Abstract
Botulinum neurotoxins (BoNT) possess an analgesic effect through several mechanisms including an inhibition of acetylcholine release from the neuromuscular junction as well as an inhibition of specific pain transmitters and mediators. Animal studies have shown that a peripheral injection of BoNTs impairs the release of major pain transmitters such as substance P, calcitonin gene related peptide (CGRP) and glutamate from peripheral nerve endings as well as peripheral and central neurons (dorsal root ganglia and spinal cord). These effects lead to pain relief via the reduction of peripheral and central sensitization both of which reflect important mechanisms of pain chronicity. This review provides updated information about the effect of botulinum toxin injection on local pain caused by cancer, painful muscle spasms from a remote cancer, and pain at the site of cancer surgery and radiation. The data from the literature suggests that the local injection of BoNTs improves muscle spasms caused by cancerous mass lesions and alleviates the post-operative neuropathic pain at the site of surgery and radiation. It also helps repair the parotid damage (fistula, sialocele) caused by facial surgery and radiation and improves post-parotidectomy gustatory hyperhidrosis. The limited literature that suggests adding botulinum toxins to cell culture slows/halts the growth of certain cancer cells is also reviewed and discussed.
Keywords: botulinum toxin, botulinum neurotoxin, cancer, cancer cells, neuropathic pain, post-surgical pain, parotid gland, submaxillary gland, gustatory hyperhidrosis, sialocele, parotid fistula
1. Introduction
Currently, there are vast indications for the use of botulinum neurotoxins (BoNT) type A and B in clinical medicine. Their specific inhibitory action on cholinergic synapses makes them desirable for the treatment of several hyperkinetic movement disorders as well as symptoms caused by glandular hyperactivity (sialorrhea and hyperhidrosis) and bladder dysfunction [1]. Disease-oriented reviews indicate that these agents are frequently used for the treatment of spasticity in several common disease conditions such as stroke, cerebral palsy, multiple sclerosis, cerebral, and spinal cord injury [2]. The efficacy of BoNT therapy in migraine headaches, predicted by early investigators [3], has been proven via two large, multicenter clinical trials leading to the approval of onabotulinumtoxinA for the treatment of chronic migraine [4]. Animal and human studies have shown that the local injection of botulinum toxins has an analgesic effect and can relieve several forms of neuropathic pain [5,6,7]. The data indicate an analgesic activity for BoNTs in a wide range of pain disorders that include both neuropathic and non-neuropathic pain.
In recent years, several publications have drawn attention to the utility of BoNT injections in cancer-related pain syndromes arising either by direct pressure from a neoplastic mass or from neuropathic pain at the site of cancer surgery or radiation [8]. Aside from pain, BoNT injection into parotid or submaxillary glands has been shown to reduce symptoms such as sialorrhea resulting from gland injury as well as healing surgical complications such as fistula and sialocele [9]. BoNT injections have been reported to relieve gustatory hyperhidrosis resulting from parotid and oral surgery in cancer patients [10]. The limited literature also suggests that adding BoNT to the culture of cancer cell lines slows growth and mitotic activity of certain cancer cells and promotes apoptosis [11].
This review is based on a literature search using the search engines of Pub Med, Ovid embrace, and Google Scholar from 1989 to 1 September 2019. The terms botulinum toxin, botulinum neurotoxin, onabotulinumtoxinA, incobotulinumtoxinA, abobotulinumtoxinA, and rimabotulinumtoxinB were crossed with cancer pain, postsurgical cancer pain, post-radiation cancer pain, salivary glands, sialorrhea, gustatory sweating, cancer cells, and cancer cell line. Book chapters that were written over the past 10 years focusing on the subject of botulinum toxin therapy in cancer patients were also reviewed. The inclusion criteria encompassed all articles found via those three afore-mentioned search engines using the above-mentioned search words. Inclusion required that the manuscript’s abstract contain both words cancer (or neoplasm) and botulinum toxin (or botulinum neurotoxin) therapy. Manuscripts that did not have both cancer and botulinum toxin therapy noted in the abstract were excluded. Manuscripts with benign mass lesions were also excluded.
2. Results
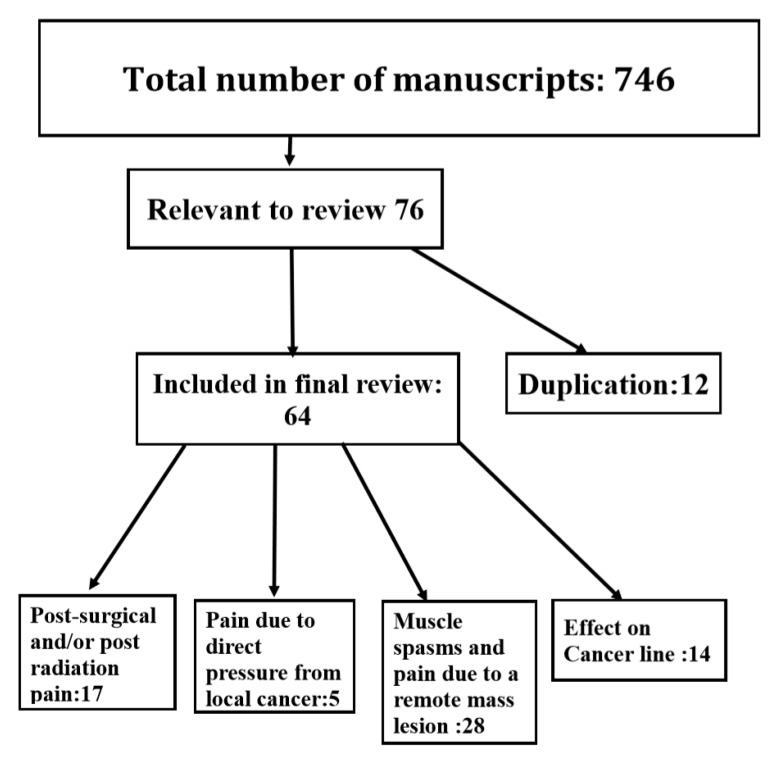
The search identified 746 manuscripts from which 76 were relevant to the subject of botulinum toxins and cancer (see flow chart in Figure 1). After eliminating 12 duplications (due to an overlap between MedLine and Google Scholar), 64 manuscripts remained for final analysis. The collected data can be classified under 3 categories: (1) The role of botulinum toxins in post-radiation and post-surgical cancer pain; (2) the repairing and healing function of BoNT injections upon parotid gland damaged by radiation or surgery; and (3) the effect of botulinum toxins on cancer cell line, cell growth, and apoptosis.
Figure 1.
Flow chart of the reviewed manuscripts on cancer and botulinum neurotoxin therapy.
2.1. Botulinum Neurotoxins Therapy for Post-Radiation And/Or Post-Surgical Cancer Pain
This heading includes six prospective clinical trials, four retrospective studies, one double blind placebo-controlled study, and six single case reports (Table 1). A majority of patients had burning and searing pain along the region of fibrosis and keloid formation (neuropathic pain). Some also experienced additional local muscle spasms close to the scarred tissue affecting the neck and shoulder muscles. The most affected muscles were sternocleidomastoid, splenius capitus, trapezius, and levator scapulae. Injections were either subcutaneous (close or at the area of keloid and post-surgical scars, see Figure 2) or both subcutaneous and intramuscular.
Table 1.
Published studies on the effect of botulinum neurotoxins (BoNT) on local pain resulting from radiation and/or surgery *.
| Authors | Pts Study | Toxin | Dose Units | Treatment | Location of Cancer | Primary Outcome | Result |
|---|---|---|---|---|---|---|---|
| Van Daele et al., 2002 [12] | 6 Retro |
OnaA | 20–25 | Radiation chemotherapy | Head and neck | Pain VAS | Complete pain relief in four of six patients. Significant improvement of quality of life using SF36, EQ-5D scales |
| Layeeque et al., 2004 [13] | 48 Pro |
OnaA | 100 | Mastectomy, assessed for pain after expander placement | Breast | (1) Pain assessed by VAS (2) Narcotic use |
Less pain in BoNT group (p < 0.00001) Less narcotic use in BoNT group (p < 0.0001) |
| Vasan et al., 2004 [14] | 16 Pro |
AboA | 100 to 320 | Surgery | Head and neck | Pain (VAS- days 3 and 4 weeks), global. Quality of life |
Significant pain reduction (p = 0.05); Quality of life improved (p = 0.7) |
| Wittekindt et al., 2006 [15] | 23 Pro |
OnaA | 60–120 160–240 |
Radiation; surgery |
Head and neck | Pain VAS: 28 weeks |
Significant reduction of pain (<0.05) |
| Hartl et al., 2008 [16] | 19 Pro |
OnaA AboA |
50 250 |
Chemotherapy; radiation |
Head and neck | Pain: (VAS) Function: At 4 weeks |
Improved pain (p = 0.02) Function (p = 0.04) |
| Stubblefield et al., 2008 [17] | 23 Retro |
OnaA | 25–200 | Radiation; surgery |
Head, neck, breast | Pain (VAS) | Pain improved in Improved in 85% of patients |
| Mittal et al., 2012 [18] | 7 Retro |
OnaA | 100 | Radiation; surgery |
Head, neck, breast | Pain (VAS) PGIC At 4 weeks |
VAS: Six of seven patients improved: p < 0.05 PGIC: Six of seven, very satisfied QoL: Six of seven improved (p < 0.05) |
| Bach et al., 2012 [19] | 9 Pro |
OnaA | 100–400 | Radiation and surgery |
Head and neck | Pain (VAS) and FDSNP at 4 weeks | Both pain and FDSNP improved (p < 0.01) |
| Rostami et al., 2014 [20] | 12 Pro |
IncoA | 100 | Radiation and surgery |
Head neck breast | Pain (VAS) and PGIC at week 6 | VAS improved (p < 0.05) PGIC: Very satisfied QoL improved in 38% of patients (p < 0.05) |
| De Groef et al. 2018 [21] | 50 DBPC |
onaA | 100 | Surgery | Breast | Pain measured by VAS | Pain reduction 60% in the BoNT and 40% in saline group (statistically ns) |
| Mailly et al., 2019 [22] | 16 Retro |
incoA aboA |
20 40 |
Radiation and surgery | Head and neck | Pain (VAS) | VAS improved p < 0.01 |
* Case reports are not included. onaA: OnabotulinumtoxinA; aboA: AbobotulinumtoxinA; incoA: IncobotulinumtoxinA; VAS: Visual Analogue Scale; PGIC: Patient Global Impression of Change; FDSNP: Functional Disability Scale for Neck Pain; Pro: Prospective; Retro: Retrospective; DBPC: double-blind, placebo-controlled; QoL: Quality of Life.
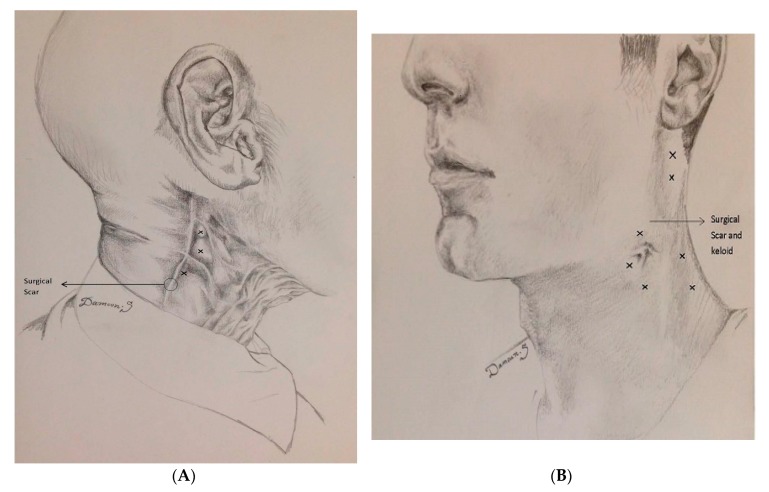
Figure 2.
Post-surgical and post-radiation pain treated with BoNT. Example of two patients. From Jabbari B. Botulinum Toxin Treatment in Pain Disorders. Springer, New York 2015. Printed with permission from the publisher. (A) A 47-year-old man had undergone right neck dissection and radiotherapy for cancer of the tongue and cervical adenopathy 6 years prior to visiting the Yale clinic. A year following surgery and radiotherapy, severe pain (VAS 9–10, both sharp and deep) developed over the right side of the neck which was mostly felt below the mandible and anterior to the angle of the jaw. Injecting onabotulinumtoxinA into the areas designated by X on the figure, (30, 30, and 20 units) reduced the pain significantly (VAS 1) within a week after injection. He remained responsive and satisfied (assessed by PGIC) receiving injections every 4–6 months over 7 years of follow-up. (B) A 48-year old man with squamous cell carcinoma of piriform sinus had supraglottic laryngectomy. Two years following neck dissection and radiotherapy, he developed severe pain (VAS 9) over the left side of the neck. The pain was deep as well as sharp and superficial. Injection of onabotulinumtoxinA, 20 units into each superficial pain region (Xs around the jaw) and 30 units into nearby posteriorly located muscles (splenius and trapezius) designated by X reduced the pain to VAS 0–1 level. The total dose was 200 units. The patient enjoyed pain relief with repeat injections over the 3 years of follow-up. Drawings courtesy of Damoun Safarpour M.D.
A total of 10 of 11 studies used a standardized scale for pain measurement (Visual Analogue Scale: VAS) which in 9 of 10 demonstrated statistically significant improvement of local pain at 4–8 post-injection weeks compared to baseline (p < 0.05) (Table 1). Two studies included patient global impression of change (PGIC) in the evaluation, using a 7-grade scale ranging from “very unsatisfied” to “very satisfied”. In both studies, patients expressed significant satisfaction with the results [18,20]. Four studies used a scale for evaluating quality of life. Three of 4 demonstrated significant improvement of quality of life after BoNT-A (onaA and incoA) injection therapy [14,18,20]. One study demonstrated significant reduction of daily opioid use after BoNT therapy [13]. One prospective study provided long-term follow up of up to 82 months [20]. Side effects consisted mainly of transient pain at the site of injection(s) and minor local bleeding. None of the 229 patients, reported in Table 1, demonstrated any serious side effect following BoNT injections.
Clinical data from case reports includes six single case reports. One publication reported a 50 year-old man with adenocarcinoma of the soft palate, who following radiotherapy, developed trismus and myokymia of the masseter muscles. Trismus and myokymia improved after injection of 25 units of onaA into each masseter muscle [23]. Two other manuscripts described improvement of central neuropathic pain in association with a mass lesion. One described a 55 year-women who developed severe burning pain and allodynia in the distribution of T1 dermatomes bilaterally following partial resection of an angioma at the C7–C8 region. Subcutaneous injection of onaA at 25 sites into T1 dermatomes (100 units on each sides) resulted in a marked reduction of neuropathic pain and allodynia. This effect was sustained with repeated injections over a follow up period of three years [24]. A similar experience with central pain was reported by Nam et al. [25], in a 62-year old man who had developed severe allodynia and neuropathic pain over the posterior aspect of the left thigh contralateral to a frontal lobe malignant brain tumor. A subcutaneous injection of onaA with a total dose of 100 units at 16 sites substantially improved the patient’s neuropathic pain and allodynia over the affected region. In another patient, radiation of a left submandibular chondrosarcoma resulted in hyperactivity of the spinal accessory nerve and gradual painful hypertrophy of the left trapezius muscle. An injection of 90 units of onaA resulted in a substantial reduction of left shoulder pain and diminished the involuntary myokymic movements of the left trapezius muscle [26]. Boukovalas et al. [27] reported a patient with squamous cell carcinoma of the anterior mandible who, following mandibulectomy, bilateral neck dissection, and radiotherapy, gradually developed pain and tightness of the sternocleidomastoid and platysmal muscles associated with Raynaud phenomenon of the lower face. Injection of botulinum toxin (type and dose not mentioned) into the above-mentioned muscles improved painful muscle tightness and reduced the Raynaud phenomena. Schuler et al. [28] described a 47-year old female who, at the scarred skin site of resected melanoma, developed severe neuropathic pain. Injection of onaA, 50 units in a grid-like pattern (injection sites were 1.5 cm apart), resulted in 50% reduction of pain four weeks after BoNT injection.
The duration of action of BoNT injections for pain relief in the above-mentioned studies was 3–6 months (mean 3.9 month). In most studies, the follow up was short term, not exceeding 6–12 months. In some cases, however, patients were followed-up for years with repeated injections. Two patients described in Figure 1 were followed-up for 3 and 7 years (see figure legend). Nine of 11 studies reported no side effects. One study reported increased pain for a few days at the site of injection in one patient, which was followed by baseline pain improvement [14]. One study reported the occurrence of a diffuse maculo-papular rash in one patient 2–3 days after the botulinum neurotoxin injection after which the rash disappeared over a month [20].
2.2. Botulinum Neurotoxins Therapy for Post-Radiation or Postsurgical Damage to Parotid Gland
This category includes six prospective clinical trials, 10 retrospective studies, and 12 single case reports (Table 2). All prospective studies are open label. Botulinum toxin treatment was used for the remedy of post-parotidectomy complications such as gustatory hyperhidrosis (GH), post-parotidectomy sialorrhea, fistula, and sialocele formation.
Table 2.
BoNT therapy for post-parotidectomy gustatory hyperhidrosis, fistula, sialocele formation, and for post-parotidectomy sialorrhea.
| Authors | Design | Pts # | Clinical Problem | Injection Site |
Toxin and Dose | Result |
|---|---|---|---|---|---|---|
| Laskawi et al., 2013 [29] | R | 10 | Post-parotidectomy fistula | Parotid gland | OnaA 30–50 units |
Treated within 6 weeks of surgery: Fistulas healed in 9 of 10 patients |
| Marchese-Ragona et al., 2006 [30] | R | 3 | Post-parotidectomy fistula | Parotid gland | OnaA 15–20 units |
Complete healing of fistula with follow ups 12,18, and 14 months |
| Nolte et al., 2004 [31] | P | 20 | Gustatory sweating after parotidectomy | Facial skin | OnaA 3 units/cm |
Complete loss of sweating for 12 months |
| Kuttner et al., 2001 [32] | R | 8 | GH after parotidectomy | Face | BoNT-A 0.5 units/cm |
Stopped facial sweating within one week |
| Vargas et al., 2000 [33] | P | 4 | Post-parotidectomy sialocele-pain |
Parotid gland | OnaA 30–50 units |
Total resolution in 4 weeks in all patients |
| Steffen et al., 2014 [34] | R | 25 | Head and neck cancer FHS: (19) Fistula (6) |
Parotid gland | OnaA and incoA: Par: 30 U SM: 20 U | FHS: 11 of 19 improved. Fistula: 4 of 6 improved |
| Machese et al., 2008 [35] | R | 8 | Head and neck cancer sialorrhea: 6, fistula: 1, and sialocele: 1 | Parotid gland | AboA: 100 U |
Fistulas healed. Sialorrhea stopped |
| Eckardt et al., 2003 [36] | R | 33 | GH after parotidectomy | Face | OnaA 16 to 80 units |
Facial sweating disappeared within a week after injections |
| Cantarella and Barbieri [37] |
R | 7 | GH after parotidectomy | Face | RimaB 2200 units |
Cessation of sweating in 6 of 7 patients 4 weeks after injection |
| Matos Dias et al., 2008 [38] | R | 10 | GH after parotidectomy | Face | Ona-A 38 units |
Sweating stopped |
| Hatrl et al., 2008 [39] | R | 7 | GH after parotidectomy | Face | BoNT-A | Sweating and quality of life improved |
| Pomprasit et al., 2007 [40] | P | 9 | GH after Parotidectomy | Face | Ona-A 10.6 units |
Sweating stopped in 5 and reduced in 4 |
| Cavalot et al., 2000 [41] | P | 40 | GH after parotidectomy | Face | Ona-A, 2.5/cm2 | 100% response in severe group, 72% response in moderate group |
| Von Lindern et al., 2000 [42] | R | 7 | GH after parotidectomy | Face | Ona-A | Sweating stopped after BoNT injection |
| Laccourreye et al., 1998 [43] | P | 14 | GH after parotidectomy | Face | Ona-A | All showed total cessation of sweating |
| Bjerkhoel et al., 1997 [44] | P | 15 | GH after parotidectomy | Face | Ona-A | Total cessation of facial sweating in 13 patients |
Case reports are not included for salivary gland problems related to cancer surgery or cancer irradiation. R: Retrospective; P: Prospective; onaA: OnabotulinumtoxinA; incoA: IncobotulinumtoxinA; aboA: AbobotulinumtoxinA; FHS: Functional hypersalivation; Par: Parotid, SM: Submandibular.
The positive information of these studies has been supported by several case reports [45,46,47,48,49,50,51,52,53,54,55,56]. Among these 12 case reports, six described healing of post-parotidectomy fistula and four reported on healing of sialocele. One case reported improvement of gustatory hyperhidrosis and another one reported improvement of post-parotidectomy sialorrhea. A total of 11 studies had used type-A and one had used type B toxin.
In these 11 studies, no serious side effects were reported. One study reported a patient on anticoagulation in whom a small hematoma developed at the site of injection [34]. One study reported dry mouth as the only side effect [38]. One study cited non-specified, minor issues limited to the site of injection [41]. One study mentioned mild transient weakness of the upper lip in two patients [43] and another study described transient weakness of orbicularis oris muscle in one patient [44].
The clinical studies cited above investigating the analgesic effect of BoNTs in patients after surgery or radiation therapy and BoNT’s healing effect on parotid glands injured by surgery or radiation strongly suggest the efficacy of BoNTs in cancer patients affected by surgical and radiation side effects. All three type-A FDA approved BoNTs seem to have analgesic effect in post-surgical and post-radiation pain. In case of parotid injury, at least one study (Table 2) suggests that type B is also effective. Although anecdotal observations have demonstrated safety over 3 to 7 years of treatment (cases presented in Figure 1), the long term safety of BoNT therapy in cancer patients needs to be further investigated through controlled, prospective clinical trials.
2.3. The Effects of Botulinum Neurotoxins Injections on Malignant Tumors and Cancer Cell Line
This category includes 14 studies. In three studies, investigators injected BoNT into a malignant tumor and demonstrated cellular apoptosis and reduction of tumor size [57,58,59] (Table 3). In another six studies, adding BoNT-A to cancer cell cultures reduced cell growth, induced apoptosis, and inhibited mitosis in various cancer cell lines: Prostate, breast, colon, and pancreatic tumors [60,61,62,63,64,65]. In one study, transfection of insulin secreting cells by BoNT-A reduced insulin secretion, suggesting a potential for treatment of insulinomas [66]. In another study, the addition of BoNT-A to Her2 positive breast cancer cell line increased Herceptin efficacy [67]. In one study, authors reported no effect on prostate tumor growth and LNCaP and PC3 cancer cells after exposure to BoNT [68]. In one study, increased tumor oxygenation after the injection of BoNT-A into hepatic sarcoma and fibrosarcoma suggested that the BoNT injection potentially made these tumors more susceptible to chemotherapy [69]. In another study, the injection of onaA into one side of cancerous human prostate increased apoptosis on the injected side (compare to saline injected into other side) [70].
Table 3.
In vivo and in vitro effects of BoNT injection on malignant tumors and cancer cell lines.
| Authors | Study Type | Type of Cells or Tissue | Study Design | Results |
|---|---|---|---|---|
| Vezdrevanis 2011 [57] |
In vivo | Prostatic cancer | Injected BoNT into prostate | Tumor size reduction |
| Ulloa et al., 2015 [58] | In vivo | Glioblastoma cells | Cells with or without transfection by BoNT-C1 injected into mice striatum | By BoNT-C1 blocks the growth of Glioblastoma cells via blocking Syntaxin1 |
| He et al., 2016 [59] |
In vivo | Mice with pancreatic tumor | Injected onaA or saline into tumor | Reduced tumor size; increased apoptosis |
| Karsenty et al., 2009 [60] | In vitro | Prostate LNCaP and PC-3 cell lines | LNCaP and PC-3 cell lines were exposed to onaA | OnaA inhibited LNCasP cell proliferation; had no effect on PC-3 cell |
| Nam et al., 2012 [61] | In vitro | Breast and colorectal cancer | PLC-γl-transformed cells were exposed to BoNT-A (difficile) | Caused apoptosis and mitotic inhibition |
| Proietti et al., 2012 [62] | In vitro | Prostate LNCaP and PC-3 cell lines | Prostate cancer cell lines were exposed to incoA | Tumor cell growth slowed down probably due to toxin effect on SV2 receptors |
| Bandala et al., 2013 [63] | In vitro | Breast T47D cancer cells | Breast T47D cancer cells were exposed to diverse dilutions of BoNT | BoNT via caspase 3, slow down the growth of T47d cells and caused apoptosis |
| Bandala et al., 2015 [64] | In vitro | Breast cancer cell line | Added BoNT-A to breast cancer cell line | BoNT-A diminished SV2 protein on the surface of breast cancer cells |
| Rust et al., 2016 [65] | In vitro | Human neuroblastoma cells | Added BoNT-C to human neuroblastoma cell culture | Apoptosis of neuroblastoma cells |
| Huang et al., 1998 [66] |
Invitro | Insulin secreting HIT-T15 cells | Insulin secreting cells were transfected by BoNT-A | Marked reduction of insulin secretion- potential to treat insulinoma |
| Hajighasemlou et al., 2015 [67] | In vitro | Her2 positive breast cancer cell line | Assessed the effect of BoNT-A on Her2 positive cells responsive to Herceptin | Herceptin efficacy significantly improved |
| Cheng et al., 2013 [68] | In vitro and in vivo | Prostate cancer cell line in Mice |
LNCaP and PC3 cancer cells were exposed to 1 to 10 units of onaA | No effect on tumor growth in LNCaP and PC3 cancer cells |
| Ansiaux et al., 2006 [69] | In vivo | Fibrosarcoma, hepatosarcoma | BoNT-A injected into the tumor | Increased oxygenation of the tumor and made it more susceptible to chemo and radiotherapy |
| Coarfa et al., 2017 [70] | In vivo | Prostate of 250 nude mice Four human cancerous prostates |
Effect of onaA versus saline injection into cancer cells implanted into rodent’s prostate Assessed the effect of onaA versus saline injection in cancerous prostate before prostatectomy |
Increased apoptosis; slowed cancer progression Increased apoptosis in ona-A injected side of prostate |
OnaA: OnabotulinumtoxinA (Botox). IncoA: IncobotulinumtoxinA (Xeomin).
3. Discussion
Botulinum neurotoxins exert their analgesic effect through two known mechanisms. The inhibitory effect of the BoNTs upon the release of acetylcholine at the neuromuscular junction is mostly responsible for the relief of pain caused by muscle spasms. In the case of neuropathic pain, it is currently believed that the analgesic effect of botulinum injections predominantly results from inhibition of pain neurotransmitters both at peripheral and at central sensory levels [5,6,71,72]. The peripheral injection of botulinum toxin-A into the muscle or close to peripheral nerve endings reduces the release of calcitonin gene related peptide, a major pain transmitter from trigeminal ganglion [73]. Direct exposure of dorsal root ganglia to botulinum toxin-A significantly reduces the thermal sensitivity in the animal model of thermal pain [74]. In the formalin pain model, injection botulinum toxin B into the rat’s paw reduced substance P release from ipsilateral sensory spinal neurons and prevented spinal sensory neuron activation (c-Fos) which occurred after formalin injection [75]. Injection of botulinum toxins into mice hind paw reduces glutamate release from spinal sensory neurons [76]. Intra-articular injection of botulinum toxin in animal models of pain reduces upregulation of transient receptor potential cation channel subfamily V member 1 (TrpV1), a protein closely associated with pain pathophysiology [77]. A central analgesic function for botulinum toxins has been suggested by studies that have shown the presence of cleaved SNAP-25 in medullary and midbrain sensory regions following the peripheral injection of botulinum toxins [78,79]. Further suggestion for central effects of BoNTs comes from the studies that have demonstrated bilateral improvement of pain sensations after the unilateral injection of botulinum toxin in animal models of diabetic neuropathy and acidic saline injection [80,81]. The analgesic effect of BoNTs results from their direct and indirect effects since patients experience analgesia prior to the muscle relaxation [82].
Pain is a common symptom in cancer patients and when present often impairs the patient’s quality of life [83]. Approximately 20–60% of the patients with breast cancer and 30% of the patients with head and neck cancer experience chronic pain localized to the site of radiation or surgery [84]. Post-radiation/surgical pain may be treated with the topical application of a hyaluronic acid, calendula officinalis, trolamine, and lidocaine patch [85,86]. However, sustained relief from pain happens only in 25% of the patients using these remedies [87]. Potent systemic analgesic agents such as opioids provide pain relief in many patients but the development of undesirable side effects including nausea, somnolence, constipation, and addiction complicates their use [88]. Botulinum toxin treatment has two major advantages over these pharmacological remedies. Firstly, the effects of the BoNT-A and B injection lasts 3–6 months. Secondly, the BoNT injection has fewer side effects and is safer when compared to potent analgesic agents. Lack of any serious side effect in the studies cited above supports this statement.
Gustatory hyperhidrosis (Frey syndrome) can be congenital or acquired. Acquired gustatory hyperhidrosis results from injury to the parotid gland or face as well as conditions such as diabetic autonomic neuropathy. Gustatory hyperhidrosis after parotidectomy results from the aberrant innervation of sweat glands from parasympathetic nerves of the parotid region. Facial sweating during chewing and eating is often a cause of social embarrassment. Gustatory hyperhidrosis (GH) is common after parotidectomy and about half of the patients complained of this symptom after surgery [89]. Botulinum neurotoxins via blocking acetylcholine release at autonomic synapses are highly effective in treatment of autonomic dysfunctions such as sialorrhea and hyperhidrosis [90]. In a meta-analysis of literature on Frey syndrome (multiple etiologies) treated with BoNTs, Xie et al. found the effectiveness of BoNT therapy to be present in 98% of the patients [10].
Fistula with sialorrhea and sialocele (entrapped saliva with cyst formation) are two common complications of parotidectomy. Treatment of post-parotidectomy fistula consist of pressure dressing, systemic anticholinergic drugs, suction drain insertion, tympanic neurectomy, and surgery [91]. Overall, the results of the above mentioned surgical and medical strategies in the treatment of parotid fistula is disappointing [92]. Furthermore, side effects of anticholinergic therapy such as memory loss, blurring of vision, dryness of the mouth, and urinary dysfunction are not well tolerated, especially in the elderly. Botulinum toxin injections provides a safe and effective way to suppress sialorrhea and to help heal the fistula.
4. Conclusions
The studies of botulinum toxins in post-surgical and post-radiation pain indicated that the local injection of BoNT improved neuropathic pain and local muscle spasm at/or close to the site of surgery and radiation. The proof of efficacy of botulinum toxin therapy in this form of cancer-related pain, however, awaits the results of blinded and placebo-controlled studies. The same conclusion applies to the use of botulinum neurotoxins in gustatory hyperhidrosis and in the management of post-parotidectomy fistula and sialocele where all open-label studies suggest efficacy. The positive effect of BoNTs on different cancer cell lines and their direct effects upon certain cancerous tumors is encouraging. More studies are necessary to verify these results and if verified to devise a methodology through which BoNT injections can safely be used for the treatment of certain human cancers.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This review demonstrates that local injection of botulinum toxins can alleviate neuropathic pain experienced at the site of surgery and radiation in cancer patients. Local injection of botulinum neurotoxins can also heal fistula and sialocele in the parotid gland of cancer patients damaged by surgical trauma and radiation.
References
- 1.Jankovic J. Botulinum toxin: State of the Art. Mov. Disord. 2017;32:1131–1138. doi: 10.1002/mds.27072. [DOI] [PubMed] [Google Scholar]
- 2.Jabbari B., editor. Botulinum Toxin Treatment in Clinical Medicine. Springer; New York, NY, USA: 2018. A disease- oriented approach. [Google Scholar]
- 3.Aoki K.R. Evidence for antinociceptive activity of botulinum toxin type A in pain management. Headache. 2003;43(Suppl. S1):9–15. doi: 10.1046/j.1526-4610.43.7s.3.x. [DOI] [PubMed] [Google Scholar]
- 4.Dodick D.W., Turkel C.C., DeGryse R.E., Aurora S.K., Silberstein S.D., Lipton R.B., Diener H.C., Brin M.F. OnabotulinumtoxinA for treatment of chronic migraine: Pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50:921–936. doi: 10.1111/j.1526-4610.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 5.Oh H.M., Chung M.E. Botulinum Toxin for Neuropathic Pain: A Review of the Literature. Toxins. 2015;7:3127–3154. doi: 10.3390/toxins7083127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J., Park H.J. Botulinum Toxin for the Treatment of Neuropathic Pain. Toxins. 2017;9:260. doi: 10.3390/toxins9090260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safarpour Y., Jabbari B. Botulinum toxin treatment of pain syndromes—An evidence based review. Toxicon. 2018;147:120–128. doi: 10.1016/j.toxicon.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Shaw L., Bazzell A.F., Dains J.E. Botulinum Toxin for Side-Effect Management and Prevention of Surgical Complications in Patients Treated for Head and Neck Cancers and Esophageal Cancer. J. Adv. Pract. Oncol. 2019;10:40–52. [PMC free article] [PubMed] [Google Scholar]
- 9.Melville J.C., Stackowicz D.J., Jundt J.S., Shum J.W. Use of Botox (OnabotulinumtoxinA) for the Treatment of Parotid Sialocele and Fistula After Extirpation of Buccal Squamous Cell Carcinoma with Immediate Reconstruction Using Microvascular Free Flap: A Report of 3 Cases. J. Oral Maxillofac. Surg. 2016;74:1678–1686. doi: 10.1016/j.joms.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Xie S., Wang K., Xu T., Guo X.S., Shan X.F., Cai Z.G. Efficacy and safety of botulinum toxin type A for treatment of Frey’s syndrome: Evidence from 22 published articles. Cancer Med. 2015;4:1639–1650. doi: 10.1002/cam4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matak I., Lacković Z. Botulinum neurotoxin type A: Actions beyond SNAP-25? Toxicology. 2015;335:79–84. doi: 10.1016/j.tox.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Van Daele D.J., Finnegan E.M., Rodnitzky R.L., Zhen W., McCulloch T.M., Hoffman H.T. Head and neck muscle spasm after radiotherapy: Management with botulinum toxin A injection. Arch. Otolaryngol. Head Neck Surg. 2002;128:956–959. doi: 10.1001/archotol.128.8.956. [DOI] [PubMed] [Google Scholar]
- 13.Layeeque R., Hochberg J., Siegel E., Kunkel K., Kepple J., Henry-Tillman R.S., Dunlap M., Seibert J., Klimberg V.S. Botulinum toxin infiltration for pain control after mastectomy and expander reconstruction. Ann. Surg. 2004;240:608–613. doi: 10.1097/01.sla.0000141156.56314.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasan C.W., Liu W.C., Klussmann J.P., Guntinas-Lichius O. Botulinum toxin type A for the treatment of chronic neck pain after neck dissection. Head Neck. 2004;26:39–45. doi: 10.1002/hed.10340. [DOI] [PubMed] [Google Scholar]
- 15.Wittekindt C., Liu W.C., Preuss S.F., Guntinas-Lichius O. Botulinum toxin A for neuropathic pain after neck dissection: A dose-finding study. Laryngoscope. 2006;116:1168–1171. doi: 10.1097/01.mlg.0000217797.05523.75. [DOI] [PubMed] [Google Scholar]
- 16.Hartl D.M., Cohen M., Juliéron M., Marandas P., Janot F., Bourhis J. Botulinum toxin for radiation-induced facial pain and trismus. Otolaryngol. Head Neck Surg. 2008;138:459–463. doi: 10.1016/j.otohns.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Stubblefield M.D., Levine A., Custodio C.M., Fitzpatrick T. The role of botulinum toxin type A in the radiation fibrosis syndrome: A preliminary report. Arch. Phys. Med. Rehabil. 2008;89:417–421. doi: 10.1016/j.apmr.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Mittal S., Machado D.G., Jabbari B. OnabotulinumtoxinA for treatment of focal cancer pain after surgery and/or radiation. Pain Med. 2012;13:1029–1033. doi: 10.1111/j.1526-4637.2012.01437.x. [DOI] [PubMed] [Google Scholar]
- 19.Bach C.A., Wagner I., Lachiver X., Baujat B., Chabolle F. Botulinum toxin in the treatment of post-radiosurgical neck contracture in head and neck cancer: A novel approach. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2012;129:6–10. doi: 10.1016/j.anorl.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Rostami R., Mittal S.O., Radmand R., Jabbari B. Incobotulinum Toxin-A Improves Post-Surgical and Post-Radiation Pain in Cancer Patients. Toxins. 2016;8:22. doi: 10.3390/toxins8010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Groef A., Devoogdt N., Van Kampen M., Nevelsteen I., Smeets A., Neven P., Geraerts I., Dams L., Van der Gucht E., Debeer P. Effectiveness of Botulinum Toxin A for Persistent Upper Limb Pain After Breast Cancer Treatment: A Double-Blinded Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018;99:1342–1351. doi: 10.1016/j.apmr.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 22.Mailly M., Benzakin S., Chauvin A., Brasnu D., Ayache D. Douleurs post-radiques après radiothérapie pour cancer des voies aérodigestives superieures: Traitement par injections de toxine botulique A [Radiation-induced head and neck pain: Management with botulinum toxin a injections] Cancer Radiother. 2019;23:312–315. doi: 10.1016/j.canrad.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Lou J.S., Pleninger P., Kurlan R. Botulinum toxin A is effective in treating trismus associated with postradiation myokymia and muscle spasm. Mov. Disord. 1995;10:680–681. doi: 10.1002/mds.870100527. [DOI] [PubMed] [Google Scholar]
- 24.Jabbari B., Maher N., Difazio M.P. Botulinum toxin a improved pain and allodynia in two patients with intracranial pathology. Pain Med. 2003;4:206–210. doi: 10.1046/j.1526-4637.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 25.Nam K.E., Kim J.S., Hong B.Y. Botulinum Toxin Type A Injection for Neuropathic Pain in a Patient With a Brain Tumor: A Case Report. Ann. Rehabil. Med. 2017;41:1088. doi: 10.5535/arm.2017.41.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippakis A., Ho D.T., Small J.E., Small K.M., Ensrud E.R. Radiation-induced painful neurogenic hypertrophy Treated with Botulinum Toxin, A. J. Clin. Neuromuscul. Dis. 2018;19:135–137. doi: 10.1097/CND.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 27.Boukovalas S., Mays A.C., Selber J.C. Botulinum Toxin Injection for Lower Face and Oral Cavity Raynaud Phenomenon after Mandibulectomy, Free Fibula Reconstruction, and Radiation Therapy. Ann. Plast. Surg. 2019;82:53–54. doi: 10.1097/SAP.0000000000001622. [DOI] [PubMed] [Google Scholar]
- 28.Schuler A., Veenstra J., Ozog D. Battling Neuropathic Scar Pain with Botulinum Toxin. J. Drugs Dermatol. 2019;18:937–938. [PubMed] [Google Scholar]
- 29.Laskawi R., Winterhoff J., Köhler S., Kottwitz L., Matthias C. Botulinum toxin treatment of salivary fistulas following parotidectomy: Follow-up results. Oral Maxillofac. Surg. 2013;17:281–285. doi: 10.1007/s10006-012-0375-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchese-Ragona R., Marioni G., Restivo D.A., Staffieri A. The role of botulinum toxin in postparotidectomy fistula treatment. A technical note. Am. J. Otolaryngol. 2006;27:221–224. doi: 10.1016/j.amjoto.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Nolte D., Gollmitzer I., Loeffelbein D.J., Hölzle F., Wolff K.D. Botulinumtoxin zur Behandlung des gustatorischen Schwitzens. Eine prospektive randomisierte Therapiestudie [Botulinum toxin for treatment of gustatory sweating. A prospective randomized study] Mund Kiefer Gesichtschir. 2004;8:369–375. doi: 10.1007/s10006-004-0575-3. [DOI] [PubMed] [Google Scholar]
- 32.Kuttner C., Tröger M., Dempf R., Eckardt A. Effektivität von Botulinumtoxin A in der Behandlung des gustatorischen Schwitzens [Effectiveness of botulinum toxin A in the treatment of gustatory sweating] Nervenarzt. 2001;72:787–790. doi: 10.1007/s001150170035. [DOI] [PubMed] [Google Scholar]
- 33.Vargas H., Galati L.T., Parnes S.M. A pilot study evaluating the treatment of postparotidectomy sialoceles with botulinum toxin type A. Arch. Otolaryngol. Head Neck Surg. 2000;126:421–424. doi: 10.1001/archotol.126.3.421. [DOI] [PubMed] [Google Scholar]
- 34.Steffen A., Hasselbacher K., Heinrichs S., Wollenberg B. Botulinum toxin for salivary disorders in the treatment of head and neck cancer. Anticancer Res. 2014;34:6627–6632. [PubMed] [Google Scholar]
- 35.Marchese M.R., Almadori G., Giorgio A., Paludetti G. Post-surgical role of botulinum toxin-A injection in patients with head and neck cancer: Personal experience. Acta Otorhinolaryngol. Ital. 2008;28:13–16. [PMC free article] [PubMed] [Google Scholar]
- 36.Eckardt A., Kuettner C. Treatment of gustatory sweating (Frey′s syndrome) with botulinum toxin A. Head Neck. 2003;25:624–628. doi: 10.1002/hed.10262. [DOI] [PubMed] [Google Scholar]
- 37.Cantarella G., Berlusconi A., Mele V., Cogiamanian F., Barbieri S. Treatment of Frey′s syndrome with botulinum toxin type B. Otolaryngol. Head Neck Surg. 2010;143:214–218. doi: 10.1016/j.otohns.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Martos Díaz P., Bances del Castillo R., Mancha de la Plata M., Gías L., Nieto C.M., Lee G.Y., Guerra M.M. Clinical results in the management of Frey′s syndrome with injections of Botulinum toxin. Med. Oral Patol. Oral Cir. Bucal. 2008;13:E248–E252. [PubMed] [Google Scholar]
- 39.Hartl D.M., Julieron M., LeRidant A.M., Janot F., Marandas P., Travagli J.P. Botulinum toxin A for quality of life improvement in post-parotidectomy gustatory sweating (Frey′s syndrome) J. Laryngol. Otol. 2008;122:1100–1104. doi: 10.1017/S0022215108001771. [DOI] [PubMed] [Google Scholar]
- 40.Pomprasit M., Chintrakarn C. Treatment of Frey′s syndrome with botulinum toxin. J. Med. Assoc. Thail. 2007;90:2397–2402. [PubMed] [Google Scholar]
- 41.Cavalot A.L., Palonta F., Preti G., Nazionale G., Ricci E., Staffieri A., Di Girolamo S., Cortesina G. Sindrome di Frey post parotidectomia. Trattamento con tossina botulinica di tipo A [Post-parotidectomy Frey′s syndrome. Treatment with botulinum toxin type A] Acta Otorhinolaryngol. Ital. 2000;20:187–191. [PubMed] [Google Scholar]
- 42.Von Lindern J.J., Niederhagen B., Bergé S., Hägler G., Reich R.H. Frey syndrome: Treatment with type A botulinum toxin. Cancer. 2000;89:1659–1663. doi: 10.1002/1097-0142(20001015)89:8<1659::AID-CNCR2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 43.Laccourreye O., Muscatelo L., Naude C., Bonan B., Brasnu D. Botulinum toxin type A for Frey′s syndrome: A preliminary prospective study. Ann. Otol. Rhinol. Laryngol. 1998;107:52–55. doi: 10.1177/000348949810700110. [DOI] [PubMed] [Google Scholar]
- 44.Bjerkhoel A., Trobbe O. Frey′s syndrome: Treatment with botulinum toxin. J. Laryngol. Otol. 1997;111:839–844. doi: 10.1017/S0022215100138769. [DOI] [PubMed] [Google Scholar]
- 45.Ferron C., Cernea S.S., Almeida A.R.T., Cesar D.V.G. Primary treatment of early fistula of parotid duct with botulinum toxin type A injection. An. Bras. Dermatol. 2017;92:864–866. doi: 10.1590/abd1806-4841.20175848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Philouze P., Vertu D., Ceruse P. Bilateral gustatory sweating in the submandibular region after bilateral neck dissection successfully treated with botulinum toxin. Br. J. Oral Maxillofac. Surg. 2014;52:761–763. doi: 10.1016/j.bjoms.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Ihler F., Laskawi R., Matthias C., Rustenbeck H.H., Canis M. Botulinumtoxin A gegen Hypersalivation. Einsatz bei Wundheilung nach Resektion eines Zungenkarzinoms [Botulinum toxin A after microvascular ALT flap in a patient with (corrected) squamous cell carcinoma of the tongue] [published correction appears in HNO. 2012 Oct; 60, 905] HNO. 2012;60:524–527. doi: 10.1007/s00106-011-2476-8. [DOI] [PubMed] [Google Scholar]
- 48.Pantel M., Volk G.F., Guntinas-Lichius O., Wittekindt C. Botulinum toxin type b for the treatment of a sialocele after parotidectomy. Head Neck. 2013;35:E11–E12. doi: 10.1002/hed.21778. [DOI] [PubMed] [Google Scholar]
- 49.Steffen A., Wollenberg B., Schönweiler R., Brüggemann N., Meyners T. Drooling nach Strahlentherapie. Botulinumtoxin als erfolgreiches Therapieverfahren [Drooling following radiation. Botulinum toxin as a successful treatment modality] HNO. 2011;59:115–117. doi: 10.1007/s00106-010-2232-5. [DOI] [PubMed] [Google Scholar]
- 50.Hill S.E., Mortimer N.J., Hitchcock B., Salmon P.J. Parotid fistula complicating surgical excision of a basal cell carcinoma: Successful treatment with botulinum toxin type A. Dermatol. Surg. 2007;33:1365–1367. doi: 10.1097/00042728-200711000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Kizilay A., Aladağ I., Ozturan O. Parotidektomi sonrasi gelişen tükürük fistülünün botulinum toksini ile iyileşmesi [Successful use of botulinum toxin injection in the treatment of salivary fistula following parotidectomy] Kulak Burun Bogaz Ihtis Derg. 2003;10:78–81. [PubMed] [Google Scholar]
- 52.Guntinas-Lichius O., Sittel C. Treatment of postparotidectomy salivary fistula with botulinum toxin. Ann. Otol. Rhinol. Laryngol. 2001;110:1162–1164. doi: 10.1177/000348940111001214. [DOI] [PubMed] [Google Scholar]
- 53.Marchese Ragona R., Blotta P., Pastore A., Tugnoli V., Eleopra R., De Grandis D. Management of parotid sialocele with botulinum toxin. Laryngoscope. 1999;109:1344–1346. doi: 10.1097/00005537-199908000-00032. [DOI] [PubMed] [Google Scholar]
- 54.Báez A., Paleari J., Durán M.N., Rudy T., Califano I., Barbosa N., Casas Parera I. Síndrome de Frey por submaxilectomía y tratamiento con toxina botulínica [Frey syndrome secondary to submaxillectomy and botulinic treatment] Medicina (B Aires) 2007;67:478–480. [PubMed] [Google Scholar]
- 55.Hatzis G.P., Finn R. Using botox to treat a mohs defect repair complicated by a parotid fistula. J. Oral Maxillofac. Surg. 2007;65:2357–2360. doi: 10.1016/j.joms.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 56.Birch J.F., Varma S.K., Narula A.A. Botulinum toxoid in the management of gustatory sweating (Frey′s syndrome) after superficial parotidectomy. Br. J. Plast. Surg. 1999;52:230–231. doi: 10.1054/bjps.1998.3058. [DOI] [PubMed] [Google Scholar]
- 57.Vezdrevanis K. Prostatic carcinoma shrunk after intraprostatic injection of botulinum toxin. Urol. J. 2011;8:239–241. [PubMed] [Google Scholar]
- 58.Ulloa F., Gonzàlez-Juncà A., Meffre D., Barrecheguren P.J., Martínez-Mármol R., Pazos I., Olivé N., Cotrufo T., Seoane J., Soriano E. Blockade of the SNARE protein syntaxin 1 inhibits glioblastoma tumor growth. PLoS ONE. 2015;10:e0119707. doi: 10.1371/journal.pone.0119707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He D., Manzoni A., Florentin D., Fisher W., Ding Y., Lee M., Ayala G. Biologic effect of neurogenesis in pancreatic cancer. Hum. Pathol. 2016;52:182–189. doi: 10.1016/j.humpath.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Karsenty G., Rocha J., Chevalier S., Scarlata E., Andrieu C., Zouanat F.Z., Rocchi P., Giusiano S., Elzayat E.A., Corcos J. Botulinum toxin type A inhibits the growth of LNCaP human prostate cancer cells in vitro and in vivo. Prostate. 2009;69:1143–1150. doi: 10.1002/pros.20958. [DOI] [PubMed] [Google Scholar]
- 61.Nam H.J., Kang J.K., Chang J.S., Lee M.S., Nam S.T., Jung H.W., Kim S.K., Ha E.M., Seok H., Son S.W., et al. Cells transformed by PLC-gamma 1 overexpression are highly sensitive to clostridium difficile toxin A-induced apoptosis and mitotic inhibition. J. Microbiol. Biotechnol. 2012;22:50–57. doi: 10.4014/jmb.1107.07018. [DOI] [PubMed] [Google Scholar]
- 62.Proietti S., Nardicchi V., Porena M., Giannantoni A. Attività della tossina botulinica A in linee cellulari di cancro prostatico [Botulinum toxin type-A toxin activity on prostate cancer cell lines] Urologia. 2012;79:135–141. doi: 10.5301/RU.2012.9254. [DOI] [PubMed] [Google Scholar]
- 63.Bandala C., Perez-Santos J.L., Lara-Padilla E., Delgado Lopez G., Anaya-Ruiz M. Effect of botulinum toxin A on proliferation and apoptosis in the T47D breast cancer cell line. Asian Pac. J. Cancer Prev. 2013;14:891–894. doi: 10.7314/APJCP.2013.14.2.891. [DOI] [PubMed] [Google Scholar]
- 64.Bandala C., Cortés-Algara A.L., Mejía-Barradas C.M., Ilizaliturri-Flores I., Dominguez-Rubio R., Bazán-Méndez C.I., Floriano-Sánchez E., Luna-Arias J.P., Anaya-Ruiz M., Lara-Padilla E., et al. Botulinum neurotoxin type A inhibits synaptic vesicle 2 expression in breast cancer cell lines. Int. J. Clin. Exp. Pathol. 2015;8:8411–8418. [PMC free article] [PubMed] [Google Scholar]
- 65.Rust A., Leese C., Binz T., Davletov B. Botulinum neurotoxin type C protease induces apoptosis in differentiated human neuroblastoma cells. Oncotarget. 2016;7:33220–33228. doi: 10.18632/oncotarget.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang X., Wheeler M.B., Kang Y.H., Sheu L., Lukacs G.L., Trimble W.S., Gaisano H.Y. Truncated SNAP-25 (1-197), like botulinum neurotoxin A, can inhibit insulin secretion from HIT-T15 insulinoma cells. Mol. Endocrinol. 1998;12:1060–1070. doi: 10.1210/mend.12.7.0130. [DOI] [PubMed] [Google Scholar]
- 67.Hajighasemlou S., Alebouyeh M., Rastegar H., Manzari M.T., Mirmoghtadaei M., Moayedi B., Ahmadzadeh M., Parvizpour F., Johari B., Naeini M.M., et al. Preparation of Immunotoxin Herceptin-Botulinum and Killing Effects on Two Breast Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2015;16:5977–5981. doi: 10.7314/APJCP.2015.16.14.5977. [DOI] [PubMed] [Google Scholar]
- 68.Cheng Y.T., Chung Y.H., Kang H.Y., Tai M.H., Chancellor M.B., Chuang Y.C. OnobotulinumtoxinA Has No Effects on Growth of LNCaP and PC3 Human Prostate Cancer Cells. Low. Urin. Tract Symptoms. 2013;5:168–172. doi: 10.1111/luts.12003. [DOI] [PubMed] [Google Scholar]
- 69.Ansiaux R., Gallez B. Use of botulinum toxins in cancer therapy. Expert Opin. Investig. Drugs. 2007;16:209–218. doi: 10.1517/13543784.16.2.209. [DOI] [PubMed] [Google Scholar]
- 70.Coarfa C., Florentin D., Putluri N., Ding Y., Au J., He D., Ragheb A., Frolov A., Michailidis G., Lee M., et al. Influence of the neural microenvironment on prostate cancer. Prostate. 2018;78:128–139. doi: 10.1002/pros.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matak I., Bölcskei K., Bach-Rojecky L., Helyes Z. Mechanisms of Botulinum Toxin Type A Action on Pain. Toxins. 2019;11:459. doi: 10.3390/toxins11080459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mittal S.O., Safarpour D., Jabbari B. Botulinum Toxin Treatment of Neuropathic Pain. Semin. Neurol. 2016;36:73–83. doi: 10.1055/s-0036-1571953. [DOI] [PubMed] [Google Scholar]
- 73.Kitamura Y., Matsuka Y., Spigelman I., Ishihara Y., Yamamoto Y., Sonoyama W., Kamioka H., Yamashiro T., Kuboki T., Oguma K. Botulinum toxin type a (150 kDa) decreases exaggerated neurotransmitter release from trigeminal ganglion neurons and relieves neuropathy behaviors induced by infraorbital nerve constriction. Neuroscience. 2009;159:1422–1429. doi: 10.1016/j.neuroscience.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 74.Omoto K., Maruhama K., Terayama R., Yamamoto Y., Matsushita O., Sugimoto T., Oguma K., Matsuka Y. Cross-Excitation in Peripheral Sensory Ganglia Associated with Pain Transmission. Toxins. 2015;7:2906–2917. doi: 10.3390/toxins7082906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marino M.J., Terashima T., Steinauer J.J., Eddinger K.A., Yaksh T.L., Xu Q. Botulinum toxin B in the sensory afferent: Transmitter release, spinal activation, and pain behavior. Pain. 2014;155:674–684. doi: 10.1016/j.pain.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong B., Yao L., Ni L., Wang L., Hu X. Antinociceptive effect of botulinum toxin A involves alterations in AMPA receptor expression and glutamate release in spinal dorsal horn neurons. Neuroscience. 2017;357:197–207. doi: 10.1016/j.neuroscience.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Fan C., Chu X., Wang L., Shi H., Li T. Botulinum toxin type A reduces TRPV1 expression in the dorsal root ganglion in rats with adjuvant-arthritis pain. Toxicon. 2017;133:116–122. doi: 10.1016/j.toxicon.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 78.Restani L., Antonucci F., Gianfranceschi L., Rossi C., Rossetto O., Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A) J. Neurosci. 2011;31:15650–15659. doi: 10.1523/JNEUROSCI.2618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matak I., Bach-Rojecky L., Filipović B., Lacković Z. Behavioral and immunohistochemical evidence for central antinociceptive activity of botulinum toxin A. Neuroscience. 2011;186:201–207. doi: 10.1016/j.neuroscience.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 80.Bach-Rojecky L., Salković-Petrisić M., Lacković Z. Botulinum toxin type A reduces pain supersensitivity in experimental diabetic neuropathy: Bilateral effect after unilateral injection. Eur. J. Pharmacol. 2010;633:10–14. doi: 10.1016/j.ejphar.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 81.Bach-Rojecky L., Lacković Z. Central origin of the antinociceptive action of botulinum toxin type A. Pharmacol. Biochem. Behav. 2009;94:234–238. doi: 10.1016/j.pbb.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 82.Arezzo J.C. Possible mechanisms for the effects of botulinum toxin on pain. Clin. J. Pain. 2002;18(Suppl. S6):S125–S132. doi: 10.1097/00002508-200211001-00003. [DOI] [PubMed] [Google Scholar]
- 83.Neufeld N.J., Elnahal S.M., Alvarez R.H. Cancer pain: A review of epidemiology, clinical quality and value impact. Future Oncol. 2017;13:833–841. doi: 10.2217/fon-2016-0423. [DOI] [PubMed] [Google Scholar]
- 84.Schreiber K.L., Kehlet H., Belfer I., Edwards R.R. Predicting, preventing and managing persistent pain after breast cancer surgery: The importance of psychosocial factors. Pain Manag. 2014;4:445–459. doi: 10.2217/pmt.14.33. [DOI] [PubMed] [Google Scholar]
- 85.Fisher J., Scott C., Stevens R., Marconi B., Champion L., Freedman G.M., Asrari F., Pilepich M.V., Gagnon J.D., Wong G. Randomized phase III study comparing best supportive care to Biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group [RTOG] 97–13. Int. J. Radiat. Oncol. Biol. Phys. 2000;48:1307–1310. doi: 10.1016/S0360-3016(00)00782-3. [DOI] [PubMed] [Google Scholar]
- 86.Chargari C., Fromantin I., Kirova Y.M. Importance of local skin treatments during radiotherapy for prevention and treatment of radio-induced epithelitis. Cancer Radiother. 2009;13:259–266. doi: 10.1016/j.canrad.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 87.Kirova Y.M., Fromantin I., De Rycke Y., Fourquet A., Morvan E., Padiglione S., Falcou M.C., Campana F., Bollet M.A. Can we decrease the skin reaction in breast cancer patients using hyaluronic acid during radiation therapy-results of phase III randomised trial. Radiother. Oncol. 2011;100:205–209. doi: 10.1016/j.radonc.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 88.Fleming J.A., O’Connor B.D. Use of lidocaine patches for neuropathic pain in a comprehensive cancer center. Pain Res. Manag. 2009;14:381–388. doi: 10.1155/2009/723179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wiffen P.J., Derry S., Moore R.A. Impact of morphine, fentanyl, oxycodone or codeine on patient consciousness, appetite and thirst when used to treat cancer pain. Cochrane Database Syst. Rev. 2014;5:CD011056. doi: 10.1002/14651858.CD011056.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lakraje A.A., Moghimi N., Jabbari B. Sialorrhea, anatomy, physiology and treatment with emphasis on the role of botulinum toxins. Toxins. 2013;5:1010–1031. doi: 10.3390/toxins5051010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schindel J., Markowicz H., Levie B. Combined surgical-radiological treatment of parotid gland fistulae. J. Laryngol. Otol. 1968;82:867–870. doi: 10.1017/S0022215100069607. [DOI] [PubMed] [Google Scholar]
- 92.Lovato A., Restivo D.A., Ottaviano G., Marioni G., Marchese-Ragona R. Botulinum toxin therapy: Functional silencing of salivary disorders. Terapia con tossina botulinica: Silenziamento funzionale dei disordini salivari. Acta Otorhinolaryngol. Ital. 2017;37:168–171. doi: 10.14639/0392-100X-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]