Abstract
Globally, tobacco use is a major modifiable risk factor and leading cause of many forms of cancer and cancer death. Tobacco use contributes to poorer prognosis in cancer care. This article reviews the current state of tobacco cessation treatment in oncology. Effective behavioral and pharmacological treatments exist for tobacco cessation, but are not being widely used in oncology treatment settings. Comprehensive tobacco treatment increases success with quitting smoking and can improve oncological and overall health outcomes. This article describes the components of a model treatment program, which includes automatic referrals for all current tobacco users and recent quitters, motivational interviewing during initial and follow-up contacts, combined behavioral and pharmacological interventions for cessation, and systematic follow-up phone calls for relapse prevention.
Keywords: Smoking, Tobacco, Nicotine, Cessation, Cancer, Oncology, Prevention, Treatment
Advances in cancer prevention, screening, early detection, and treatment over the past 40 years have resulted in declines in cancer mortality and improved prognosis. The 5-year overall survival rates for cancer in the United States rose from 49% in 1975–1977 to 67% in 2007–2013 [1], with a similar increase in survival rates seen in high-and low-income countries worldwide [2].
Though mortality rates are declining, cancer incidence continues to rise, both in the United States [1] and globally [3]. Where cancer incidence trends have declined, for instance in lung cancer and colorectal cancer, this is due in large part to reduced smoking prevalence [3]. Hence, there is a renewed focus on prevention, particularly modifiable risk factors, such as smoking [4].
Smoking is responsible for 22% of cancer deaths globally [5], and nearly a third of all cancer deaths in the United States [6]. Cancer survivors who smoke are at increased risk for recurrence of primary and secondary cancers, diminished quality of life, and cancer death [7, 8]. Smokers have a poorer response to radiation therapy and more radiation-related side effects when compared to former smokers and recent quitters who stopped smoking before the treatment [9, 10].
Smokers also have worse surgical outcomes [10]. For lung cancer patients undergoing resection, smoking increases the risk of in-hospital mortality threefold and greatly increases the rate of pulmonary complications [11]. In a randomized trial, a smoking cessation intervention for lung cancer patients reduced postsurgery complications by half, compared to a no treatment control group (21 and 41%, respectively) [12]. For breast cancer patients undergoing surgery, smoking increased postmastectomy wound infection, skin flap necrosis, and epidermolysis, even after controlling for other potential risk factors [13] Finally, in leukemia patients undergoing bone marrow transplants, current smokers were hospitalized twice as long as nonsmokers, former smokers, and recent quitters [14]. Apart from cancer site and the stage at the time of diagnosis, abstinence from smoking is the strongest predictor of survival in cancer patients [15].
Tobacco use is common among cancer patients. In a cohort of 5,185 cancer patients in New York state, 17.6% reported regular tobacco use within a month following diagnosis and an additional 10.1% reported use within the last 12 months, placing them at high risk for relapse [16]. Current smoking prevalence is elevated further among head and neck (26.4%) [17] and thoracic (50%) cancer patients [10]. These numbers do not account for the estimated 10% of cancer patients who misrepresent their smoking status at their oncology visits, mostly due to shame and censure [18].
Cigarettes are designed to initiate and sustain addiction, delivering nicotine rapidly to the brain via smoke inhaled into the lung [19]. No other drug is dosed as frequently as nicotine is by a daily smoker, and the sustained use over time with exposure to numerous carcinogens leads to cancer. Notably, cancer patients have more severe nicotine addiction than smokers without cancer [20]. Cigarettes per day and time to first cigarette, both key indicators of nicotine addiction, also predict the development of lung cancer [21].
Smoking Cessation Treatment in Cancer Patients
A cancer diagnosis can be life-altering, and smokers with cancer report higher motivation to quit relative to the general population [22]. This increased motivation, however, has not translated into higher quit rates among cancer patients compared to the general population [15]. About half of cancer patients who smoked prior to diagnosis continue to smoke [23]. Given the elevated prevalence of use and significant health harms related to smoking in the context of oncology treatment, tobacco screening and cessation interventions are recommended as an essential part of the National Comprehensive Cancer Network (NCCN) guidelines for comprehensive oncology care [24]. The NCCN guidelines recommend 12 weeks of cessation behavioral therapy combined with cessation medications for all patients seen in oncology care who are interested in quitting smoking. The few exceptions are pregnant smokers, nondaily smokers, and smokers under 18 years of age, for whom cessation medication should be a second-line option of care [24]. Comprehensive treatment of tobacco use within cancer care has proven cost-effective [25]; yet, only 40% of oncologists discuss medications with patients who smoke and only 38% actively treat their patients for tobacco dependence [26]. Critical opportunities to improve oncological outcomes and extend patient survival are being missed.
Minimal Intervention
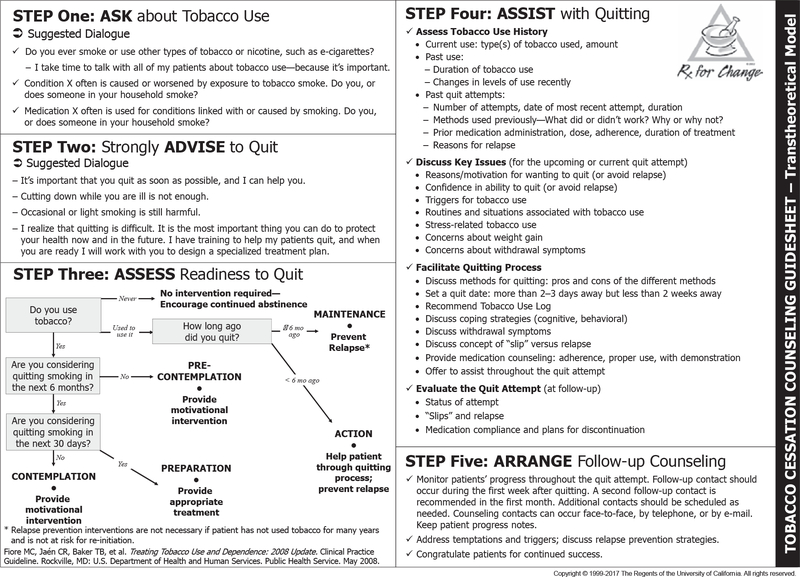
In general practice, minimal smoking cessation interventions typically consist of only clinician advice to quit. Despite its widespread use, brief advice is not enough for most smokers, and should really be viewed as the initial step on the path to more comprehensive and effective tobacco treatment [27]. The US Public Health Service has issued guidelines for treating tobacco dependence in the general population beyond simply brief advice to quit. Recommended are the “5 As” to: ask all patients about tobacco use, advise smokers to quit, assess readiness to make a quit attempt, assist patients with quitting smoking, and arrange follow-up [27] (Fig. 1). The National Institute for Health Care and Excellence (NICE) in the UK issued a similar quality standard guidance regarding tobacco cessation [28].
Fig. 1.
The “5 As.”
In recognition that it may be infeasible or impractical for oncologists to provide ongoing tobacco cessation treatment, research supports modification of the “5 As” approach to: ask, advise, connect (AAC) [29]. With AAC, the oncology team would ask about tobacco use, advise patients who smoke to quit, and then actively link the patients to other programs through the electronic health record or a fax referral (e.g., outpatient quit smoking group, quitline) to provide cessation assistance and arrange follow-up.
There is a dose-response relationship between clinical attention to tobacco and successful quitting in the general population [27]. Compared to no intervention, physician advice increases the likelihood of a quit attempt by 24%; providing medication by 68%; and providing behavioral support by 117%; compared to physician advice, providing medication increases quit attempts by 39% and behavioral support increases quit attempts by 69% [30]. However, these numbers only represent quit attempts, and relapse is common. To achieve improved health outcomes, long-term abstinence is needed. High-level care that includes combined medication and behavioral support can greatly improve the odds of long-term cessation [31].
Treating the Biopsychosocial Aspects of Tobacco Use and Addiction
Tobacco use disorder is a chronic relapsing disease that needs to be treated as a chronic illness with biological, psychological, and social components similar to treatment methods for diabetes, hypertension, and cancer [10]. Therefore, the language used to describe tobacco treatment outcomes should be the same as the language used in cancer care; for example, “complete response,” “can benefit from long-term follow-up and regular monitoring,” and “partial remission.” Patients who relapse to smoking should not be viewed as “treatment failures.” If there is a “recurrence” of smoking, a nonjudgmental reassessment and restructuring of the treatment plan will be needed to achieve sustained abstinence. Further, part of recovering from an addiction is the recognition that relapse is a real risk and preventing relapse is an active and ongoing process. This “recovery” mindset closely parallels recovery pathways in cancer care. Oncology treatment and recovery pathways parallel existing recovery pathways to addiction and other chronic diseases, spanning prevention, early intervention efforts, active treatment, and then posttreatment recovery, self-management, and relapse prevention [32].
The physiological elements of tobacco addiction and nicotine withdrawal are effectively treated by medications (comprehensive information on types, dosing, and precautions for all pharmacological treatments for smoking cessation is presented in online suppl. Table 1; see www.karger.com/doi/10.1159/000489266 for all online suppl. Material). There are 7 pharmacological treatments: 5 nicotine replacement therapies or NRTs (nicotine gum, inhaler, lozenge, nasal spray, and patch) and 2 nonnicotine medications (bupropion SR and varenicline) that have efficacy in increasing long-term quit rates [24]. A Cochrane review of pharmacological interventions for smoking cessation in the general population concluded that varenicline and combination NRT (i.e., combining slow-acting patch plus faster-acting gum or lozenge) have the strongest and comparable treatment effects followed by bupropion and single forms of NRT [33]. In an open-label study of varenicline among treatment-seeking smokers with cancer, 40% were abstinent at 12 weeks [34]. For oncology patients, the NCCN considers bupropion an effective second-line treatment [24].
When treating the biological aspects of tobacco addiction, the psychological and social aspects should not be overlooked. Effective psychosocial therapies for smoking cessation generally work by identifying high-risk situations for smoking, problem-solving strategies to manage these situations, and providing ongoing motivational enhancement [24]. According to recent Cochrane reviews, individual counseling is more effective than brief contact [35], and group therapy cessation interventions outperform self-help programs (RR 1.88, 95% CI 1.52–2.33, 13 studies, n = 4,395) and brief support from a health care provider (RR 1.22, 95% CI 1.03–1.43, 14 studies, n = 7,286) [36]. Many groups exist, such as the American Lung Association’s Freedom from Smoking program (http://www.lung.org/stop-smoking/join-freedom-from-smoking/). Cochrane reviews have also shown effectiveness for telephone quit lines [37] and web-based interventions [38]. Oncologists in the United States with limited referral options for smoking cessation treatment can direct their patients to resources such as 1–800-QUIT- NOW or smokefree.gov. Similar resources are available in the United Kingdom (www.nhs.uk/smokefree) and Australia (www.quitnow.gov.au).
In oncology, there are important psychological components to consider in addition to traditional tobacco-focused behavioral interventions. Anxiety, stress, and depression are common side effects of cancer treatment and nicotine withdrawal [39]. These concerns warrant attention on their own merit and should be addressed during tobacco addiction treatment to help sustain abstinence [40]. Stigma and self-blame are also relevant clinical issues to consider both with regard to smoking and cancer diagnosis [10]. Smokers have become increasingly marginalized in society, and feelings of shame and stigma are common among lung cancer patients, regardless of smoking status [10]. Tobacco cessation treatments ought to incorporate mood and stress management coping strategies and provide additional support and psychological treatment referrals as needed [10].
Mindfulness training for smoking cessation warrants a brief discussion here, as it is an emerging behavioral therapy for smoking cessation. Mindfulness practices teach patients to take a nonjudgmental, nonreactive stance toward present-moment experiences [41]. Mindfulness practices, which are centered on teaching awareness and nonreactivity toward craving states, are especially relevant in the treatment of addictive behaviors [42]. Patients practice and learn specific interventions such as breath meditation, mindful eating, and urge surfing, a mindfulness practice applied specifically to cravings [42, 43]. In one study, patients who were randomized to mindfulness training for tobacco cessation achieved abstinence rates of 31% at the 17-week follow-up, compared to only 6% of those in the American Lung Association’s Freedom from Smoking program [44]. In another randomized controlled trial, mindfulness training for smokers achieved a 6-month abstinence rate of 39% compared to 21% in a telephone quit-line control group [45]. At least two other randomized trials found positive results for mindfulness training for smoking cessation, producing abstinence rates of between 20 and 30% [46, 47]. For oncology patients who are long-term smokers and who have failed to quit with traditional treatments, a quit smoking program that incorporates mindfulness training may instill hope and raise motivation. Mindfulness treatments have been developed for reducing stress, depression, anxiety, fatigue, and sleep problems [48], and may therefore yield positive benefits above and beyond smoking cessation for cancer care.
Combined Treatments for Smoking Cessation
As mentioned prior, the NCCN guidelines recommend combining behavioral and pharmacological cessation interventions [24]. The 2008 US Public Health Service Clinical Guidelines found that combining evidence- based counseling and pharmacotherapy doubled the long-term quit rates over either modality alone and tripled those rates over unassisted quit attempts in the general public [27].
Unfortunately, combined cessation treatments are not being readily disseminated or evaluated in oncology settings. A 2013 meta-analysis of 13 studies (10 randomized trials and 3 prospective cohort studies) summarized the evidence from tobacco treatment interventions for cancer patients [49]. The behavioral components ranged from physician advice, counseling and informational booklets, to motivational interviewing, and cognitive-behavioral therapy. Six of the studies included NRT, 3 included other cessation medications, and 5 did not include any pharmacotherapy. Overall, smoking cessation treatment effects were not significant at short- (5 weeks) or long-term (6 months or more) follow-up. Examined by treatment type, abstinence outcomes were not significant for counseling-only but were significant for combination treatments (medication plus counseling). In terms of timing, smoking cessation interventions in the perioperative period were found to double the odds of quitting. In general, the behavioral interventions were fairly brief in contact and do not represent best evidence-based protocols for tobacco cessation. More intensive treatments are anticipated to improve long-term success rates [49]. The importance of the perioperative period as an ideal window for addressing tobacco is noted. As mentioned prior, smoking is associated with poor surgical outcomes including increased risks of general anesthesia, poor wound healing, and cardiovascular events [10–12].
Worth highlighting is an exemplary study testing a behavioral and pharmacologic cessation treatment in an oncology patient population. Duffy et al. [50] evaluated 9–11 sessions of telephone-delivered cognitive-behavioral therapy with bupropion plus NRT in a randomized-controlled design for up to 6 months of follow-up in cancer patients. The control group in this study reflected real-world usual care with participants assigned to this condition receiving a one-time assessment; advice to quit smoking; and a list of referrals for local, state, and national resources for smoking cessation. At the 6-month follow-up, with significant effects, 47% of intervention participants were abstinent compared to 31% of control participants [50]. A second, more recent meta-analysis further underscores the importance of combining behavioral counseling with pharmacologic cessation interventions: among 1,239 patients with head and neck cancer, behavioral counseling plus NRT significantly improved cessation rates compared to NRT alone [51].
Comprehensive Treatment for Smoking Cessation
Most oncology providers encounter smokers at a point in their lives when their tobacco addiction is now longstanding; this chronicity often indicates an addiction that is difficult to treat. With the experience of past failed quit attempts, patients may feel defeated in their ability to quit. However, oncology providers are in a unique position to provide intervention because motivation to quit can increase at the time of a cancer diagnosis. It is important to optimize this opportunity and create a comprehensive smoking cessation treatment plan. Box 1 presents a real-world case example where an oncologist partners with a tobacco cessation program and uses the NCI’s “5 As” with attention to motivation and readiness to quit.
Box 1. Case example – Thomas and Jennifer.
Thomas is a 56-year-old male who has recently been diagnosed with lung cancer. He has a 20-pack-year smoking history and currently smokes 20 cigarettes per day. He was advised to quit at the time of his diagnosis, and was referred to the Stanford Tobacco Cessation Program. At that time, he declined the referral, stating he would try to quit on his own. He successfully quit for a few weeks while receiving radiation therapy. However, when he was contacted at the 3-month follow-up, he had resumed smoking. While still unsure about joining the tobacco cessation program, he agreed to talk to his oncologist.
At his next oncology appointment, his wife, Jennifer, accompanied him to provide support. The astute oncologist asked Jennifer whether she was also smoking. Indeed, she is a 15-pack-year smoker and currently smoking 10 cigarettes per day. At the urging of their oncologist, the couple decided to quit together and enter treatment. At intake, they both decided to join the 8-week psychotherapy group, discovered the utility of the urge surfing skill to cope with cravings, and accepted referrals to medication consultation.
Thomas began taking varenicline and using nicotine lozenges, and Jennifer started using the patch and lozenges. Both incorporated skills learned in the psychotherapy group. Both set quit dates while in group and quit smoking within 1 month. They attended the group a few more times to help others quit – and stay quit themselves. Nine months after their intake appointment (15 months after initial referral to the program), both have remained tobacco-free.
Universal screening and assessment provide the entry into comprehensive tobacco treatment care. For patients not ready to make a quit attempt, motivational interviewing (MI) can help resolve ambivalence about quitting smoking. MI is based on the principles of expressing empathy, developing discrepancy, rolling with resistance, and supporting self-efficacy [52]. In the general population, the overall effect of MI on tobacco abstinence at 6 months is a modest improvement over brief advice or usual care (RR 1.27, 95% CI 1.12–1.43) [53].
Patients ready to quit smoking ought to be encouraged to set a quit date, ideally within the next 2 weeks; should be offered counseling and support; provided cessation medications, unless contraindicated; and referred for additional behavioral intervention, either individual, group, or virtual (phone, web-based, and/or texting). Prior quit attempts should be discussed with plans for overcoming barriers to quitting (e.g., nicotine withdrawal, stress, weight gain, lack of social support) and triggers to use (e.g., coffee, alcohol, advertising, other smokers). Patients early in a quit attempt should be assessed for nicotine withdrawal symptoms, compliance with cessation medication, and any lapses to use of tobacco.
Relapse prevention and ongoing monitoring are key for sustaining abstinence. In a nonclinical sample, compared to a control group that received 12 weeks of bupropion, NRT, and 5 weeks of behavioral counseling, Hall et al. [54] examined the efficacy of extended treatment contact to address relapse prevention. They spaced out 11 additional counseling sessions during weeks 12–52, with goals of quit maintenance, relapse prevention, and motivational enhancement. The extended counseling condition increased the likelihood of abstinence at weeks 64 and 104 by 54%, with an impressive 48% still quitting at week 104 [54]. In the clinical setting, long-term follow-ups could be delivered by phone or by inviting patients to return to group or individual counseling for relapse management. An added benefit of group treatments is that the successful quitter can assist and give confidence to other smokers trying to quit, which also helps the former smoker stay quit. This may be one explanation for why some studies show that group counseling is more effective than individual counseling for quitting smoking [55].
Who Provides the Cessation Treatment?
It is unreasonable to expect highly specialized oncologists to provide comprehensive smoking cessation treatment to their patients given competing demands for their time, the cost of their clinical care, and the general lack of tobacco cessation training in oncology. Less than 1% of physicians at cancer care centers prefer to provide cessation assistance themselves [56]. Yet, oncologists are central leaders and valuable allies for supporting the development and growth of cessation services in-house. The most successful cessation programs are referral-based, dedicated comprehensive treatment programs, to which oncologists can easily refer their patients who smoke [57]. In addition to comprehensive treatment, two other key elements for successful referral-based programs are automatic referral systems and systematic telephone follow-up [20]. These technologies reduce physician burden further, help to achieve the highest possible success for all patients who endorse current smoking or recent quitting within a medical system, and dovetail well with hospital initiatives to report on tobacco-related metrics for the merit-based incentive payment system as well as meet the Joint Commission standards.
Stepped Care Models
For some patients, a stepped care tobacco treatment approach may be warranted. This may be particularly true for cancer patients with higher levels of nicotine addiction and with co-occurring psychiatric disorders. In stepped care models, if a patient demonstrates an inability to quit or experiences repeated relapse to smoking with minimal intervention, providers should intervene with a higher level of care instead of recommending that patients continue to try the same approaches [24, 27]. The American Society of Addiction Medicine (ASAM) has an established set of criteria to determine an optimal level of care that is widely applied in the treatment of addictive disorders [58]. Williams et al. [59] make a convincing argument for these criteria to be applied to tobacco addiction treatment as well. For example, patients who cannot quit smoking with standard outpatient treatment, despite severe medical problems, may require a more intensive outpatient program, or perhaps even a higher level of care such as residential or inpatient [59]. Many cancer patients who smoke may meet such criteria for placement in higher levels of care. In contrast to treatment for other addictions, there are no known intensive outpatient programs and few residential programs that exist for the primary purpose of tobacco treatment. An exception is the Mayo Clinic, which has an 8-day residential treatment program for tobacco cessation and reports 6-month quit rates of 52% [60].
Model Tobacco Treatment Programs
Quitting smoking is a difficult process for many tobacco users; it can add additional stress to an already stressful cancer treatment regimen. However, given the impact of cessation on cancer treatment efficacy and survival, quitting smoking is a task well worth pursuing even during this stressful time. Because of the added stress of cancer care, it is important for the care team to support patients as they quit smoking and to ensure that an adequate level of individualized tobacco treatment is offered, integrated, and easily accessible. Established tobacco treatment programs in oncology clinics demonstrate high efficacy and utilization rates, debunking common myths that cancer patients cannot quit smoking because it is too stressful or patients are unavailable to engage in effective tobacco treatment.
The most successful programs provide a combination of MI, behavioral skills training, pharmacological interventions, and long-term follow-up [24, 27]. It is also helpful to provide education to oncology providers and patients about the importance of quitting smoking during cancer treatments. A few programs have specifically tailored their interventions to reflect the treatment recommendations of the US Public Health Service [27] and the NCCN [24] while also meeting the needs of large, hospital-based cancer centers.
The Tobacco Treatment Program at the University of Texas MD Anderson Cancer Center provides a helpful model for understanding how comprehensive tobacco treatment can serve oncology patients. The program was founded in 2006 with the central philosophy of individualized care tailored to each patient’s level of motivation, pharmacologic needs and preferences, and environmental situation [20, 57]. The program begins with a provider referral or proactive outreach via an automated referral system whereby all patients identified as current tobacco users or recent quitters are contacted. Following outreach, the program has a number of different treatment pathways to engage patients, including self-help materials mailed to the homes of those who cannot be reached or who stated they were not interested in quitting at that time, telephone counseling and over-the-phone prescribing for those who cannot access in-person services, and face-to-face evaluation and counseling [57]. A comprehensive interview covers smoking history, previous attempts to quit and methods used, as well as a detailed psychosocial history. An in-house medical provider also reviews each case to determine the best medication option. Behavioral counseling consists of 15- to 45-min sessions weekly for 10–12 weeks, with additional sessions as needed. During the active treatment, for those who do not quit within the first few weeks, tobacco treatment providers tailor medications and behavioral skills training as needed to help the patient quit. Follow-up sessions are conducted in person or by telephone according to patient preference and are aimed at preventing relapse to smoking. This program, including pharmacotherapy, is free to all MD Anderson Cancer Center patients and justified by cost savings.
The MD Anderson Tobacco Treatment Program reports impressive effectiveness data [15, 57]. For patients who had at least one in-person appointment from January 2006 through August 2013 and were reached at follow-up (n = 2,085 individuals, response rate 75%), the 9-month abstinence rate was 47%. Using a modified in- tent-to-treat model (i.e., counting those lost to follow-up as smokers), the 9-month abstinence rate was 38%. When MD Anderson instituted an automatic, proactive referral system, participation dramatically increased, resulting in over 5,000 automatic referrals per year, with approximately 1,100 individuals entering face-to-face treatment [15].
Similar efforts are being initiated at cancer care centers across the country, all with the goal of improving tobacco treatment in oncology care. For example, the Tobacco Cessation Program at the Stanford Cancer Center is in an early stage of development. The program similarly begins with a thorough in-person assessment to obtain smoking history, improve motivation, monitor breath carbon monoxide, and determine an individualized treatment plan.
Patients are then referred to a weekly psychotherapy and skills training group as well as a medication consultation, if appropriate. The psychotherapy group consists of once-a-week, hour-long sessions for at least 8 weeks aimed at teaching mindfulness and cognitive behavioral skills to make a quit attempt and cope with cigarette cravings. The program also includes educational sessions on pharmacotherapy, and a medication consultation is available immediately after every group. For some patients, hearing others’ experiences with medications in the group provides the impetus to get started. All patients referred to the program, regardless of whether they engaged in treatment, are contacted for telephone follow-up at 3 and 9 months following referral. The follow-up calls consist of brief questions to assess current smoking and recent quit attempts, as well as motivational interviewing to encourage re-engagement in treatment if they are smoking. Box 1 provides a case example of the program at work. Planned future enhancements to the program include automatic referral via the electronic medical record and telemedicine services offered to those unable to come to treatment in person.
Conclusion
Given the significant impact of smoking on cancer prognosis and the cost of oncology treatment, treating tobacco dependence needs to be an essential part of cancer treatment. The advice to quit smoking that often takes place in an oncologist’s office is laudable and necessary, but generally not sufficient to promote long-term abstinence among tobacco-dependent cancer patients. Comprehensive tobacco treatment that addresses the psychosocial, behavioral, and biological aspects of a tobacco use disorder can produce impressive quit rates among the oncology population and thus warrants greater institutional support and dissemination into practice. Automatic referrals reduce the need to rely on individual provider referrals and greatly expand the program impact. Continued follow-up by phone, with referrals for additional support if a relapse occurs, will help sustain the initial success of a quit attempt, promote long-term abstinence, and help patients recover quickly if they slip back to smoking. Systematic follow-up is necessary and addresses the reality of tobacco dependence as a treatable, chronic, relapsing condition. Further, oncology providers who help link their patients to the appropriate level of addiction treatment can help their cancer patients quit smoking and ultimately improve their cancer prognosis and quality of life. Free and accessible tobacco treatment programs provide an opportunity for significant cost savings, especially as systems transition to value-based care or bundled payment models.
Supplementary Material
Acknowledgments
Funding Sources
Ms. Kaiser and Dr. Kendra’s time on this review was supported by the Stanford Cancer Institute Clinical Innovation Fund. Dr. Prochaska’s time on this review was supported by the National Cancer Institute, grant No. R01CA204356.
Footnotes
Disclosure Statement
Dr. Prochaska has served as an expert witness in lawsuits against the tobacco companies and has provided consultation to pharmaceutical and technology companies that make medications and other treatments for quitting smoking. The other authors have no potential conflicts of interest to disclose.
References
- 1.Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Kosary C, et al. (eds): SEER Cancer Statistics Review, 1975–2014. Bethesda, National Cancer Institute, 2017. [Google Scholar]
- 2.Hashim D, Boffetta P, La Vecchia C, Rota M, Bertuccio P, Malvezzi M, et al. : The global decrease in cancer mortality: trends and disparities. Ann Oncol 2016;27:926–933. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Siegel RL, Ward EM, Jemal A: Global cancer incidence and mortality rates and trends - an update. Cancer Epidemiol Biomarkers Prev 2016;25:16–27. [DOI] [PubMed] [Google Scholar]
- 4.Moyer VA: Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330–338. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2015 Risk Factors Collaborators: Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United States Department of Health and Human Services: The health consequences of smoking - 50 years of progress a report of the surgeon general. Rep Surg Gen 2014;1081. [Google Scholar]
- 7.Jensen K, Jensen aB, Grau C: Smoking has a negative impact upon health related quality of life after treatment for head and neck cancer. Oral Oncol 2007;43:187–192. [DOI] [PubMed] [Google Scholar]
- 8.Parsons A, Daley A, Begh R, Aveyard P: Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 2010;340:b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browman GP, Mohide EA, Willan A, Hodson I, Wong G, Grimard L, et al. : Association between smoking during radiotherapy and prognosis in head and neck cancer: a follow-up study. Head Neck 2002;24:1031–1037. [DOI] [PubMed] [Google Scholar]
- 10.Cataldo JK, Dubey S, Prochaska JJ: Smoking cessation: an integral part of lung cancer treatment. Oncology 2010;78:289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason DP, Subramanian S, Nowicki ER, Grab JD, Murthy SC, Rice TW, et al. : Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg 2009;88:362–371. [DOI] [PubMed] [Google Scholar]
- 12.Lindstrom D, Sadr Azodi O, Wladis A, T0nnesen H, Linder S, Nàsell H, et al. : Effects of a perioperative smoking cessation intervention on postoperative complications. Ann Surg 2008;248:739–745. [DOI] [PubMed] [Google Scholar]
- 13.S0rensen LT, H0rby J, Friis E, Pilsgaard B, J0rgensen T: Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol 2002;28:815–820. [DOI] [PubMed] [Google Scholar]
- 14.Ehlers SL, Gastineau DA, Patten CA, Decker PA, Rausch SM, Cerhan JR, et al. : The impact of smoking on outcomes among patients undergoing hematopoietic SCT for the treatment of acute leukemia. Bone Marrow Transplant 2011;46:285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karam-Hage M, Cinciripini PM, Gritz ER: Tobacco use and cessation for cancer survivors: an overview for clinicians. CA Cancer J Clin 2014;64:272–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR: Smoking at diagnosis and survival in cancer patients. Int J Cancer 2013; 132:401–410. [DOI] [PubMed] [Google Scholar]
- 17.Duffy SA, Ronis DL, McLean S, Fowler KE, Gruber SB, Wolf GT, et al. : Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol 2009;27:1969–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin DW, Park JH, Kim SY, Park EW, Yang HK, Ahn E, et al. : Guilt, censure, and concealment of active smoking status among cancer patients and family members after diagnosis: a nationwide study. Psychooncology 2014;23: 585–591. [DOI] [PubMed] [Google Scholar]
- 19.Prochaska JJ, Benowitz NL: The past, present, and future of nicotine addiction therapy. Annu Rev Med 2016;67:467–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabius V, Karam-Hage M, Blalock JA, Cinciripini PM: “Meaningful use” provides a meaningful opportunity. Cancer 2014; 120: 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flanders WD, Lally CA, Zhu B-P, Henly J, Thun MJ: Lung cancer mortality in relation to age, duration of smoking, and daily cigarette consumption: results from Cancer Prevention Study II. Cancer Res 2003;63:6556–6562. [PubMed] [Google Scholar]
- 22.Walker MS, Vidrine DJ, Gritz ER, Larsen RJ, Yan Y, Govindan R, et al. : Smoking relapse during the first year after treatment for early- stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev 2006;15:2370–2377. [DOI] [PubMed] [Google Scholar]
- 23.Cox L, Africano N, Tercyak K, Taylor K: Nicotine dependence treatment for patients with cancer. Cancer 2003;98:632–644. [DOI] [PubMed] [Google Scholar]
- 24.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Smoking Cessation Version 1.2017. 2017. DOI: 10.7326/AITC201603010. [DOI] [Google Scholar]
- 25.Slatore CG, Au DH, Hollingworth W: Cost- effectiveness of a smoking cessation program implemented at the time of surgery for lung cancer. J Thorac Oncol 2009;4:499–504. [DOI] [PubMed] [Google Scholar]
- 26.Warren GW, Marshall JR, Cummings KM, Toll B a, Gritz ER, Hutson A, et al. : Addressing tobacco use in patients with cancer: a survey of American Society of Clinical Oncology members. J Oncol Pract 2013;9:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. : A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 2008;35:158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institute for Health Care Excellence: Smoking: reducing and preventing tobacco use. 2015. https://www.nice.org.uk/guidance/qs82 (accessed June 4, 2018).
- 29.Vidrine JI, Shete S, Cao Y, Greisinger A, Harmonson P, Sharp B, et al. : Ask-advise-connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med 2013;173:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aveyard P, Begh R, Parsons A, West R: Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction 2012;107:1066–1073. [DOI] [PubMed] [Google Scholar]
- 31.Stead LF, Koilpillai P, Fanshawe T, Lancaster T: Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2016; 3: CD008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foster C, Fenlon D: Recovery and self-management support following primary cancer treatment. Br J Cancer 2011;105:S21–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cahill K, Stevens S, Perera R, Lancaster T: Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2013; 5:CD009329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price S, Hitsman B, Veluz-Wilkins A, Blazekovic S, Brubaker TR, Leone F, et al. : The use of varenicline to treat nicotine dependence among patients with cancer. Psychooncology 2017;26:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lancaster T, Stead LF: Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2017;CD001292. [DOI] [PubMed] [Google Scholar]
- 36.Stead LF, Carroll AJ, Lancaster T: Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2017;CD001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T: Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2013; 8:CD002850. [DOI] [PubMed] [Google Scholar]
- 38.Taylor GMJ, Dalili MN, Semwal M, Civljak M, Sheikh A, Car J: Internet-based interventions for smoking cessation. Cochrane Database Syst Rev 2017;9:CD007078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehnert A, Brahler E, Faller H, Harter M, Keller M, Schulz H, et al. : Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol 2014;32:3540–3546. [DOI] [PubMed] [Google Scholar]
- 40.Gritz ER, Fingeret MC, Vidrine DJ, Lazev AB, Mehta NV, Reece GP: Smoking cessation in cancer patients. Cancer 2006;106:17–27. [DOI] [PubMed] [Google Scholar]
- 41.Kabat-Zinn J, Hanh TN: Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York, Delta Trade Paperbacks, 2009. [Google Scholar]
- 42.Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, et al. : Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: a randomized clinical trial. J Consult Clin Psychol 2016;84:824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowen S, Marlatt A: Surfing the urge: brief mindfulness-based intervention for college student smokers. Psychol Addict Behav 2009; 23:666–671. [DOI] [PubMed] [Google Scholar]
- 44.Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, et al. : Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend 2011;119:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis JM, Goldberg SB, Anderson MC, Manley AR, Smith SS, Baker TB: Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Subst Use Misuse 2014;49:571–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis JM, Manley AR, Goldberg SB, Smith SS, Jorenby DE: Randomized trial comparing mindfulness training for smokers to a matched control. J Subst Abuse Treat 2014; 47:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis JM, Mills DM, Stankevitz KA, Manley AR, Majeskie MR, Smith SS: Pilot randomized trial on mindfulness training for smokers in young adult binge drinkers. BMC Complement Altern Med 2013;13:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlson LE: Mindfulness-based cancer recovery. The development of an evidence-based psychosocial oncology intervention. Oncol Exch 2013;12:21–25. [Google Scholar]
- 49.Nayan S, Gupta MK, Strychowsky JE, Sommer DD: Smoking cessation interventions and cessation rates in the oncology population: an updated systematic review and meta-analysis. Otolaryngol Head Neck Surg 2013; 149:200–211. [DOI] [PubMed] [Google Scholar]
- 50.Duffy SA, Ronis DL, Valenstein M, Lambert MT, Fowler KE, Gregory L, et al. : A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol Biomarkers Prev 2006;15: 2203–2208. [DOI] [PubMed] [Google Scholar]
- 51.Klemp I, Steffenssen M, Bakholdt V, Thygesen T, Sorensen JA: Counseling is effective for smoking cessation in head and neck cancer patients - a systematic review and meta-analysis. J Oral Maxillofac Surg 2016; 74: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 52.Miller WR, Rollnick S: Motivational Interviewing: Helping People Change. New York, Guilford Press, 2012. [Google Scholar]
- 53.Lai DT, Cahill K, Qin Y, Tang JL: Motivational interviewing for smoking cessation. Cochrane database Syst Rev 2010;1:CD006936. [DOI] [PubMed] [Google Scholar]
- 54.Hall SM, Humfleet GL, Muñoz RF, Reus VI, Prochaska JJ, Robbins JA: Using extended cognitive behavioral treatment and medication to treat dependent smokers. Am J Public Health 2011;101:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobbie F, Hiscock R, Leonardi-Bee J, Murray S, Shahab L, Aveyard P, et al. : Evaluating long-term outcomes of NHS stop smoking services (ELONS): a prospective cohort study. Health Technol Assess (Rockv) 2015;19:9–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pommerenke A, Alberg A, Brandon TH, Croghan I, Cummings MK, Dresler C, et al. : Physician preferences in tobacco cessation support for cancer patients: a survey of physicians at National Cancer Institute Designated Cancer Centers. Cancer Res 2014;74:5049. [Google Scholar]
- 57.Karam-Hage M, Oughli HA, Rabius V, Beneventi D, Wippold RC, Blalock JA, et al. : Tobacco cessation treatment pathways for patients with cancer: 10 years in the making. JNCCN J Natl Compr Cancer Netw 2016;14: 1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mee-Lee DE (ed): The ASAM Criteria: Treatment Criteria for Addictive, Substance-Related and Co-occurring Conditions, ed 3 Carson City, The Change Companies, 2013. [Google Scholar]
- 59.Williams JM, Steinberg ML, Kenefake AN, Burke MV: An argument for change in tobacco treatment options guided by the ASAM criteria for patient placement. J Addict Med 2016;10:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayo Foundation for Medical Education and Research: Nicotine Dependence Center in Minnesota - Mayo Clinic 2017. https://www.mayoclinic.org/departments-centers/nico-tine-dependence-center/minnesota/services/residential-treatment-program (accessed December 2, 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.