Abstract
Background:
Cerebellar mutism (CM), pseudobulbar palsy, posterior fossa syndrome (PFS), and cerebellar cognitive affective syndrome (CCAS) are terms that have been used, sometimes interchangeably, to refer to the complex neurological constellation that occurs following surgical removal of cerebellar and fourth ventricular tumors, mostly in children, but also sometimes in adults.
Methods:
This paper reviews the origins of what is now regarded as pediatric post-operative cerebellar mutism, the cerebellar cognitive affective syndrome, and the neurological manifestations of injury to or disruption of brainstem and cerebellar structures. It examines the specific components of each of these phenomena in the context of the evolving understanding of the role of the cerebellum in nervous system function.
Results:
Children undergoing surgical management of tumors in the posterior cranial fossa are at risk of experiencing cranial neuropathies, corticospinal damage, cerebellar ataxia and related motor disorders, neuropsychiatric and cognitive changes, and in some patients, mutism. These clinical presentations are differentiated from each other and examined in the context of the relevant anatomical stuctures and distributed neural circuits. The term posterior fossa syndrome is not sufficiently helpful in distinguishing the different elements of the clinical phenomena from each other, and because of this lack of precision and specificity there is consensus among investigators in the international Posterior Fossa Society that the designation be retired.
Conclusions:
Using contemporary brain imaging methods and guided by careful clinical observation and meticulous definition of clinical phenomenology, it is now feasible to perform detailed structure function correlation analyses to achieve two critical goals in the care of children with tumors in the posterior cranial fossa. The first goal is to identify and understand the neural circuits responsible for the different manifestations – arousal, cranial neuropathies, long tract signs, cerebellar motor syndrome, cerebellar vestibular syndrome, cerebellar cognitive affective syndrome including emotional dyscontrol, and mutism. The second goal is to transform this knowledge into practical clinical intervention, preventing the complications inherent in the necessary surgery whenever possible, and developing new approaches to treatment with methods including brain modulation targeting interconnected nodes of the damaged neural circuits.
Keywords: Cerebellar cognitive affective syndrome, cerebellar mutism, posterior fossa syndrome, emotion, cognition, behavior
Preamble
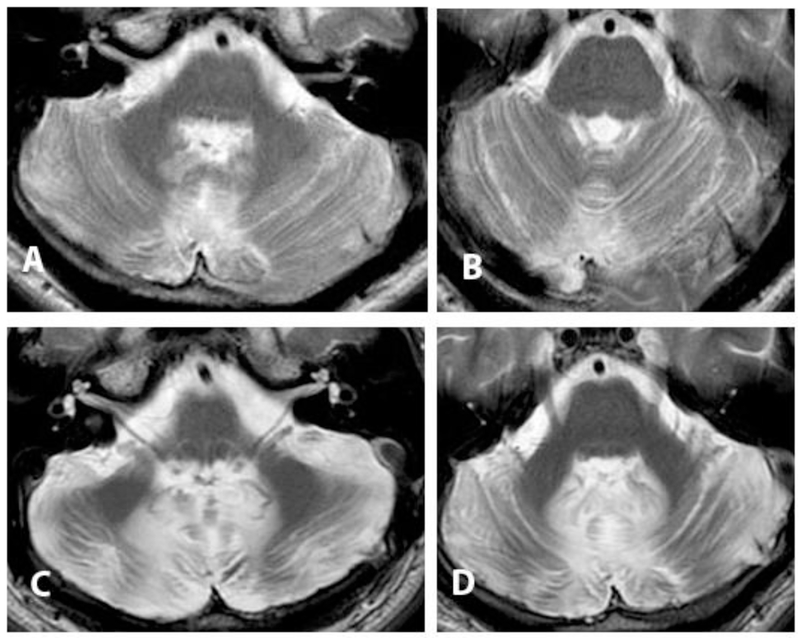
Andrew is a 35-year-old man who underwent resection of a choroid plexus papilloma at age 19, heralded by progressive school failure, attentional deficit and emotional dysregulation and then headache that prompted brain imaging which showed the tumor. Resection via a vermis splitting approach was complicated by mutism for two months, before speech started to return with a dysarthric quality. He had a severe cerebellar motor syndrome affecting gait, trunk and extremities, prominent nystagmus and internuclear ophthalmoplegia, mild right hemiparesis, and complete bilateral facial paralysis. He underwent successful gracilis muscle transplant to enable facial expression. Sequential brain imaging revealed progressive cerebellar volume loss, and white matter signal hyperintensity on T2-weighted magnetic resonance imaging which was consistent with gliosis (Figure 1). He experienced incremental but meaningful improvement over the ensuing 15 years with physical interventions, graduating from wheelchair to two-person assisted gait and then independent but slow use of a walker, still with a moderately severe cerebellar motor syndrome attested to by his Brief Ataxia Rating Scale [1] score of 18 / 30. Medications helped control resting appendicular and truncal tremor. For the first year after surgery, Andrew had a major personality change with irritability, aggression, impulsivity, explosiveness, irrationality and child-like regression. His parents reported that his thinking would “go around in circles” in an obsessive manner. Neuropsychological testing 2 years after surgery revealed lowered overall intellectual function compared to presurgical scores, including deficits in working memory and verbal fluency. The cognitive and neuropsychiatric constellation improved with the aid of targeted therapeutic interventions. When asked in retrospect what he recalls as the major life-affecting traits at the time that he was so impaired, Andrew recalled that for about 8 to 10 years after surgery he was not able to appreciate social cues. “If I did something wrong I was not able to pick up on it, so I kept doing the wrong thing over and over again”. He needed to be coached about social cues repeatedly, as it “did not stick with just one coaching session”. He lives in a separate apartment in his parents’ home and is engaged in social and community causes.
Figure.
T2-weighted brain MRI axial sections though the caudal and mid-pons following resection via vermis spitting approach of a choroid plexus papilloma. A, B: 2 years post-operatively, age 19. C, D: 15 years post-operatively, age 34. There is progressive volume loss, and signal hyperintensity in cerebellar white matter consistent with gliosis. The patient experienced cerebellar mutism, cerebellar cognitive affective syndrome, cerebellar motor syndrome, cranial neuropathies and corticospinal signs.
Introduction
Cerebellar mutism (CM), pseudobulbar palsy, posterior fossa syndrome (PFS), and cerebellar cognitive affective syndrome (CCAS) are the terms that have been used, sometimes interchangeably, to refer to the complex neurological constellation that occurs following a delay after surgical removal of cerebellar and fourth ventricular tumors, comprising variably mutism, cranial neuropathies, long tract signs, cerebellar motor phenomena, and neuropsychiatric and cognitive changes. The paper reviews the origins of what we now regard as pediatric post-operative cerebellar mutism, the cerebellar cognitive affective syndrome, and the neurological manifestations of injury to or disruption of brainstem and cerebellar structures that are the basis of the deficits that occur in this patient population. It harmonizes with the contributions of other investigators in the international Posterior Fossa Society by providing historical context to the decision to retire the PFS terminology.
The original reports
The title of the paper by Wisoff and Epstein [2], Pseudobulbar palsy after posterior fossa operation In children, reflected their original observation of an unusual postoperative syndrome in 7 children occurring after extirpation of large midline vermian or 4th ventricular tumors. Seven patients were described with neurological abnormalities that set in between 24 and 72 hours after surgery. In case 3, the findings were confined to cranial neuropathy and truncal ataxia, while these brainstem and cerebellar or long tract motor findings were noted variably in the remaining patients together with the other principal features described in the report. Four patients (cases 1, 2, 6 and 7) developed mutism or aphonia, becoming progressively unable to produce words or sounds. Four patients (cases 4 through 7) demonstrated extreme irritably and marked emotional lability. All improved to what was described as a normal neurological baseline, mostly between 2 weeks and 6 months later, and 18 months in case 2. The authors noted that Baily et al [3] reported a child with stupor, dysarthria and dysphagia (but not mutism) coming on 3 days following otherwise uneventful excision of a 4th ventricle ependymoma. An early report of mutism following cerebellar injury was included in the study of Guidetti and Fraioli [4] who were treating spasticity with bilateral simultaneous lesions of the dentate nuclei, in which two patients were reported to have developed total inability to speak for up to 3 months. The study of medulloblastomas in childhood by Hirsch et al [5] has passing mention of post-operative mutism but this was not further discussed. Mutism was therefore part of the Wisoff and Epstein report, even though the title emphasized the behavioral change within the context of what was regarded at the time as pseudobulbar affect and/or emotional lability.
Rekate and colleagues [6] in their paper Muteness of cerebellar origin drew attention specifically to the delayed onset mutism following cerebellar tumor excision in two patients. In an 8-year old girl with a vermis predominant medulloblastoma whose excision was complicated by hemorrhage in the tumor bed, mutism developed 24 hours after the second surgery to evacuate the hemorrhage. A 6-year-old boy with a midline and left cerebellar hemisphere astrocytoma became withdrawn on the fourth day after surgical excision and responded to questions only with a whine. As he started to recover 4 weeks later, he had difficulty finding correct articulatory postures which the authors felt were indicative of dyspraxia, at 6 weeks post-surgery he demonstrated what was felt to be characteristic cerebellar dysarthria, and he was described as returning to normal by 6 months.
Confirmatory reports
Subsequent case reports and larger patient series demonstrated the accuracy of these early observations. Yonemasu [7] is reported to have presented an abstract at the 13th annual meeting of the Japanese Society for Pediatric Neurosurgery describing four patients with large fourth ventricle tumor resections who developed delayed post-operative mutism which evolved into cerebellar dysarthria within 1 to 3 months and subsequently to near or complete resolution. In a letter in response to the Rekate et al report, Volcan et al [8] described an 8-year-old girl with a fourth ventricle medulloblastoma resected via a vermis splitting approach, who developed mutism and evolving hemiparesis the day following surgery, and a shrill, whining cry when people in white coats appeared at her bedside. In his book review of Concepts in Pediatric Neurosurgery (1989) containing the proceedings of the 11th Annual Meeting of the American Society for Paediatric Neurosurgery that was held in February 1988, Hayward [9] writes that the volume contained a “good description by Robin Humphreys of Toronto of the strange behavioural state (including mutism) that can complicate an otherwise apparently successful removal of a large tumour from the fourth ventricle.” In this report, Humphreys [10] described 5 patients with mutism in children recovering from posterior fossa surgery, which was delayed in onset in 3 and associated with neurological decline, and which evolved into cerebellar dysarthria with apparent subsequent complete or near complete recovery. Ammirati et al [11] reported a 14-year old boy who underwent removal of a midline grade 1 cystic astrocytoma through a vermis-splitting approach, and developed mutism 24 hours later, together with appendicular dysmetria and ataxia. As the mutism started to resolve he had dysarthric speech which improved as he recovered over the ensuing 4 months. Some of the six children (6 to 16 years of age) studied by Hudson et al [12] at least one year after surgical excision of posterior fossa tumors had dysarthria and/or language impairment. They concluded that muteness immediately post-surgery indicated a poor prognosis for speech, and they posited a link between post-operative radiation and long-term language disabilities.
Dietze and Mickle [13] described a 7-year old boy with a medulloblastoma and a 15-year-old girl with a ruptured cerebellar arteriovenous malformation, both of whom demonstrated the cerebellar motor syndrome and mutism. This was the first report of cerebellar hemorrhage causing mutism. Mutism in the post-operative case evolved to dysarthria which resolved almost completely by 3 months, but the patient with cerebellar hemorrhage “had a monotonous, slightly labored, and bradykinetic dysarthria” (page 27).
Additional case reports made it clear that the mutism following surgery in the posterior fossa in children was an entity to be recognized and understood and shared with families as a potential outcome following the necessary surgical intervention for treatment of the tumor. These included the reports by Ferrante et al (3 cases)[14], Nagatani et al (1 case)[15], Catsman-Berrevoets et al (3 cases)[16], Herb and Thyen (1 case)[17], Boratynski and Wocjan (2 cases)[18], Al-Jarallah et al (3 cases)[19], Kingma et al (4 cases)[20], Crutchfield et al (1 case of medulloblastoma)[21], Asamoto et al (1 case)[22], Aguiar et al (2 cases)[23], and Ersahin et al (7 cases)[24]. The series of van Dongen et al (15 patients) [25], regarded the phenomenon as the syndrome of ‘cerebellar’ mutism and subsequent dysarthria. Post-operative mutism in adults was noted early on as well, as in Salvati et al [26] whose 20-year old patient with medulloblastoma has a narrative that is indistinguishable from that of the younger children.
Dailey et al [27] reviewed 110 children who had undergone resection of posterior fossa tumors, 9 of whom developed post-operative mutism, a phenomenon they believed to represent “an apraxia, or inability to execute complex motor movements of the oral pharyngeal musculature as manifested postoperatively by mutism and difficulty in swallowing” (page 467). They believed this complication was related to the use of a vermal spitting approach to the tumor, and citing the work of Leiner et al [28, 29] and Schmahmann [30] they considered the possibility that it could be nested within the evolving understanding of the cerebellum “in higher functions such as learning, initiation, and cognitive processing” (page 472).
The neurobehavioral / neuropsychiatric component of the post-operative syndrome
Pollack et al [31] described 12 children out of 142 reviewed who manifested mutism and pseudobulbar symptoms. All their 12 patients manifested marked neurobehavioral abnormalities with “bizarre personality changes, emotional lability, and/or decreased initiation of voluntary movements” (page 885). Eleven exhibited an almost stereotypical response, remaining curled up in bed and whining inconsolably, with emotional lability and no intelligible speech. Neuropsychological testing demonstrated impairment in initiation and completion of age-appropriate motor activities, and impairments in recent memory, attention span, and problem-solving ability. The difficulties with cognition and initiation of activities ultimately cleared in each of these patients. The focus of Pollack and colleagues on the intellectual and emotional functioning of this cohort brought to the fore the pseudobulbar palsy features noted by Wisoff and Epstein [2]. They also re-introduced the problems highlighted by Hirsch et al [5] who concluded that the lives of children surviving medulloblastoma surgery are “frequently impaired by mental or behavioural disturbances. IQ varies from 70 to 90 in 58% of the children; it is below 70 in 31%. Behavioural disturbances are found in 93% of cases. 82% have defective spatial orientation, dysphasia, or dysgraphia” (page 1). Speech disorders in this cohort included “postoperative mutism, articulatory problems, slowness of speech, and speech retardation” (page 8). Hirsch and colleagues considered it “likely that the large prevalence of neuropsychological sequelae in medulloblastomas is due to the X-ray therapy which is given in these cases” (page 12).
Introduction and retirement of the term, posterior fossa syndrome
Dailey et al [27] seem to have been the first to use the designation posterior fossa syndrome (page 472) to refer to the post-operative mutism. Prior to that, reports described posterior fossa compression syndrome [32, 33], posterior cranial fossa syndrome [34, 35], posterior cerebral fossa tumoral syndrome [36], and posterior fossa syndrome in the setting of xanthomatosis of the central nervous system [37], histiocytosis X ([38], and Hand-Schüller-Christian disease [39].
The first appearance of posterior fossa syndrome as the title of a manuscript in the setting of children having undergone posterior fossa brain surgery was a nursing study of Kirk et al. [40] published in the Journal of Pediatric Oncology Nursing. Here the authors reviewed 121 children with posterior fossa brain tumors and found a behavioral change in 19 cases. They reported: “a postoperative syndrome, labeled posterior fossa syndrome, has been identified in certain children. This syndrome involves a variety of signs and symptoms including mutism or speech disturbances, dysphagia, decreased motor movement, cranial nerve palsies and, emotional lability. These signs and symptoms develop from an average range of 24 to 107 hours after surgery and may take weeks to months to resolve”. They urged “early recognition of this syndrome to facilitate preventive and restorative patient care, prevent subsequent complications, decrease length of hospital stays, and promote patient and family understanding of and coping with the syndrome” (page 181).
The next appearance of the term posterior fossa syndrome in this context was the title of an invited chapter by Ian Pollack in the monograph, the Cerebellum and Cognition, completed in 1996 and published the following year [41]. The chapter title was chosen by the volume editor. The term was then used over the ensuing two decades, and a large number of papers on the topic have been published during this period using the various nomenclatures – CMS, PFS, pseudobulbar palsy, CCAS, in attempts to further elucidate the neurobiological underpinnings of the syndrome (see, e.g., http://www.posteriorfossa.org/posterior-fossa-syndrome-cerebellar-mutism-articles). It became apparent, however, that the detailed neurological and neurobehavioral aspects of the phenomenology resulting from the tumors and their treatment could not adequately be conceptualized within one overarching term (PFS), that patients did not always experience the same manifestations, and that more precision was needed to define the entity. The retirement of the term posterior fossa syndrome, and the development of the consensus definition of the post-operative pediatric cerebellar mutism syndrome and related conditions [42] represents an important precursor to improved diagnosis, targeted scientific inquiry, treatment and prevention. Notably, as defined by consensus, the discrete aspects of the post-operative syndrome include delayed onset mutism/reduced speech and emotional lability, hypotonia and oropharyngeal dysfunction/dysphagia, the cerebellar motor syndrome, cerebellar cognitive affective syndrome, and brainstem dysfunction including long tract signs and cranial neuropathies.
The cerebellar cognitive affective syndrome in relation to cerebellar mutism and pseudobulbar palsy
The field of the cognitive neuroscience of the cerebellum commenced in earnest in the 1980s and 1990s. This was sparked by the dysmetria of thought hypothesis [30] and its examination using tract tracing studies in animal models [43–48], and theoretical notions of the cerebellar role in cognition and emotion [30, 49–52].
The synopsis of the field of cerebellar cognition may be stated as follows (from [53]): What the cerebellum does to sensorimotor and vestibular control, it also does to cognition, emotion, and autonomic function. This hypothesis is based on the theories of dysmetria of thought and the universal cerebellar transform, which hold that the cerebellum maintains behavior around a homeostatic baseline, automatically, without conscious awareness, informed by implicit learning, and performed according to context. Functional topography within the cerebellum facilitates the modulation of distributed networks subserving multiple different functions. The sensorimotor cerebellum is represented in the anterior lobe with a second representation in lobule VIII, and lesions of these areas lead to the cerebellar motor syndrome of ataxia, dysmetria, dysarthria and impaired oculomotor control. The cognitive / limbic cerebellum is in the cerebellar posterior lobe, with current evidence pointing to three separate topographic representations, the nature of which remain to be determined. Posterior lobe lesions result in the cerebellar cognitive affective syndrome (CCAS), the hallmark features of which include deficits in executive function, visual spatial processing, linguistic skills and regulation of affect. The affective dyscontrol manifests in autism spectrum and psychosis spectrum disorders, and disorders of emotional control, attentional control, and social skill set. Together with the cerebellar motor syndrome and the cerebellar vestibular syndrome, the CCAS is thus conceptualized as the third cornerstone of clinical ataxiology (Schmahmann syndrome, [54]).
The CCAS was described first in adults in preliminary form by Schmahmann and Sherman [55] in the same edited monograph in which Pollack’s [41] posterior fossa syndrome chapter appeared, before its peer-reviewed publication [56]. At this time, Levisohn et al [57] were investigating the neuropsychological consequences of children who had undergone tumor resection, but without the complicating factor of radiation therapy, a potential critical confound as noted before by Hirsch et al [5] and others, or of methotrexate that similarly impairs cognition through its impact on myelinated pathways [58,59]. The Levisohn et al [57] study identified the CCAS in children with tumor excisions and noted the cerebellar mutism syndrome (then called posterior fossa syndrome) in some of these children with the social-emotional-cognitive impairments. The core elements of the CCAS were deficits in executive function, including planning and sequencing, and in visual-spatial function, expressive language, verbal memory and modulation of affect. These deficits were common and, in some cases, could be dissociated from motor deficits. Lesions of the vermis in particular were associated with dysregulation of affect. Behavioral deficits were more apparent in older than younger children. These results revealed that clinically relevant neuropsychological changes may occur following cerebellar tumor resection in children in the absence of radiation treatment of chemotherapeutic toxicity. This first demonstration was relevant also for its prediction that cerebellar damage in childhood may influence a wide range of psychological processes, both as an immediate consequence, and as these processes fail to develop normally later, reflecting the sustaining trophic influence of the cerebellum in typical cognitive and motor development. Riva and Giorgi [60] reported a similar experience. Children surviving resection of tumors in the right cerebellar hemisphere presented with disturbances of auditory sequential memory and language processing; those with left cerebellar tumors showed deficits on tests of spatial and visual sequential memory; and children with vermal lesions had post-surgical mutism which evolved into dysarthria and/ or agrammatism, and behavioral disturbances ranging from irritability to features reminiscent of autism.
Attempts to reconcile the syndromes and terminologies
Initial suggestions that postoperative mutism was a psychiatric / behavioral response to the major trauma of the diagnosis and the fact of neurosurgery are no longer entertained, but the underlying nature of the disorder is not yet settled. Early in the discussion some argued that it is a severe form of dysarthria [61], but this view has not prevailed.
Sadeh and Cohen [62] considered the postoperative mutism as one aspect of the larger neuropsychological entity - the CCAS. They suggested that the plasticity in PFS/CMS/CCAS might be explained by the feed-forward and feedback interconnections within the homologous areas of the cerebrocerebellar circuit, and these interconnections could be critical in the neural plasticity that would facilitate recovery of the cognitive and behavioral processes.
In their discussion of the relation of CMS to CCAS, Wells et al. [63] regarded CMS as consisting of diminished speech output, hypotonia, ataxia, and emotional lability. They emphasized its long-term adverse neurological, cognitive, and psychological sequelae, concluding that CMS shares many similarities with the CCAS, although it remained to be shown why some children undergoing surgery for posterior fossa tumors are more vulnerable to developing mutism.
These views were expanded upon by neurolinguist Peter Mariën [64], who expressed in committee at the Posterior Fossa Society Iceland Delphi Consensus Conference in Reykjavik in 2015 that the mutism and the wider behavioral syndrome are a form of acute presentation of the CCAS. Notably, marked diminution of semantic and phonemic verbal fluency was observed in patients in the original series of adults and children with CCAS [56, 57] and in subsequent studies (e.g., [65], [66]). In agreement with this notion is the fact that transient mutism occurs also, although more rarely, in adults with the CCAS after surgical interventions for cerebellar lesions (medulloblastoma [26]; metastatic carcinoma of the lungs [67]; hemangioblastoma and metastatic tumor [68]; hemorrhage [69]), and even without surgical intervention (hemangioblastoma [70]; hemorrhage [71]).
Proposed resolution of the entities and terminology as a guide for future study
The hypotheses of dysmetria of thought and the universal cerebellar transform (UCT) are based on the contrasting but complementary realties of (i) an essentially consistent cerebellar architecture (the basis of the constant computation by the cerebellar cortex, that is, the UCT), and (ii) the complex heterogeneity and precise topographic arrangement of the cerebellar connections with extracerebellar structures [30, 50, 81]. These connections are the underpinnings of multiple, highly organized anatomical subsystems in the cerebrocerebellar circuit which facilitate the contribution of the UCT to many different functional domains – sensorimotor, vestibular, cognitive, affective, and autonomic. The dysmetria of thought theory holds that the incorporation of the cerebellum and its neural computation (the UCT) into these distributed neural circuits modulates and optimizes harmonious motor, cognitive, and affective behaviors implicitly without conscious awareness, and according to context. Damage to the cerebellar component of these neural circuits produces dysmetria of thought that results in the cerebellar cognitive affective syndrome. The elucidation of the different manifestations of cerebellar injury following posterior fossa surgery is therefore best understood by defining where the lesion is located, and which interconnected circuits are affected.
Children undergoing resection of cerebellar tumors may experience the following difficulties in varying combinations and degrees of severity. Conclusions regarding level of arousal, cranial neuropathies, and long tract signs reflect well-established neurological and neurosurgical principles. The understanding of the cerebellar role in the remaining features has become clearer in recent years through a steady program of structure-function correlation in tract tracing experiments, animal models, patient studies and functional imaging investigations in clinics and laboratories throughout the world.
Neurological – neuropsychiatric manifestations of children undergoing resection of tumors in the posterior fossa
The range of disorders experienced by patients with tumors in the posterior fossa include the following:
Impaired level of arousal when it occurs is a complex combination of lesions of the brainstem reticular nuclei and related widespread interconnections with the diencephalon and cerebral hemispheres.
Cranial neuropathies arise from damage to brainstem nuclei and tracts as part of the underlying pathology or as consequence of the surgical approach to the management of the disease.
Long tract signs of weakness, hemiparesis, spasticity, hyper-reflexia and extensor plantar responses, arise from lesions of the descending pyramidal / corticospinal tracts in the midbrain, pons or medulla.
The cerebellar motor syndrome. This includes gait ataxia, truncal instability, extremity dysmetria, dysarthria, and oculomotor disorders. These motor control impairments result from damage to the cerebellar motor system and/or its connections. The cerebellar motor system is located in lobules III-V of the anterior lobe, extending into lobule VI, with a second representation in lobule VIII. It includes the deep cerebellar nuclei, mainly the interpositus (globose and emboliform) and the dorsal part of the dentate nucleus. Its spinocerebellar and pontocerebellar mossy fiber afferents and climbing fiber afferents from the accessory olivary nuclei, and its efferent connections linking the cerebellar motor system with the spinal cord, brainstem and cerebral hemispheres are included in this conceptualization. Cerebellar dysarthria – a motor phenomenon of articulatory clarity, is included in the motor system, and is distinct from mutism. Dysphagia is also common in patients with cerebellar disorders [72], and while oral pharyngeal apraxia may be part of the post-surgical constellation in children with posterior fossa tumors, there may be overlap with true dysphagia from incoordination of the voluntary muscles of deglutition.
The cerebellar vestibular syndrome. Severe oculomotor control disorders may be associated with marked truncal and gait instability. These arise from damage to the vestibulocerebellum including lobules IX and X (flocculonodular lobe) and the oculomotor vermis in lobule VII, the vestibular relevant regions of the fastigial nuclei and the brainstem vestibular nuclei that are essentially extracerebellar deep nuclei, and the spinal cord and brainstem afferent and efferent connections of these nuclei and cerebellar cortical regions.
-
The cerebellar cognitive affective syndrome is characterized by impaired control of executive function (planning, set-shifting, abstract reasoning, verbal fluency, working memory), often with preserveration, distractibility, or inattention; visual-spatial disorganization and impaired visual-spatial memory; personality change with blunting of affect or disinhibited and inappropriate behavior; and difficulties with language production, including dysprosodia, agrammatism, and mild anomia. Since the original description, it has been shown that the neuropsychiatric features include impairments in the modulation of five domains of behavior – attention, mood, social skill set, psychosis spectrum and autism spectrum disorders. The language impairments include metalinguistics which is the way we use language to communicate with each other and to understand nuance – such as metaphor, ambiguity, and inference, and express thoughts and ideas in a logically coherent and appropriately sequentially ordered stream of content [73]. The social skill set reflects theory of mind and social cognition – the ability to interact with others and in society with empathy, understanding, and awareness of boundaries, with a sense of the needs and feelings of others, and of our place in society [74]. These challenges inherent in the CCAS are faced by adults and children with a range of cerebellar disorders and occur with or without cerebellar mutism.
Most of the human cerebellum is unrelated to motor control [75–77]. There is a triple representation of cognition and emotion in anatomically distinct regions of the human cerebellar posterior lobe, lobules VI through IX, with some studies implicating vestibulocerebellar lobule X having a cognitive role as well [76, 78]. This conclusion derives from the converging lines of evidence from anatomy, physiology, lesion studies, and resting state and task based functional imaging experiments. This includes the deep cerebellar nuclei – the fastigial nucleus involved in limbic and autonomic circuits, and the ventral part of the dentate nucleus involved in cognitive domains. Corticopontine afferents from associative, limbic, and autonomic areas synapse around pontine neurons that then send their mossy fibers to the cerebellum through the middle cerebellar peduncles. Climbing fiber afferents from the principal inferior olivary nucleus engage in reverberating circuits of cognitive control with the dentate nucleus and cognitive cerebellum. Feedback circuits to the cerebral association and paralimbic areas arise from the ventral dentate and the fastigial nucleus and travel in the superior cerebellar peduncles to the thalamus where they synapse before projecting back to the cerebral cortices. These have been likened to closed loop circuits [79], although perhaps these loops are not exclusively closed.
Brainstem circuits other than the pontine and inferior olivary nuclei are also highly relevant in their influence on states of cognition and emotion as well. These include dopaminergic, serotonergic and noradrenergic systems, all of which have reciprocal connections with the cerebellum and are therefore relevant in the consideration of the cerebellar contribution to higher function, and to the clinical manifestations of children with tumors whose diseases often involve or invade brainstem and in whom the surgical approach frequently encroaches upon these brainstem systems.
Emotional lability is a frequent feature of the post-operative syndrome and also occurs in patients with midline cerebellar damage. Emotional lability was fully conceptualized within the original description of the CCAS in adults and children. It was defined as reflecting damage to the limbic cerebellum, namely, the vermis and related structures [30, 80, 81]. Cerebellar connections with the hypothalamus, amygdala, parahippocampal gyrus, and brainstem structures including the ventral tegmental area [81] that influence social and emotional behavior [82] provide the anatomical and neurobehavioral correlates of this clinical reality in experimental models.
-
In contrast to dysarthria which is impaired articulation of spoken words, mutism is the absence of sounds. For our purposes, given that children with CM often have high pitched whining, CM may be regarded as the phenomenon of being nonverbal, producing no words, but understanding spoken language. Mutism is also distinguished from aphasia, which is the impaired understanding of the symbols of language. Mutism often coexists with difficulty handling secretions and swallowing, which in this context has been regarded as an oral motor apraxia. Apraxia is a higher order motor disorder [83, 84] manifesting as the inability to perform a previously learned motor act despite the presence of a functioning motor system and comprehension of the required task. In CM, the patient lacks the ability to speak, yet is awake, not volitionally withholding speech, and is not in an akinetic mute state where there is no or minimal motor output of any kind. Cerebellar mutism, therefore, most closely resembles an apraxia of speech because it is a motor speech planning and programming disorder which selectively disrupts speech movements [85]. This view is consistent with the conclusion of Dailey et al. [27], and of Rekate et al [6] whose Case 1 had “full range of lip, tongue, and palatal movements on a non-voluntary basis, but no ability to imitate tongue and lip movements on command” (page 698), a description confirmed by others with almost exactly the same wording [60, page 1055]. CMS therefore falls within the CCAS as a deficit in cognitive initiation or cognitive control and may be regarded as a maximal manifestation of apraxia of speech.
The CCAS can occur without passing through a stage of mutism, as has been noted in both medical and surgical diseases of the cerebellum since the description of the CCAS. In contrast, patients like Andrew in our prologue who experience mutism with or without emotional lability are at high risk for the cognitive and emotional disturbances that characterize the CCAS. The fact that mutism is not a prerequisite to develop the CCAS implies that there are different circuits involved in the generation of these behaviors. The anatomy and circuit disruptions that result in mutism therefore need to be distinguished from the circuits that cause the remainder of the CCAS without mutism. This is a finely sculpted challenge and presents an opportunity for future study.
Defining the neural systems responsible for the mutism, CCAS, ataxia, and related disorders
Detailed knowledge of the cerebellum and related structures is critical to advancing the understanding and developing successful approaches to management of these many neurological and neuropsychiatric manifestations of children undergoing surgical treatments of posterior fossa tumors. In the search for etiological factors in the development of pediatric postoperative cerebellar mutism, investigators have pointed to vermal splitting, deep cerebellar nuclear involvement, and disruption of efferent pathways in the superior cerebellar peduncle. Mutism is described also following lesions of the supplementary and pre-motor cerebral cortices, descending corticospinal pathways, basis pontis, pontocerebellar pathways, cerebellum itself, and thalamus. All of these constitute the cerebrocerebellar circuit, and contemporary lesion network analysis makes it clear that damage to any node of an interconnected functional network may impair the outcome or behavioral response [86]. It is likely that each element of the clinical syndrome may have its own pathophysiological mechanism related to the neural circuits which are affected, either alone or in combination. It is essential to link the anatomy and systems pathophysiology with the different components of these presentations, and this exciting area of investigation is now coalescing around these new insights and appreciation of the clinical neuroscience.
Conclusion
Using contemporary brain imaging methods and guided by careful clinical observation and meticulous definition of clinical phenomenology, it is now feasible to perform detailed structure function correlation analyses to achieve two critical goals in the care of children with tumors in the posterior cranial fossa. First, to identify and understand the neural circuits responsible for the different manifestations – arousal, cranial neuropathies, long tract signs, cerebellar motor syndrome, cerebellar vestibular syndrome, cerebellar cognitive affective syndrome including emotional dyscontrol, and mutism. Second, to transform this knowledge into practical clinical intervention, preventing the complications inherent in the necessary surgery whenever possible, and developing new approaches to treatment with methods including brain modulation targeting interconnected nodes of the damaged neural circuits.
Acknowledgements:
Supported in part by NIH 1U01NS104326-01 and 1R01NS080816-01A1, the National Ataxia Foundation, Ataxia Telangiectasia Children’s Project, and the MINDlink Foundation.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest Statement:
Dr. Schmahmann discloses that he consults for Cadent Therapeutics, and is site Principal Investigator for Biohaven Pharma. He declares no conflict of interest relevant to content of this manuscript.
REFERENCES
- 01.Schmahmann JD, Gardner R, MacMore J, Vangel MG (2009) Development of a brief ataxia rating scale (BARS) based on a modified form of the ICARS. Mov Disord September 15;24(12):1820–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 02.Wisoff JH, Epstein FJ (1984) Pseudobulbar palsy after posterior fossa operation in children. Neurosurgery November;15(5):707–709. [DOI] [PubMed] [Google Scholar]
- 03.Baily P, Buchanan DN, Bucy PC (1939) Intracranial Tumors of Infancy and Childhood. Chicago, University of Chicago Press. [Google Scholar]
- 04.Guidetti B, Fraioli B (1977) Neurosurgical treatment of spasticity and dyskinesias. Acta Neurochir (Wien) (Suppl 24):27–39. [DOI] [PubMed] [Google Scholar]
- 05.Hirsch JF, Renier D, Czernichow P, Benveniste L, Pierre-Kahn A (1979) Medulloblastoma in childhood. Survival and functional results. Acta Neurochir (Wien) 48(1-2):1–15. [DOI] [PubMed] [Google Scholar]
- 06.Rekate HL, Grubb RL, Aram DM, Hahn JF, Ratcheson RA (1985) Muteness of cerebellar origin. Arch Neurol July;42(7):697–698. [DOI] [PubMed] [Google Scholar]
- 07.Yonemasu Y (1985) Cerebellar mutism and speech disturbance as a complication of posterior fossa surgery in children. 13th Annual Meeting of the Japanese Society of Pediatric Neurosurgery, Tsukuba, Japan [Google Scholar]
- 08.Volcan I, Cole GP, Johnston K (1986) A case of muteness of cerebellar origin. Arch Neurol. April;43(4):313–314. [DOI] [PubMed] [Google Scholar]
- 09.Hayward R (1989) Journal of Neurology, Neurosurgery & Psychiatry, 1 July, Vol.52(7):932. [Google Scholar]
- 10.Humphreys RP (1989) Mutism after posterior fossa tumor surgery In: Marlin AE (ed) Concepts in Pediatric Neurosurgery. Karger, Basel, pp 57–64. [Google Scholar]
- 11.Ammirati M, Mirzai S, Samii M (1989) Transient mutism following removal of a cerebellar tumor. A case report and review of the literature. Childs Nerv Syst February;5(1):12–14. [DOI] [PubMed] [Google Scholar]
- 12.Hudson LJ, Murdochj BE, Ozanne AE (1989) Posterior fossa tumours in choildhood: Associated speech and language disorders post-surgery. Aphasiology 3(1):1–18. [Google Scholar]
- 13.Dietze DD Jr, Mickle JP (1990-1991) Cerebellar mutism after posterior fossa surgery. Pediatr Neurosurg 16(1):25–31; discussion 31. [DOI] [PubMed] [Google Scholar]
- 14.Ferrante L, Mastronardi L, Acqui M, Fortuna A (1990) Mutism after posterior fossa surgery in children. Report of three cases. J Neurosurg June;72(6):959–963. [DOI] [PubMed] [Google Scholar]
- 15.Nagatani K, Waga S, Nakagawa Y (1991) Mutism after removal of a vermian medulloblastoma: cerebellar mutism. Surg Neurol October;36(4):307–309. [DOI] [PubMed] [Google Scholar]
- 16.Catsman-Berrevoets CE, van Dongen HR, Zwetsloot CP (1992) Transient loss of speech followed by dysarthria after removal of posterior fossa tumour. Dev Med Child Neurol December;34(12):1102–1109. [DOI] [PubMed] [Google Scholar]
- 17.Herb E, Thyen U (1992) Mutism after cerebellar medulloblastoma surgery. Neuropediatrics June;23(3):144–146. [DOI] [PubMed] [Google Scholar]
- 18.Boratyński W, Wocjan J (1993) Mutism after surgeries with removal of posterior cranial fossa neoplasms. [Article in Polish] Neurol Neurochir Pol Mar-Apr;27(2):261–265. [PubMed] [Google Scholar]
- 19.al-Jarallah A, Cook JD, Gascon G, Kanaan I, Sigueira E (1994) Transient mutism following posterior fossa surgery in children. J Surg Oncol February;55(2):126–131. [DOI] [PubMed] [Google Scholar]
- 20.Kingma A, Mooij JJ, Metzemaekers JD, Leeuw JA (1994) Transient mutism and speech disorders after posterior fossa surgery in children with brain tumours. Acta Neurochir (Wien) 131(1-2):74–79. [DOI] [PubMed] [Google Scholar]
- 21.Crutchfield JS, Sawaya R, Meyers CA, Moore BD 3rd (1994) Postoperative mutism in neurosurgery. Report of two cases. J Neurosurg July;81(l):115–121. [DOI] [PubMed] [Google Scholar]
- 22.Asamoto M, Ito H, Suzuki N, Oiwa Y, Saito K, Haraoka J (1994) Transient mutism after posterior fossa surgery. Childs Nerv Syst May;10(4):275–278. [DOI] [PubMed] [Google Scholar]
- 23.Aguiar PH, Plese JP, Ciquini O, Marino R (1995) Transient mutism following a posterior fossa approach to cerebellar tumors in children: a critical review of the literature. Childs Nerv Syst May;11(5):306–310. [DOI] [PubMed] [Google Scholar]
- 24.Ersahin Y, Mutluer S, Cağli S, Duman Y (1996) Cerebellar mutism: report of seven cases and review of the literature. Neurosurgery. 38(1):60–5 [DOI] [PubMed] [Google Scholar]
- 25.van Dongen HR, Catsman-Berrevoets CE, van Mourik M (1994) The syndrome of ‘cerebellar’ mutism and subsequent dysarthria. Neurology November;44(11):2040–2046. [DOI] [PubMed] [Google Scholar]
- 26.Salvati M, Missori P, Lunardi P, Orlando ER (1991) Transient cerebellar mutism after posterior cranial fossa surgery in an adult. Case report and review of the literature. Clin Neurol Neurosurg 93(4):313–316. [DOI] [PubMed] [Google Scholar]
- 27.Dailey AT, McKhann GM 2nd Berger MS (1995) The pathophysiology of oral pharyngeal apraxia and mutism following posterior fossa tumor resection in children. J Neurosurg September;83(3):467–475. [DOI] [PubMed] [Google Scholar]
- 28.Leiner HC, Leiner AL, Dow RS (1989) Reappraising the cerebellum: what does the hindbrain contribute to the forebrain? Behav Neurosci 103:998–1008. [DOI] [PubMed] [Google Scholar]
- 29.Leiner HC, Leiner AL, Dow RS (1991) The human cerebro-cerebellar system: its computing, cognitive, and language skills. Behav Brain Res 44:113–128. [DOI] [PubMed] [Google Scholar]
- 30.Schmahmann JD (1991) An emerging concept: The cerebellar contribution to higher function. Arch Neurol 48:1178–1187. [DOI] [PubMed] [Google Scholar]
- 31.Pollack IF, Polinko P, Albright AL, Towbin R, Fitz C (1995) Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery November;37(5):885–893. [DOI] [PubMed] [Google Scholar]
- 32.Rogers L (1933) The posterior fossa compression syndrome. Br Med J July 15;2(3784):100–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendelowitsch A, Radue EW, Gratzl O (1990) Aneurysm, arteriovenous malformation and arteriovenous fistula in posterior fossa compression syndrome. Eur Neurol 30(6):338–342. [DOI] [PubMed] [Google Scholar]
- 34.Maneke M (1953) [Posterior cranial fossa syndrome in childhood; arachnoid cyst or adhesive meningopathy], Monatsschr Kinderheilkd April;101(4):239–243. [PubMed] [Google Scholar]
- 35.Brobeil A, Maneke M (1954) [Clinical aspects and pathogenesis of the so-called arachnoidal cysts of the cisterna magna; differential diagnosis of the posterior cranial fossa syndrome in infants]. Monatsschr Psychiatr Neurol Feb-Mar;127(2-3):103–121. [PubMed] [Google Scholar]
- 36.Pascu I (1965) [Thrombosis of the postero-inferior cerebellar artery after a cranial injury which resembles a posterior cerebral fossa tumoral syndrome (clinical case surgically verified)]. Neurol Psihiatr Neurochir Sep-Oct;10(5):429–432. [PubMed] [Google Scholar]
- 37.Beard W, Foster DB, Kepes JJ, Guillan RA (1970) Xanthomatosis of the central nervous system. Clinical and pathological observations of a case with a posterior fossa syndrome. Neurology April;20(4):305–314. [DOI] [PubMed] [Google Scholar]
- 38.Faivre J, Pecker J, Ferrand B (1975) [Posterior fossa syndrome terminating the course of histiocytosis X. Study of lesions of the central nervous system. Association with polyvinylpyrrolidone thesaurismosis]. [Article in French] Sem Hop September 10-20;51(37-38):2229–2237. [PubMed] [Google Scholar]
- 39.Pascual ML, Rodríguez C, García-Merino JA, López F, Liaño H, Barceló B (1984) [Posterior fossa syndrome in Hand-SchUller-Christian disease]. [Article in Spanish] Med Clin (Barc) May 19;82(19):863. [PubMed] [Google Scholar]
- 40.Kirk EA, Howard VC, Scott CA (1995) Description of posterior fossa syndrome in children after posterior fossa brain tumor surgery. J Pediatr Oncol Nurs October;12(4):181–187. [DOI] [PubMed] [Google Scholar]
- 41.Pollack IF (1997) Posterior fossa syndrome In: Schmahmann JD (ed) The Cerebellum and Cognition. Academic Press, San Diego, Int Rev Neurobiol 41:411–32. [DOI] [PubMed] [Google Scholar]
- 42.Gudrunardottir T, Morgan AT, Lux AL, et al. (2016) Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst July;32(7):1195–1203. [DOI] [PubMed] [Google Scholar]
- 43.Schmahmann JD, Pandya DN (1987) Posterior parietal projections to the basis pontis in rhesus monkey: Possible anatomical substrate for the cerebellar modulation of complex behavior? Neurology 37(Suppl. 1):291. [Google Scholar]
- 44.Schmahmann JD, Pandya DN (1989) Anatomical investigation of projections to the basis pontis from posterior parietal association cortices in rhesus monkey. J Comp Neurol 289:53–73. [DOI] [PubMed] [Google Scholar]
- 45.Schmahmann JD, Pandya DN (1993) Prelunate, occipitotemporal, and parahipppocampal projections to the basis pontis in rhesus monkey. J Comp Neurol 337:94–112. [DOI] [PubMed] [Google Scholar]
- 46.Schmahmann JD, Pandya DN (1997) Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci 17:438–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Middleton FA, Strick PL (1994) Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science October 21;266(5184):458–461. [DOI] [PubMed] [Google Scholar]
- 48.Schmahmann JD, Guell X, Stoodley CJ, Halko MA (2019) The theory and neuroscience of cerebellar cognition. Ann Rev Neurosci 42:In press. [DOI] [PubMed] [Google Scholar]
- 49.Leiner HC, Leiner AL, Dow RS (1986) Does the cerebellum contribute to mental skills? Behav Neurosci August;100(4):443–454. [DOI] [PubMed] [Google Scholar]
- 50.Schmahmann JD (1996) From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping 4:174–198. [DOI] [PubMed] [Google Scholar]
- 51.Schmahmann JD (1998) Dysmetria of thought. Clinical consequences of cerebellar dysfunction on cognition and affect. Trends in Cognitive Sciences 2:362–370. [DOI] [PubMed] [Google Scholar]
- 52.Ito M (1993) Movement and thought: identical control mechanisms by the cerebellum. Trends Neurosci November;16(11):448–450. [DOI] [PubMed] [Google Scholar]
- 53.Schmahmann JD (2019) The cerebellum and cognition. Neurosci Lett January 1;688:62–75. [DOI] [PubMed] [Google Scholar]
- 54.Manto M, Mariën P (2015) Schmahmann’s syndrome - identification of the third cornerstone of clinical ataxiology. Cerebellum Ataxias February 27;2:2. doi: 10.1186/s40673-015-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmahmann JD, Sherman JC (1997) The cerebellar cognitive affective syndrome In: Schmahmann JD (ed) The Cerebellum and Cognition. Academic Press, San Diego, Int Rev Neurobiol 41:433–440. [DOI] [PubMed] [Google Scholar]
- 56.Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 121:561–579. [DOI] [PubMed] [Google Scholar]
- 57.Levisohn L, Cronin-Golomb A, Schmahmann JD (2000) Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain May;123(Pt 5):1041–1050. [DOI] [PubMed] [Google Scholar]
- 58.Montour-Proulx I, Kuehn SM, Keene DL, Barrowman NJ, Hsu E, Matzinger MA, Dunlap H, Halton JM (2005) Cognitive changes in children treated for acute lymphoblastic leukemia with chemotherapy only according to the Pediatric Oncology Group 9605 protocol. J Child Neurol February;20(2):129–133. [DOI] [PubMed] [Google Scholar]
- 59.Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, Woo PJ, Barres BA, Liddelow S, Vogel H, Monje M (2019) Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell January 10;176(1-2):43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riva D, Giorgi C (2000) The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain May;123(Pt 5):1051–1061. [DOI] [PubMed] [Google Scholar]
- 61.Van Calenbergh F, Van de Laar A, Plets C, Goffin J, Casaer P (1995) Transient cerebellar mutism after posterior fossa surgery in children. Neurosurgery November;37(5):894–898. [DOI] [PubMed] [Google Scholar]
- 62.Sadeh M, Cohen I (2001) Transient loss of speech after removal of posterior fossa tumors--one aspect of a larger neuropsychological entity: the cerebellar cognitive affective syndrome. Pediatr Hematol Oncol Oct-Nov;18(7):423–426. [DOI] [PubMed] [Google Scholar]
- 63.Wells EM, Walsh KS, Khademian ZP, Keating RF, Packer RJ (2008) The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev Disabil Res Rev 14(3):221–228. [DOI] [PubMed] [Google Scholar]
- 64.Mariën P, De Smet HJ, Wijgerde E, Verhoeven J, Crols R, De Deyn PP (2013) Posterior fossa syndrome in adults: a new case and comprehensive survey of the literature. Cortex January;49(1):284–300. [DOI] [PubMed] [Google Scholar]
- 65.Stoodley CJ, Schmahmann JD (2009) The cerebellum and language: evidence from patients with cerebellar degeneration. Brain Lang September;110(3):149–153. [DOI] [PubMed] [Google Scholar]
- 66.Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD (2018) The cerebellar cognitive affective / Schmahmann syndrome scale. Brain January 1;141(l):248–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cakir Y, Karakişi D, Koçanaoğullari O (1994) Cerebellar mutism in an adult: case report. Surg Neurol April;41(4):342–344. [DOI] [PubMed] [Google Scholar]
- 68.Kai Y, Kuratsu J, Suginohara K, Marubayashi T, Ushio Y (1997) Cerebellar mutism after posterior fossa surgery--two case reports. Neurol Med Chir (Tokyo) December;37(12):929–933. [DOI] [PubMed] [Google Scholar]
- 69.De Smet HJ, Mariën P (2012) Posterior fossa syndrome in an adult patient following surgical evacuation of an intracerebellar haematoma. Cerebellum June;11(2):587–592. [DOI] [PubMed] [Google Scholar]
- 70.Akil H, Statham PF, Götz M, Bramley P, Whittle IR (2006) Adult cerebellar mutism and cognitive-affective syndrome caused by cystic hemangioblastoma. Acta Neurochir (Wien) May;148(5):597–598. [DOI] [PubMed] [Google Scholar]
- 71.Mariën P, Verslegers L, Moens M, Dua G, Herregods P, Verhoeven J (2013) Posterior fossa syndrome after cerebellar stroke. Cerebellum October;12(5):686–691. [DOI] [PubMed] [Google Scholar]
- 72.Stephen CD, Brizzi KT, Bouffard MA, Gomery P, Sullivan SL, Mello J, MacLean J, Schmahmann JD (2019) The Comprehensive Management of Cerebellar Ataxia in Adults. Curr Treat Options Neurol February 21;21(3):9. [DOI] [PubMed] [Google Scholar]
- 73.Guell X, Hoche F, Schmahmann JD (2015) Metalinguistic deficits in patients with cerebellar dysfunction: empirical support for the dysmetria of thought theory. Cerebellum February;14(1):50–58. [DOI] [PubMed] [Google Scholar]
- 74.Hoche F, Guell X, Sherman JC, Vangel MG, Schmahmann JD (2016) Cerebellar contribution to social cognition. Cerebellum December;15(6):732–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stoodley G, Schmahmann JD (2009) Functional topography in the human cerebellum: A metaanalysis of neuroimaging studies. Neuroimage 44:489–501. [DOI] [PubMed] [Google Scholar]
- 76.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol November;106(5):2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stoodley G, Valera EM, Schmahmann JD (2012) Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 59(2):1560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guell X, Gabrieli JDE, Schmahmann JD (2018) Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage May 15;172:437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strick PL, Dum RP, Fiez JA (2009) Cerebellum and nonmotor function. Annu Rev Neurosci 32:413–434. [DOI] [PubMed] [Google Scholar]
- 80.Heath RG (1977) Modulation of emotion with a brain pacemaker. Treatment for intractable psychiatric illness. J Nerv Ment Dis 165:300–317. [PubMed] [Google Scholar]
- 81.Schmahmann JD (2000) The role of the cerebellum in affect and psychosis. Journal of Neurolinguistics 13:189–214. [Google Scholar]
- 82.Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K (2019) Cerebellar modulation of the reward circuitry and social behavior. Science January 18;363(6424). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paillard J (1982) Apraxia and the neurophysiology of motor control. Philos Trans R Soc Lond B Biol Sci June 25;298(1089):111–134. [DOI] [PubMed] [Google Scholar]
- 84.Goldenberg G (2003) Apraxia and beyond: life and work of Hugo Liepmann. Cortex June;39(3):509–524. [DOI] [PubMed] [Google Scholar]
- 85.Mariën P, van Dun K, Verhoeven J (2015) Cerebellum and apraxia. Cerebellum February;14(1):39–42. [DOI] [PubMed] [Google Scholar]
- 86.Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS Jr, Fox MD (2015) Network localization of neurological symptoms from focal brain lesions. Brain October;138(Pt 10):3061–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]