Abstract
Background:
Medical care, public health, and criminal justice systems encounters could serve as touchpoints to identify and intervene with individuals at high-risk of opioid overdose death. The relative risk of opioid overdose death and proportion of deaths that could be averted at such touchpoints are unknown.
Methods:
We used 8 individually linked data sets from Massachusetts government agencies to perform a retrospective cohort study of Massachusetts residents ages 11 and older. For each month in 2014, we identified past 12-month exposure to 4 opioid prescription touchpoints (high dosage, benzodiazepine co-prescribing, multiple prescribers, or multiple pharmacies) and 4 critical encounter touchpoints (opioid detoxification, nonfatal opioid overdose, injection-related infection, and release from incarceration). The outcome was opioid overdose death. We calculated Standardized Mortality Ratios (SMRs) and Population Attributable Fractions (PAFs) associated with touchpoint exposure.
Results:
The cohort consisted of 6,717,390 person-years of follow-up with 1315 opioid overdose deaths. We identified past 12-month exposure to any touchpoint in 2.7% of person-months and for 51.8% of opioid overdose deaths. Opioid overdose SMRs were 12.6 (95% CI: 11.1, 14.1) for opioid prescription and 68.4 (95% CI: 62.4, 74.5) for critical encounter touchpoints. Fatal opioid overdose PAFs were 0.19 (95% CI: 0.17, 0.21) for opioid prescription and 0.37 (95% CI: 0.34, 0.39) for critical encounter touchpoints.
Conclusions:
Using public health data, we found eight candidate touchpoints were associated with increased risk of fatal opioid overdose, and collectively identified more than half of opioid overdose decedents. These touchpoints are potential targets for development of overdose prevention interventions.
Keywords: Opioid overdose, Standardized mortality ratio, Population attributable fraction, Opioid prescribing
1. Introduction
The United States is experiencing a surge in opioid overdose deaths.(Rudd et al., 2016) Several overdose risk factors are well-established and can be identified through medical care, public health, or criminal justice systems encounters. These encounters could serve as “touchpoints” – opportunities to identify individuals at high-risk of opioid overdose death in order to deliver harm-reduction services (e.g. overdose education and naloxone rescue kits) to them, and engage them in evidence-based treatment (e.g. medication for opioid use disorders).
Known opioid prescription touchpoints associated with increased risk of fatal opioid overdose include high opioid dosage, co-prescribing opioids and benzodiazepines, and receiving or filling opioid prescriptions from multiple providers or pharmacies (Baumblatt et al., 2014; Bohnert et al., 2011; Dunn et al., 2010; Garg et al., 2017; Park et al., 2015; Rose et al., 2018). The development and implementation of prescription monitoring programs and safer opioid prescribing guidelines are efforts to identify and reduce high-risk opioid prescribing (Dowell et al., 2016; Haffajee et al., 2015). After decades of increases, prescription opioid supply has leveled off and is decreasing; however, increases in opioid-related deaths have accelerated because of increased use of heroin and illicit fentanyl (Guy et al., 2017; Jeffery et al., 2018; Jones et al., 2014; O’Donnell et al., 2017). Thus, critical encounter touchpoints beyond opioid prescribing are also needed such as nonfatal opioid overdose and release from incarceration which are each associated with marked increase in the risk of opioid overdose death (Binswanger et al., 2013; Caudarella et al., 2016; Darke et al., 2011; Larochelle et al., 2018; Merrall et al., 2010). Opportunities also arise when persons who use opioids seek short-term inpatient detoxification, or seek care for complications related to opioid use, such as injection-related infections (Bailey et al., 2013; Davoli et al., 2007; Kim et al., 2016; Ronan and Herzig, 2016; Rosenthal et al., 2016; Stein et al., 2017).
The relative public health burden and potential for reduction in population opioid overdose deaths attributable to opioid prescription and critical encounter touchpoints have not been described. These data may form a roadmap for policy makers to identify the highest yield opportunities for programmatic interventions to deliver harm-reduction services and engage individuals with opioid use disorder (OUD) in treatment. The Massachusetts Public Health Data Warehouse (PHD), containing individual-level linked data from 16 government agencies and programs, provides a unique opportunity to identify such touchpoints and examine their association with opioid-related death in a highly impacted state (MDPH, 2016, 2018). For this analysis, we calculated opioid overdose Standardized Mortality Ratios (SMRs) and Population Attributable Fractions (PAFs) associated with four opioid prescription and four critical encounter touchpoints. SMRs identify the relative mortality of those exposed to a touchpoint compared with those not exposed, and PAFs identify the proportion of opioid overdose deaths in the population that potentially could have been averted by interventions to reduce the risk of opioid overdose death following a touchpoint.
2. Methods
2.1. Study design and data source
We conducted a retrospective cohort study using the Massachusetts Public Health Data Warehouse. This dataset includes data between 2011 and 2015 for residents aged 11 years or older with health insurance as identified in the All-Payer Claims Database (APCD), estimated to represent more than 98% of Massachusetts residents. Records from the APCD were linked at the individual level with records from other data sets using a multistage deterministic linkage process. For this study, we used data from 8 linked databases: the APCD, the Registry of Vital Records and Statistics (RVRS), the Prescription Monitoring Program (PMP), the Acute Care Hospital Case Mix (Case Mix), the Massachusetts Ambulance Trip Record Information System (MATRIS), the Bureau of Substance Addiction Services’ (BSAS) licensed treatment encounters, and the Department of Corrections (DOC) and Houses of Corrections (HOC). Detailed descriptions of the datasets and linkage process have been previously published, and linkage rates are summarized in Appendix A (MDPH, 2017). This work was mandated by Massachusetts law and conducted by a public health authority that required no institutional board review. The Boston University Medical Campus Institutional Review Board also determined that this study was not human subjects research.
2.2. Cohort selection
We included Massachusetts residents aged 11 years or older. Individuals entered the cohort on January 2014 and were followed until the earlier of December 2014 or their month of death as identified through RVRS. This period allowed for at least a 12-month historical exposure window (back to January 2013) for assessment of touchpoint exposure within all component datasets. We excluded 44,680 individuals (0.7%) with missing age or sex, resulting in a final cohort of 6,741,707 individuals.
2.3. Key variables
The outcome was opioid overdose death based on medical examiner determination or standardized assessment by the Massachusetts Department of Public Health as previously described.(Larochelle et al., 2018) We identified four opioid prescription and four critical encounter touchpoints that have been previously associated with increased risk of opioid overdose death (see introduction), and identifiable in the data warehouse. In each person-month we assessed touchpoint exposure using a window from the current month through 12 months prior.
We examined four high-risk opioid prescribing touchpoints identified with PMP data. “High dosage” was defined as an average daily dosage of 100 mg morphine-equivalents or more in three or more months of the exposure window. Daily dosage calculation is detailed in a prior study (Rose et al., 2018). “Benzodiazepine co-prescribing” was defined as having a prescription for both an opioid and a benzodiazepine in three or more months of the exposure window. “Multiple prescribers” and “multiple pharmacies” were defined as having three or more opioid prescribers or opioid-prescription-filling pharmacies respectively in a quarter. Individuals were considered exposed to “multiple prescribers” or “multiple pharmacies” if the exposure window included at least one month of a quarter where criteria were met.
We also examined four critical encounter touchpoints. “Opioid detoxification” was identified as an inpatient withdrawal management episode (BSAS). “Nonfatal opioid overdose” was identified as one of two types of encounters without death from any cause in the subsequent 7 days (Appendix A). First, we identified emergency department, outpatient observation, or inpatient discharges with validated diagnosis codes for opioid poisoning (Case Mix) (Green et al., 2017). Second, we identified ambulance encounters for opioid overdose using a validated algorithm developed by Centers for Disease Control and Prevention and Massachusetts Department of Public Health (MATRIS). A potential “injection-related infection” was identified as an emergency department, observation, or inpatient discharge with a diagnosis for infectious endocarditis, osteomyelitis, or skin-soft tissue infection, among individuals with evidence of injection drug use in the same or prior 12 months via a diagnosis of hepatitis C (Case Mix) or opioid related disorder (APCD) (Case Mix; Appendix B). A validation study found inclusion of hepatitis C diagnosis improved the sensitivity and specificity of an algorithm to identify inpatient cases of drug injection-related endocarditis (Ball et al., 2017). We excluded individuals who died within 7 days of discharge, as these may represent fatal infections. “Release from incarceration” was identified as release from state prison or county jail from DOC and HOC data respectively.
2.4. Statistical analysis
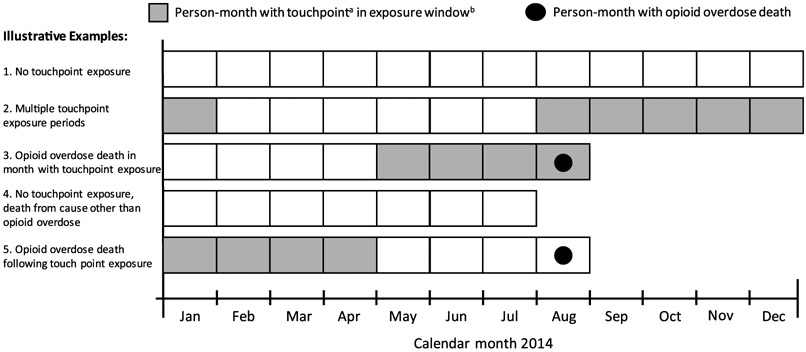
The unit of analysis was the person-month. For each person-month in 2014, we identified exposure to each of the eight touchpoints in the prior 12-month exposure window and whether the outcome had been experienced (Fig. 1). We identified the crude opioid overdose death incidence rates per 100,000 person-years of follow-up for the entire cohort and each touchpoint. We calculated standardized mortality ratios (SMRs) and population attributable fractions (PAFs) associated with touchpoint exposure compared with no touchpoint exposure. SMRs and PAFs were standardized by age group (11–29 years, 30–49 years, 50–64 years, > 65 years) and sex. We repeated all analyses stratifying by sex and age. We stratified into two age categories (11–49 years and > 50 years) to minimize suppression due to small cell sizes and because of data suggesting increasing rates of prescription opioid-related events and decreasing rates of heroin-related events after age 50 (Unick and Ciccarone, 2017).
Fig. 1.
Data structure to identify exposure to touchpointsa and opioid overdose by month, used to calculate Standardized Mortality Ratios (SMRs) and Population Attributable Fractions (PAFs) for opioid overdose death among person-months exposed compared with not exposed to touchpoints.
a Touchpoints include: 4 opioid prescription touchpoints (high dosage, benzodiazepine co-prescribing, multiple prescribers, or multiple pharmacies) and 4 critical encounter touchpoints (opioid detoxification, nonfatal opioid overdose, injection-related infection, and release from incarceration)
b Exposure window for base case is in current or past 12 months and varied in sensitivity analyses.
2.5. Sensitivity analyses
We varied the 12-month exposure window used in primary analyses to consider impact on two questions. First, we examined whether or not mortality risk changes with time from touchpoint exposure. Such a change would have implications for when best to intervene with at-risk patients. To examine the impact of time from touchpoint exposure, we examined four exposure windows: 0–3 months, 4–12 months, 13–24 months, and 25–36 months. We calculated SMRs for individuals exposed in each lookback period, considering individuals exposed only in the earliest lookback period for which criteria for a respective touchpoint were met. Second, we examined the degree to which extending the exposure window captured additional individuals at risk prior to opioid overdose death. We calculated the cumulative PAFs considering individuals with exposure windows up to 3, 12, 24, and 36 months. Due to data availability, we were unable to examine a full 36 month look back period for some of the non-prescribing touchpoints (Appendix C).
Finally, recognizing that high-risk opioid prescribing may precede high-risk opioid use tied to critical encounter touchpoints, we examined the proportion of individuals with past 12-month critical encounter touchpoint exposure that were also exposed to a opioid prescription touchpoint in the past 36 months. We used SAS Studio, version 3.5 (SAS Institute; Cary, NC), for all analyses and PROC STDRATE to calculate SMRs and PAFs.
3. Results
The cohort consisted of 6,717,390 person-years of follow-up in 2014. We identified 1315 opioid overdose decedents. The opioid overdose death incidence rate was 19.6 per 100,000 person-years. We identified past 12-month exposure to one or more of the eight touchpoints in 2.7% of person-months. We found that 51.8% of opioid overdose deaths were preceded by at least one touchpoint in the prior 12 months. The fatal opioid overdose incidence rate following any touchpoint exposure was 372 per 100,000 person-years. Opioid prescription and critical encounter touchpoints preceded 20.5% and 37.3% of fatal opioid overdoses respectively. Opioid overdose incidence rates following opioid prescription and critical encounter touchpoints were 181 per 100,000 person-years and 1261 per 100,000 person-years respectively (Table 1).
Table 1.
Fatal opioid overdose standardized mortality ratios (SMR) and population attributable fractions (PAF) associated with exposure to opioid prescription and critical encounter touchpoints in past 12 months, Massachusetts, 2014.
| Touchpoint | Person years (%)a | Opioid deaths (%)a | Opioid death incidence rate per 100,000 person years |
SMR (95% CI)b | PAF (95% CI)b |
|---|---|---|---|---|---|
| All residentsc | 6,717,390 (100%) | 1315 (100%) | 19.6 | N/A | N/A |
| Any touchpoint | 183,089 (2.7%) | 681 (51.8%) | 372 | 35.2 (32.6, 37.9) | 0.50 (0.47, 0.53) |
| Any opioid prescription touchpointd | 148,535 (2.2%) | 269 (20.5%) | 181 | 12.6 (11.1, 14.1) | 0.19 (0.17, 0.21) |
| High dosage | 36,330 (0.5%) | 100 (7.6%) | 275 | 15.1 (12.1, 18.0) | 0.07 (0.06, 0.09) |
| Benzodiazepine co-prescribing | 50,130 (0.7%) | 132 (10.0%) | 263 | 18.0 (14.9, 21.1) | 0.09 (0.08, 0.11) |
| Multiple prescribers | 95,656 (1.4%) | 165 (12.5%) | 172 | 10.5 (8.9, 12.1) | 0.11 (0.10, 0.13) |
| Multiple pharmacies | 39,890 (0.6%) | 114 (8.7%) | 286 | 14.4 (11.8, 17.1) | 0.08 (0.07, 0.10) |
| Any critical encounter touchpoint | 38,948 (0.6%) | 491 (37.3%) | 1261 | 68.4 (62.4, 74.5) | 0.37 (0.34, 0.39) |
| Opioid detoxification | 16,541 (0.2%) | 259 (19.7%) | 1,844 | 66.1 (58.0, 74.1) | 0.19 (0.17, 0.22) |
| Nonfatal opioid overdosee | 9,208 (0.1%) | 223 (17.0%) | 2,422 | 111 (96.7, 126) | 0.17 (0.15, 0.19) |
| Injection-related infectionf | 5,752 (0.1%) | 81 (6.4%) | 1,408 | 54.1 (42.43 65.8) | 0.06 (0.05, 0.07) |
| Release from incarceration | 14,686 (0.2%) | 126 (9.6%) | 858 | 30.0 (24.8, 35.3) | 0.09 (0.08, 0.11) |
Percentages are relative to all residents; the categories identified are not mutually exclusive and column percentages do not add to 100%.
Standardized for age group and sex.
Data for Massachusetts residents ages 11 years or older in 2014.
Opioid prescription touchpoints identified as: three or more months in past year with more than 100 mg average daily morphine equivalents or opioid and benzodiazepine co-prescribing, or one or more quarters in past year with three or more different opioid prescribers or pharmacies dispensing opioids.
Nonfatal opioid overdose identified from ambulance, emergency department, outpatient observation, or inpatient discharge for opioid overdose without death in subsequent 7 days.
Emergency department, outpatient observation, or inpatient discharge for cellulitis, abscess, osteomyelitis, or endocarditis likely due to injection based on past 12-month history of diagnosis of opioid use disorder or hepatitis C virus.
3.1. Standardized mortality ratios (SMRs)
The opioid overdose SMR for individuals exposed to any opioid prescription touchpoint in the prior 12 months was 12.6 (95% CI: 11.1, 14.1). Thus, the risk of opioid overdose death was 12.6 times higher for those exposed to a opioid prescription touchpoint compared with those not exposed. All four high-risk opioid prescription touchpoints were associated with an elevated SMR, ranging from 10.5 (95% CI: 8.9, 12.1) for 3 or more opioid prescribers in a quarter to 18.0 (95% CI: 14.9, 21.1) for opioid and benzodiazepine co-prescribing. For individuals exposed to any critical encounter touchpoint in the prior 12 months, the opioid overdose SMR was 68.4 (95% CI: 62.4, 74.5). All four critical encounter touchpoints were associated with an elevated SMR ranging from 30.0 (95% CI: 24.8, 35.3) for individuals released from prison or jail to 111.3 (95% CI: 96.7, 125.9) for individuals surviving an opioid overdose (Table 1).
3.2. Population attributable fractions (PAFs)
The PAF for individuals experiencing any of the eight candidate touchpoints in the prior 12 months was 0.50 (95% CI: 0.47, 0.53), this indicates the potential to have prevented up to 50% of opioid overdose deaths in the population, if interventions were deployed at touchpoints that reduced overdose mortality risk. The PAF for individuals experiencing any opioid prescription touchpoint was 0.19 (95% CI: 0.17, 0.21). The PAF associated with individual opioid prescription touchpoints ranged from 0.07 (95% CI: 0.06, 0.09) for those with high dose prescribing to 0.11 (95% CI: 0.10, 0.13) for those with multiple prescribers. The PAF for individuals exposed to any critical encounter touchpoint was 0.37 (95% CI: 0.34, 0.39). The PAF for individual critical encounter touchpoints ranged from 0.06 (95% CI: 0.05, 0.07) for potentially injection-related infections to 0.19 (0.17, 0.22) for patients with a detoxification episode (Table 1).
3.3. Age
Crude opioid overdose mortality rates were 24.5 per 100,000 person-years for individuals aged 11–49 and 12.2 per 100,000 person-years for individuals aged 50+ (Table 2). The SMRs associated with each touchpoint were similar across age groups; however, there was a marked difference in the PAF of prescription versus critical encounter touchpoints by age (Table 2). Opioid prescription touchpoints were associated with PAFs of 0.14 (95% CI: 0.12, 0.17) for individuals aged 11–49 and 0.32 (95% CI: 0.27, 0.37) for individuals aged 50+. Non-prescribing touchpoints were associated with PAFs of 0.42 (95% CI: 0.38, 0.45) for 11–19 year olds and 0.22 (95% CI: 0.18, 0.27) for age 50+.
Table 2.
Fatal opioid overdose standardized mortality ratios (SMR) and population attributable fractions (PAF) associated with exposure to opioid prescription and critical encounter touchpoints in past 12 months, Massachusetts, 2014, by age group.
| Touchpoint | 11-49 years |
≥50 years |
||||||
|---|---|---|---|---|---|---|---|---|
| Opioid deathsa | Opioid death incidence rate per 100,000 person years |
SMR (95% CI)b | PAF (95% CI)b | Opioid deathsa |
Opioid death incidence rate per 100,000 person years |
SMR (95% CI)b | PAF (95% CI)b | |
| All residentsc | 984 (100%) | 24.5 | N/A | N/A | 331 (100%) | 12.2 | N/A | N/A |
| Any touchpoint | 515 (52%) | 620 | 41.5 (37.9, 45.1) | 0.51 (0.48, 0.54) | 166 (50%) | 166 | 23.9 (20.3, 27.6) | 0.48 (0.42, 0.53) |
| Any opioid prescription touchpointd | 154 (16%) | 287 | 11.8 (9.91, 13.6) | 0.14 (0.12, 0.17) | 115 (34%) | 121 | 13.8 (11.3, 16.3) | 0.32 (0.27, 0.37) |
| High dosage | 47 (5%) | 430 | 13.8 (9.8, 17.7) | 0.04 (0.03, 0.06) | 53 (16%) | 209 | 16.5 (12.0, 20.9) | 0.15 (0.11, 0.19) |
| Benzodiazepine co-prescribing | 69 (7%) | 455 | 18.4 (14.1, 22.8) | 0.07 (0.05, 0.08) | 63 (19%) | 180 | 17.6 (13.2, 21.9) | 0.18 (0.14, 0.22) |
| Multiple prescribers | 100 (10%) | 272 | 10.4 (8.4, 12.5) | 0.09 (0.07, 0.11) | 65 (19%) | 110 | 10.7 (8.1, 13.2) | 0.18 (0.13, 0.22) |
| Multiple pharmacies | 72 (7%) | 378 | 13.9 (10.7, 17.1) | 0.07 (0.05, 0.08) | 42 (13%) | 202 | 15.4 (10.8, 20.1) | 0.12 (0.08, 0.15) |
| Any critical encounter touchpoint | 416 (42%) | 1,295 | 68.1 (61.5, 74.6) | 0.42 (0.38, 0.45) | 75 (23%) | 1,100 | 70.5 (54.5, 86.4) | 0.22 (0.18, 0.27) |
| Opioid detoxification | 235 (24%) | 1,553 | 65.0 (56.7, 73.3) | 0.24 (0.21, 0.26) | 24 (7%) | 1,703 | 79.5 (47.7, 111) | 0.07 (0.04, 0.10) |
| Nonfatal opioid overdosee | 182 (18%) | 2,648 | 109 (93.2, 125) | 0.18 (0.16, 0.21) | 41 (12%) | 1,755 | 123 (85.0, 160) | 0.12 (0.09, 0.16) |
| Injection-related infectionf | 55 (6%) | 1,307 | 45.2 (33.3, 57.2) | 0.05 (0.04, 0.07) | 26 (8%) | 1,684 | 92.1 (56.7-128) | 0.08 (0.05, 0.11) |
| Release from incarceration | 113 (11%) | 901 | 30.3 (24.7, 35.9) | 0.11 (0.09, 0.13) | 13 (4%) | 606 | 27.8 (12.7, 42.9) | 0.04 (0.02, 0.06) |
Percentages are relative to all residents; touchpoints are not mutually exclusive and column percentages do not add to 100%.
Standardized for age group and sex.
Data for Massachusetts residents ages 11 years or older in 2014.
Opioid prescription touchpoints identified as: three or more months in past year with more than 100 mg average daily morphine equivalents or opioid and benzodiazepine co-prescribing, or one or more quarters in past year with three or more different opioid prescribers or pharmacies dispensing opioids.
Nonfatal opioid overdose identified from ambulance, emergency department, outpatient observation, or inpatient discharge for opioid overdose without death in subsequent 7 days.
Emergency department, outpatient observation, or inpatient discharge for cellulitis, abscess, osteomyelitis, or endocarditis likely due to injection based on past 12-month history of diagnosis of opioid use disorder or hepatitis C virus.
3.4. Sex
Opioid-related mortality was 29.4 per 100,000 person-years for males and 10.9 per 100,000 person-years for females. Despite having lower overall mortality rates, touchpoint exposure was associated with higher SMRs and PAFs for females compared with males (Table 3). The SMR following any touchpoint was 50.5 (95% CI: 43.9, 57.0) for females and 30.6 (95% CI: 27.8, 33.4) for males. The PAF following any touchpoint was 0.58 (95% CI: 0.52, 0.62) for females and 0.47 (95% CI: 0.44, 0.50) for males.
Table 3.
Fatal opioid overdose standardized mortality ratios (SMR) and population attributable fractions (PAF) for fatal opioid overdoses associated with exposure to high-risk opioid prescribing and non-prescribing touchpoints in past 12 months, Massachusetts, 2014, by sex.
| Touchpoint | Female |
Male |
||||||
|---|---|---|---|---|---|---|---|---|
| Opioid Deathsa | Opioid death incidence rate per 100,000 person years |
SMR (95% CI)b | PAF (95% CI)b | Opioid Deathsa | Opioid death incidence rate per 100,000 person years |
SMR (95% CI)b | PAF (95% CI)b | |
| All residentsc | 386 (100%) | 10.9 | N/A | N/A | 929 (100%) | 29.4 | N/A | N/A |
| Any touchpoint | 227 (59%) | 241 | 50.5 (43.9, 57.0) | 0.58 (0.52, 0.62) | 454 (49%) | 510 | 30.6 (27.8, 33.4) | 0.47 (0.44, 0.50) |
| Any opioid prescription touchpointd | 111 (29%) | 132 | 18.0 (14.7, 21.4) | 0.27 (0.22, 0.32) | 158 (17%) | 244 | 10.4 (8.7, 12.0) | 0.15 (0.13, 0.18) |
| High dosage | 39 (10%) | 215 | 22.1 (15.2, 29.0) | 0.10 (0.07, 0.13) | 61 (7%) | 335 | 12.5 (9.4, 15.7) | 0.06, 0.04, 0.08) |
| Benzodiazepine co-prescribing | 65 (17%) | 200 | 22.6 (17.1, 28.1) | 0.16 (0.12, 0.20) | 67 (7%) | 381 | 15.1 (11.5, 18.7) | 0.07 (0.05, 0.08) |
| Multiple prescribers | 67 (17%) | 124 | 14.1 (10.7, 17.5) | 0.16 (0.12, 0.20) | 98 (11%) | 235 | 9.0 (7.2, 10.7) | 0.09 (0.07, 0.11) |
| Multiple pharmacies | 44 (11%) | 203 | 18.5 (13.1, 24.0) | 0.11 (0.08, 0.14) | 70 (8%) | 385 | 12.7 (9.7, 15.6) | 0.07 (0.05, 0.09) |
| Any critical encounter touchpoint | 158 (41%) | 1,304 | 181 (153, 209) | 0.41 (0.36, 0.45) | 333 (36%) | 1,241 | 52.8 (47.2, 58.5) | 0.35 (0.32, 0.38) |
| Opioid detoxification | 75 (19%) | 1,446 | 151 (117, 185) | 0.19 (0.15, 0.23) | 184 (20%) | 1,621 | 53.8 (46.0, 61.5) | 0.19 (0.17, 0.22) |
| Nonfatal opioid overdosee | 72 (19%) | 2,025 | 207 (159, 255) | 0.19 (0.15, 0.22) | 151 (16%) | 2,672 | 91.2 (76.7, 106) | 0.16 (0.14, 0.18) |
| Injection-related infectionf | 29 (8%) | 1,206 | 95.5 (60.7, 130) | 0.07 (0.05, 0.10) | 52 (6%) | 1,554 | 43.5 (31.7, 55.4) | 0.05 (0.04, 0.07) |
| Release from incarceration | 39 (10%) | 1,082 | 92.4 (63.4, 121) | 0.10 (0.07, 0.13) | 87 (9%) | 785 | 23.0 (18.2, 27.9) | 0.09 (0.07, 0.11) |
Percentages are relative to all residents; touchpoints are not mutually exclusive and column percentages do not add to 100%.
Standardized for age group and sex.
Data for Massachusetts residents ages 11 years or older in 2014.
Opioid prescription touchpoints identified as: three or more months in past year with more than 100 mg average daily morphine equivalents or opioid and benzodiazepine co-prescribing, or one or more quarters in past year with three or more different opioid prescribers or pharmacies dispensing opioids.
Nonfatal opioid overdose identified from ambulance, emergency department, outpatient observation, or inpatient discharge for opioid overdose without death in subsequent 7 days.
Emergency department, outpatient observation, or inpatient discharge for cellulitis, abscess, osteomyelitis, or endocarditis likely due to injection based on past 12-month history of diagnosis of opioid use disorder or hepatitis C virus.
3.5. Varied exposure window
For individuals exposed to an opioid prescription touchpoint in the past three months, the SMR was 12.3 (95% CI: 10.6, 14.1); the SMR decreased to 6.9 (95% CI: 5.3, 8.6) for those last exposed 25–36 months prior. Following opioid prescription touchpoints, cumulative PAF increased from 0.13 (95% CI: 0.11, 0.15) to 0.30 (0.27, 0.32) for three-month and three-year exposure windows, respectively (Table 4). Following opioid detoxification treatment, the SMR was 61.3 (95% CI: 50.2, 72.3) in first three months, decreasing to 16.8 (95% CI: 11.0, 22.6) for those last exposed 25–36 months prior. Cumulative PAF increased from 0.09 (95% CI: 0.07, 0.10) to 0.28 (95% CI: 0.26, 0.31) for three-month and three-year exposure windows, respectively. Similar patterns were seen in other critical encounter touchpoints (Table 4).
Table 4.
Fatal opioid overdose standardized mortality ratios and population attributable fractions associated with high-risk prescribing and non-prescribing touchpoints in Massachusetts, 2014, by varied exposure windows.
| Touchpoint | Standardized Mortality Ratioa (95% CI) |
Population Attributable Fraction, Cumulative (95% CI) |
||||||
|---|---|---|---|---|---|---|---|---|
| 0-3 mos. | 4-12 mos. & NOT 0-3 mos. | 13-24 mos. & NOT 0-12 mos. | 25-36 mos. & NOT 0-24 mos. | 0-3 mos. | 0-12 mos. | 0-24 mos. | 0-36 mos. | |
| Any touchpoint | 27.9 (25.3, 30.6) | 19.2 (16.8, 21.6) | N/A | N/A | 0.32 (0.29 0.34) | 0.50 (0.47, 0.53) | N/A | N/A |
| Any opioid prescription touchpointb | 12.3 (10.6, 14.1) | 9.0 (7.1, 11.0) | 8.1 (6.4, 9.8) | 6.9 (5.2, 8.6) | 0.13 (0.11, 0.15) | 0.19 (0.17, 0.21) | 0.25 (0.23, 0.27) | 0.30 (0.27, 0.32) |
| High dose | 14.0 (10.9, 17.0) | 17.7 (9.94, 25.4) | 14.4 (8.97, 19.8) | 14.7 (8.7, 20.7) | 0.06 (0.04, 0.07) | 0.07 (0.06, 0.09) | 0.09 (0.07, 0.11) | 0.11 (0.09, 0.12) |
| Benzodiazepine co-prescribing | 18.0 (14.6, 21.3) | 13.9 (7.8, 20.0) | 13.1 (8.8, 17.4) | 13.7 (8.9, 18.6) | 0.08 (0.07, 0.10) | 0.09 (0.08, 0.11) | 0.12 (0.10, 0.14) | 0.14 (0.12, 0.16) |
| Multiple prescribers | 8.3 (6.4, 10.3) | 11.1 (8.9, 13.3) | 9.8 (7.8, 11.7) | 7.1 (5.3, 8.9) | 0.05 (0.03, 0.06) | 0.11 (0.10, 0.13) | 0.18 (0.16, 0.20) | 0.22 (0.20, 0.25) |
| Multiple pharmacies | 11.8 (8.5, 15.1) | 15.6 (11.8, 19.3) | 13.9 (10.4, 17.3) | 15.8 (11.9, 19.9) | 0.03 (0.02, 0.04) | 0.08 (0.07. 0.10) | 0.12 (0.11, 0.14) | 0.17 (0.15, 0.19) |
| Any critical encounter touchpoint | 72.2 (63.7, 80.7) | 38.3 (33.2, 43.5) | N/A | N/A | 0.21 (0.18, 0.23) | 0.37 (0.34, 0.39) | N/A | N/A |
| Opioid detoxification | 61.3 (50.2, 72.3) | 55.6 (46.4, 64.8) | 39.5 (31.3, 47.6) | 16.8 (11.0, 22.6) | 0.09 (0.07, 0.10) | 0.19 (0.17, 0.22) | 0.26 (0.24, 0.28) | 0.28 (0.26, 0.31) |
| Nonfatal opioid overdosec | 124 (101, 148) | 85.5 (69.8, 101) | N/A | N/A | 0.08 (0.07, 0.10) | 0.17 (0.15, 0.19) | N/A | N/A |
| Injection-related infectiond | 56.9 (39.1, 74.8) | 48.8 (34.1, 63.6) | 66.5 (44.1, 88.8) | N/A | 0.03 (0.02, 0.04) | 0.06 (0.05, 0.07) | 0.09 (0.07, 0.10) | N/A |
| Release from incarceration | 43.2 (32.6, 53.8) | 21.0 (15.8, 26.2) | 16.6 (12.3 (20.9) | 13.2 (8.9, 17.6) | 0.05 (0.04, 0.06) | 0.09 (0.08, 0.11) | 0.13 (0.11, 0.15) | 0.16 (0.14, 0.18) |
Standardized mortality ratios are calculated for those exposed to the touchpoint versus not exposed within the exposure window.
Opioid prescription touchpoints identified as: three or more months in past year with more than 100 mg average daily morphine equivalents or opioid and benzodiazepine co-prescribing, or one or more quarters in past year with three or more different opioid prescribers or pharmacies dispensing opioids.
Nonfatal opioid overdose identified from ambulance, emergency department, outpatient observation, or inpatient discharge for opioid overdose without death in subsequent 7 days.
Emergency department, outpatient observation, or inpatient discharge for cellulitis, abscess, osteomyelitis, or endocarditis likely due to injection based on past 12-month history of diagnosis of opioid use disorder or hepatitis C virus.
3.6. Opioid prescription touchpoints prior to critical encounter touchpoints
Of 38,949 person-years of follow-up with past 12-month exposure to a critical encounter touchpoint, 7779 person-years (20%) were also exposed to a opioid prescription touchpoint in the past 36 months (Appendix D).
4. Discussion
In a population-level cohort of Massachusetts residents ages 11 and older in 2014, we calculated fatal opioid overdose SMRs and PAFs associated with eight public health, criminal justice system, or health care system touchpoints. Opioid prescription touchpoints and critical encounter touchpoints were associated with 13-fold and 68-fold increases in opioid overdose death respectively. Effective interventions deployed at these touchpoints would have had the potential to eliminate up to 50% of opioid overdose deaths.
Our study has several strengths based on the unique characteristics of the Massachusetts Public Health Data Warehouse that includes near population-level data for an entire state that allowed us to identify opioid overdose death SMRs and PAFs across eight clinically relevant touchpoints. Presenting them side by side and stratified by age and sex allows public health policy makers and practitioners to see both the absolute and relative importance of these touchpoints as targets to develop and implement opioid overdose death prevention efforts.
In order to leverage these touchpoints to reduce opioid overdose deaths, effective interventions are needed. Following non-prescribing touchpoints, interventions should include proven harm reduction and treatment engagement. Naloxone distribution programs and treatment with medications for opioid use disorder (MOUD) are associated with reduced opioid overdose mortality (McDonald and Strang, 2016; Sordo et al., 2017; Walley et al., 2013). More specifically, studies among overdose survivors and individuals released from incarceration have shown MOUD are associated with reduced opioid overdose death yet a minority receive them (Green et al., 2018; Larochelle et al., 2018). Although less is known about linkage to treatment and outcomes following opioid detoxification and injection-drug associated infections, 43–78% of inpatient detoxification clients indicated a preference for engagement with MOUD following detoxification (Bailey et al., 2013; Stein et al., 2015, 2017; Uebelacker et al., 2016). At a Massachusetts academic medical center, fewer than 10% of patients with injection drug-related endocarditis had a plan for offering MOUD at discharge (Rosenthal et al., 2016). Though not currently widely accessible, buprenorphine initiation within emergency department and inpatient hospitals has been demonstrated to be feasible, to increase linkage to addiction treatment and to reduce illicit opioid use (D’Onofrio et al., 2017, 2015; Liebschutz et al., 2014).
Effective interventions for individuals exposed to opioid prescription touchpoints are less clear. The CDC opioid prescribing guideline recommends prescribing naloxone to high-risk individuals, and engaging individuals identified to have OUD in treatment, typically with MOUD (Dowell et al., 2016). A systematic review suggested opioid tapering may improve patient outcomes; however, evidence quality was low and most studies were limited to individuals interested in tapering (Frank et al., 2017). We found that opioid overdose death risk remained 7 times higher than in the general population 2–3 years after discontinuation of high-risk opioid prescribing. Interventions beyond stopping opioid prescribing are needed.
New approaches and interventions are needed to reach individuals at high risk of opioid overdose death following critical encounter touchpoints. Project Lazarus was a seven-pronged strategy enacted in North Carolina that included efforts to reduce opioid prescribing, and increase access to naloxone and MOUD. Unfortunately, the program had limited impact on overdose deaths and prescribing of opioid analgesics and MOUD, highlighting the need for rigorous evaluation prior to widespread adoption (Alexandridis et al., 2019, 2018). A number of program innovations are actively being implemented. Emergency department based opioid overdose prevention education and naloxone distribution to high-risk individuals has been shown to be feasible (Dwyer et al., 2015). Inpatient addiction consult services are increasingly being deployed to engage individuals with OUD while hospitalized (Priest and McCarty, 2019). An evaluation of one program demonstrated feasibility in initiating MOUD with successful linkage to post-discharge care, and a pragmatic multi-site trial of inpatient addiction consult services is underway (McNeely et al., 2019; Trowbridge et al., 2017). Public health and public safety agencies are increasingly deploying post-overdose outreach programs to engage opioid overdose survivors with varied approaches (Formica et al., 2018).
Further, our findings suggest the need for targeted prevention efforts by age and sex. Opioid prescription touchpoints were more prevalent among opioid overdose deaths in older adults whereas critical encounter touchpoints were more common in younger adults. This is consistent with national data showing that overdose deaths due to prescription opioids are more prevalent in older adults, and heroin and fentanyl more prevalent among younger adults (McBain et al., 2018). We also found that touchpoints could be a particularly salient opportunity for interventions among females, who had much higher subsequent opioid mortality than males.
Our findings are consistent with past studies identifying increased overdose mortality risk from individual touchpoints. An analysis of prescription monitoring program data linked to death records in Tennessee found adjusted odds ratios of 11.2 for high dosage, 6.5 for 4 or more prescribers in a year, and 6.0 for 4 or more pharmacies in a year (Baumblatt et al., 2014). We found SMRs of 12.6 for high dose, and 10.5 and 14.4 for multiple prescribers and pharmacies, respectively. In Medicaid data, the SMR for drug use associated deaths in the first year after a nonfatal opioid overdose was 132.1, similar to our finding of an SMR of 111 (Olfson et al., 2018). In Washington State, the relative risk of drug overdose death compared to the general population was 129 in the first two weeks following prison release, dropping to 12.2 over a median follow-up of 1.9 years (Binswanger et al., 2007). In Massachusetts, we identified an SMR of 43.2 within three months of release from incarceration, dropping to 13.2 2–3 years post release.
Data on relative frequency of touchpoints prior to overdose death are less available in other studies. In Washington State, 8.3% of overdose deaths among 15 to 84 year olds occurred among former prisoners, which is quite similar to the PAF of 0.09 in our Massachusetts study (Binswanger et al., 2013). In Tennessee between 2007 and 2011, 55% of opioid overdose deaths were preceded by high dose, multiple pharmacy or multiple prescriber prescribing touchpoints (Baumblatt et al., 2014). This is much greater than the PAF of 0.19 for any opioid prescribing touchpoint identified in our study. This may reflect the ongoing shift from prescription opioids to heroin and fentanyl among opioid overdose decedents (O’Donnell et al., 2017).
Other studies have successfully identified high rates of emergency department and other acute care utilization for individuals prior to opioid overdose death (Brady et al., 2015; Maeng et al., 2017). Our study extends beyond healthcare utilization to include criminal justice encounters and inpatient detoxification episodes. Our study also focused on touchpoints that are quite specific for identifying individuals with high-risk opioid use relative to studies examining healthcare utilization patterns only. The latter approaches may be less effective as they require an extra step of screening individuals meeting utilization thresholds for which to deliver services.
Our study has several limitations. First, our analysis focused on opioid overdose deaths in Massachusetts in a single year, 2014. Notably this study overlapped a time of increasing fentanyl involvement in opioid overdose deaths in Massachusetts and elsewhere (Somerville et al., 2017). While we used the most recent data available, further analyses are needed to determine the extent to which these results may not generalize to other states or more recent years. Second, our exposure and outcome variables may have been misclassified through either lack of capture or linkage error, though such misclassification would likely bias SMR results toward the null. Third, while we standardized for age and sex, many other characteristics may also be associated with both the touchpoint exposures and outcome. However, our intent was not to demonstrate causality, but to identify encounters associated with subsequent opioid overdose death.
5. Conclusions
Using public health data, we found that eight candidate touchpoints were each associated with marked subsequent increase in opioid overdose death risk. These findings provide a roadmap enabling policy makers, public health authorities, and health care systems to prioritize deployment of resources toward the opportunities with highest potential to reduce opioid overdose death.
Supplementary Material
Acknowledgments
Role of funding source
Dr. Larochelle was supported by NIDA (K23 DA042168) and a Boston University School of Medicine Department of Medicine Career Investment Award. Dr. Stopka was supported by the GE Foundation.
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declaration of Competing Interest
Dr. Larochelle reports a research grant from Optum Labs. The authors have no other conflicts of interest or financial disclosures to report.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2019.06.039.
References
- Alexandridis AA, Dasgupta N, McCort AD, Ringwalt CL, Rosamond WD, Chelminski PR, Marshall SW, 2019. Associations between implementation of Project Lazarus and opioid analgesic dispensing and buprenorphine utilization in North Carolina, 2009-2014. Inj. Epidemiol 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandridis AA, McCort A, Ringwalt CL, Sachdeva N, Sanford C, Marshall SW, Mack K, Dasgupta N, 2018. A statewide evaluation of seven strategies to reduce opioid overdose in North Carolina. Inj. Prev 24, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey GL, Herman DS, Stein MD, 2013. Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. J. Subst. Abuse Treat 45, 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LJ, Sherazi A, Laczko D, Gupta K, Koivu S, M AW, Mele T, Tirona R, McCormick JK, Silverman M, 2017. Validation of an algorithm to identify infective endocarditis in people who inject drugs. Med. Care [DOI] [PubMed] [Google Scholar]
- Baumblatt J, Wiedeman C, Dunn JR, Schaffner W, Paulozzi LJ, Jones TF, 2014. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern. Med 174, 796–801. [DOI] [PubMed] [Google Scholar]
- Binswanger IA, Blatchford PJ, Mueller SR, Stern MF, 2013. Mortality after prison release: opioid overdose and other causes of death, risk factors, and time trends from 1999 to 2009. Ann. Intern. Med 159, 592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD, 2007. Release from prison — a high risk of death for former inmates. N. Engl. J. Med 356, 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert AB, Valenstein M, Bair MJ, et al. , 2011. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 305, 1315–1321. [DOI] [PubMed] [Google Scholar]
- Brady JE, DiMaggio CJ, Keyes KM, Doyle JJ, Richardson LD, Li G, 2015. Emergency department utilization and subsequent prescription drug overdose death. Ann. Epidemiol 25, 613–619 e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudarella A, Dong H, Milloy MJ, Kerr T, Wood E, Hayashi K, 2016. Non-fatal overdose as a risk factor for subsequent fatal overdose among people who inject drugs. Drug Alcohol Depend. 162, 51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio G, Chawarski MC, O’Connor PG, Pantalon MV, Busch SH, Owens PH, Hawk K, Bernstein SL, Fiellin DA, 2017. Emergency department-initiated buprenorphine for opioid dependence with continuation in primary care: outcomes during and after intervention. J. Gen. Intern. Med 32, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio G, O’Connor PG, Pantalon MV, Chawarski MC, Busch SH, Owens PH, Bernstein SL, Fiellin DA, 2015. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA 313, 1636–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Mills KL, Ross J, Teesson M, 2011. Rates and correlates of mortality amongst heroin users: findings from the Australian Treatment Outcome Study (ATOS), 2001-2009. Drug Alcohol Depend. 115, 190–195. [DOI] [PubMed] [Google Scholar]
- Davoli M, Bargagli AM, Perucci CA, Schifano P, Belleudi V, Hickman M, Salamina G, Diecidue R, Vigna-Taglianti F, Faggiano F, Group, V.E.S., 2007. Risk of fatal overdose during and after specialist drug treatment: the VEdeTTE study, a national multi-site prospective cohort study. Addiction 102, 1954–1959. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. Cdc guideline for prescribing opioids for chronic pain—united states, 2016. JAMA 315, 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Saunders KW, Rutter CM, et al. , 2010. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann. Intern. Med 152, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer K, Walley AY, Langlois BK, Mitchell PM, Nelson KP, Cromwell J, Bernstein E, 2015. Opioid education and nasal naloxone rescue kits in the emergency department. West. J. Emerg. Med 16, 381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formica SW, Apsler R, Wilkins L, Ruiz S, Reilly B, Walley AY, 2018. Post opioid overdose outreach by public health and public safety agencies: exploration of emerging programs in Massachusetts. Int. J. Drug Policy 54, 43–50. [DOI] [PubMed] [Google Scholar]
- Frank JW, Lovejoy TI, Becker WC, et al. , 2017. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann. Intern. Med 167, 181–191. [DOI] [PubMed] [Google Scholar]
- Garg RK, Fulton-Kehoe D, Franklin GM, 2017. Patterns of opioid use and risk of opioid overdose death among medicaid patients. Med. Care 55, 661–668. [DOI] [PubMed] [Google Scholar]
- Green CA, Perrin NA, Janoff SL, Campbell CI, Chilcoat HD, Coplan PM, 2017. Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol. Drug Saf 26, 509–517. [DOI] [PubMed] [Google Scholar]
- Green TC, Clarke J, Brinkley-Rubinstein L, et al. , 2018. Postincarceration fatal overdoses after implementing medications for addiction treatment in a statewide correctional system. JAMA Psychiatry 75, 405–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP Jr, Zhang K, Bohm MK, Losby J, Lewis B, Young R, Murphy LB, Dowell D, 2017. Vital signs: changes in opioid prescribing in the United States, 2006-2015. Morbl. Mortall. Wklyl. Rep 66, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffajee RL, Jena AB, Weiner SG, 2015. Mandatory use of prescription drug monitoring programs. JAMA 313, 891–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery MM, Hooten WM, Henk HJ, Bellolio MF, Hess EP, Meara E, Ross JS, Shah ND, 2018. Trends in opioid use in commercially insured and Medicare Advantage populations in 2007-16: retrospective cohort study. BMJ 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Paulozzi LJ, Mack KA, 2014. Sources of prescription opioid pain relievers by frequency of past-year nonmedical use: united states, 2008-2011. JAMA Intern. Med 174, 802–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Ejiofor JI, Yammine M, Ando M, Camuso JM, Youngster I, Nelson SB, Kim AY, Melnitchouk SI, Rawn JD, MacGillivray TE, Cohn LH, Byrne JG, Sundt TM, 2016. Surgical outcomes of infective endocarditis among intravenous drug users. J. Thorac. Cardiovasc. Surg 152, 832–841 e831. [DOI] [PubMed] [Google Scholar]
- Larochelle MR, Bernson D, Land T, Stopka TJ, Wang N, Xuan Z, Bagley SM, Liebschutz JM, Walley AY, 2018. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann Internl Med. 169, 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebschutz JM, Crooks D, Herman D, Anderson B, Tsui J, Meshesha LZ, Dossabhoy S, Stein M, 2014. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern. Med 174, 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng DD, Han JJ, Fitzpatrick MH, Boscarino JA, 2017. Patterns of health care utilization and cost before and after opioid overdose: findings from 10-year longitudinal health plan claims data. Subst. Abuse Rehabil 8, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain R, Rose AJ, LaRochelle MR, 2018. The U.S. Opioid epidemic: one disease, diverging tales. Prev. Med 112, 176–178. [DOI] [PubMed] [Google Scholar]
- McDonald R, Strang J, 2016. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 111, 1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely J, Troxel AB, Kunins HV, Shelley D, Lee JD, Walley A, Weinstein ZM, Billings J, Davis NJ, Marcello RK, Schackman BR, Barron C, Bergmann L, 2019. Study protocol for a pragmatic trial of the Consult for Addiction Treatment and Care in Hospitals (CATCH) model for engaging patients in opioid use disorder treatment. Addict. Sci. Clin. Pract 14, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MDPH, 2016. An Assessment of Opioid-related Deaths in Massachusetts (2013-2014). (Accessed August 9 2017). http://www.mass.gov/eohhs/docs/dph/stop-addiction/dph-legislative-report-chapter-55-opioid-overdose-study-9-15-2016.pdf.
- MDPH, 2017. An Assessment of Fatal and Nonfatal Opioid Overdoses in Massachusetts (2011-2015). (Accessed March 4 2019). https://pilot.mass.gov/files/documents/2017/08/31/legislative-report-chapter-55-aug-2017.pdf.
- MDPH, 2018. Data Brief: Opioid-Related Overdose Deaths Among Massachusetts Residents. (Accessed March 28 2018). https://www.mass.gov/files/documents/2018/02/14/data-brief-overdose-deaths-february-2018.pdf.
- Merrall ELC, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, Hutchinson SJ, Bird SM, 2010. Meta-analysis of drug-related deaths soon after release from prison. Addiction 105, 1545–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell JK, Gladden RM, Seth P, 2017. Trends in deaths involving heroin and synthetic opioids excluding methadone, and law enforcement drug product reports, by census region — united States, 2006–2015. MMWR Morb. Mortal. Wkly. Rep 66, 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Crystal S, Wall M, Wang S, Liu S, Blanco C, 2018. Causes of death after nonfatal opioid overdose. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert ASB, 2015. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ : British Medical Journal 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest KC, McCarty D, 2019. Role of the hospital in the 21st century opioid overdose epidemic: the addiction medicine consult service. J. Addict. Med 13, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan MV, Herzig SJ, 2016. Hospitalizations related to opioid Abuse/Dependence and associated serious infections increased sharply, 2002-12. Health Aff. (Millwood) 35, 832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Bernson D, Chui KKH, Land T, Walley AY, LaRochelle MR, Stein BD, Stopka TJ, 2018. Potentially inappropriate opioid prescribing, overdose, and mortality in Massachusetts, 2011-2015. J. Gen. Intern. Med 33, 1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF, 2016. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am. J. Med 129, 481–485. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L, 2016. Increases in drug and opioid-involved overdose deaths - United States, 2010-2015. MMWR Morb. Mortal. Wkly. Rep 65, 1445–1452. [DOI] [PubMed] [Google Scholar]
- Somerville NJ, O’Donnell J, Gladden RM, Zibbell JE, Green TC, Younkin M, Ruiz S, Babakhanlou-Chase H, Chan M, Callis BP, Kuramoto-Crawford J, Nields HM, Walley AY, 2017. Characteristics of fentanyl overdose - Massachusetts, 2014-2016. MMWR Morb. Mortal. Wkly. Rep 66, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R, 2017. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ 357, j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Anderson BJ, Bailey GL, 2015. Preferences for aftercare among persons seeking short-term opioid detoxification. J. Subst. Abuse Treat 59, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Flori JN, Risi MM, Conti MT, Anderson BJ, Bailey GL, 2017. Overdose history is associated with postdetoxification treatment preference for persons with opioid use disorder. Subst. Abus 38, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge P, Weinstein ZM, Kerensky T, Roy P, Regan D, Samet JH, Walley AY, 2017. Addiction consultation services - linking hospitalized patients to outpatient addiction treatment. J. Subst. Abuse Treat 79, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebelacker LA, Bailey G, Herman D, Anderson B, Stein M, 2016. Patients’ beliefs about medications are associated with stated preference for methadone, buprenorphine, naltrexone, or no medication-assisted therapy following inpatient opioid detoxification. J. Subst. Abuse Treat 66, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unick GJ, Ciccarone D, 2017. US regional and demographic differences in prescription opioid and heroin-related overdose hospitalizations. Int. J. Drug Policy 46, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A, Ruiz S, Ozonoff A, 2013. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.