Abstract
Maize (Zea mays L.) is an important component of global food security but its production is threatened by abiotic stresses in climate change scenarios, especially drought stress. Many multinational companies have introduced maize hybrids worldwide which have variable performance under diverse environmental conditions. The maize production is likely to be affected by a future water crisis. Potassium (K) is a well-known macronutrient which improves the performance of cereals under abiotic stresses. In this field experiment, we assessed the influence of soil applied K on the productivity of diverse maize hybrids grown under well-watered and drought stress conditions. The study consisted of three K levels viz., control (no KCl), KCl at 50 kg ha−1, and KCI at 75 kg ha−1 factorally combined with two irrigation levels (i.e., normal recommended irrigation, well-watered condition, and half of the recommended irrigation, drought stress condition) and eight maize hybrids. Irrigation was kept in main plots, potassium in subplot, and maize hybrids in sub-subplots. The results revealed that performance of the maize hybrids was significantly influenced by all three factors, and the interaction of irrigation with potassium and irrigation with hybrids was significant; results being non-significant for all other interactions. Potassium application improved yield traits and water productivity under both normal and water stress conditions but effect was more prominent under water stress conditions than normal conditions. Potassium application also alleviated drought susceptibility of all maize hybrids. In all cases, the performance of maize hybrids was maximum under potassium application at 75 kg ha−1.
Keywords: water deficit, biological yield, grain yield, drought susceptibility index
1. Introduction
Maize (Zea mays L.) is the third most important cereal for global food security after wheat and rice [1,2]. Among cereal crops, maize plays a productive role in a cropping system by producing good economic return to farmers and having higher yield potential, shorter growing period, and growing ability in varying environments. It also serves as a cheaper feed source for livestock and its seeds are used in different agro-based industries. Nevertheless, in climate change scenarios, maize productivity is threatened by abiotic stresses, especially heat and drought [3,4,5,6] which in turn is threatening the global food security.
Among the abiotic stresses, drought has emerged as a serious production constraint in maize, especially in arid and semiarid regions [2,3,5,7]. The severity of water stress not only depends on the duration and intensity [8] but also on the growth stage when plants are affected, i.e., seedling, vegetative, or reproductive stage [9,10,11], all of which have differential responses but ultimately all lead to yield loss. At the early stage of water stress, losses are due to reduced growth, development, and CO2 fixation, while water stress at the reproductive stage leads to reproductive failure, less allocation of assimilates to the grains, and reduced grain filling period which results in shrunk grains [10,11].
Potassium is known as a stress alleviator plant nutrient which alleviates the negative consequences of abiotic stresses by regulating the physiological and biochemical process in plants [12,13,14]. Aslam et al. [12] reported that potassium improves drought tolerance by improving root growth, cell turgor pressure, and osmotic pressure. Waraich et al. [14] reported that potassium nutrition keeps a balance between antioxidant enzymes and reactive oxygen species and regulates osmotic and turgor pressure in order to avoid yield losses from drought. Potassium is also an integral part of many metabolic activities of the plants [13,15,16] and proper availability of potassium keeps the plants normal, even under drought stress, which ultimately leads to higher yield and water productivity.
Various formulations of potassium are available in the global markets. Among these, muriate of potash (KCl) is commonly used as a source of K. It contains a maximum amount of K2O, however, chloride (Cl−) in access amounts in KCl inhibits nitrification and affects uptake of nitrogen, potassium, phosphorus, and calcium [17] which may also result in nonsignificant effect of fertilizers on crop performance [18]. These negative consequences of Cl− are more obvious in acidic soils [19,20] and are negligible in soils with high pH (≥8). Thus, application of KCl should be avoided in acidic soils. In this study, the soil had a pH > 8.0, and therefore we used KCl as a source of potassium.
A wide range of studies have investigated the impact of water stress and potassium nutrition on different field crops, including maize [12,13,15]. All crops and genotypes do not response in the same way to potassium application under all agro-climatic regions [21,22,23]. Hussain et al. [21] reported a significant interaction between mung bean genotypes and potassium application with respect to protein content and seed yield. Amanullah et al. [24] reported that foliar and soil applied potassium improved maize performance under moisture stress. Nevertheless, there is a limited research on interactive response of potassium application and maize genotypes to water stress for yield related traits and drought susceptibility index especially under arid conditions. Keeping in mind these facts, we studied the interactive response of potassium application and maize hybrids for improving drought tolerance. The specific objective of this study was to evaluate the response of diverse maize hybrids from different multinational companies to potassium application under drought stress, in terms of grain yield, water productivity, and drought susceptibility.
2. Materials and Methods
2.1. Experimental Site and Treatments
The experiment was conducted in the experimental area of the College of Agriculture, Bahauddin Zakariya University, Bahadur Sub-Campus Layyah, Pakistan. The soil of the experimental location was sandy loam with a pH of 8.1. Physiochemical properties of soil and irrigation water are presented in Table 1. The area falls in a subtropical climate characterized by warm summers and cool winters having a long-term average rainfall of ≤200 mm. Weather data of experimental duration is presented in Supplementary Table S1.
Table 1.
Physiochemical properties of soil and irrigation water used for the experiment.
| Parameter | Unit | Value | Status | |
|---|---|---|---|---|
| 2017 | 2018 | |||
| Physical Parameters | ||||
| Sand | % | 61.6 | 61.4 | |
| Silt | % | 21.9 | 21.3 | |
| Clay | % | 16.5 | 16.3 | |
| Bulk Density | g cm−3 | 1.28 | 1.28 | |
| Texture Class | Sandy Loam | Sandy Loam | ||
| Chemical Parameters of Soil | ||||
| pH | 8.1 | 8.1 | ||
| EC | µS/cm | 1.9 | 1.8 | |
| Total Nitrogen | mg kg−1 | 432 | 421 | Very Low |
| Available Phosphorus | mg kg−1 | 6.9 | 6.8 | Low |
| Available Potassium | mg kg−1 | 124 | 118 | Adequate |
| Chemical Parameters of Water | ||||
| EC | µS/cm | 309 | 321 | |
| Ca+Mg | meq L−1 | 2.12 | 2.08 | |
| Na | meq L−1 | 1.21 | 1.09 | |
| HCO−3 | meq L−1 | 1.88 | 1.82 | |
| SAR | Cmolc L−1 | 1.01 | 0.96 | |
Experimental treatments include eight single cross maize hybrids (Table 2) factorally combined with three levels of potassium (control, 50 kg KCl ha−1, and 75 KCl kg ha−1) and two levels of water stress (i.e., normal irrigation of 75 mm applied every 10 days and water stress, where alternate irrigation was skipped). The maize hybrids used in study are widely grown by farmers in Pakistan. The characteristics of these maize hybrids are given in Table 2. Potassium was applied as muriate of potash (KCl). Experimental treatments were placed in a randomized complete block design with three replications and split-split plot arrangement where irrigation was kept in main plots, Potassium application was kept in subplots and maize hybrids were placed in sub-subplots.
Table 2.
Characteristics of maize hybrids used in the study.
| Maize Hybrids | Source of Seed | Characteristics |
|---|---|---|
| 30Y87 | Pioneer Pakistan | Stay green character, single cross, adopted to all types of weather conditions and a yield potential of 10.4 t ha−1 |
| 30T60 | Pioneer Pakistan | Strong stem and root, single cross, reddish seeds and a yield potential of 10.9 t ha−1 |
| 30R50 | Pioneer Pakistan | Appropriate only for average environments |
| S-7720 | Syngenta Pakistan | Single cross, well suited product for high density planting environments. responds very well to high management conditions with a yield potential of 9.8 t ha−1 |
| DK-6714 | Monsanto Pakistan | Single cross, flex ear with 117 days relative maturity and a yield potential of 11.4 t ha−1 |
| DK-6789 | Monsanto Pakistan | Single cross and a yield potential of 10.4 t ha−1 |
| DK-Garanon | Monsanto Pakistan | White hybrid well adapted to diverse climatic conditions |
| Gorrila | Monsanto Pakistan | White seeded maize hybrid |
All treatments were kept in the same plot for both years. The seed of all maize hybrids was sown by dibbler keeping an R × R and P × P distance of 45 and 15 cm, respectively, on 1 July 2017 and 2018. The nitrogen and phosphorus were applied, at the recommended rates of 170 and 115 kg ha−1, using urea (46% N) and diammonium phosphate (46% N, 18% P2O5) as the source. All treatments received an equal amount nitrogen and phosphors, where potassium was applied as per treatment. All fertilizers were applied on the basis of a soil analysis report. Four lines of each hybrid were sown in each replication. All the potassium and phosphorus was applied at the time of land preparation, whereas the nitrogen was applied in three equal splits (at the time of land preparation, 2 weeks after germination, and 5 weeks after germination). The total applied irrigation water was calculated as water received through irrigation and rainfall. In the well-watered condition, the total water supplied to maize crop was 600 mm, while in the drought treatment, it was 350 mm. For each rainfall event, the incident amount of rainfall was recorded through a weather station. This rainfall was subtracted from the total irrigation water applied at that interval, and the remaining amount of water was supplied through irrigation water. Cutthroat flumes were installed in the experimental plot to provide for the harvest of the calculated crop on October 20 for both years.
2.2. Data Collection
On maturity, data were collected for 1000-grain weight, biological yield, grain yield, and drought susceptibility index for grain yield. The 1000 grains were counted using a grain counter and weighed on an electric balance. Two central rows of each replication were harvested and oven dried until a constant weight, for the biological yield. All cobs of the same two rows were threshed to measure grain yield. Data for grain yield and biological yield was then converted into yield per hectare. Water productivity was calculated as per the following formula:
| (1) |
where total water received is water received by the crop from irrigation and rainfall. Drought susceptibility index was calculated based on grain yield as per the following formula proposed by Grzesiak et al. [25]:
| (2) |
where DSIGY is drought susceptibility index for grain yield, (grain yield)D is grain yield under drought, and (grain yield)IR is grain yield under normal irrigated conditions. DS stands for drought severity index, which is calculated by the following formula:
| (3) |
where DS is drought severity index, (total water)IR is total water received by the crop under normal irrigation, and (total water)D is total water received by the crop under water stress conditions. Total water received stands for cumulative water received by the irrigation and rainfall under specific irrigation treatment.
3. Statistical Analysis
Data collected on growth and yield parameters was analyzed statistically by using Fischer’s analysis of variance technique and HSD test at 5% probability was applied to compare the treatment means [26]. Data were analyzed using software Statistix 8.1. The year effect was found non-significant, and therefore the average of two years was used in the presentation of data.
4. Results
Both irrigation regimes significantly affected the 1000-grain weight, grain yield, biological yield, water productivity, and drought susceptibility index of maize hybrids. Likewise, potassium application also had a significant effect on 1000-grain weight, grain yield, biological yield, water productivity, and drought susceptibility index. Maize hybrids also differed for grain yield, biological yield, water productivity, and drought susceptibility index; results being non-significant for 1000-grain weight. The interaction of irrigation regimes with maize hybrids, and irrigation regimes with potassium application were significant for 1000-grain weight, grain yield, biological yield, water productivity, and drought susceptibility index. Two-way interaction of maize hybrids with potassium application was only significant for biological/grain yield and water productivity. All the other two-, three-, and four-way interactions were non-significant for all the studied traits (Supplementary Table S2).
Under irrigated conditions, the highest 1000-grain weight, biological yield, grain yield, and water productivity were recorded for the potassium application at 75 kg ha−1 and this was similar for the potassium application at 50 kg ha−1 for 1000-grain weight and biological yield. A similar trend was observed for potassium application under drought stress for biological yield, grain yield, and water productivity; 1000-grain weight was not significantly affected by potassium application under drought stress (Table 3).
Table 3.
Influence of various potassium levels on 1000-grain weight, biological yield, grain yield, and water productivity of various maize hybrids under well-watered and drought stress conditions.
| Well-Watered | Drought | |||||||
|---|---|---|---|---|---|---|---|---|
| Hybrids | K0 | K1 | K2 | Mean (G) | K0 | K1 | K2 | Mean (G) |
| 1000-grain weight (g) | ||||||||
| 30T60 | 192.0 | 207.0 | 201.3 | 200.1 | 175.3 | 190.0 | 199.7 | 188.3 |
| 30Y87 | 181.7 | 194.7 | 207.0 | 194.5 | 186.0 | 194.0 | 196.3 | 192.1 |
| 30R50 | 185.0 | 188.3 | 196.3 | 189.9 | 173.0 | 180.7 | 165.3 | 173.0 |
| DK-6714 | 196.7 | 196.3 | 204.0 | 199.0 | 184.7 | 185.7 | 195.7 | 188.7 |
| S-7720 | 190.3 | 197.3 | 195.3 | 194.3 | 188.3 | 194.0 | 193.3 | 191.9 |
| DK-Garanon | 194.3 | 200.5 | 192.7 | 195.8 | 175.0 | 183.0 | 186.5 | 181.5 |
| DK-6789 | 187.5 | 202.5 | 190.7 | 193.6 | 182.5 | 185.0 | 174.3 | 180.6 |
| Gorrila | 187.0 | 187.5 | 202.5 | 192.3 | 181.0 | 184.5 | 193.0 | 186.2 |
| Mean (K) | 189.3B | 196.8A | 198.7A | 180.7 | 187.1 | 188.0 | ||
| HSD (p ≤ 0.05) | K = 6.7 | K = 9.7 | ||||||
| Biological Yield (t ha−1) | ||||||||
| 30T60 | 12.3bc | 12.3bc | 13.4a | 12.7A | 9.8gh | 10.3e–h | 11.2a–d | 10.4BC |
| 30Y87 | 10.5g–j | 10.7d–i | 11.6c–f | 10.9C | 8.5i | 10.3d–h | 11.4ab | 10.1CD |
| 30R50 | 10.0h–j | 10.8d–h | 10.5g–j | 10.4CD | 8.6i | 10.1f–h | 10.3d–h | 9.7D |
| DK-6714 | 13.1ab | 12.2bc | 12.4a–c | 12.6A | 10.3c–g | 11.3a–c | 11.7a | 11.1A |
| S-7720 | 11.7cd | 11.6c–e | 11.5c–g | 11.6B | 9.4hi | 10.2f–h | 10.6b–g | 10.1CD |
| DK-Garanon | 11.1d–h | 11.5c–g | 12.3bc | 11.6B | 9.9f–h | 10.7b–g | 11.2a–e | 10.6B |
| DK-6789 | 10.4h–j | 11.1d–h | 10.5f–j | 10.7C | 10.0f–h | 10.4b–g | 10.3e–h | 10.2BC |
| Gorrila | 9.7ij | 10.67e–i | 9.5j | 10.0D | 8.6i | 10.2f–h | 9.4hi | 10.1CD |
| Mean (K) | 11.1B | 11.4A | 11.5A | 9.4C | 10.4B | 10.8A | ||
| HSD (p ≤ 0.05) | G = 0.5; K = 0.3; G × K = 1.03 | G = 0.5; K = 0.20; G × K = 0.90 | ||||||
| Grain Yield (t ha−1) | ||||||||
| 30T60 | 4.9d | 5.5b | 5.8a | 5.4A | 3.0hi | 3.5d–g | 4.2b | 3.6B |
| 30Y87 | 4.5h | 4.7fg | 4.8ef | 4.7E | 2.7ij | 3.3f–h | 4.1b | 3.3CD |
| 30R50 | 3.7l | 3.8k | 3.9k | 3.8G | 2.5jk | 3.2gh | 3.5e–g | 3.1E |
| DK-6714 | 5.2c | 5.2c | 5.4b | 5.3B | 3.5e–g | 4.1b | 4.8a | 4.1A |
| S-7720 | 4.3i | 4.9de | 5.1c | 4.8D | 2.2k | 3.1hi | 3.9bc | 3.1E |
| DK-Garanon | 4.7fg | 4.8de | 5.2c | 4.9C | 3.0hi | 3.8b-e | 4.1b | 3.7B |
| DK-6789 | 4.1j | 4.6g | 4.7fg | 4.5F | 3.0hi | 3.5efg | 3.9b–d | 3.5BC |
| Gorrila | 3.5m | 3.81k | 3.9k | 3.7G | 2.9h–j | 3.1gh | 3.6c–f | 3.2DE |
| Mean (K) | 4.3C | 4.7B | 4.9A | 2.9C | 3.5B | 4.0A | ||
| HSD (p ≤ 0.05) | G = 0.06; K = 0.02; G × K = 0.13 | G = 0.19; K = 0.09; G × K = 0.40 | ||||||
| Water Productivity (kg ha−1 mm−1) | ||||||||
| 30T60 | 8.2d | 9.2b | 9.7a | 9.0A | 8.6hi | 10.0d–f | 12.0b | 10.2B |
| 30Y87 | 7.5h | 7.8fg | 8.0ef | 7.8C | 7.7ij | 9.4fgh | 11.7b | 9.6D |
| 30R50 | 6.2l | 6.3k | 6.5k | 6.3E | 7.1jk | 9.1gh | 10.0e–g | 8.8F |
| DK-6714 | 8.7c | 8.7c | 9.0b | 8.8A | 10.0e–g | 11.7b | 13.7a | 11.8A |
| S-7720 | 7.2i | 8.2de | 8.5c | 7.9C | 6.3k | 8.9hi | 11.1bc | 8.8F |
| DK-Garanon | 7.8fg | 8.0de | 8.7c | 8.2B | 8.6hi | 10.9b–d | 11.7b | 10.4B |
| DK-6789 | 6.8j | 7.7g | 7.8fg | 7.4D | 8.6hi | 10.0e–g | 11.1bc | 9.9C |
| Gorrila | 5.8m | 6.4k | 6.5k | 6.2E | 8.3h–j | 8.9gh | 10.3c–e | 9.1E |
| Mean (K) | 7.3C | 7.8B | 8.1A | 8.1C | 9.9B | 11.5A | ||
| HSD (p ≤ 0.05) | G = 0.18; K = 0.15 | G = 0.19; K = 0.17 | ||||||
K0: no potassium application; K1: 50 kg KCl ha−1; K2: 75 kg KCl ha−1.
Under well-watered conditions, the highest biological yield, grain yield, and water productivity were recorded in maize hybrid “30T60” and that was statistically similar in maize hybrid “DK-6714” for biological yield and water productivity, however, maize hybrid “DK-6714” produced significantly higher biological yield, grain yield, and water productivity under drought stress (Table 3).
The interactions showed that the highest biological yield, grain yield, and water productivity were recorded in maize hybrid “30T60” for potassium application at 75 kg ha−1 under well-watered conditions. Under drought stress, the biological yield, grain yield, and water productivity were higher in maize hybrid “DK-6714” for potassium application at 75 kg ha−1 and this was similar in maize hybrid ”30Y87” for biological yield with potassium application of 75 kg ha−1 (Table 3).
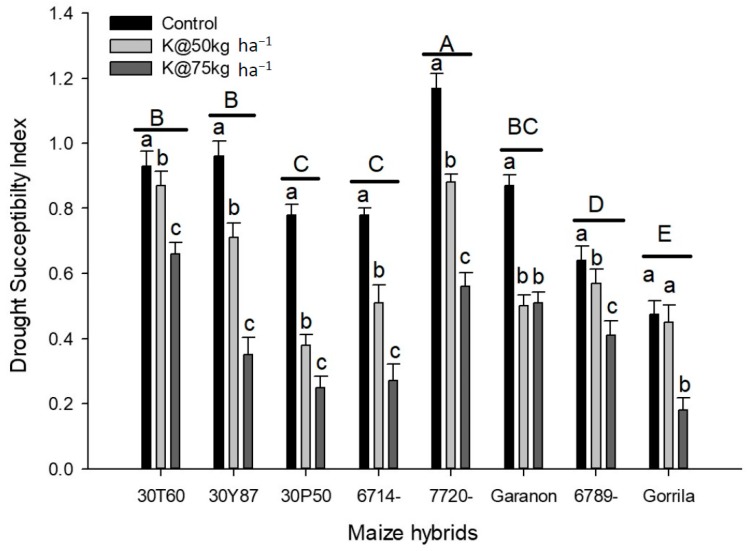
The drought susceptibility index was the highest in maize hybrid “S-7720” and lowest in maize hybrid “Gorrila”. The highest drought susceptibility index was recorded with potassium application at 75 kg ha−1 which was followed by potassium application at 50 kg ha−1 in all studied hybrids except the Gorilla hybrid. For the DK-Garamon hybrid, the drought susceptibility index for potassium application at 50 kg ha−1 was statistically similar to the control treatment (Figure 1).
Figure 1.
Drought susceptibility index of different maize hybrids under different treatments. Data are an average of two years and three replications. Small letters show the comparison between treatments of each hybrid and capital letters shows the comparison of different hybrids.
5. Discussion
Drought stress had a negative impact on performance of all maize hybrids which was indicated through reduced values of 1000-grain weight, biological yield, and grain yield as compared with well-watered conditions. Indeed, drought has emerged as a serious production constraint in maize especially in arid and semiarid regions [2,4,5,7] which affects the crops at seedling, vegetative, and reproductive stages [9,10,11], and thus leading towards yield losses. At early-stage water stress, losses are due to reduced growth, development, and CO2 fixation and water stress at the reproductive stage leads to reproductive failure, less allocation to assimilate to the grains and reduced grain filling period, and less grain set which result in reduced yields [27].
However, potassium application at either rate increased the 1000-grain weight, biological yield, grain yield, water productivity, and alleviated the drought susceptibility of maize hybrids. Indeed, potassium is a stress alleviator plant nutrient which alleviates the negative impacts of abiotic stresses by regulating the physiological and biochemical process [12,13,14], including improvement in root growth, cell turgor pressure, and osmotic pressure [12], and a stable balance between antioxidant enzymes and reactive oxygen species [14].
For accumulation of dry matter in crop plants, the process of photosynthesis is of key importance, and a decrease in photosynthesis under drought stress leads to closing of stomata due to a decrease in leaf internal CO2 concentration and leaf transpiration rate, however, addition of K enhances photosynthesis and carbohydrate metabolism under drought stress [28,29], by improving the leaf internal CO2 concentration and leaf stomatal conductance which regulates the stomatal opening. The stomatal oscillations also have a strong impact on the concentration of abscisic acid in cells, and water relation of plants [30,31].
Potassium is also part of many metabolic activities of plants [13,15,16] and proper availability of potassium keeps the plant nearly normal even under drought which ultimately leads to higher yield and water productivity, as was observed in this study. Input of K improves the water relations under water stress by helping the plant absorb more water to attain turgidity. In a study, Subbarao et al. [32] found that the osmotic potential and relative water contents were decreased at low K input. Our findings of a positive role of K in alleviation of drought stress in maize can be related to the enhancement in leaf K due to an external input of K which enhances relative water contents and leaf turgor in maize [33] and sunflower [34]. Indeed, the application of K enhances the activities of different antioxidant enzymes (e.g., superoxide dismutase, catalase, and peroxidase) [35] and improves tolerance to osmotic stress. In a study, it was reported that external K input ameliorated the negative impacts of salt stress by improving the activities of antioxidant enzymes [36]. In another study, it was reported that the scavenging of reactive oxygen species through antioxidants (e.g., SOD, CAT, and GPX) can be improved by exogenous potassium application [37].
Potassium also plays key roles in sugar metabolism and sugar remobilization under drought stress. For example, Martineau et al. [38] reported that the sugars are accumulated during drought stress in maize which are reallocated with optimum potassium nutrition, possibly due to an improvement in photosynthesis and phloem transport of carbohydrates from leaves to roots. They also found that sugar transport was 35% lower in potassium deficit plants than potassium sufficient plants [38]. This decrease in assimilate transport is attributed to the decreased activity of sucrose phosphate synthase, a key enzyme in sucrose formation [39]. According to Cakmak et al. [40], the potassium effect on sucrose transport accounts for most of the plant responses undergoing potassium stress which include: sugar accumulation, negative feedback on photosynthesis [41], and lower growth rates. Potassium application also improves the zeatin, Z ribiside, and abscisic acid contents during grain filling which increase the sink strength [28,42], and thus result in better grain filling and grain yield under drought stress, as was observed in this study. Increase in grain weight due to potassium application in this study might be attributed to increased activity of photosynthesis which finally improves the source-sink relationship resulting in better grain development [24,43]. In a previous study, Amanullah et al. [24] reported that foliar and soil applied potassium improved the growth, yield, and yield components of maize under drought stress conditions. Many other studies in Pakistan [44,45,46,47], China [48,49,50,51], and USA [52] have reported an improvement in maize performance due to potassium application under optimal and suboptimal conditions. Thus, improvement in grain yield in maize hybrids under drought stress due to potassium application was possibly due to an improvement in osmolyte accumulations, activation of antioxidants, or improved sugar metabolism which improves the grain weight ultimately resulting in better grain yield and water productivity.
Maize hybrids also differed for 1000-grain weight, biological yield, grain yield, water productivity, and drought susceptibility. These differences in the grain weight, grain yield, water productivity, and drought susceptibility are desirable for cereals [53] and could be useful to identify better maize hybrids to cope with drought stress in the future. These differences are due to the diverse origin and the variation in the genetic makeup of each hybrid.
6. Conclusions
Potassium application improved yield traits and water productivity under both normal and water stress conditions, but effect was more prominent under water stress conditions than normal conditions. Potassium application also alleviated drought susceptibility of all hybrids. The performance of maize hybrids was maximum under potassium application at 75 kg ha−1, and therefore maize farmers in arid regions with a problem of water stress may get higher productions under potassium application at 75 kg ha−1.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/1/75/s1, Table S1: Weather data during the period of experimentation, Table S2: Significance terms for irrigation, potassium, maize hybrids and year on 1000 grain weight, grain yield, biological yield, water productivity and drought susceptibility index as obtained in Layyah, Pakistan.
Author Contributions
S.U.-A. and M.I. floated the idea of research and supervised work. A.N., A.S. (Abdul Sattar), and A.S. (Ahmed Sher) arranged the experimental materials and worked on different preliminary drafts of paper. M.N., U.F., and F.N. collected data and were involved in crop husbandry. U.S. and K.M. proofread the paper and helped in paper revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Shiferaw B., Prasanna B.M., Hellin J., Bänziger M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011;3:307. doi: 10.1007/s12571-011-0140-5. [DOI] [Google Scholar]
- 2.Tesfaye K., Gbegbelegbe S., Cairns J.E., Shiferaw B., Prasanna B.M., Sonder K., Boote K., Makumbi D., Robertson R. Maize systems under climate change in sub-Saharan Africa: Potential impacts on production and food security. Int. J. Clim. Chang. Strat. Manag. 2015;7:247–271. doi: 10.1108/IJCCSM-01-2014-0005. [DOI] [Google Scholar]
- 3.Jin C., Luo X., Xiao X., Dong J., Li X., Yang J., Zhao D. The 2012 Flash Drought Threatened US Midwest Agroecosystems. Chin. Geogr. Sci. 2019:1–15. doi: 10.1007/s11769-019-1066-7. [DOI] [Google Scholar]
- 4.Jin Z., Zhuang Q., Tan Z., Dukes J.S., Zheng B., Melillo J.M. Do maize models capture the impacts of heat and drought stresses on yield? Using algorithm ensembles to identify successful approaches. Glob. Chang. Biol. 2016;22:3112–3126. doi: 10.1111/gcb.13376. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q., Hu Z. Assessment of drought during corn growing season in Northeast China. Theor. Appl. Climatol. 2018;133:1315–1321. doi: 10.1007/s00704-018-2469-6. [DOI] [Google Scholar]
- 6.Zhang Q., Yang Z. Impact of Extreme Heat on Corn Yield in Main Summer Corn Cultivating Area of China at Present and Under Future Climate Change. Int. J. Plant Prod. 2019:1–8. doi: 10.1007/s42106-019-00052-w. [DOI] [Google Scholar]
- 7.Ul-Allah S., Khan A.A., Burkert A., Wachendorf M. Socio-economic aspects of fodder production in urban and peri-urban areas of Faisalabad. Pak. J. Agric. Sci. 2014;51:483–490. [Google Scholar]
- 8.Samarah N.H., Alqudah A.M., Amayreh J.A., McAndrews G.M. The effect of late-terminal drought stress on yield components of four barley cultivars. J. Agron. Crop Sci. 2009;195:427–441. doi: 10.1111/j.1439-037X.2009.00387.x. [DOI] [Google Scholar]
- 9.Ali Q., Ashraf M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: Growth, photosynthesis, water relations and oxidative defence mechanism. J. Agron. Crop Sci. 2011;197:258–271. doi: 10.1111/j.1439-037X.2010.00463.x. [DOI] [Google Scholar]
- 10.Daryanto S., Wang L., Jacinthe P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE. 2016;11:e0156362. doi: 10.1371/journal.pone.0156362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge T., Sui F., Bai L., Tong C., Sun N. Effects of water stress on growth, biomass partitioning, and water-use efficiency in summer maize (Zea mays L.) throughout the growth cycle. Acta Physiol. Plant. 2012;34:1043–1053. doi: 10.1007/s11738-011-0901-y. [DOI] [Google Scholar]
- 12.Aslam M., Zamir M.S.I., Afzal I., Yaseen M., Mubeen M., Shoaib A. Drought stress, its effect on maize production and development of drought tolerance through potassium application. Cercet. Agron. Mold. 2013;46:99–114. [Google Scholar]
- 13.Wang M., Zheng Q., Shen Q., Guo S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013;14:7370–7390. doi: 10.3390/ijms14047370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waraich E.A., Ahmad R., Ashraf M.Y. Role of mineral nutrition in alleviation of drought stress in plants. Aust. J. Crop Sci. 2011;5:764. [Google Scholar]
- 15.Abbasi H., Jamil M., Haq A., Ali S., Ahmad R., Malik Z. Salt stress manifestation on plants, mechanism of salt tolerance and potassium role in alleviating it: A review. Zemdirb. Agric. 2016;103:229–238. doi: 10.13080/z-a.2016.103.030. [DOI] [Google Scholar]
- 16.Soleimani B. Genetic Regulation of Juvenile Plant Growth under Contrasting Levels of Phosphorus, Potassium and Carbon Dioxide Nutrition in the Wild Barley Introgression Library S42IL. Martin-Luther-University; Halle-Wittenberg, Germany: 2018. [Google Scholar]
- 17.Farooq M., Hussain M., Wakeel A., Siddique K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015;35:461–481. doi: 10.1007/s13593-015-0287-0. [DOI] [Google Scholar]
- 18.Parker B., Gaines T.P., Gascho G.J. Chloride effects on corn. Commun. Soil Sci. Plant Anal. 2008;16:1319–1333. doi: 10.1080/00103628509367690. [DOI] [Google Scholar]
- 19.Christensen N.W., Roseberg R.J., Brett M., Jackson T.L. Chloride inhibition of nitrification as related to take-all disease of wheat. In: Jackson T.L., editor. Special Bulletin on Chloride and Crop Production. 2nd ed. Potash & Phosphate Institute; Atlanta, Georgia: 1986. pp. 22–39. [Google Scholar]
- 20.Du Z., Zhou J., Wang H., Chen X., Wang Q. Soil pH changes from fertilizer site as affected by application of monocalcium phosphate and potassium chloride. Commun. Soil Sci. Plant Anal. 2010;41:1779–1788. doi: 10.1080/00103624.2010.492064. [DOI] [Google Scholar]
- 21.Hussain F., Malik A.U., Haji M.A., Malghani A.L. Growth and yield response of two cultivars of mungbean (Vigna radiata L.) to different potassium levels. J. Anim. Plant Sci. 2011;21:622–625. [Google Scholar]
- 22.Anser A.M., Hussain S. Nutritional and physiological significance of potassium application in maize hybrid crop production. Pak. J. Nutr. 2012;11:187–202. [Google Scholar]
- 23.Singh H., Verma A., Ansari M.W., Shukla A. Physiological response of rice (Oryza sativa L.) genotypes to elevated nitrogen applied under field conditions. Plant Sign. Behav. 2014;9:e29015. doi: 10.4161/psb.29015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amanullah, Iqbal A., Irfanullah, Hidayat Z. Potassium management for improving growth and grain yield of maize (Zea mays L.) under moisture stress condition. Sci. Rep. 2016;6:34627. doi: 10.1038/srep34627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grzesiak M.T., Marcińska I., Janowiak F., Rzepka A., Hura T. The relationship between seedling growth and grain yield under drought conditions in maize and triticale genotypes. Acta Physiol. Plant. 2012;34:1757–1764. doi: 10.1007/s11738-012-0973-3. [DOI] [Google Scholar]
- 26.Steel R.G.D., Torrie J.H., Dickey D.A. Principles and Procedures of Statistics: A Biological Approach. McGraw-Hill; New York, NY, USA: 1997. [Google Scholar]
- 27.Nawaz A., Farooq M., Cheema S.A., Yasmeen A., Wahid A. Stay green character at grain filling ensures resistance against terminal drought in wheat. Int. J. Agric. Biol. 2013;15:1272–1276. [Google Scholar]
- 28.Lv X., Li T., Wen X., Liao Y., Liu Y. Effect of potassium foliage application post-anthesis on grain filling of wheat under drought stress. Field Crops Res. 2017;206:95–105. doi: 10.1016/j.fcr.2017.02.015. [DOI] [Google Scholar]
- 29.Zahoor R., Dong H., Abid M., Zhao W., Wang Y., Zhou Z. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 2017;137:73–83. doi: 10.1016/j.envexpbot.2017.02.002. [DOI] [Google Scholar]
- 30.Athar H.R., Ashraf M. Photosynthesis under drought stress. In: Pessarakli M., Marcel D., editors. Handbook of Photosynthesis. 2nd ed. Taylor and Francis, Inc.; New York, NY, USA: 2005. pp. 793–809. [Google Scholar]
- 31.Negin B., Moshelion M. The evolution of the role of ABA in the regulation of water-use efficiency: From biochemical mechanisms to stomatal conductance. Plant Sci. 2016;251:82–89. doi: 10.1016/j.plantsci.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Subbarao G.V., Wheeler R.M., Stutte G.W., Levine L.H. Low potassium enhances sodium uptake in red-beet under moderate saline conditions. J. Plant Nutr. 2000;23:1449–1470. doi: 10.1080/01904160009382114. [DOI] [PubMed] [Google Scholar]
- 33.Cerda A., Pardines J., Botella M.A., Martinez V. Effect of potassium on growth, water relations, and the inorganic and organic solute contents for two maize cultivars grown under saline conditions. J. Plant Nutr. 1995;18:839–851. doi: 10.1080/01904169509364942. [DOI] [Google Scholar]
- 34.Hussain R.A., Ahmad R., Nawaz F., Ashraf M.Y., Waraich E.A. Foliar NK application mitigates drought effects in sunflower (Helianthus annuus L.) Acta Physiol. Plant. 2016;38:83. doi: 10.1007/s11738-016-2104-z. [DOI] [Google Scholar]
- 35.Jan A.U., Hadi F., Nawaz M.A., Rahman K. Potassium and zinc increase tolerance to salt stress in wheat (Triticum aestivum L.) Plant Physiol. Biochem. 2017;116:139–149. doi: 10.1016/j.plaphy.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y., Jia A., Ning T., Xu J., Li Z., Jiang G. Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J. Plant Physiol. 2008;165:1455–1465. doi: 10.1016/j.jplph.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Soleimanzadeh H., Habibi D., Ardakani M.R., Paknejad F., Rejali F. Effect of potassium levels on antioxidant enzymes and malondialdehyde content under drought stress in sunflower (Helianthus annuus L.) Am. J. Agric. Biol. Sci. 2010;5:56–61. doi: 10.3844/ajabssp.2010.56.61. [DOI] [Google Scholar]
- 38.Martineau E., Domec J.C., Bosc A., Dannoura M., Gibon Y., Bénard C., Jordan-Meille L. The role of potassium on maize leaf carbon exportation under drought condition. Acta Physiol. Plant. 2017;39:219. doi: 10.1007/s11738-017-2515-5. [DOI] [Google Scholar]
- 39.Huber S.C. Biochemical basis for effects of K-deficiency on assimilate export rate and accumulation of soluble sugars in soybean leaves. Plant Physiol. 1984;76:424–430. doi: 10.1104/pp.76.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cakmak I., Hengeler C., Marschner H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J. Exp. Bot. 1994;45:1251–1257. doi: 10.1093/jxb/45.9.1251. [DOI] [Google Scholar]
- 41.Ainsworth E.A., Bush D.R. Carbohydrate export from the leaf: A highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011;155:64–69. doi: 10.1104/pp.110.167684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lv X., Han J., Liao Y., Liu Y. Effect of phosphorus and potassium foliage application post-anthesis on grain filling and hormonal changes of wheat. Field Crops Res. 2017;214:83–93. doi: 10.1016/j.fcr.2017.09.001. [DOI] [Google Scholar]
- 43.White A.C., Rogers A., Rees M., Osborne C.P. How can we make plants grow faster? A source–sink perspective on growth rate. J. Exp. Bot. 2015;67:31–45. doi: 10.1093/jxb/erv447. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad M., Riaz A., Ishaque M., Malik A.U. Response of maize hybrids to varying potassium application in Pakistan. Pak. J. Agric. Sci. 2009;46:179–184. [Google Scholar]
- 45.Jan M.F., Khan A.A., Wahab F., Liaqat W., Ahmad H., Rehan W. Phenology and productivity response of maize (Zea mays L.) hybrids to different levels of mineral potassium under semiarid climate. Middle East J. Agric. Res. 2018;7:287–291. [Google Scholar]
- 46.Aslam M., Zamir I., Shahid M., Afzal I., Yaseen M. Morphological and physiological response of maize hybrids to potassium application under drought stress. J. Agric. Res. 2013;51:443–454. [Google Scholar]
- 47.Sadiq G., Khan A.A., Inamullah A.R., Fayyaz H., Naz G., Nawaz H., Ali I., Raza H., Amin J., Ali S., et al. Impact of phosphorus and potassium levels on yield and yield components of maize. Pure Appl. Biol. 2017;6:1071–1078. doi: 10.19045/bspab.2017.600114. [DOI] [Google Scholar]
- 48.Niu J., Zhang W., Chen X., Li C., Zhang F., Jiang L., Liu Z., Xiao K., Assaraf M., Imas P. Potassium fertilization on maize under different production practices in the North China Plain. Agron. J. 2011;103:822–829. doi: 10.2134/agronj2010.0471. [DOI] [Google Scholar]
- 49.Wu L.Q., Ma W.Q., Zhang C.C., Wu L., Zhang W.F., Jiang R.F., Zhang F.S., Cui Z.L., Chen X.P. Current potassium-management status and grain-yield response of Chinese maize to potassium application. J. Plant Nutr. Soil Sci. 2013;176:441–449. doi: 10.1002/jpln.201200314. [DOI] [Google Scholar]
- 50.Wu L., Cui Z., Chen X., Zhao R., Si D., Sun Y., Yue S. High-yield maize production in relation to potassium uptake requirements in China. Agron. J. 2014;106:1153–1158. doi: 10.2134/agronj13.0538. [DOI] [Google Scholar]
- 51.Qiu S., Xie J., Zhao S., Xu X., Hou Y., Wang X., Zhou W., He P., Johnston A.M., Christie P., et al. Long-term effects of potassium fertilization on yield, efficiency, and soil fertility status in a rain-fed maize system in northeast China. Field Crops Res. 2014;163:1–9. doi: 10.1016/j.fcr.2014.04.016. [DOI] [Google Scholar]
- 52.Mallarino A.P., Webb J.R., Blackmer A.M. Corn and soybean yields during 11 years of phosphorus and potassium fertilization on a high-testing soil. J. Prod. Agric. 1991;4:312-NP. doi: 10.2134/jpa1991.0312. [DOI] [Google Scholar]
- 53.Menezes C.B., Ticona-Benavente C.A., Tardin F.D., Cardoso M.J., Bastos E.A., Nogueira D.W., Portugal A.F., Santos C.V., Schaffert R.E. Selection indices to identify drought-tolerant grain sorghum cultivars. Genet. Mol. Res. 2014;13:9817–9827. doi: 10.4238/2014.November.27.9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.