Abstract
Microglia are the resident immune cells and professional phagocytes of the central nervous system. However, little is known about the contribution of their phagocytic signaling to the neuropathology and pathophysiology of epilepsy. Here, we summarize and discuss the implications of recent evidence supporting that aberrant microglia phagocytic activity and alterations in phagocytosis signaling molecules occur in association with microglia–neuronal contacts, neuronal/synaptic loss, and spontaneous recurrent seizures in human and preclinical models of epilepsy. This body of evidence provides strong support that the microglial contribution to epileptogenic networks goes beyond inflammation, and suggests that phagocytic signaling molecules may be novel therapeutic targets for epilepsy.
Keywords: microglia, phagocytosis, synaptic pruning, neuronal loss, recurrent seizures, inflammation
Introduction
Microglia are the macrophages and professional phagocytes of the central nervous system (CNS). Under physiological conditions, microglia are typically highly ramified cells with dynamic processes that actively monitor their local environment to safeguard neural homeostasis.1 Under pathological conditions, including seizures and epilepsy, microglia become reactive, develop amoeboid shapes, and produce inflammatory mediators such as cytokines, chemokines, and complement proteins.2 Depending on their intensity and duration, these inflammatory signals can have beneficial or detrimental effects on the plasticity and survival of nearby cells.3 For example, short-lasting inflammation can promote neuroprotection by attracting microglia to remove (phagocytose) dead/apoptotic cells, a process that suppresses production of pro-inflammatory cytokines, stimulates release of anti-inflammatory mediators, and promotes tissue repair.3,4 In contrast, exacerbated long-lasting inflammation is linked to pathological consequences including neurodegeneration, cognitive decline, seizures, and epilepsy.2,3 Interestingly, new findings support that in addition to inflammatory molecules, signals regulating microglial phagocytic and proliferating properties are altered in response to seizures and may play important roles in epileptogenic processes. Here, we summarize and discuss the implications of these new discoveries.
Phagocytic Signaling
Phagocytosis is the process in which phagocytes, such as microglia, engulf and remove unwanted particles and dead cells. Phagocytosis can be performed by ramified and amoeboid “reactive” microglia, and is orchestrated by an assortment of molecules which regulate chemoattraction, engulfing, and degradation, also known as “find-me,” “eat-me,” and “digest-me” signals, each recognized by specialized receptors (Figure 1).4,5 “Find-me” signals such as nucleotides (e.g., ATP) are sensed by purinergic receptors (P2Y12) and guide microglia to the location of altered neuronal homeostasis. “Eat-me” signals include phosphatidylserine (PS), which is typically externalized to the outer leaflet of the plasma membrane in cells undergoing apoptosis; Protein S (ProS), an opsonin that binds to PS; and complements C1q and C3b. The receptor Mer Tyrosine Kinase (MerTK) recognizes ProS, while complement receptors 1 and 3 (CR1, CR3) recognize C1q and C3b, respectively. These receptors along with the triggering receptor expressed in myeloid cells 2 (Trem2) aid in engulfment and phagocytosis through remodeling the actin cytoskeleton.4,5 An additional set of signals referred to as “don’t-eat-me” signals include the integrin associated protein CD47 and its receptor the signal regulatory protein α (SIRP-α). It is well-known that phagocytosis of apoptotic cells is anti-inflammatory and contributes to the resolution of inflammation in injured tissues.4 However, molecules such as C1q, C3b, CR3, and Trem2 can crosstalk with other receptors/pathways to also regulate microglial inflammatory responses,4-7 suggesting that depending on the target and context (healthy vs injured) these signals can mediate production of pro- or anti-inflammatory cytokines. Interestingly, a number of studies support that microglial phagocytic signaling is essential for the establishment and maturation of neural networks.1,7 Importantly, new evidence indicates that dysregulation of these signaling cascades is associated with the pathology of neurodegenerative disorders1,7 and epilepsy.8 Recent histological and transcriptomic immune profiling of microglia from patients with drug-resistant seizures showed that microglia have high expression of CR3, Trem2, and MerTK9-12 suggesting a robust phagocytic phenotype. In human focal cortical dysplasia (FCD), we found increases in C1q, C3b, and MerTK that paralleled decreases in ProS and Trem2.13 In addition, decreased levels of CD47 and SIRP-α were found in human FCD and tuberous sclerosis complex (TSC).14 Taken together these findings suggest that microglia may have altered phagocytic functions in the human epileptic brain.
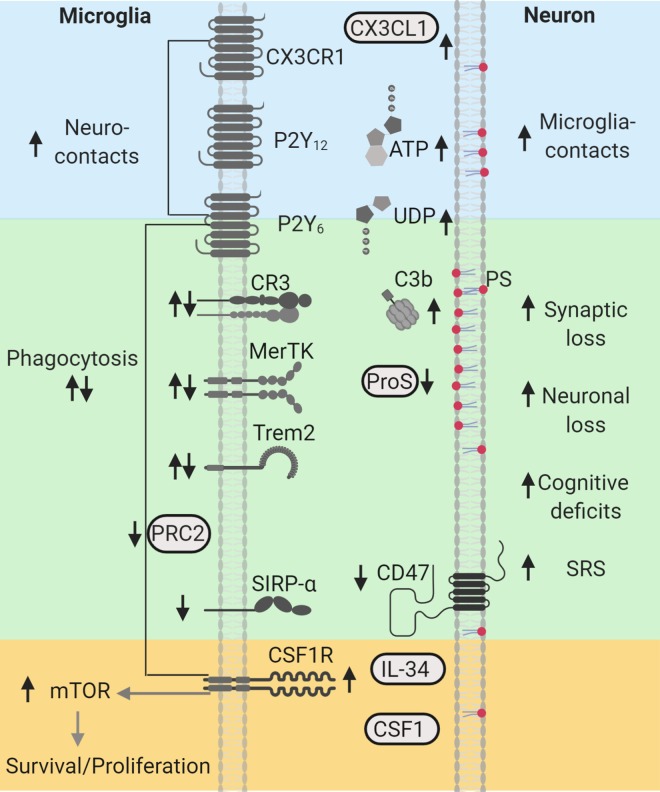
Figure 1.
Phagocytic signaling molecules altered in human and experimental epilepsy. “Find-me” signals CX3CL1/CX3CR1, ATP/P2Y12, and UDP/P2Y6, shown in blue, are associated with increased neuroimmune interactions during seizures. Microglia clearance/phagocytic activity controlled by PRC2 and mediated by “eat-me” signals PS (red), C3b/CR3, ProS/MerTK, and Trem2, shown in green, are associated with neuronal/synapse loss, cognitive deficits, and spontaneous recurrent seizures (SRS). “Don’t-eat-me” signals, CD47 and SIRP-α, shown in green, are reduced in human epilepsy. CSF1R-mTOR signaling activated by CSF1/interleuklin-34 (IL34), shown in yellow, regulate microglial survival, proliferation, and phagocytic microglial properties, and are associated with synaptic loss, cognitive decline, and SRS. Arrows indicate the direction of the changes reported in human and experimental models. This diagram was created with Biorender.com. CR indicates complement receptor; CSF1R, colony stimulating factor 1 receptor; MerTK, Mer Tyrosine Kinase; mTOR, mechanistic target of rapamycin; P2Y12, purinergic receptors; ProS, Protein S; PRC2, Polycomb repressive complex 2; PS, phosphatidylserine; SIRP-α, signal regulatory protein α; SRS, spontaneous recurrent seizures; Trem2, triggering receptor expressed in myeloid cells 2.
Activity-Dependent Microglia–Neuronal Interactions
Microglia sense neuronal activity through the purinergic receptor P2Y12R, which responds to microgradients of ATP.15 Under normal conditions, active neurons release ATP. However, following seizures ATP release increases.15 Consequently, microglial–neuronal interactions occur in an activity-dependent manner16-18 and have been observed in epilepsy. In human epilepsy, microglial processes were found in close proximity and apposed along cortical apical dendrites.13,19 In experimental models, pronounced microglial interactions with hippocampal CA1 dendrites were observed during and after status epilepticus (SE).17,20,21 Studies using 2-photon live imaging of in vivo and ex vivo systems demonstrated that microglial processes move rapidly toward dendrites in response to seizures, a process that is dependent upon neuronal NMDA receptor activation along with microglial P2Y12R and fractalkine (CX3CL1-CX3CR1; “find-me” signal) signaling.17,22 These studies also reported that mice lacking either P2Y12R or CX3CR1 exhibited higher seizure severity during SE,17,22,23 thereby suggesting a potential role for microglial–dendritic interactions in controlling neuronal excitability. Although the functional impact of these contacts in epilepsy is not definitively known, emerging evidence suggests that microglial contacts can alter the structure of synaptic sites. Live and electron microscope imaging of microglia in cortex and hippocampus demonstrated that direct interactions with synaptic structures can result in the disappearance or growth of pre and postsynaptic elements.18,24-26 For example, some microglia contacts were followed by the loss of spines, axonal boutons, or spine head tips (by trogocytosis),18,24-26 while others stimulated filopodia growth on dendrites26 and spine heads.24 Taken together, these findings suggest that activity-dependent increases in physical microglia–dendritic/synaptic interactions may contribute to circuit remodeling in epilepsy. Thus, determining the functional impact of these neuroimmune interactions may lead to novel treatment strategies to control neuronal hyperexcitability.
Phagocytosis of Synapses
During synaptogenesis in the healthy brain, microglia eliminate extranumerary synapses in an activity-dependent manner to allow for stronger synapses to form functional connections.7,18,27-29 Microglial synaptic pruning in developing networks is mediated by “eat-me” signals C1q and C3b27,28,30,31 and by receptors CR3,27,28 CX3CR1,29 and Trem2.32 CX3CR1, Trem2, C3, or C1q knockout (KO) mice display either reduced microglial engulfment of synaptic material or higher densities of spines/synapses and excessive innervation in cortical or hippocampal networks.27-29,32 Functional implications of failed microglial synaptic pruning during development include altered synaptic plasticity, neuronal hyperexcitability, and seizures.7,30,31 For instance, insufficient synaptic pruning in C1q KO mice is associated with increases in spine density, synaptic connectivity, excitability in the somatosensory cortex, and absence seizures.30,31 However, higher levels of phagocytic signaling molecules are also associated with synaptic dysfunction. For example, increases in complement C1q and C3/CR3 in mature/adult systems are linked to exacerbated synaptic pruning and cognitive decline, including learning and memory impairments, in models of neurodegenerative disorders.7 These findings are relevant because increased levels of C1q and C3 are consistently found in human and experimental models of epilepsy, thereby suggesting a potential role for complement proteins in activity-dependent microglial synaptic pruning in seizure disorders. Elevated levels of C1q and C3 occur in human epilepsies with drug-resistant seizures including temporal lobe epilepsy (TLE),9,33 FCD,13 and TSC.34 Similarly, long-lasting increases in the levels of C1q-C3 that correlate with seizure severity occur in adult rodent models of SE and acquired TLE.33,35,36 It is possible that a complement-dependent microglial elimination of synapses may contribute to the exacerbated synaptic loss, seizures, and memory impairments that occur after SE and in epilepsy.35 The overall functional impact of aberrant C1q-C3 signaling to epileptic networks would depend on the proportion of excitatory or inhibitory cells/synapses being phagocytosed—an idea that requires further investigation.
Phagocytosis of Neurons
During neurogenesis in the healthy developing brain, a high number of cells undergo apoptosis as neuronal networks develop and mature, and microglia are tasked with their removal. Clearance of apoptotic newborn cells continues in the mature hippocampus, where neurogenesis persists through adulthood.37 In the adult hippocampal subventricular zone, noninflammatory microglia help maintain homeostasis by rapidly removing excess newborn cells through apoptosis-coupled phagocytosis.37 However, following SE-induced epilepsy, microglia failed to remove newborn apoptotic cells allowing their accumulation throughout the dentate gyrus (DG).16 Using 2-photon microscopy along with quantitative reverse transcription-polymerase chain reaction of microglia, Abiega et al showed that this phagocytic impairment paralleled decreased motility, reduced levels of Trem2, MerTK, and CR3, and increased levels of inflammatory cytokines.16 Despite the reduced phagocytosis of apoptotic cells, microglial engulfment of nonapoptotic viable cells was observed in the DG during epileptogenesis.16,38 In fact, inflammatory microglia can engulf and “kill” stressed but otherwise healthy neurons in proximity to injured/apoptotic cells.5 Phagoptosis of viable cells occurs through the transient exposure of PS,5 which could take place in association with seizures and exacerbate cell loss in epilepsy. Although it is not known whether this contributes to epilepsy, evidence that PS supplements are linked to reduced seizure frequency in aged rats39 and epileptic patients40 suggest a potential role. Nevertheless, in contrast to Abiega’s findings, Koizumi et al reported that kainate-induced seizure activity provoked an increase in UDP/P2Y6–dependent microglial phagocytic activity in the hippocampal CA3 region.41 Because microglial immune properties including clearance/phagocytic activity are region- and context-specific,8,42 it is possible that these differences may be due to a higher load of dead/dying cells in the CA3 as opposed to DG, where neurogenesis occurs. Lastly, a recent study showed that disinhibition of microglial clearance activity due to ablation of the Polycomb repressive complex 2 resulted in decreased spine density in cortical neurons and spontaneous recurrent seizures (SRS) in aged mice,42 thereby suggesting that enhanced phagocytic activity may be epileptogenic. Further research is needed to determine how seizures alter the phagocytic profiles of microglia in different brain areas and how these relate to regional neuropathology.
Microglial Proliferation
Microgliosis is the proliferation and accumulation of reactive microglia. Microgliosis is widely observed in human epilepsy as well as in experimental models.8 To determine how microgliosis contributes to epileptogenesis and seizure generation, it is necessary to examine the regulatory signaling pathways. Activation of the colony stimulating factor 1 receptor (CSF1R) signaling pathway in microglia, by CSF1 or interleukin-34, leads to the downstream activation of a number of molecules including the mechanistic target of rapamycin (mTOR) to regulate survival, proliferation, and phagocytic properties.6,43 Because mTOR is ubiquitously expressed in neurons and microglia, recent studies have specifically targeted mTOR hyperactivation in microglia44 as well as CSF1R signaling.45-47 A microglia-specific TSC1 KO mouse model produced an mTOR hyperactivation phenotype that resulted in an increase in the number of microglia with enhanced phagocytic activity in the hippocampus.44 In these mice, the altered microglial properties correlated with reduced densities of excitatory and inhibitory synapses, and with the development of SRS.44 In a rat model of acquired TLE, we found that at the peak of SE-induced hippocampal microgliosis these cells had activated mTOR signaling and were localized to areas with severe spine/dendritic loss.21,48,49 Treatment with the mTOR inhibitor rapamycin attenuated the SE-induced microgliosis, dendritic/spine loss, and memory deficits,21 suggesting that microgliosis contributes to the epilepsy dendritic and cognitive pathology. Neuroprotective and antiepileptic outcomes were also reported when inhibiting CSF1R signaling in mouse models of SE.45,46 Blocking CSF1R signaling with either CSF1 antibodies or the CSF1R antagonist GW2580 attenuated microglial proliferation and neuronal loss in hippocampi of mice that sustained kainate-induced SE.46 Similarly, treatment with the CSF1R inhibitor PLX3397 decreased both the number of IBA1-positive microglial cells in the hippocampus and seizure frequency in a mouse model of pilocarpine-induced SE.45 Although these studies suggest that suppressing microgliosis during epileptogenesis is beneficial, recent studies also showed that total ablation is detrimental,47,50 thereby suggesting that microglia have multiple roles. Ablation of microglia with the CSF1R inhibitor PLX5622 exacerbated the seizure phenotype and the mortality rate in epileptic mice induced with the Theiler murine encephalomyelitis virus.47 Moreover, homozygous mutations in the human CSF1R gene are associated with a drastic loss of microglia and brain structural malformations.50 Of two patients described in this study, one died prematurely at age one and the other presented with developmental delay and epilepsy.50 Taken together, these data put forward the idea that the wide range of rapamycin-mediated outcomes described in the epilepsy literature may also have do to with the modulation of mTOR-dependent microglial proliferating and phagocytic properties. In addition, these findings suggest that inhibition of microgliosis through the CSF1R pathway during epileptogenesis may be a novel approach to prevent or reduce SRS.
Conclusion
Although microglia are the professional phagocytes of the CNS, relatively little is known regarding their phagocytic profiles in epilepsy or their contribution to the neuropathology and pathophysiology of this disorder. The studies described here are the first to show that seizures promote alterations in phagocytosis-associated signaling molecules that result in neuroimmune interactions,13,17,19-22 increases in “find-me”17,22,41 and “eat-me”9,13,33-36 signals, decreases in the phagocytosis of newborn apoptotic cells,16 phagocytosis of viable newborn cells,16,38 and increases in microglial phagocytic activity and/or proliferation that parallel neuronal/synaptic loss, seizures, and cognitive decline.21,35,41,42,44-46 Thus, it is possible that microglia may modulate synaptic circuitries in epilepsy by improperly phagocytosing synaptic structures and neurons. However, whether this is a pro- or anti-epileptogenic mechanism, or if this is a cause or consequence of seizures and epilepsy requires further investigation. Overall, this body of evidence provides strong support that the microglial contribution to the epileptogenic networks goes beyond inflammation, and suggests that phagocytic signaling molecules may be novel therapeutic targets for epilepsy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Amy L. Brewster  https://orcid.org/0000-0002-3677-410X
https://orcid.org/0000-0002-3677-410X
References
- 1. Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179(2):292–311. [DOI] [PubMed] [Google Scholar]
- 2. van Vliet EA, Aronica E, Vezzani A, Ravizza T. Review: neuroinflammatory pathways as treatment targets and biomarker candidates in epilepsy: emerging evidence from preclinical and clinical studies. Neuropathol Appl Neurobiol. 2018;44(1):91–111. [DOI] [PubMed] [Google Scholar]
- 3. DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139(suppl 2):136–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15(4):209–216. [DOI] [PubMed] [Google Scholar]
- 6. Konishi H, Kiyama H. Microglial TREM2/DAP12 signaling: a double-edged sword in neural diseases. Front Cell Neurosci. 2018;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hammond TR, Robinton D, Stevens B. Microglia and the brain: complementary partners in development and disease. Annu Rev Cell Dev Biol. 2018;34:523–544. [DOI] [PubMed] [Google Scholar]
- 8. Brewster AL. Human microglia seize the chance to be different. Epilepsy Curr. 2019;19(3):190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gosselin D, Skola D, Coufal NG, et al. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356(6344). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morin-Brureau M, Milior G, Royer J, et al. Microglial phenotypes in the human epileptic temporal lobe. Brain J Neurol. 2018;141(12):3343–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dachet F, Bagla S, Keren-Aviram G, et al. Predicting novel histopathological microlesions in human epileptic brain through transcriptional clustering. Brain J Neurol. 2015;138(pt 2):356–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bottcher C, Schlickeiser S, Sneeboer MAM, et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat Neurosci. 2019;22(1):78–90. [DOI] [PubMed] [Google Scholar]
- 13. Wyatt SK, Witt T, Barbaro NM, Cohen-Gadol AA, Brewster AL. Enhanced classical complement pathway activation and altered phagocytosis signaling molecules in human epilepsy. Expe Neurol. 2017;295:184–193. [DOI] [PubMed] [Google Scholar]
- 14. Sun FJ, Zhang CQ, Chen X, et al. Downregulation of CD47 and CD200 in patients with focal cortical dysplasia type IIb and tuberous sclerosis complex. J Neuroinflam. 2016;13(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beamer E, Conte G, Engel T. ATP release during seizures—a critical evaluation of the evidence. Brain Res Bull. 2019;151:65–73. [DOI] [PubMed] [Google Scholar]
- 16. Abiega O, Beccari S, Diaz-Aparicio I, et al. Neuronal hyperactivity disturbs ATP microgradients, impairs microglial motility, and reduces phagocytic receptor expression triggering apoptosis/microglial phagocytosis uncoupling. PLoS biol. 2016;14(5):e1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ. Neuronal hyperactivity recruits microglial processes via neuronal NMDA receptors and microglial P2Y12 receptors after status epilepticus. J Neurosci. 2014;34(32):10528–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8(11):e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wirenfeldt M, Clare R, Tung S, Bottini A, Mathern GW, Vinters HV. Increased activation of Iba1+ microglia in pediatric epilepsy patients with Rasmussen’s encephalitis compared with cortical dysplasia and tuberous sclerosis complex. Neurobiol Dis. 2009;34(3):432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hasegawa S, Yamaguchi M, Nagao H, Mishina M, Mori K. Enhanced cell-to-cell contacts between activated microglia and pyramidal cell dendrites following kainic acid-induced neurotoxicity in the hippocampus. J Neuroimmunol. 2007;186(1-2):75–85. [DOI] [PubMed] [Google Scholar]
- 21. Brewster AL, Lugo JN, Patil VV, et al. Rapamycin reverses status epilepticus-induced memory deficits and dendritic damage. PLoS one 2013;8(3): e57808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eyo UB, Peng J, Murugan M, et al. Regulation of physical microglia-neuron interactions by fractalkine signaling after status epilepticus. eNeuro. 2016;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eyo UB, Murugan M, Wu LJ. Microglia-neuron communication in epilepsy. Glia. 2016;65(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weinhard L, Di Bartolomei G, Bolasco G, et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun. 2018;9(1):1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyamoto A, Wake H, Ishikawa AW, et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schafer DP, Lehrman EK, Kautzman AG, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stevens B, Allen NJ, Vazquez LE, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–1178. [DOI] [PubMed] [Google Scholar]
- 29. Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. [DOI] [PubMed] [Google Scholar]
- 30. Ma Y, Ramachandran A, Ford N, Parada I, Prince DA. Remodeling of dendrites and spines in the C1q knockout model of genetic epilepsy. Epilepsia. 2013;54(7):1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chu Y, Jin X, Parada I, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U.S.A. 2010;107(17):7975–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Filipello F, Morini R, Corradini I, et al. The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity. 2018;48(5):979–991 e8. [DOI] [PubMed] [Google Scholar]
- 33. Aronica E, Boer K, Van Vliet EA, et al. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol Dis. 2007;26(3):497–511. [DOI] [PubMed] [Google Scholar]
- 34. Boer K, Jansen F, Nellist M, et al. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epil Res. 2008;78(1):7–21. [DOI] [PubMed] [Google Scholar]
- 35. Schartz ND, Wyatt-Johnson SK, Price LR, Colin SA, Brewster AL. Status epilepticus triggers long-lasting activation of complement C1q-C3 signaling in the hippocampus that correlates with seizure frequency in experimental epilepsy. Neurobiol Dis. 2018;109(pt A):163–173. [DOI] [PubMed] [Google Scholar]
- 36. Kharatishvili I, Shan ZY, She DT, Foong S, Kurniawan ND, Reutens DC. MRI changes and complement activation correlate with epileptogenicity in a mouse model of temporal lobe epilepsy. Brain Struc Func. 2014;219(2):683–706. [DOI] [PubMed] [Google Scholar]
- 37. Sierra A, Encinas JM, Deudero JJ, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luo C, Koyama R, Ikegaya Y. Microglia engulf viable newborn cells in the epileptic dentate gyrus. Glia. 2016;64(9):1508–1517. [DOI] [PubMed] [Google Scholar]
- 39. Aporti F, Borsato R, Calderini G, et al. Age-dependent spontaneous EEG bursts in rats: effects of brain phosphatidylserine. Neurobiol Aging. 1986;7(2):115–120. [DOI] [PubMed] [Google Scholar]
- 40. Loeb C, Benassi E, Bo GP, Cocito L, Maffini M, Scotto P. Preliminary evaluation of the effect of GABA and phosphatidylserine in epileptic patients. Epil Res. 1987;1(3):209–212. [DOI] [PubMed] [Google Scholar]
- 41. Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nat. 2007;446(7139):1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ayata P, Badimon A, Strasburger HJ, et al. Epigenetic regulation of brain region-specific microglia clearance activity. Nat Neurosci. 2018;21(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ulland TK, Wang Y, Colonna M. Regulation of microglial survival and proliferation in health and diseases. Semin Immunol. 2015;27(6):410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao X, Liao Y, Morgan S, et al. Noninflammatory changes of microglia are sufficient to cause epilepsy. Cell Rep. 2018;22(8):2080–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Srivastava PK, van Eyll J, Godard P, et al. A systems-level framework for drug discovery identifies Csf1 R as an anti-epileptic drug target. Nat Commun. 2018;9(1):3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Feng L, Murugan M, Bosco DB, et al. Microglial proliferation and monocyte infiltration contribute to microgliosis following status epilepticus. Glia. 2019;67(8):1434–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sanchez JMS, DePaula-Silva AB, Doty DJ, Truong A, Libbey JE, Fujinami RS. Microglial cell depletion is fatal with low level picornavirus infection of the central nervous system. J Neurovirol. 2019;25(3):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schartz ND, Herr SA, Madsen L, et al. Spatiotemporal profile of Map2 and microglial changes in the hippocampal CA1 region following pilocarpine-induced status epilepticus. Sci Rep. 2016;6:24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wyatt-Johnson SK, Herr SA, Brewster AL. Status epilepticus triggers time-dependent alterations in microglia abundance and morphological phenotypes in the hippocampus. Front Neurol 2017;8:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oosterhof N, Chang IJ, Karimiani EG, et al. Homozygous mutations in CSF1 R cause a pediatric-onset leukoencephalopathy and can result in congenital absence of microglia. Am J Hum Genet. 2019;104(5):936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]