Pogostol | 0.9 |
| 1659 | 1668 | ar-Turmerone | 0.3 |
| 1667 | 1668 | 14-Hydroxy-9-epi-(E)-caryophyllene | 1.1 |
| 1676 | 1678 | 9-Tetradecyn-1-ol | 0.4 |
| 1681 | 1683 | epi-α-Bisabolol | 0.8 |
| 1683 | 1685 | α-Bisabolol | 1.8 |
| 1722 | --- | Unidentified g | 1.4 |
| 1809 | --- | Unidentified h | 1.3 |
| 1830 | 1836 | Neophytadiene | 0.2 |
| 1835 | 1841 | Phytone | 0.8 |
| 1939 | 1947 | iso-Phytol | 0.1 |
| 1652 | 1958 | Palmitic acid | 0.2 |
| 2101 | 2109 | (E)-Phytol | 3.5 |
| 2131 | --- | Unidentified i | 1.7 |
| Monoterpene hydrocarbons | 1.0 | ||
| Oxygenated monoterpenoids | 0.1 | ||
| Sesquiterpene hydrocarbons | 28.0 | ||
| Oxygenated sesquiterpenoids | 50.5 | ||
| Diterpenoids | 4.7 | ||
| Others | 1.5 | ||
| Total Identified | 85.9 |
a RI = Retention Index determined with respect to a homologous series of n-alkanes on a ZB-5 column. b Retention indices from the databases. c tr = trace (<0.05%). d MS: 205(5%), 187(5%), 176(7%), 163(9%), 149(12%), 138(23%), 120(22%), 109(28%), 107(37%), 106(91%), 93(50%), 91(68%), 79(100%), 69(33%), 67(32%), 55(30%), 43(61%), 41(65%). e MS: 205(11%), 187(10%), 159(34%), 148(16%), 131(19%), 121(32%), 119(39%), 105(41%), 93(68%), 91(43%), 81(34%), 79(52%), 69(34%), 67(34%), 59(35%), 43(100%), 41(44%). f MS: 202(6%), 187(4%), 159(26%), 134(67%), 132(25%), 121(30%), 119(63%), 105(50%), 93(63%), 91(53%), 79(100%), 67(45%), 59(39%), 43(31%). g MS: 218(3%), 203(3%), 175(13%), 148(36%), 135(25%), 121(18%), 109(45%), 69(100%), 41(77%). h MS: 220(5%), 105(8%), 202(10%), 187(12%), 179(33%), 161(35%), 127(74%), 123(90%), 109(100%), 95(66%), 93(47%), 81(93%), 69(68%), 55(94%), 43(85%), 41(85%). i MS: 281(0.5%), 263(1%), 179(1%), 163(3%), 149(8%), 140(8%), 121(9%), 111(20%), 109(10%), 97(28%), 95(17%), 84(100%), 71(25%), 69(25%), 57(28%), 55(28%), 43(40%), 41(27%).
2.1.4. Callicarpa longifolia
Leaf essential oils of C. longifolia were obtained from Son Tra Peninsula (Da Nang City) and from Nghia Dan district (Nghe An province). Sesquiterpene hydrocarbons and oxygenated sesquiterpenoids dominated both essential oils (Table 5). There were, however, notable differences in the chemical profiles. For example, β-selinene was relatively abundant in the Nghia Dan sample (13.2%), but much less in the sample from Da Nang (3.2%). Conversely, trans-β-guaiene was abundant in the Da Nang sample (22.2%), but much lower in the Nghia Dan sample (0.4%). To our knowledge, there are no previous reports on the essential oil of C. longifolia.
Table 5.
Chemical compositions of Callicarpa longifolia leaf essential oils from Vietnam.
| RI a | RI b | Compound | % | |
|---|---|---|---|---|
| Da Nang | Nghia Dan | |||
| 931 | 932 | α-Pinene | 0.4 | 0.1 |
| 1007 | 1008 | δ-3-Carene | --- | tr c |
| 1022 | 1024 | p-Cymene | --- | tr |
| 1028 | 1024 | Limonene | 0.5 | 0.1 |
| 1033 | 1032 | (Z)-β-Ocimene | --- | tr |
| 1043 | 1044 | (E)-β-Ocimene | --- | tr |
| 1097 | 1095 | Linalool | --- | 1.0 |
| 1101 | 1104 | Hotrienol | --- | tr |
| 1103 | 1100 | Nonanal | --- | tr |
| 1105 | 1110 | Octen-3-yl acetate | --- | tr |
| 1110 | 1113 | 4,8-Dimethylnona-1,3,7-triene | --- | tr |
| 1116 | 1118 | 3-Octyl acetate | --- | tr |
| 1190 | 1190 | Methyl salicylate | --- | 0.9 |
| 1192 | 1197 | (2E)-Hexenyl butyrate | --- | tr |
| 1193 | 1186 | α-Terpineol | --- | 0.1 |
| 1221 | 1227 | Nerol | --- | tr |
| 1234 | 1226 | (2E)-Hexenyl 2-methylbutyrate | --- | tr |
| 1247 | 1249 | Geraniol | --- | 0.1 |
| 1290 | 1287 | Dihydroedulan IIA | --- | 0.1 |
| 1295 | 1294 | (Z)-Theaspirane | --- | 0.2 |
| 1311 | 1298 | (E)-Theaspirane | --- | 0.2 |
| 1330 | 1334 | Bicycloelemene | 0.2 | 0.4 |
| 1333 | 1335 | δ-Elemene | 0.1 | 2.7 |
| 1349 | 1352 | Tricyclosantalal A | --- | 0.2 |
| 1374 | 1374 | α-Copaene | 0.4 | 0.6 |
| 1375 | 1383 | (E)-β-Damascenone | --- | 0.2 |
| 1387 | 1389 | β-Elemene | 0.5 | 0.4 |
| 1401 | 1408 | (Z)-Caryophyllene | --- | 0.1 |
| 1403 | 1409 | α-Gurjunene | --- | 0.2 |
| 1407 | 1415 | β-Maaliene | 0.7 | --- |
| 1409 | 1411 | cis-α-Bergamotene | --- | 0.1 |
| 1418 | 1417 | (E)-Caryophyllene | 11.8 | 28.0 |
| 1427 | 1434 | γ-Elemene | 0.6 | 1.3 |
| 1429 | 1432 | trans-α-Bergamotene | --- | 0.5 |
| 1431 | 1438 | α-Maaliene | --- | 0.1 |
| 1436 | 1439 | Aromadendrene | 0.2 | 0.5 |
| 1438 | 1442 | 6,9-Guaiadiene | --- | 0.5 |
| 1447 | 1445 | Myltayl-4(12)-ene | 0.7 | --- |
| 1449 | 1457 | Sesquisabinene | --- | 0.3 |
| 1452 | 1454 | (E)-β-Farnesene | 1.6 | --- |
| 1454 | 1452 | α-Humulene | 1.9 | 1.6 |
| 1458 | 1458 | allo-Aromadendrene | 1.4 | 0.8 |
| 1472 | 1478 | γ-Muurolene | --- | 0.2 |
| 1473 | 1475 | γ-Gurjunene | --- | 0.6 |
| 1479 | 1484 | Germacrene D | 0.3 | 0.2 |
| 1483 | 1488 | δ-Selinene | --- | 0.7 |
| 1484 | 1476 | β-Chamigrene | 4.0 | --- |
| 1488 | 1489 | β-Selinene | 3.2 | 13.2 |
| 1489 | 1491 | Eremophilene | 4.3 | --- |
| 1492 | 1500 | Bicyclogermacrene | --- | 5.9 |
| 1494 | 1496 | Valencene | 1.4 | --- |
| 1494 | 1500 | α-Muurolene | --- | 0.2 |
| 1500 | 1502 | trans-β-Guaiene | 22.2 | 0.4 |
| 1510 | 1505 | β-Bisabolene | 1.2 | 0.3 |
| 1504 | 1507 | Eremophila-1(10),8,11-triene | 0.3 | --- |
| 1511 | 1513 | γ-Cadinene | 0.2 | 0.2 |
| 1510 | 1508 | 6-epi-Shyobunone | 0.3 | --- |
| 1516 | 1522 | δ-Cadinene | 0.4 | 0.2 |
| 1520 | 1520 | 7-epi-α-Selinene | 3.5 | --- |
| 1535 | 1540 | Selina-4(15),7(11)-diene | 0.2 | 0.3 |
| 1538 | 1544 | α-Calacorene | --- | 0.2 |
| 1557 | 1559 | Germacrene B | 1.3 | 2.1 |
| 1557 | 1561 | (E)-Nerolidol | --- | 0.1 |
| 1570 | 1567 | Palustrol | 0.9 | --- |
| 1576 | 1577 | Spathulenol | 1.1 | 5.3 |
| 1581 | 1582 | Caryophyllene oxide | 1.7 | 6.1 |
| 1584 | 1590 | Globulol | 0.2 | 0.2 |
| 1593 | 1592 | Viridiflorol | 0.3 | 0.2 |
| 1598 | 1596 | trans-β-Elemenone | 0.6 | --- |
| 1605 | 1602 | Ledol | 2.4 | --- |
| 1606 | --- | Unidentified d | 2.5 | --- |
| 1610 | 1608 | Humulene epoxide II | 0.3 | --- |
| 1616 | --- | Unidentified e | 0.2 | 2.7 |
| 1623 | 1624 | Selina-6-en-4β-ol | --- | 0.4 |
| 1627 | 1629 | iso-Spathulenol | 0.2 | 4.2 |
| 1629 | 1642 | Caryophylla-4(12),8(13)-dien-5α-ol | --- | 0.6 |
| 1632 | 1637 | Dehydroxycalamendiol | 0.7 | --- |
| 1634 | 1644 | Caryophylla-4(12),8(13)-dien-5β-ol | --- | 0.2 |
| 1652 | 1649 | β-Eudesmol | --- | 0.9 |
| 1655 | 1652 | α-Cadinol | 0.7 | --- |
| 1662 | 1658 | Selin-11-en-4α-ol | 8.0 | 7.4 |
| 1668 | --- | Unidentified f | 1.4 | --- |
| 1670 | --- | Unidentified g | 1.2 | --- |
| 1679 | 1685 | Germacra-4(15),5,10(14)-trien-1α-ol | --- | 0.4 |
| 1685 | 1685 | α-Bisabolol | 0.8 | --- |
| 1686 | 1690 | (Z)-trans-α-Bergamotol | --- | 0.5 |
| 1693 | 1693 | Germacrone | 2.7 | --- |
| 1704 | 1706 | (E)-trans-α-Bergamotol | --- | 0.3 |
| 1711 | 1715 | Pentadecanal | --- | 0.3 |
| 1723 | 1729 | Isobicyclogermacrenal | 0.7 | --- |
| 1738 | 1734 | 1(10),11-Eremophiladien-9-one | 6.7 | --- |
| 1747 | 1744 | Isocalamenediol | 0.5 | --- |
| 1765 | 1766 | β-Costol | --- | 0.4 |
| 1768 | 1773 | α-Costol | --- | 0.4 |
| 1777 | 1786 | trans-Isovalencenol | 0.2 | --- |
| 1886 | 1891 | (E)-Hexadecantrienal | --- | 0.2 |
| 2045 | 2046 | Kaur-16-ene | 0.3 | --- |
| 2101 | 2109 | (E)-Phytol | --- | 0.5 |
| Monoterpene hydrocarbons | 0.9 | 0.1 | ||
| Oxygenated monoterpenoids | 0.0 | 1.2 | ||
| Sesquiterpene hydrocarbons | 63.0 | 62.9 | ||
| Oxygenated sesquiterpenoids | 29.4 | 27.8 | ||
| Diterpenoids | 0.3 | 0.5 | ||
| Others | 0.0 | 2.3 | ||
| Total identified | 93.5 | 94.8 | ||
a RI = Retention Index determined with respect to a homologous series of n-alkanes on a ZB-5 column. b Retention indices from the databases. c tr = trace (<0.05%). d MS: 220(10%), 205(20%), 178(21%), 177 (47%), 153(19%), 140(30%), 135(20%), 121(17%), 107(47%), 97(100%), 93(44%), 81(73%), 79(65%), 69(57%), 67(31%), 55(56%), 41(53%). e MS: 222(3%), 207(38%), 204(42%), 189(40%), 161(33%), 147(25%), 137(27%), 135(55%), 133(25%), 121(28%), 109(42%), 107(35%), 105(26%), 95(47%), 93(41%), 81(94%), 71(54%), 67(41%), 55(41%), 43(100%), 41(39%). f MS: 220(16%), 205(96%), 202(20%), 187(35%), 177(35%), 163(40%), 159(100%), 151(50%), 149(40%), 145(57%), 131(52%), 121(59%), 119(88%), 109(55%), 107(81%), 105(85%), 93(98%), 91(90%), 79(70%), 67(69%), 55(73%), 41(73%). g MS: 220(3%), 205(64%), 189(33%), 177(21%), 162(29%), 147(100%), 138(40%), 133(66%), 119(45%), 109(44%), 107(50%), 105(64%), 93(73%), 91(79%), 79(71%), 67(54%), 55(60%), 41(57%).
2.1.5. Callicarpa nudiflora
Unlike the essential oils of other Callicarpa species in this investigation, the leaf essential oil of C. nudiflora was dominated by the monoterpenes α-pinene (8.1%) and β-pinene (34.2%). Caryophyllene oxide (20.1%) was also an abundant component (Table 6). The chemical composition of Vietnamese C. nudiflora is markedly different from the leaf essential oil from China [46]. The Chinese sample showed only small quantities of α- and β-pinene (0.1% and 1.6%, respectively) and caryophyllene oxide was not observed. Conversely, humulene epoxide II was abundant in the sample from China (17.3%), but relatively minor in the sample from Vietnam (0.5%). Bisabolene oxide was abundant in the Chinese essential oil (10.5%), but was not detected in the sample from Vietnam.
Table 6.
Chemical composition of Callicarpa nudiflora leaf essential oil from Son Tra Peninsula, Da Nang City, Vietnam.
| RI a | RI b | Compound | % |
|---|---|---|---|
| 920 | 921 | Tricyclene | tr c |
| 923 | 924 | α-Thujene | 0.4 |
| 931 | 932 | α-Pinene | 8.1 |
| 945 | 945 | α-Fenchene | tr |
| 947 | 946 | Camphene | 0.5 |
| 951 | 953 | Thuja-2,4(10)-diene | tr |
| 970 | 969 | Sabinene | 0.6 |
| 977 | 974 | β-Pinene | 34.2 |
| 983 | 979 | Octan-3-one | tr |
| 989 | 988 | Myrcene | 0.2 |
| 988 | 988 | Dehydro-1,8-cineole | tr |
| 995 | 988 | 3-Octanol | tr |
| 1023 | 1024 | p-Cymene | 2.3 |
| 1027 | 1024 | Limonene | 1.0 |
| 1029 | 1025 | β-Phellandrene | 0.1 |
| 1030 | 1026 | 1,8-Cineole | 1.1 |
| 1033 | 1032 | (Z)-β-Ocimene | 0.1 |
| 1098 | 1099 | α-Pinene oxide | 0.4 |
| 1117 | 1114 | endo-Fenchol | 0.2 |
| 1123 | 1118 | cis-p-Menth-2-en-1-ol | 0.1 |
| 1125 | 1122 | α-Campholenal | 0.3 |
| 1137 | 1135 | Nopinone | 0.5 |
| 1139 | 1135 | trans-Pinocarveol | 2.0 |
| 1141 | 1136 | trans-p-Menth-2-en-1-ol | tr |
| 1144 | 1140 | trans-Verbenol | 0.1 |
| 1153 | 1145 | Camphene hydrate | 0.1 |
| 1156 | 1154 | Sabina ketone | 0.1 |
| 1159 | 1158 | trans-Pinocamphone | tr |
| 1160 | 1160 | Pinocarvone | 0.3 |
| 1170 | 1165 | Borneol | 0.2 |
| 1179 | 1174 | Terpinen-4-ol | 1.0 |
| 1185 | 1183 | Cryptone | tr |
| 1186 | 1179 | p-Cymen-8-ol | 0.2 |
| 1187 | 1182 | cis-Pinocarveol | tr |
| 1194 | 1195 | Myrtenal | 6.8 |
| 1217 | 1215 | trans-Carveol | 0.1 |
| 1273 | 1266 | trans-Ascaridol glycol | 0.1 |
| 1274 | 1269 | Perilla aldehyde | 0.1 |
| 1276 | 1277 | Phellandral | 0.1 |
| 1281 | 1287 | Bornyl acetate | 0.1 |
| 1296 | 1295 | Thujyl acetate | 0.2 |
| 1297 | 1294 | Perilla alcohol | 0.4 |
| 1304 | --- | Unidentified d | 1.0 |
| 1320 | 1324 | Myrtenyl acetate | 0.1 |
| 1373 | 1374 | α-Copaene | 0.3 |
| 1376 | 1383 | (E)-β-Damascenone | tr |
| 1386 | 1389 | β-Elemene | tr |
| 1417 | 1417 | (E)-Caryophyllene | 2.9 |
| 1436 | 1439 | Aromadendrene | 0.4 |
| 1452 | 1452 | α-Humulene | 0.2 |
| 1457 | 1458 | allo-Aromadendrene | 1.4 |
| 1484 | 1491 | Eremophilene | 0.2 |
| 1486 | 1489 | β-Selinene | 0.1 |
| 1488 | 1496 | Viridiflorene | 0.1 |
| 1510 | 1513 | γ-Cadinene | 0.1 |
| 1575 | 1577 | Spathulenol | 2.9 |
| 1581 | 1582 | Caryophyllene oxide | 20.1 |
| 1583 | 1590 | Globulol | 0.2 |
| 1607 | 1608 | Humulene epoxide II | 0.5 |
| 1631 | 1642 | Caryophylla-4(12),8(13)-dien-5α-ol | 0.4 |
| 1634 | 1644 | Caryophylla-4(12),8(13)-dien-5β-ol | 1.7 |
| 1653 | 1656 | 14-Hydroxy-9-epi-(Z)-caryophyllene | 0.8 |
| 1668 | 1668 | 14-Hydroxy-9-epi-(E)-caryophyllene | 0.5 |
| 1677 | 1678 | 9-Tetradecyn-1-ol | 0.1 |
| 1989 | 1987 | Manoyl oxide | 0.3 |
| 2103 | 2106 | (E)-Phytol | 0.4 |
| Monoterpene hydrocarbons | 47.5 | ||
| Oxygenated monoterpenoids | 14.6 | ||
| Sesquiterpene hydrocarbons | 5.7 | ||
| Oxygenated sesquiterpenoids | 27.1 | ||
| Diterpenoids | 0.7 | ||
| Others | 0.1 | ||
| Total identified | 95.8 |
a RI = Retention Index determined with respect to a homologous series of n-alkanes on a ZB-5 column. b Retention indices from the databases. c tr = trace (<0.05%). d MS: 135(10%), 119(12%), 107(18%), 93(36%), 92(51%), 91(45%), 79(45%), 69(100%), 55(30%), 53(31%), 43(27%), 41(78%).
2.1.6. Callicarpa petelotii
Leaves of C. petelotii were collected from Tay Giang district, Quang Nam province, Vietnam. The leaf essential oil was dominated by the sesquiterpene hydrocarbons α-humulene (53.8%) and α-selinene (12.8%), in addition to humulene epoxide II (8.1%) (Table 7). There are no previous reports on the essential oil of C. petelotii.
Table 7.
Chemical composition of Callicarpa petelotii leaf essential oil from Tay Giang District, Quang Nam province, Vietnam.
| RI a | RI b | Compound | % |
|---|---|---|---|
| 921 | 924 | α-Thujene | tr c |
| 927 | 932 | 2-Methyl-5-isopropenylfuran | tr |
| 929 | 932 | α-Pinene | 0.4 |
| 945 | 946 | Camphene | tr |
| 972 | 969 | Sabinene | 0.1 |
| 973 | 974 | β-Pinene | 0.4 |
| 975 | 974 | 1-Octen-3-ol | tr |
| 981 | 979 | Octan-3-one | tr |
| 984 | 988 | Myrcene | 0.1 |
| 985 | 984 | 2-Pentylfuran | tr |
| 993 | 988 | 3-Octanol | tr |
| 1003 | 1002 | α-Phellandrene | 0.5 |
| 1005 | 1008 | δ-3-Carene | tr |
| 1013 | 1014 | α-Terpinene | tr |
| 1020 | 1024 | p-Cymene | 0.5 |
| 1025 | 1024 | Limonene | 0.4 |
| 1026 | 1025 | β-Phellandrene | 1.5 |
| 1031 | 1032 | (Z)-β-Ocimene | 0.9 |
| 1041 | 1044 | (E)-β-Ocimene | 0.1 |
| 1053 | 1054 | γ-Terpinene | tr |
| 1081 | 1086 | Terpinolene | tr |
| 1095 | 1095 | Linalool | 0.4 |
| 1099 | 1104 | Hotrienol | 0.1 |
| 1101 | 1100 | Nonanal | tr |
| 1109 | 1113 | 4,8-Dimethylnona-1,3,7-triene | tr |
| 1124 | 1128 | allo-Ocimene | tr |
| 1141 | 1139 | (E)-Tagetone | tr |
| 1183 | 1183 | Cryptone | tr |
| 1188 | 1190 | Methyl salicylate | 0.4 |
| 1191 | 1186 | α-Terpineol | 0.1 |
| 1246 | 1249 | Geraniol | tr |
| 1274 | 1277 | Phellandral | tr |
| 1279 | 1287 | Bornyl acetate | tr |
| 1283 | 1287 | Dihydroedulan IA | tr |
| 1288 | 1294 | Dihydroedulan IIA | tr |
| 1293 | 1299 | (Z)-Theaspirane | tr |
| 1309 | 1303 | (E)-Theaspirane | tr |
| 1329 | 1335 | δ-Elemene | tr |
| 1341 | 1345 | α-Cubebene | tr |
| 1363 | 1369 | Cyclosativene | tr |
| 1370 | 1374 | α-Copaene | 0.1 |
| 1373 | 1383 | (E)-β-Damascenone | tr |
| 1378 | 1387 | β-Bourbonene | 0.1 |
| 1382 | 1390 | 7-epi-Sesquithujene | tr |
| 1383 | 1389 | β-Elemene | 0.4 |
| 1408 | 1407 | Longifolene | tr |
| 1413 | 1417 | (E)-Caryophyllene | 2.7 |
| 1424 | 1430 | β-Copaene | 0.1 |
| 1429 | 1437 | α-Guaiene | tr |
| 1443 | 1447 | iso-Germacrene D | tr |
| 1447 | 1454 | (E)-β-Farnesene | tr |
| 1452 | 1452 | α-Humulene | 53.8 |
| 1456 | 1456 | Nootkatene | tr |
| 1467 | 1476 | Selina-4,11-diene | 0.1 |
| 1469 | 1478 | γ-Muurolene | tr |
| 1475 | 1484 | Germacrene D | 0.5 |
| 1483 | 1489 | β-Selinene | 4.0 |
| 1491 | 1498 | α-Selinene | 12.8 |
| 1511 | 1518 | δ-Cadinene | 0.1 |
| 1571 | 1577 | Spathulenol | 0.1 |
| 1575 | 1582 | Caryophyllene oxide | 2.0 |
| 1587 | 1590 | cis-β-Elemenone | 0.3 |
| 1592 | 1592 | Humulene epoxide I | 1.1 |
| 1604 | 1608 | Humulene epoxide II | 8.1 |
| 1626 | 1642 | Caryophylla-4(12),8(13)-dien-5α-ol | 1.5 |
| 1631 | 1644 | Caryophylla-4(12),8(13)-dien-5β-ol | 0.5 |
| 1648 | 1649 | β-Eudesmol | 0.3 |
| 1652 | 1658 | Selin-11-en-4α-ol | 1.3 |
| 1664 | 1656 | 14-Hydroxy-9-epi-(Z)-caryophyllene | 0.1 |
| 1675 | 1685 | Germacra-4(15),5,10(14)-trien-1α-ol | 0.7 |
| 1679 | 1668 | epi-Zizanone | 0.3 |
| 1708 | 1715 | Pentadecanal | 0.1 |
| 2012 | 2026 | (E,E)-Geranyl linalool | 0.3 |
| Monoterpene hydrocarbons | 4.8 | ||
| Oxygenated monoterpenoids | 0.5 | ||
| Sesquiterpene hydrocarbons | 74.7 | ||
| Oxygenated sesquiterpenoids | 16.3 | ||
| Diterpenoids | 0.3 | ||
| Others | 0.5 | ||
| Total identified | 97.0 |
a RI = Retention Index determined with respect to a homologous series of n-alkanes on a ZB-5 column. b Retention indices from the databases. c tr = trace (<0.05%).
2.1.7. Callicarpa rubella
The leaf essential oils of C. rubella were obtained from three different sites in central Vietnam, Nậm Giải Commune (Quế Phong district, Pu Hoat Nature Reserve, Nghe An province), Bach Ma National Park (Phu Loc district, Thua Thien Hue province), and Tay Giang district (Quang Nam province). The essential oil compositions showed very different profiles (Table 8). The leaf essential oil from Nam Giai was dominated by caryophyllene oxide (25.1%), cis-thujopsenol (8.8%), and corymbolone (5.6%); β-bisabolene (25.0%), germacrone (22.1%), and (E)-caryophyllene (7.1%) were the major components of the leaf essential oil from Bach Ma; and the essential oil from Tay Giang was rich in (E)-caryophyllene (18.0%) and α-cubebene (17.4%). The volatiles, obtained by head-space solid-phase microextraction (HS-SPME) techniques, of C. rubella from China showed α-cubebene (8.7%), palmitic acid (5.4%), epizonarene (4.8%), heptadecane (4.8%), and spathulenol (4.5%) as the major components [47]. Thus, there is wide variation in the chemical compositions of C. rubella leaf essential oils. In addition to geographical and climatic effects, genetic differences may be responsible for the wide variation in essential oil composition; the Missouri Botanical Garden lists 11 subordinate taxa for C. rubella [3]. The stem bark essential oil from Bach Ma National Park was similar in composition to the leaf essential oil from that collection site. The major components in the bark essential oil were germacrone (23.9%), β-bisabolene (17.9%), germacrene B (8.4%), and (E)-caryophyllene (7.3%) (Table 9).
Table 8.
Chemical compositions of Callicarpa rubella leaf essential oils from Vietnam.
| RI a | RI b | Compound | % | ||
|---|---|---|---|---|---|
| Nam Giai | Bach Ma | Tay Giang | |||
| 923 | 924 | α-Thujene | --- | 0.1 | tr c |
| 930 | 932 | α-Pinene | 0.1 | 0.5 | 1.1 |
| 946 | 946 | Camphene | --- | --- | tr |
| 971 | 969 | Sabinene | --- | 0.1 | 0.1 |
| 975 | 974 | β-Pinene | 0.3 | 2.4 | 1.7 |
| 977 | 974 | 1-Octen-3-ol | 0.4 | 0.1 | tr |
| 982 | 979 | 3-Octanone | 0.2 | --- | tr |
| 986 | 988 | Myrcene | --- | 0.1 | 0.2 |
| 987 | 984 | 2-Pentylfuran | --- | --- | tr |
| 995 | 988 | 3-Octanol | 0.4 | 0.1 | tr |
| 1005 | 1002 | α-Phellandrene | --- | 0.3 | 3.0 |
| 1007 | 1008 | δ-3-Carene | --- | 0.3 | tr |
| 1015 | 1014 | α-Terpinene | --- | --- | tr |
| 1022 | 1024 | p-Cymene | tr | 0.7 | 1.0 |
| 1027 | 1024 | Limonene | 0.1 | 0.4 | 0.8 |
| 1028 | 1025 | β-Phellandrene | --- | 0.9 | 2.6 |
| 1030 | 1026 | 1,8-cineole | --- | 0.1 | --- |
| 1033 | 1032 | (Z)-β-Ocimene | --- | --- | 0.1 |
| 1043 | 1044 | (E)-β-Ocimene | --- | --- | tr |
| 1055 | 1054 | γ-Terpinene | --- | --- | tr |
| 1067 | 1067 | cis-Linalool oxide (furanoid) | 0.2 | --- | --- |
| 1083 | 1086 | Terpinolene | --- | 0.1 | 0.1 |
| 1084 | 1084 | trans-Linalool oxide (furanoid) | 0.2 | --- | --- |
| 1098 | 1095 | Linalool | 1.4 | 0.1 | tr |
| 1104 | 1100 | Nonanal | --- | 0.1 | tr |
| 1123 | 1118 | cis-p-Menth-2-en-1-ol | --- | tr | --- |
| 1137 | 1134 | Benzeneacetonitrile | 0.1 | --- | --- |
| 1139 | 1135 | trans-Pinocarveol | 0.1 | tr | --- |
| 1141 | 1136 | trans-p-Menth-2-en-1-ol | --- | tr | --- |
| 1161 | 1160 | Pinocarvone | --- | tr | --- |
| 1170 | 1165 | Borneol | --- | tr | --- |
| 1179 | 1174 | Terpinen-4-ol | --- | 0.1 | tr |
| 1185 | 1183 | Cryptone | --- | 0.2 | --- |
| 1185 | 1184 | (3Z)-Hexenyl butyrate | 0.2 | --- | --- |
| 1189 | 1191 | Hexyl butyrate | tr | --- | --- |
| 1191 | 1190 | Methyl salicylate | 0.2 | --- | tr |
| 1192 | 1193 | (2E)-Hexenyl butyrate | 0.1 | --- | --- |
| 1193 | 1195 | Myrtenal | 0.1 | --- | --- |
| 1194 | 1186 | α-Terpineol | --- | 0.3 | 0.1 |
| 1201 | 1202 | cis-Sabinol | --- | 0.1 | --- |
| 1221 | 1222 | 2-Hydroxycineole | --- | 0.1 | --- |
| 1285 | 1287 | Dihydroedulan IA | 0.1 | --- | tr |
| 1290 | 1294 | Dihydroedulan IIA | 0.1 | --- | tr |
| 1295 | 1299 | (Z)-Theaspirane | --- | --- | 0.1 |
| 1312 | 1303 | (E)-Theaspirane | --- | --- | tr |
| 1318 | 1318 | 3-Hydroxycineole | --- | 0.3 | --- |
| 1328 | 1334 | Bicycloelemene | --- | 0.2 | 0.3 |
| 1332 | 1335 | δ-Elemene | --- | 0.2 | 0.2 |
| 1344 | 1345 | α-Cubebene | 0.1 | 0.4 | 17.4 |
| 1350 | 1356 | Eugenol | --- | --- | 0.1 |
| 1366 | 1373 | α-Ylangene | 0.1 | --- | tr |
| 1372 | 1374 | α-Copaene | 0.4 | 0.1 | 4.6 |
| 1377 | 1383 | (E)-β-Damascenone | --- | --- | tr |
| 1380 | 1382 | β-Bourbonene | 3.2 | 0.1 | 4.1 |
| 1383 | 1385 | α-Bourbonene | 0.3 | --- | --- |
| 1384 | 1387 | β-Cubebene | --- | --- | 4.3 |
| 1386 | 1389 | β-Elemene | 0.5 | 1.3 | 0.5 |
| 1400 | 1408 | (Z)-Caryophyllene | --- | --- | 0.1 |
| 1403 | 1409 | α-Gurjunene | --- | --- | 0.1 |
| 1414 | 1419 | β-Ylangene | 0.3 | --- | --- |
| 1417 | 1417 | (E)-Caryophyllene | 0.3 | 7.1 | 18.0 |
| 1426 | 1430 | β-Copaene | 0.3 | --- | 0.7 |
| 1426 | 1427 | γ-Elemene | --- | 2.5 | --- |
| 1430 | 1432 | trans-α-Bergamotene | --- | 0.1 | --- |
| 1431 | 1437 | α-Guaiene | 2.8 | --- | 3.0 |
| 1435 | 1439 | Aromadendrene | 0.1 | --- | 0.3 |
| 1441 | 1447 | iso-Germacrene D | 0.2 | --- | 0.3 |
| 1445 | 1448 | cis-Murrola-3,5-diene | --- | --- | 0.6 |
| 1449 | 1454 | (E)-β-Farnesene | --- | 0.5 | 0.1 |
| 1452 | 1452 | α-Humulene | 0.1 | 0.9 | 2.0 |
| 1456 | 1458 | allo-Aromadendrene | --- | --- | 0.2 |
| 1459 | 1465 | cis-Muurola-4(14),5-diene | --- | --- | 0.1 |
| 1465 | 1461 | cis-Cadina-1(6),4-diene | --- | --- | 0.2 |
| 1466 | 1473 | Drima-7,9(11)-diene | 0.1 | --- | --- |
| 1468 | 1475 | trans-Cadina-1(6),4-diene | --- | --- | 0.8 |
| 1471 | 1478 | γ-Muurolene | 0.5 | --- | 0.5 |
| 1476 | 1475 | γ-Gurjunene | 0.2 | --- | 0.4 |
| 1478 | 1479 | ar-Curcumene | --- | 0.7 | --- |
| 1478 | 1484 | Germacrene D | --- | --- | 4.2 |
| 1486 | 1489 | β-Selinene | 1.6 | 0.9 | 0.8 |
| 1488 | 1495 | γ-Amorphene | --- | --- | 1.2 |
| 1492 | 1500 | Bicyclogermacrene | --- | --- | 4.6 |
| 1493 | 1498 | α-Selinene | --- | 0.7 | --- |
| 1495 | 1500 | α-Muurolene | --- | 0.1 | 0.9 |
| 1495 | --- | Unidentified d | 2.7 | --- | --- |
| 1498 | 1509 | α-Bulnesene | 1.7 | --- | 1.8 |
| 1500 | 1505 | (E,E)-α-Farnesene | --- | --- | 0.1 |
| 1505 | 1505 | β-Bisabolene | 0.7 | 25.0 | 0.1 |
| 1510 | 1513 | γ-Cadinene | --- | 0.1 | 0.3 |
| 1512 | 1514 | Cubebol | --- | 0.5 | 1.0 |
| 1515 | 1518 | δ-Cadinene | --- | 0.2 | 4.6 |
| 1519 | 1521 | trans-Calamenene | --- | 0.2 | 0.4 |
| 1520 | 1528 | Zonarene | --- | --- | 0.2 |
| 1521 | 1521 | β-Sesquiphellandrene | --- | 0.5 | --- |
| 1529 | 1533 | trans-Cadina-1,4-diene | --- | --- | 0.4 |
| 1534 | 1540 | Selina-4(15),7(11)-diene | --- | 0.5 | --- |
| 1539 | 1545 | Selina-3,7(11)-diene | --- | 0.3 | --- |
| 1545 | 1548 | α-Elemol | --- | 0.2 | --- |
| 1556 | 1559 | Germacrene B | --- | 4.6 | 0.1 |
| 1564 | --- | Unidentified e | 3.2 | --- | --- |
| 1568 | --- | Unidentified f | 7.2 | --- | --- |
| 1574 | 1577 | Spathulenol | 3.9 | 0.2 | 2.7 |
| 1579 | 1582 | Caryophyllene oxide | 25.1 | 3.0 | 2.7 |
| 1581 | --- | Unidentified g | 1.7 | --- | 0.3 |
| 1590 | 1590 | cis-β-Elemenone | --- | 0.4 | --- |
| 1595 | 1596 | trans-β-Elemenone | --- | 4.2 | --- |
| 1607 | 1608 | Humulene epoxide II | 3.8 | 0.4 | 0.2 |
| 1625 | 1629 | iso-Spathulenol | --- | 0.6 | --- |
| 1625 | 1627 | 1-epi-Cubenol | --- | --- | 0.8 |
| 1634 | 1644 | Caryophylla-4(12),8(13)-dien-5β-ol | --- | --- | 0.2 |
| 1640 | 1645 | Cubenol | --- | --- | 0.5 |
| 1641 | 1640 | τ-Muurolol | --- | --- | 0.1 |
| 1644 | 1644 | α-Muurolol (= δ-Cadinol) | --- | --- | 0.3 |
| 1653 | 1652 | α-Cadinol | --- | 0.4 | 0.2 |
| 1655 | 1651 | Pogostol | 1.6 | --- | 0.2 |
| 1661 | --- | Unidentified h | --- | 2.2 | --- |
| 1668 | 1668 | 14-Hydroxy-9-epi-(E)-caryophyllene | 1.2 | --- | 0.1 |
| 1677 | --- | Unidentified i | 1.1 | --- | --- |
| 1684 | 1685 | α-Bisabolol | --- | 0.2 | --- |
| 1691 | 1693 | Germacrone | --- | 22.1 | --- |
| 1698 | 1704 | cis-Thujopsenol | 8.8 | --- | --- |
| 1709 | --- | Unidentified j | --- | 1.8 | --- |
| 1715 | --- | Unidentified k | 2.1 | --- | --- |
| 1766 | --- | Unidentified l | 1.8 | --- | --- |
| 1768 | --- | Unidentified m | --- | 1.0 | --- |
| 1792 | --- | Unidentified n | 4.0 | --- | --- |
| 1802 | --- | Unidentified o | 2.5 | --- | --- |
| 1809 | 1806 | Nootkatone | 1.6 | --- | --- |
| 1815 | --- | Unidentified p | --- | 1.8 | --- |
| 1834 | --- | Unidentified q | --- | 3.0 | --- |
| 1849 | --- | Unidentified r | 1.6 | --- | --- |
| 1885 | 1884 | Corymbolone | 5.6 | --- | --- |
| 2049 | 2055 | Abietatriene | --- | 1.3 | 0.1 |
| Monoterpene hydrocarbons | 0.5 | 5.9 | 10.5 | ||
| Oxygenated monoterpenoids | 2.0 | 1.2 | 0.1 | ||
| Sesquiterpene hydrocarbons | 13.5 | 47.1 | 78.5 | ||
| Oxygenated sesquiterpenoids | 45.9 | 32.3 | 9.0 | ||
| Diterpenoids | 5.6 | 1.3 | 0.1 | ||
| Others | 1.8 | 0.2 | 0.1 | ||
| Total identified | 69.2 | 88.0 | 98.3 | ||
a RI = Retention Index determined with respect to a homologous series of n-alkanes on a ZB-5 column. b Retention indices from the databases. c tr = trace (<0.05%). d MS: 202(24%), 189(12%), 187(14%), 159(28%), 147(66%), 145(53%), 134(30%), 133(31%), 131(35%), 121(37%), 119(59%), 107(68%), 105(99%), 93(92%), 91(79%), 81(100%), 80(69%), 79(74%), 77(42%), 67(35%), 55(40%), 41(55%). e MS: 220(3%), 205(13%), 187(18%), 162(34%), 147(27%), 145(30%), 135(35%), 121(67%), 107(85%), 95(74%), 93(77%), 81(81%), 79(67%), 69(68%), 67(82%), 55(87%)41(100%). f MS: 220(0.5%), 205(4%), 187(12%), 177(6%), 162(9%), 149(9%), 147(20%), 145(13%), 123(26%), 122(24%), 111(44%), 107(78%), 95(42%), 93(42%), 83(40%), 81(58%), 79(38%), 67(47%), 55(43%), 43(100%), 41(49%). g MS: 220(3%), 205(4%), 202(12%), 187(24%), 159(25%), 146(29%), 145(23%), 133(22%), 131(20%), 123(18%), 121(19%0, 119(18%), 109(18%), 107(27%), 105(28%), 95(29%), 93(35%), 91(30%), 81(35%), 79(36%), 69(27%), 55(32%), 43(100%), 41(33%). h MS: 218(4%), 203(5%), 175(14%), 136(68%), 135(68%), 121(25%), 107(100%), 91(29%), 79(22%), 67(50%), 53(18%), 41(28%). i MS: 218(7%), 203(8%), 175(12%), 161(17%), 160(18%), 147(22%), 145(15%), 135(28%), 134(29%), 121(32%), 119(33%), 109(42%), 107(35%), 105(33%), 95(59%), 93(41%), 81(34%), 79(30%), 69(30%), 67(37%),55(29%), 53(19%), 43(100%), 41(43%). j MS: 220(35%), 205(17%), 202(27%), 187(27%), 162(88%), 159(32%), 149(55%), 147(57%), 145(30%), 131(30%), 121(71%), 119(79%), 107(66%), 105(68%), 97(43%), 93(57%), 91(67%), 43(100%), 41(63%). k MS: 218(2%), 200(3%), 185(5%), 160(13%), 145(9%), 121(25%), 120(27%), 98(22%), 97(23%), 83(100%), 67(18%), 55(34%), 43(96%), 41(25%). l MS: 220(1%), 205(4%), 179(10%), 161(4%), 147(10%), 137(10%), 133(14%), 121(17%), 119(20%), 108(32%), 95(35%), 93(53%), 91(42%), 81(34%), 79(61%), 69(32%), 67(38%), 55(45%), 43(100%), 41(58%). m MS: 220(62%), 202(11%), 187(15%), 177(100%), 159(74%), 135(49%), 123(75%), 107(76%), 93(64%), 91(52%), 81(61%), 67(54%), 55(57%), 43(62%), 41(68%). n MS: 234(8%), 219(30%), 216(8%), 201(8%), 191(12%), 177(15%), 176(13%), 163(14%), 159(16%), 152(21%), 137(16%), 133(16%), 111(27%), 105(20%), 91(25%), 79(19%), 77(17%), 67(17%), 55(17%), 43(100%), 41(26%). o MS: 234(1%), 216(16%), 188(8%), 177(9%), 173(8%), 163(10%), 161(14%), 159(13%), 133(22%), 111(26%), 105(24%), 95(47%), 91(23%), 81(23%), 79(24%), 77(20%), 67(22%), 55(18%), 43(100%), 41(28%). p MS: 234(2%), 219(4%), 201(5%), 191(5%), 177(7%), 167(12%), 149(34%), 135(51%), 121(50%), 107(100%), 93(35%), 91(37%), 79(34%), 68(36%), 67(58%), 55(34%), 43(53%), 41(60%). q MS: 167(40%), 121(32%), 68(100%), 67(58%), 43(33%), 41(39%). r MS: 236(5%), 221(8%), 218(8%), 193(15%), 180(26%), 167(30%), 149(22%), 147(18%), 136(65%), 123(74%), 110(97%), 97(84%), 69(83%), 55(80%), 43(80%), 41(100%).
Table 9.
Chemical composition of Callicarpa rubella stem bark essential oil from Bach Ma National Park, Vietnam.
| RI a | RI b | Compound | % |
|---|---|---|---|
| 933 | 932 | α-Pinene | 0.4 |
| 949 | 946 | Camphene | 0.1 |
| 972 | 969 | Sabinene | 0.1 |
| 978 | 974 | β-Pinene | 2.7 |
| 989 | 988 | Myrcene | 0.1 |
| 1007 | 1002 | α-Phellandrene | 0.3 |
| 1009 | 1008 | δ-3-Carene | 1.5 |
| 1025 | 1024 | p-Cymene | 0.6 |
| 1029 | 1024 | Limonene | 0.4 |
| 1031 | 1025 | β-Phellandrene | 0.7 |
| 1085 | 1086 | Terpinolene | 0.1 |
| 1100 | 1095 | Linalool | 0.1 |
| 1196 | 1186 | α-Terpineol | 0.1 |
| 1333 | 1334 | Bicycloelemene | 0.1 |
| 1347 | 1345 | α-Cubebene | 0.7 |
| 1376 | 1374 | α-Copaene | 0.2 |
| 1382 | 1389 | β-Elemene | 0.1 |
| 1388 | 1387 | β-Cubebene | 0.3 |
| 1389 | 1389 | β-Elemene | 2.0 |
| 1420 | 1417 | (E)-Caryophyllene | 7.3 |
| 1429 | 1427 | γ-Elemene | 4.8 |
| 1452 | 1457 | Sesquisabinene | 0.3 |
| 1456 | 1452 | α-Humulene | 1.0 |
| 1460 | 1458 | allo-Aromadendrene | 0.1 |
| 1472 | 1475 | trans-Cadina-1(6),4-diene | 0.2 |
| 1481 | 1479 | ar-Curcumene | 2.2 |
| 1489 | 1489 | β-Selinene | 0.6 |
| 1492 | 1493 | trans-Muurola-4(14),5-diene | 0.2 |
| 1496 | 1498 | α-Selinene | 1.0 |
| 1498 | 1500 | α-Muurolene | 0.3 |
| 1505 | 1501 | Aciphyllene | 0.3 |
| 1508 | 1505 | β-Bisabolene | 17.9 |
| 1513 | 1513 | γ-Cadinene | 0.3 |
| 1515 | 1514 | Cubebol | 0.3 |
| 1518 | 1518 | δ-Cadinene | 0.5 |
| 1521 | 1521 | trans-Calamenene | 0.2 |
| 1524 | 1521 | β-Sesquiphellandrene | 0.6 |
| 1537 | 1528 | Zonarene | 0.6 |
| 1542 | 1545 | Selina-3,7(11)-diene | 0.5 |
| 1548 | 1548 | Elemol | 0.3 |
| 1559 | 1559 | Germacrene B | 8.4 |
| 1577 | 1577 | Spathulenol | 0.2 |
| 1582 | 1582 | Caryophyllene oxide | 1.9 |
| 1593 | 1590 | cis-β-Elemenone | 0.4 |
| 1594 | 1592 | Viridiflorol | 0.4 |
| 1598 | 1596 | trans-β-Elemenone | 3.8 |
| 1607 | 1608 | β-Atlantol | 0.2 |
| 1610 | 1608 | Humulene epoxide II | 0.1 |
| 1628 | 1629 | iso-Spathulenol | 0.5 |
| 1632 | 1630 | γ-Eudesmol | 0.1 |
| 1643 | 1645 | Cubenol | 0.3 |
| 1647 | 1644 | α-Muurolol (=δ-Cadinol) | 0.2 |
| 1655 | 1652 | α-Cadinol | 0.7 |
| 1658 | 1658 | Selin-11-en-4α-ol | 0.1 |
| 1664 | --- | Unidentified c | 1.6 |
| 1687 | 1685 | α-Bisabolol | 0.4 |
| 1694 | 1693 | Germacrone | 23.9 |
| 2015 | 2009 | 13-epi-Manool oxide | 0.2 |
| 2053 | 2055 | Abietatriene | 1.1 |
| Monoterpene hydrocarbons | 7.1 | ||
| Oxygenated monoterpenoids | 0.9 | ||
| Sesquiterpene hydrocarbons | 50.7 | ||
| Oxygenated sesquiterpenoids | 36.1 | ||
| Diterpenoids | 1.3 | ||
| Others | 0.0 | ||
| Total identified | 93.0 |
a RI = Retention Index determined with respect to a homologous series of n-alkanes on a ZB-5 column. b Retention indices from the databases. c MS: 218(5%), 203(7%), 185(5%), 175(18%), 161(4%), 147(10%), 136(77%), 135(78%), 121(33%), 107(100%), 91(29%), 79(21%), 67(44%), 55(11%), 53(17%), 41(24%).
2.1.8. Callicarpa sinuata
The leaf essential oil of C. sinuata from Son Tra Peninsula (Da Nang City) showed α-humulene (24.8%), α-copaene (12.6%), humulene epoxide II (6.7%), and spathulenol (5.9%) as the major components (Table 10). There have been no previous reports on the essential oil composition of C. sinuata.
Table 10.
Chemical composition of Callicarpa sinuata leaf essential oil from Son Tra Peninsula, Da Nang City, Vietnam.
| RI a | RI b | Compound | % |
|---|---|---|---|
| 930 | 932 | α-Pinene | 0.1 |
| 969 | 969 | Sabinene | 0.1 |
| 975 | 974 | β-Pinene | tr c |
| 976 | 974 | 1-Octen-3-ol | tr |
| 1006 | 1008 | δ-3-Carene | tr |
| 1022 | 1024 | p-Cymene | 0.1 |
| 1026 | 1024 | Limonene | 0.1 |
| 1030 | 1026 | 1,8-Cineole | tr |
| 1103 | 1100 | Nonanal | 0.1 |
| 1295 | 1299 | (Z)-Theaspirane | 0.1 |
| 1311 | 1303 | (E)-Theaspirane | 0.1 |
| 1328 | 1334 | Bicycloelemene | 0.1 |
| 1331 | 1335 | δ-Elemene | 0.3 |
| 1343 | 1345 | α-Cubebene | 2.0 |
| 1365 | 1373 | α-Ylangene | 0.1 |
| 1372 | 1374 | α-Copaene | 12.6 |
| 1378 | 1383 | cis-β-Elemene | 0.2 |
| 1380 | 1382 | β-Bourbonene | 0.3 |
| 1384 | 1387 | β-Cubebene | 1.8 |
| 1385 | 1389 | trans-β-Elemene | 3.1 |
| 1416 | 1417 | (E)-Caryophyllene | 3.8 |
| 1426 | 1430 | β-Copaene | 0.4 |
| 1431 | 1437 | α-Guaiene | 0.2 |
| 1435 | 1439 | Aromadendrene | 0.5 |
| 1448 | 1455 | Valerena-4,7(11)-diene | 0.4 |
| 1453 | 1452 | α-Humulene | 24.8 |
| 1456 | 1458 | allo-Aromadendrene | 0.4 |
| 1466 | 1473 | 4,5-di-epi-Aristolochene | 0.4 |
| 1469 | 1476 | Selina-4,11-diene | 0.2 |
| 1471 | 1478 | γ-Muurolene | 1.3 |
| 1475 | 1483 | α-Amorphene | 0.2 |
| 1477 | 1484 | Germacrene D | 2.6 |
| 1484 | 1491 | Eremophilene | 0.3 |
| 1485 | 1489 | β-Selinene | 1.8 |
| 1488 | 1493 | trans-Muurola-4(14),5-diene | 0.3 |
| 1492 | 1500 | Bicyclogermacrene | 4.0 |
| 1494 | 1500 | α-Muurolene | 0.5 |
| 1509 | 1513 | γ-Cadinene | 0.6 |
| 1511 | 1514 | Cubebol | 0.4 |
| 1514 | 1518 | δ-Cadinene | 2.3 |
| 1517 | 1521 | trans-Calamenene | 0.4 |
| 1538 | 1544 | α-Calcorene | 0.9 |
| 1556 | 1561 | (E)-Nerolidol | 0.3 |
| 1558 | 1564 | β-Calacorene | 0.4 |
| 1573 | 1577 | Spathulenol | 5.9 |
| 1578 | 1582 | Caryophyllene oxide | 1.9 |
| 1582 | 1590 | Globulol | 0.4 |
| 1590 | 1592 | Viridiflorol | 0.5 |
| 1592 | 1600 | Guaiol | 0.2 |
| 1595 | 1592 | Humulene epoxide I | 0.6 |
| 1606 | 1608 | Humulene epoxide II | 6.7 |
| 1624 | 1627 | 1-epi-Cubenol | 0.7 |
| 1628 | 1629 | iso-Spathulenol | 2.4 |
| 1639 | 1638 | τ-Cadinol | 0.3 |
| 1640 | 1640 | τ-Muurolol | 0.3 |
| 1651 | 1652 | α-Eudesmol | 2.3 |
| 1655 | 1658 | Selin-11-en-4α-ol | 0.7 |
| 1659 | 1668 | ar-Turmerone | 0.2 |
| 1661 | 1662 | 9-Methoxycalamenene | 0.5 |
| 1669 | 1675 | Cadalene | 0.2 |
| 1734 | 1740 | Mint sulfide | 1.2 |
| 1836 | 1841 | Phytone | 0.2 |
| 2048 | 2055 | Abietatriene | 0.1 |
| 2101 | 2106 | (E)-Phytol | 3.4 |
| 2132 | 2138 | Palmitaldehyde, diallyl acetal | 0.6 |
| Monoterpene hydrocarbons | 0.3 | ||
| Oxygenated monoterpenoids | tr | ||
| Sesquiterpene hydrocarbons | 67.5 | ||
| Oxygenated sesquiterpenoids | 24.1 | ||
| Diterpenoids | 3.8 | ||
| Others | 2.1 | ||
| Total identified | 97.8 |
a RI = Retention Index determined with respect to a homologous series of n-alkanes on a ZB-5 column. b Retention indices from the databases. c tr = trace (<0.05%).
With the exception of C. nudiflora, the Callicarpa leaf essential oils are dominated by sesquiterpene hydrocarbons and oxygenated sesquiterpenoids. Overall, the most abundant sesquiterpenes were (E)-caryophyllene and caryophyllene oxide and those compounds were found in all of the Callicarpa leaf essential oil samples. α-Humulene and β-selinene were also found in all of the leaf oil samples, while α-copaene, spathulenol, and humulene epoxide II were detected in 12 of the 13 leaf essential oils sampled. The furanoid sesquiterpenoid atractylone was only found in C. candicans.
2.2. Mosquito Larvicidal Activity
The 24-h and 48-h mosquito larvicidal activities of the Callicarpa leaf essential oil are summarized in Table 11 and Table 12. As far as we are aware, there have been no previous larvicidal investigations on these Callicarpa essential oils. Due to limited supply of some of the essential oils and limited supplies of mosquito larvae, not all essential oils could be screened against both mosquito species. Dias and Moraes have concluded that plant essential oils are considered larvicidal against Ae. aegypti if the LC50 values are less than 100 μg/mL [48]. Based on these guidelines, all of the Callicarpa essential oils showed good larvicidal activity. However, the leaf essential oils of C. candicans, from Nghia Dan district, Nghe An province and from Dai Loc district, Quang Nam province were particularly active with 48-h LC50 values of 3.8 and 2.1 μg/mL, respectively, against Ae. aegypti. The leaf essential oils of C. candicans were also effective larvicidal agents against Cx. quinquefasciatus.
Table 11.
Twenty-four-hour mosquito larvicidal activity (µg/mL) of Callicarpa leaf essential oils from central Vietnam.
| Callicarpa Species | LC50 (95% Confidence Limits) | LC90 (95% Confidence Limits) | χ2 | p |
|---|---|---|---|---|
| Aedes aegypti | ||||
| C. bodinieri | 53.99 (50.29–58.32) | 76.61 (69.03–90.35) | 4.90 | 0.086 |
| C. candicans (Nghia Dan) | 5.337 (4.769–5.961) | 12.05 (10.38–14.57) | 10.10 | 0.018 |
| C. candicans (Dai Loc) | 2.695 (2.342–3.051) | 6.633 (5.685–8.107) | 70.57 | 0.000 |
| C. formosana | 31.85 (29.39–34.55) | 48.94 (44.06–56.50) | 3.74 | 0.154 |
| C. longifolia (Nghia Dan) | 37.44 (34.16–41.05) | 66.33 (58.53–78.54) | 3.86 | 0.145 |
| C. nudiflora | 37.51 (33.76–41.79) | 79.16 (67.91–97.04) | 9.47 | 0.009 |
| C. petelotii | 19.14 (17.13–21.22) | 37.87 (32.85–46.26) | 8.37 | 0.015 |
| C. rubella (Nam Giai) | 24.15 (21.33–27.13) | 57.15 (48.61–71.14) | 3.22 | 0.200 |
| C. rubella (Tay Giang) | 26.00 (24.19–28.06) | 39.42 (34.57–47.24) | 2.43 | 0.297 |
| C. sinuata | 28.69 (25.87–31.82) | 58.15 (50.38–70.31) | 7.43 | 0.024 |
| Culex quinquefasciatus | ||||
| C. candicans (Nghia Dan) | 2.041 (1.683–2.426) | 10.43 (8.14–14.46) | 5.36 | 0.252 |
| C. candicans (Dai Loc) | 1.204 (0.903–1.510 | 7.841 (6.035–11.146) | 2.01 | 0.734 |
| C. nudiflora | 108.9 (101.2–117.1) | 75.76 (66.11–85.11) | 2.34 | 0.126 |
Table 12.
Forty-eight-hour mosquito larvicidal activity (µg/mL) of Callicarpa leaf essential oils from central Vietnam.
| Callicarpa Species | LC50 (95% Confidence Limits) | LC90 (95% Confidence Limits) | χ2 | p |
|---|---|---|---|---|
| Aedes aegypti | ||||
| C. bodinieri | 52.00 (48.39–56.11) | 74.18 (66.88–87.49) | 2.43 | 0.297 |
| C. candicans (Nghia Dan) | 3.824 (3.426–4.256) | 8.165 (7.077–9.813) | 17.80 | 0.000 |
| C. candicans (Dai Loc) | 2.145 (1.998–2.301) | 2.891 (2.667–3.211) | 8.14 | 0.012 |
| C. formosana | 29.04 (26.89–31.49) | 43.37 (39.04–50.31) | 9.12 | 0.010 |
| C. longifolia (Nghia Dan) | 35.64 (32.40–39.22) | 66.15 (58.04–78.86) | 6.87 | 0.032 |
| C. nudiflora | 27.34 (23.84–31.16) | 77.02 (63.21–101.27) | 17.10 | 0.000 |
| C. petelotii | 18.49 (16.52–20.50) | 36.52 (31.69–44.62) | 6.65 | 0.036 |
| C. rubella (Nam Giai) | 17.93 (14.85–20.87) | 54.72 (45.00–72.37) | 3.61 | 0.165 |
| C. rubella (Tay Giang) | 21.73 (19.75–23.90) | 37.09 (33.44–43.74) | 8.48 | 0.014 |
| C. sinuata | 25.86 (23.20–28.77) | 54.55 (47.03–66.51) | 5.20 | 0.074 |
| Culex quinquefasciatus | ||||
| C. candicans (Nghia Dan) | 1.670 (1.425–1.929) | 5.726 (4.688–7.448) | 16.78 | 0.002 |
| C. candicans (Dai Loc) | 0.945 (0.742–1.137) | 3.537 (2.881–4.691) | 9.68 | 0.046 |
| C. nudiflora | 178.5 (148.3–240.1) | 170.6 (153.7–198.8) | 15.72 | 0.000 |
The leaf essential oils of C. candicans were rich in (E)-caryophyllene, caryophyllene oxide, β-selinene and atractylone. Both (E)-caryophyllene and caryophyllene oxide have shown only weak larvicidal activity against Ae. aegypti [48]. However, atractylone may be contributing to the larvicidal activity; the compound has shown insecticidal [49] as well as acaricidal activity [50]. β-Selinene has also shown insecticidal activity [49]. In addition to the insecticidal properties of atractylone and β-selinene, there may be synergistic effects between these components and (E)-caryophyllene, caryophyllene oxide, or other minor components. Scalerandi and co-workers have shown that Musca domestica preferentially oxidize major essential oil components in a mixture while the components in lesser concentrations act as toxicants [51]. In addition, there were several unidentified components, particularly in the Dai Loc sample, that may be contributing to the larvicidal effects.
Interestingly, C. nudiflora leaf essential oil was rich in α- and β-pinene and caryophyllene oxide but was relatively inactive (24-h LC50 = 109 μg/mL) compared to the C. candicans leaf essential oils (24-h LC50 = 2.0 and 1.2 μg/mL) against Cx. quinquefasciatus. Consistent with these results, both α-pinene and β-pinene have shown relatively weak larvicidal activity against Cx. quinquefasciatus [52]. Likewise, the seed essential oil of Psoralea corylifolia, rich in caryophyllene oxide (40.8%), also showed relatively weak larvicidal activity against Cx. quinquefasciatus [53]. C. nudiflora leaf essential oil was also less active against Ae. aegypti larvae with a 24-h LC50 value of 37.5 μg/mL. There are conflicting results regarding the larvicidal activities of α- and β-pinene on Ae. aegypti. Lucia and co-workers reported LC50 values of 15.4 and 12.1 μg/mL for α- and β-pinene, respectively, against Ae. aegypti [54], while Waliwitiya and co-workers found the pinenes to be inactive (LC50 > 500 μg/mL) against the mosquito larvae [55]. Caryophyllene oxide is apparently only weakly larvicidal (LC50 = 125 μg/mL) on Ae. aegypti [39,56].
The leaf essential oil of C. longifolia from Nghia Dan, rich in (E)-caryophyllene (28.0%) and β-selinene (13.2%), showed larvicidal activity with 24-h LC50 of 37.4 μg/mL. (E)-Caryophyllene is relatively inactive with reported LC50 values of 93.7 [57] and 1202 μg/mL [56]. Notably, Piper humaytanum leaf essential oil, with 3.5% (E)-caryophyllene and 15.8% β-selinene, was weakly larvicidal (LC50 = 156 μg/mL) against Ae. aegypti [58].
The larvicidal activity of C. bodinieri leaf essential oil was the weakest of the Callicarpa species tested with a 24-h LC50 of 54 μg/mL. Limonene was one of the major components (8.0%), and this compound had shown larvicidal activity against Ae. aegypti of around 30 μg/mL [59]. Caryophyllene oxide, another major component (9.8%) is inactive against Ae. aegypti [39]. Although apparently not tested against mosquito larvae, β-selinene (8.9% in C. bodinieri leaf essential oil) is known to be insecticidal against Drosophila melanogaster adults [49].
The leaf essential oils of C. formosana, C. rubella (Nam Giai), C. rubella (Tay Giang), and C. sinuata showed comparable larvicidal activities with 24-h LC50 ranging from 24.2 to 31.9 μg/mL. However, the chemical compositions of the essential oils were very different.
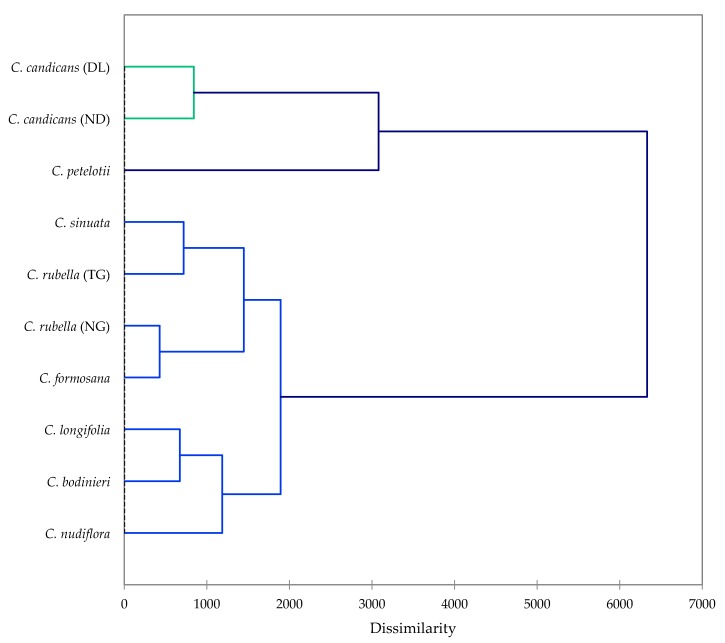
In order to evaluate potential correlation between constituents and larvicidal activities, multivariate analyses (hierarchical cluster analysis, HCA, and principal component analysis, PCA, were undertaken. The hierarchical cluster analysis (Figure 1) showed four groupings. Group 1 is made up of the two C. candicans samples and represents a very larvicidal group (average 24-h LC50 and LC90 = 4.02 and 9.34 μg/mL). The major components in this group are atractylone (average 20.9%) and caryophyllene oxide (average 8.1%). Group 2 is a single sample, C. petelotii is somewhat active with 24-h LC50 and LC90 of 19.1 and 37.9 μg/mL and α-humulene, α-selinene, and humulene epoxide II as the major components. Group 3 (C. sinuata, C. formosana, and both C. rubella samples) had average 24-h larvicidal LC50 and LC90 of 27.7 and 50.9 μg/mL, respectively. The major component in group 3 is caryophyllene oxide with an average concentration of 17.1%. Group 4 is the least active group (24-h LC50 and LC90 = 43.0 and 73.0 μg/mL) and also has caryophyllene oxide as the major component (average = 12.0%) as well as (E)-caryophyllene (average = 10.6%).
Figure 1.
Agglomerative hierarchical cluster analysis based on the major components of the Callicarpa essential oils from central Vietnam along with larvicidal activities (LC50 and LC90) against Aedes aegypti.
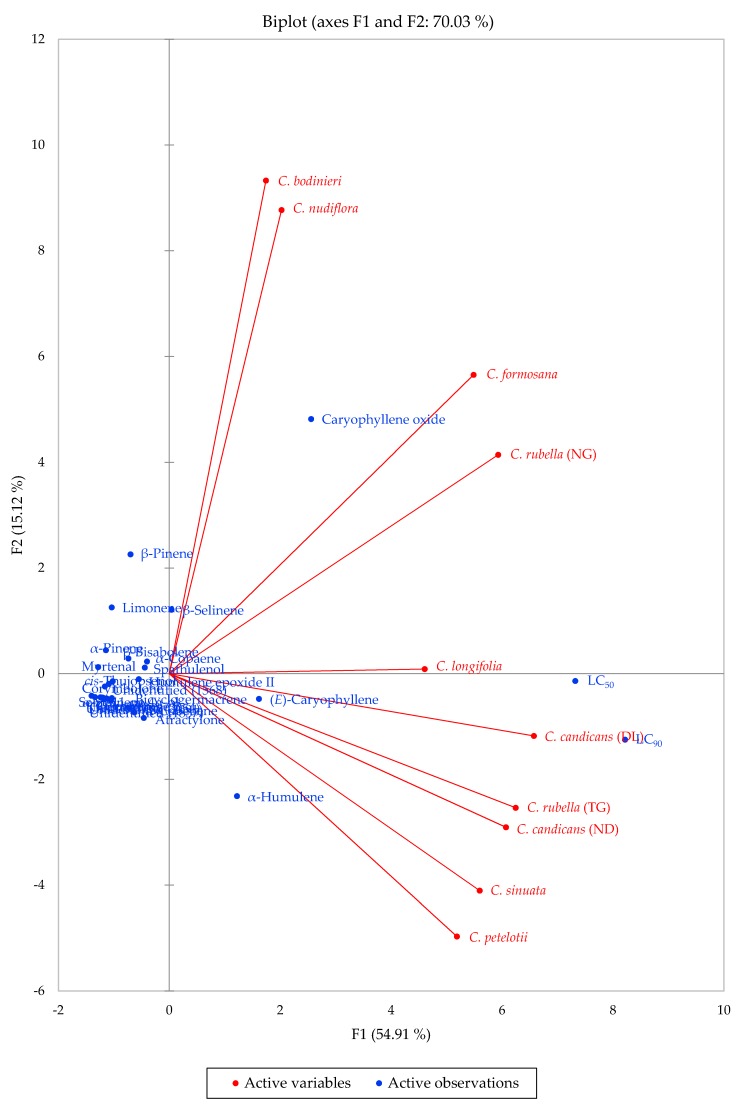
The principal component analysis (Figure 2) does not reveal any clear associations between chemical components and larvicidal activity. (E)-Caryophyllene, caryophyllene oxide, and α-humulene were found in all of the samples and therefore correlate with the essential oil samples and not necessarily with the larvicidal activities. Apparently the synergistic and antagonistic interactions of the components in these essential oils are too subtle to be parsed out with so few data.
Figure 2.
Principal component biplot of PC1 and PC2 scores and loadings indicating the correlation of chemical components of Callicarpa essential oils from central Vietnam and Aedes aegypti larvicidal activity.
3. Materials and Methods
3.1. Plant Material
Plant material (leaves and/or stem bark) from Callicarpa species was collected from several locations in central Vietnam (Table 13). The plant material from several individuals from each site were combined in order to provide enough plant material for each species. The plants were identified by Dr. Do Ngoc Dai, and voucher specimens (Table 13) have been deposited in the School of Natural Science Education, Vinh University. The fresh plant materials (2.0 kg each) were shredded and hydrodistilled for 4 h using a Clevenger type apparatus (Witeg Labortechnik, Wertheim, Germany). The yields of essential oils are summarized in Table 13.
Table 13.
Collection details and essential oil yields of Callicarpa species from central Vietnam.
| Callicarpa Species | Vietnamese Name | Collection Site | Growth Period | Voucher Number | Part | % Yield |
|---|---|---|---|---|---|---|
| Callicarpa bodinieri Lév. | Tử châu bodinier | Ngoc Linh Nature Reserve, Quang Nam Province (15°50′16.0″ N, 107°22′54.7″ E, elev. 1341 m) | Flowers and young fruits | DND-62 | Leaf | 0.1 |
| Callicarpa candicans (Burm.f.) Hochr. | Tử châu chồi trắng, Nàng nàng | Nghia Dan District, Nghe An province (19°22′24.4″ N, 105°25′15.3″ E, elev. 75 m) | Flowers, young fruits and ripe fruits | DND-17 | Leaf | 0.15 |
| Dai Loc district, Quang Nam province 15°53′16″ N, 107°59′38″ E, elev. 514 m) |
Flowers, young fruits and ripe fruits | DND-80 | Leaf | 0.18 | ||
| Hoa Vang district, Da Nang city (16°01′0.6″ N, 108°4′25.6″ E, elev. 28 m) | Flowers, young fruits and ripe fruits | NHH-57 | Leaf Bark |
0.17 0.04 |
||
| Callicarpa formosana Rolfe | Tử châu đài loan | Ngoc Linh Nature Reserve, Quang Nam Province (15°50′16.0″ N, 107°22′54.7″ E, elev. 1341 m) | Flowers and young fruits | DND-72 | Leaf | 0.11 |
| Callicarpa longifolia Lam. | Tử châu lá dài, Tu hú lá dài | Nghia Dan District, Nghe An province (19°20′6.2″ N, 105°25′58.1″ E, elev. 51 m) | Flowers, young fruits and ripe fruits | DND-31 | Leaf | 0.13 |
| Callicarpa nudiflora Hook. & Arn. | Tử châu hoa trần | Son Tra Peninsula, Da Nang City (16°07′18″ N, 108°18′07″ E, elev. 118 m) | Flowers, young fruits and ripe fruits | DND-33 | Leaf | 0.14 |
| Callicarpa petelotii Dop | Tử châu petelot | Tay Giang District, Quang Nam province (15°50′16.0″ N, 107°22′54.7″ E, elev. 1341 m) | Flowers, young fruits and ripe fruits | DND-98 | Leaf | 0.22 |
| Callicarpa rubella Lindl. | Tử châu đỏ, Tu hú hồng | Nậm Giải Commune, Quế Phong district, Pu Hoat Nature Reserve, Nghe An province (19°41′40″ N, 104°49′29″ E, elev. 671 m) | Flowers, young fruits and ripe fruits | DND-709 | Leaf | 0.15 |
| Tay Giang District, Quang Nam province (15°50′16.0″ N, 107°22′54.7″ E, elev. 1341 m) | Flowers, young fruits | DND-99 | Leaf | 0.12 | ||
| Bach Ma National Park, Phu Loc District, Thua Thien Hue province (16°11′59″ N, 107°51′25″ E, elev. 1376 m) | ripe fruits | DND-27 | Leaf Bark |
0.11 0.06 |
||
| Callicarpa sinuata Budantzev & Phuong | Tử châu răng sâu | Son Tra Peninsula, Da Nang City (16°06′00″ N, 108°18′24″ E, elev. 124 m) | Flowers, young fruits | NHH-84 | Leaf | 0.14 |
3.2. Gas Chromatography-Mass Spectrometry
Each of the Callicarpa essential oils was analyzed by GC-MS using a Shimadzu GCMS-QP2010 Ultra (Shimadzu Scientific Instruments, Columbia, MD, USA) operated in the electron impact (EI) mode (electron energy = 70 eV), scan range = 40–400 atomic mass units, scan rate = 3.0 scans/s, and GC-MS solution software. The GC column was a ZB-5 fused silica capillary column (Phenomenex, Torrance, CA, USA) (30 m length × 0.25 mm internal diameter) with a (5% phenyl)-polymethylsiloxane stationary phase and a film thickness of 0.25 μm. The carrier gas was helium with a column head pressure of 552 kPa and flow rate of 1.37 mL/min. Injector temperature was 250 °C and the ion source temperature was 200 °C. The GC oven temperature program was programmed for 50 °C initial temperature, temperature increased at a rate of 2 °C/min to 260 °C. A 5% w/v solution of the sample in CH2Cl2 was prepared and 0.1 μL was injected with a splitting mode (30:1). Identification of the oil components was based on their retention indices determined by reference to a homologous series of n-alkanes (C8-C40), and by comparison of their mass spectral fragmentation patterns with those reported in the databases [60,61,62,63]. The percentages of each component in the essential oils are reported as raw percentages based on total ion current without standardization.
3.3. Mosquito Larvicidal Assay
Eggs of Ae. aegypti were purchased from Institute of Biotechnology, Vietnam Academy of Science and Technology, and maintained at the Laboratory of Department of Pharmacy of Duy Tan University, Da Nang, Vietnam. For the assay, aliquots of the essential oils of Callicarpa species, dissolved in DMSO (1% stock solution), was placed in a 500-mL beaker and added to water that contained 20 larvae (third and early fourth instar). With each experiment, a set of controls using DMSO was also run for comparison. Mortality was recorded after 24 h and again after 48 h of exposure during which no nutritional supplement was added. The experiments were carried out 25 ± 2 °C. Each test was conducted with four replicates with several concentrations (100, 50, 25, 12.5, 6.0, 3.0, 1.5, and 0.75 μg/mL). Larvicidal activity against Culex quinquefasciatus (The larvae were fed on Koi fish food: Adults were provided with a 10% sucrose solution and a 1-week-old chick for blood feeding.) were determined similarly with concentrations of 150, 100, 50, 25, 6.0, 3.0, 1.5, and 0.75 μg/mL. Permethrin was used as a positive control. The acute larvicidal effects on Ae. aegypti, and Cx. quinquefasciatus were recorded 24 h and 48 h after treatment. The data obtained were subjected to log-probit analysis [64] to obtain LC50 values, LC90 values, and 95% confidence limits using XLSTAT v. 2018.5 (Addinsoft, Paris, France).
3.4. Statistical Analysis
Mosquito larvicidal activities (LC50 and LC90) against Ae. aegypti and Cx. quinquefasciatus were determined by log-probit analysis using XLSTAT v. 2018.5 (Addinsoft, Paris, France). The more abundant chemical components of the Callicarpa essential oils were used in the multivariate analyses. The essential oil compositions were treated as operational taxonomic units (OTUs), and the concentrations (percentages) of 26 major essential oil components and the 24-h LC50 and LC90 larvicidal activity data were used to determine the associations between the Callicarpa essential oils using agglomerative hierarchical cluster (AHC) analysis using XLSTAT Premium, version 2018.5 (Addinsoft, Paris, France). Dissimilarity was determined using Euclidean distance, and clustering was defined using Ward’s method. For the principal component analysis (PCA), the 26 major components and the larvicidal data were taken as variables using a Pearson correlation matrix using XLSTAT Premium, version 2018.5 (Addinsoft, Paris, France). A total of 280 data (28 variables × 10 samples) were used for the PCA.
4. Conclusions
There are profound chemical variations in the leaf essential oils of Callicarpa species, both between species and within species. All of the Callicarpa leaf essential oils showed larvicidal activity against Ae. aegypti. However, C. candicans showed excellent mosquito larvicidal activity against Ae. aegypti as well as Cx. quinquefasciatus, which can be attributed to atractylone and/or to unidentified components. This essential oil, therefore, may represent a low-cost and environmentally friendly mosquito control agent. Nevertheless, although the larvicidal activities of Callicarpa leaf essential oils are promising, additional screening on non-target organisms is needed [41,42].
Acknowledgments
P.S. and W.N.S. participated in this work as part of the activities of the Aromatic Plant Research Center (APRC, https://aromaticplant.org/).
Author Contributions
Conceptualization, N.H.H.; methodology, N.H.H., P.S., and W.N.S.; software, P.S.; validation, N.H.H., P.S., and W.N.S.; formal analysis, P.S. and W.N.S.; investigation, L.T.H., N.T.C., N.T.H.T., N.A.D., and T.A.T.; resources, N.H.H.; data curation, W.N.S.; writing—original draft preparation, W.N.S.; writing—review and editing, N.H.H., P.S., and W.N.S.; supervision, N.H.H.; project administration, N.H.H.; funding acquisition, N.H.H. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the NAFOSTED (Vietnam) for financial support of this study through Project No. 106.03-2019.25.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mabberley D.J. Mabberley’s Plant-Book. 3rd ed. Cambridge University Press; Cambridge, UK: 2008. [Google Scholar]
- 2.Jones W.P., Kinghorn A.D. Biologically active natural products of the genus Callicarpa. Curr. Bioact. Compd. 2008;4:15–32. doi: 10.2174/157340708784533393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Missouri Botanical Garden Tropicos. [(accessed on 21 December 2019)]; Available online: www.tropicos.org.
- 4.Leeratiwong C., Chantaranothai P., Paton A.J. A synopsis of the genus Callicarpa L. (Lamiaceae) in Thailand. Thai For. Bull. 2009;37:36–58. [Google Scholar]
- 5.Tu Y., Sun L., Guo M., Chen W. The medicinal uses of Callicarpa L. in traditional Chinese medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2013;146:465–481. doi: 10.1016/j.jep.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 6.Gao J.B., Yang S.J., Yan Z.R., Zhang X.J., Pu D.B., Wang L.X., Li X.L., Zhang R.H., Xiao W.L. Isolation, characterization, and structure-activity relationship analysis of abietane diterpenoids from Callicarpa bodinieri as spleen tyrosine kinase inhibitors. J. Nat. Prod. 2018;81:998–1006. doi: 10.1021/acs.jnatprod.7b01082. [DOI] [PubMed] [Google Scholar]
- 7.Buot I.E. An ethnobotanical study of the plant biodiversity of Mt. Mayon, Bicol Peninsula, Albay, Philippines. J. Nat. Stud. 2009;8:1–10. [Google Scholar]
- 8.Jawale C.S. Piscicidal plants of India. Trends Fish. Res. 2018;7:33–45. [Google Scholar]
- 9.Muensaen S., Ruangwises N., Jarikasem S., Sithisarn P. Isolation, structure elucidation and quantitative analysis of callicarpone and oleanolic acid in the leaves of Callicarpa candicans collected from different provinces in Thailand. Agric. Nat. Resour. 2019;53:251–259. [Google Scholar]
- 10.Lê V.T.T., Lâm Đ.T., Ính C.T., Long P.Q., Thạch T.Đ., Vân N.T.H., Minh P.T.H. Triterpenoid và flavonoid từ dịch chiết etyl axetat của cây N àng nàng (Callicarpa candicans (Burm. f.) Hochr.) Ở V iệt Nam. Vietnam J. Chem. 2018;56:341–345. [Google Scholar]
- 11.Chusri S., Chaicoch N., Thongza-ard W., Limsuwan S., Voravuthikunchai S.P. In Vitro antibacterial activity of ethanol extracts of nine herbal formulas and its plant components used for skin infections in Southern Thailand. J. Med. Plants Res. 2012;6:5616–5623. [Google Scholar]
- 12.Nandwani D., Calvo J.A., Tenorio J., Calvo F., Manglona L. Medicinal plants and traditional knowledge in the Northern Mariana Islands. J. Appl. Biosci. 2008;8:323–330. [Google Scholar]
- 13.Vo V.C. The Dictionary of Medicinal Plants of Vietnam 1. Medical Publishing House; Hanoi, Vietnam: 2012. [Google Scholar]
- 14.Dan Y., Qian Z., Liu Y., Zhou G., Peng Y., Xiao P. New collection of crude drugs in Chinese pharmacopoeia 2010 I. Callicarpa Linn. and related items. Chin. Herb. Med. 2010;2:272–288. [Google Scholar]
- 15.Ho P.-H. An Illustrated Flora of Vietnam, Vol. 3. Youth Publishing House; Ho Chi Minh City, Vietnam: 2000. [Google Scholar]
- 16.Phuông V.X. Flora of Vietnam, Volume 6—Verbenaceae. Science & Technics Publishing House; Hanoi, Vietnam: 2007. [Google Scholar]
- 17.Au D.T., Wu J., Jiang Z., Chen H., Lu G., Zhao Z. Ethnobotanical study of medicinal plants used by Hakka in Guangdong, China. J. Ethnopharmacol. 2008;117:41–50. doi: 10.1016/j.jep.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Zheng X., Xing F. Ethnobotanical study on medicinal plants around Mt. Yinggeling, Hainan Island, China. J. Ethnopharmacol. 2009;124:197–210. doi: 10.1016/j.jep.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 19.Bramley G.L.C. Distribution patterns in Malesian Callicarpa (Lamiaceae) Garden Bull. Singap. 2011;63:287–298. [Google Scholar]
- 20.Bramley G.L.C. The genus Callicarpa (Lamiaceae) in the Philippines. Kew Bull. 2013;68:369–418. doi: 10.1007/s12225-013-9456-y. [DOI] [Google Scholar]
- 21.Munir A.A. A taxonomic revision of the genus Callicarpa L. (Verbenaceae) in Australia. J. Adel. Bot. Gardens. 1982;6:5–39. [Google Scholar]
- 22.Lee C., Kim S.-Y., Eum S., Paik J.-H., Bach T.T., Darshetkar A.M., Choudhary R.K., Hai D.V., Quang B.H., Thanh N.T., et al. Ethnobotanical study on medicinal plants used by local Van Kieu ethnic people of Bac Huong Hoa nature reserve, Vietnam. J. Ethnopharmacol. 2019;231:283–294. doi: 10.1016/j.jep.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Gubler D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998;11:480–496. doi: 10.1128/CMR.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett A.D.T., Higgs S. Yellow fever: A disease that has yet to be conquered. Annu. Rev. Entomol. 2007;52:209–229. doi: 10.1146/annurev.ento.52.110405.091454. [DOI] [PubMed] [Google Scholar]
- 25.Dhimal M., Gautam I., Joshi H.D., Hara R.B.O. Risk factors for the presence of Chikungunya and dengue vectors (Aedes aegypti and Aedes albopictus), their altitudinal distribution and climatic determinants of their abundance in central Nepal. PLoS Negl. Trop. Dis. 2015;9:e0003545. doi: 10.1371/journal.pntd.0003545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benelli G., Mehlhorn H. Declining malaria, rising of dengue and Zika virus: Insights for mosquito vector control. Parasitol. Res. 2016;115:1747–1754. doi: 10.1007/s00436-016-4971-z. [DOI] [PubMed] [Google Scholar]
- 27.Quyen D., Le N.T., Van Anh C.T., Nguyen N.B., Van Hoang D., Montgomery J.L., Kutcher S.C., Le N.H., Hien N.T., Kien D.T.H., et al. Epidemiological, serological, and virological features of dengue in Nha Trang City, Vietnam. Am. J. Trop. Med. Hyg. 2018;98:402–409. doi: 10.4269/ajtmh.17-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quyen N.T.H., Kien D.T.H., Rabaa M., Tuan N.M., Vi T.T., Hung N.T., Tuan H.M., Van Tram T., Le Da Ha N., Quang H.K., et al. Chikungunya and Zika virus cases detected against a backdrop of endemic dengue transmission in Vietnam. Am. J. Trop. Med. Hyg. 2017;97:146–150. doi: 10.4269/ajtmh.16-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albuquerque C.M.R., Cavalcanti V.M.S., Melo M.A.V., Verçosa P., Regis L.N., Hurd H. Bloodmeal microfilariae density and the uptake and establishment of Wuchereria bancrofti infections in Culex quinquefasciatus and Aedes aegypti. Mem. Inst. Oswaldo Cruz. 1999;94:591–596. doi: 10.1590/S0074-02761999000500005. [DOI] [PubMed] [Google Scholar]
- 30.Turell M.J. Members of the Culex pipiens complex as vectors of viruses. J. Am. Mosq. Control Assoc. 2012;28:123–127. doi: 10.2987/8756-971X-28.4.123. [DOI] [PubMed] [Google Scholar]
- 31.Van den Hurk A.F., Hall-Mendelin S., Jansen C.C., Higgs S. Zika virus and Culex quinquefasciatus mosquitoes: A tenuous link. Lancet Infect. Dis. 2017;17:1014–1016. doi: 10.1016/S1473-3099(17)30518-2. [DOI] [PubMed] [Google Scholar]
- 32.Vontas J., Kioulos E., Pavlidi N., Morou E., della Torre A., Ranson H. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic. Biochem. Physiol. 2012;104:126–131. doi: 10.1016/j.pestbp.2012.05.008. [DOI] [Google Scholar]
- 33.Liu N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015;60:537–559. doi: 10.1146/annurev-ento-010814-020828. [DOI] [PubMed] [Google Scholar]
- 34.Smith L.B., Kasai S., Scott J.G. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 2016;133:1–12. doi: 10.1016/j.pestbp.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Yadouléton A., Badirou K., Agbanrin R., Jöst H., Attolou R., Srinivasan R., Padonou G., Akogbéto M. Insecticide resistance status in Culex quinquefasciatus in Benin. Parasites Vectors. 2015;8:17. doi: 10.1186/s13071-015-0638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lopes R.P., Lima J.B.P., Martins A.J. Insecticide resistance in Culex quinquefasciatus Say, 1823 in Brazil: A review. Parasites Vectors. 2019;12:591. doi: 10.1186/s13071-019-3850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamrin M.A. Pesticide Profiles: Toxicity, Environmental Impact, and Fate. CRC Press; Boca Raton, FL, USA: 1997. [Google Scholar]
- 38.Goulson D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013;50:977–987. doi: 10.1111/1365-2664.12111. [DOI] [Google Scholar]
- 39.Silva W.J., Dória G.A.A., Maia R.T., Nunes R.S., Carvalho G.A., Blank A.F., Alves P.B., Marçal R.M., Cavalcanti S.C.H. Effects of essential oils on Aedes aegypti larvae: Alternatives to environmentally safe insecticides. Bioresour. Technol. 2008;99:3251–3255. doi: 10.1016/j.biortech.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 40.Benelli G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015;114:2801–2805. doi: 10.1007/s00436-015-4586-9. [DOI] [PubMed] [Google Scholar]
- 41.Masetti A. The potential use of essential oils against mosquito larvae: A short review. Bull. Insectol. 2016;69:307–310. [Google Scholar]
- 42.Pavela R., Benelli G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016;21:1000–1007. doi: 10.1016/j.tplants.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Ntalli N., Koliopoulos G., Giatropoulos A., Menkissoglu-Spiroudi U. Plant secondary metabolites against arthropods of medical importance. Phytochem. Rev. 2019;18:1255–1275. doi: 10.1007/s11101-019-09647-7. [DOI] [Google Scholar]
- 44.Su X., Zhu S. Analysis of volatile compounds from Callicarpa bodinieri purple pearl. J. Jingmen Tech. Coll. 2008;23:7–10. [Google Scholar]
- 45.Lin C.Z., Zhu C.C., Zhang C.X., Zhao Z.X., He W.J., Li J.K., Chai L., Deng J.W., Wei Y.L. Chemical constituents and antioxidant activity of the essential oils from the leaves of Callicarpa formosana Rolfe. J. Trop. Subtrop. Bot. 2009;17:401–405. [Google Scholar]
- 46.Liang J., Han F., Wang Z., Yan Y., Mo F. Chemical composition of the essential oil from leaves of Callicarpa nudiflora. Chem. Nat. Compd. 2009;45:267–268. [Google Scholar]
- 47.Zhang W., Zhang J., Kang W. Volatiles of Callicarpa rubella. Chem. Nat. Compd. 2017;53:976–977. doi: 10.1007/s10600-017-2175-0. [DOI] [Google Scholar]
- 48.Dias C.N., Moraes D.F.C. Essential oils and their compounds as Aedes aegypti L. (Diptera: Culicidae) larvicide: Review. Parasitol. Res. 2014;113:565–592. doi: 10.1007/s00436-013-3687-6. [DOI] [PubMed] [Google Scholar]
- 49.Chu S.S., Jiang G.H., Liu Z.L. Insecticidal compounds from the essential oil of Chinese medicinal herb Atractylodes chinensis. Pest Manag. Sci. 2011;67:1253–1257. doi: 10.1002/ps.2180. [DOI] [PubMed] [Google Scholar]
- 50.Kim H.-K., Yun Y.-K., Ahn Y.-J. Toxicity of atractylon and atractylenolide III identified in Atractylodes ovata rhizome to Dermatophagoides farinae and Dermatophagoides pteronyssinus. J. Agric. Food Chem. 2007;55:6027–6031. doi: 10.1021/jf0708802. [DOI] [PubMed] [Google Scholar]
- 51.Scalerandi E., Flores G.A., Palacio M., Defagó M.T., Carpinella M.C., Valladares G., Bertoni A., Palacios S.M. Understanding synergistic toxicity of terpenes as insecticides: Contribution of metabolic detoxification in Musca domestica. Front. Plant Sci. 2018;9:1579. doi: 10.3389/fpls.2018.01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pavela R. Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res. 2015;114:3835–3853. doi: 10.1007/s00436-015-4614-9. [DOI] [PubMed] [Google Scholar]
- 53.Dua V.K., Kumar A., Pandey A.C., Kumar S. Insecticidal and genotoxic activity of Psoralea corylifolia Linn. (Fabaceae) against Culex quinquefasciatus Say, 1823. Parasites Vectors. 2013;6:30. doi: 10.1186/1756-3305-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lucia A., Gonzalez Audino P., Seccacini E., Licastro S., Zerba E., Masuh H. Larvicidal effect of Eucalyptus grandis essential oil and turpentine and their major components on Aedes aegypti larvae. J. Am. Mosq. Control Assoc. 2007;23:299–303. doi: 10.2987/8756-971X(2007)23[299:LEOEGE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 55.Waliwitiya R., Kennedy C.J., Lowenberger C.A. Larvicidal and oviposition-altering activity of monoterpenoids, trans-anethole and rosemary oil to the yellow fever mosquito Aedes aegypti (Diptera: Culicidae) Pest Manag. Sci. 2009;65:241–248. doi: 10.1002/ps.1675. [DOI] [PubMed] [Google Scholar]
- 56.Cheng S.S., Liu J.Y., Tsai K.H., Chen W.J., Chang S.T. Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J. Agric. Food Chem. 2004;52:4395–4400. doi: 10.1021/jf0497152. [DOI] [PubMed] [Google Scholar]
- 57.Perumalsamy H., Kim N.-J., Ahn Y.-J. Larvicidal activity of compounds isolated from Asarum heterotropoides against Culex pipiens pallens, Aedes aegypti, and Ochlerotatus togoi (Diptera: Culicidae) J. Med. Entomol. 2009;46:1420–1423. doi: 10.1603/033.046.0624. [DOI] [PubMed] [Google Scholar]
- 58.De Morais S.M., Facundo V.A., Bertini L.M., Cavalcanti E.S.B., dos Anjos Júnior J.F., Ferreira S.A., de Brito E.S., Neto M.A. Chemical composition and larvicidal activity of essential oils from Piper species. Biochem. Syst. Ecol. 2007;35:670–675. doi: 10.1016/j.bse.2007.05.002. [DOI] [Google Scholar]
- 59.Santos S.R.L., Melo M.A., Cardoso A.V., Santos R.L.C., de Sousa D.P., Cavalcanti S.C.H. Structure-activity relationships of larvicidal monoterpenes and derivatives against Aedes aegypti Linn. Chemosphere. 2011;84:150–153. doi: 10.1016/j.chemosphere.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 60.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 61.Mondello L. FFNSC 3. Shimadzu Scientific Instruments; Columbia, MD, USA: 2016. [Google Scholar]
- 62.NIST17. National Institute of Standards and Technology; Gaithersburg, MD, USA: 2017. [Google Scholar]
- 63.Satyal P. Ph.D. Thesis. University of Alabama in Huntsville; Huntsville, Alabama: 2015. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. [Google Scholar]
- 64.Finney D. Probit Analysis. Reissue ed. Cambridge University Press; Cambridge, UK: 2009. [Google Scholar]