Abstract
A prospective, observational, cross-sectional study documenting the prevalence of pain in dogs presented to the emergency service of a veterinary teaching hospital and their handling (times to triage, examination, treatment) was conducted. Pain was assessed and compared using a validated and an unvalidated pain assessment scale. Sedation was monitored using a validated scale. A first evaluation was completed in 109 dogs. A second evaluation was completed for 95 dogs: 36 (38%) were identified as painful and 53% (19/36) were provided analgesia in the clinic. The remainder either did not receive analgesia (6/36, 17%) or were prescribed an analgesic for administration at home (11/36, 31%). Of dogs receiving analgesia in the clinic, most showed a decrease in pain score (15/19, 79%). Pain assessment scales were positively correlated (r = 0.69, P < 0.0001) but the unvalidated scale was insensitive in discriminating changes. Between painful and non-painful dogs, progression did not differ: admission to treatment [P = 0.96, 95% confidence interval (CI): –23 to 22 minutes] and examination to treatment (P = 0.73, 95% CI: 14 to 20 minutes). Suboptimal analgesic use suggests focused training in pain assessment and analgesic use guided by a validated pain assessment scale, is warranted.
Résumé
Prévalence et gestion de la douleur chez des chiens présentés au service d’urgence d’un hôpital d’enseignement vétérinaire. Une étude prospective, observationnelle et transversale a été réalisée pour documenter la prévalence de la douleur chez les chiens présentés au service d’urgence d’un hôpital universitaire vétérinaire ainsi que leur gestion (délai pour le triage, examen et traitement). Une échelle validée d’évaluation de la douleur a été utilisée pour évaluer la douleur à l’admission et suivant le traitement en clinique. A titre de comparaison, une échelle non validée d’évaluation de la douleur a également été utilisé et le degré de sédation a été documenté à l’aide d’une échelle de sédation validée. Une première évaluation a été complétée chez 109 chiens. Sur les 95 chiens pour lesquels une deuxième évaluation a été complétée, 36 (38 %) ont été identifiés comme étant en douleur et 53 % (19/36) ont reçu de l’analgésie en clinique. Les chiens restants n’ont soit pas reçu d’analgésie (6/36, 17 %) ou ont reçu une prescription pour un traitement analgésique à la maison (11/36, 31 %). Pour les chiens ayant reçu un traitement analgésique en clinique, la grande majorité ont démontré une diminution de leur score de douleur (15/19, 79 %). Une corrélation positive entre les deux échelles d’évaluation de la douleur était présente (r = 0,69, P < 0,0001), mais l’échelle non validée n’était pas sensible pour distinguer les changements de score de douleur. Il n’y avait pas de différence significative entre les chiens en douleur et non en douleur concernant le délai entre l’admission et le traitement (P = 0,96, 95 % CI : –23 à 22 minutes) ou entre l’examen et le traitement (P = 0,73, 95 % CI : 14 à 20 minutes). L’administration d’analgésie était suboptimal dans la population étudiée, suggérant qu’un entraînement ciblé pour reconnaître et traiter la douleur à l’aide d’une échelle validée est recommandé.
(Traduit par Dr Frédérik Rousseau-Blass)
Introduction
Pain is considered the 5th vital sign, after body temperature, heart rate, respiratory rate, and blood pressure, and veterinarians are aware of the importance of pain recognition and its central role in patient care and welfare (1–3). However, numerous surveys have shown that pain is under-recognized and under-treated in cats and dogs (3–9). A wide variety of underlying influences have been suggested for these shortcomings in pain management, including the number of animal health technicians working in a practice, concerns regarding side effects of analgesics, perception of pain associated with different procedures, limited understanding of drug pharmacology, year of veterinary graduation, (in)ability to assess pain, and the lack of a validated pain assessment scale (3–5,7,9). With regard to the latter, a validated pain assessment scale for acute pain, the Glasgow Composite Measures Pain Scale-Short Form (CMPS-SF), has been available for dogs for over a decade, although its use in clinical practice is unknown (10). A strength of the CMPS-SF is the availability of an analgesic intervention threshold to aid decision-making in pain management and facilitate tracking of patient comfort during hospital visits (11).
The incidence of pain in dogs presenting to an emergency service has received little investigation, with 1 study reporting 56% (179/317) of dogs to be painful and with the primary causes of pain being orthopedic and dermatologic (primarily skin lacerations and bite wounds) (12). Analgesic treatment was provided in 66% (119/179) of the dogs identified as painful and therapy appeared effective in 61% (73/119). However, at the time of this study, no validated pain assessment scale was available for use in dogs and a numeric rating scale for pain assessment consisting of 3 items (behavior, movement, vocalization) was used.
The inadequate treatment of acute pain can result in chronic pain, although the underlying mechanisms of this transition are poorly characterized (13). Proposed theories for the transition to chronic pain include persistent noxious signalling from the periphery and a maladaptive response in the central nervous system, which includes descending inhibitory and facilitatory modulation dysfunction (13). There is currently insufficient evidence in veterinary medicine to establish the prevalence of this problem, though surveys of postoperative human patients suggest that prevalence may be as high as 40% in certain surgical populations and a recent feline study suggested an increased risk of chronic pain and adverse behaviors in cats that had been declawed (13,14). The timescale over which acute pain may establish the biological foundations required for the development of chronic pain syndromes is measured in hours to days (13). Therefore, early recognition and rapid and effective treatment of acute pain are important in the prevention of chronic pain syndromes.
The aims of this study were: i) to assess the prevalence of pain in dogs presenting to the emergency service, the incidence of analgesic treatment, and the evolution of pain during the observation period, and ii) to evaluate the trajectory of dogs as they entered the care pathway by quantifying the time from admission to treatment. A secondary aim was to compare the performance of an unvalidated pain scale against the CMPS-SF. We hypothesized that dogs identified as painful would receive analgesia and would be treated more rapidly than dogs with non-painful conditions.
Materials and methods
Study design and ethical approval
This cross-sectional observational study was conducted at the emergency service of the Centre Hospitalier Universitaire Vétérinaire of the Université de Montréal. The study methodology was submitted to the institutional care and use committee before beginning the study and it confirmed that ethical approval was not required. The head of the intensive care department was aware of the study, and all other veterinarians or animal health technicians were blinded to the nature of the data collected. A sample size estimation was based on preliminary observations, with a sample size of 50 animals required to identify a mean difference in time from admission to treatment between painful and non-painful groups of 20 min with a standard deviation of 30 min (alpha 0.05, 90% power).
Study population
All dogs admitted to the emergency department during 36 selected weekends (Saturday and Sunday, between 0800 and 1800) were eligible for inclusion, except for critically ill patients requiring immediate care or dogs that had already been included in the study during a previous visit. The weekends were equally distributed from January to December 2017 and between 2 raters (FRB and JM).
Data collection
The evaluation process was as follows: after admission by reception, a triage examination was done by a student, animal health technician, or veterinary intern after which the dog was brought to the study evaluation room. Evaluation was completed by 1 of 2 investigators (FRB/JM) after a waiting period of 5 min during which the dog was not restrained and was free to explore the room. Pain was assessed using the CMPS-SF (without section B, scale range 0–20, intervention threshold ≥ 5) (11) and the Colorado Canine Acute Pain Scale (CCAPS; scale range 0–5, appendix 1) (15). Sedation level was evaluated using a validated sedation scale (16). Evaluations were completed within 5 min. The second evaluation followed the same steps as the first, taking place either after completion of the initial treatment plan (managed by the emergency department) or just before the dog was due to leave the hospital. Blinding to analgesic treatment was not always possible as assessors were following case progression in the hospital. In a pilot study, inter-rater agreement between the 2 raters was confirmed as “very good” (ICCsingle > 0.81) for the CMPS-SF and sedation scales. The following information was collected for the study: age, body mass, gender, reproductive state, breed, reason for admission, and number of active cases currently managed by the attending veterinarian. The following times were recorded to track patient handling: time of admission by reception, physical examination by a veterinarian (intern or clinician), and first treatment intervention (including potential analgesics). For animals that did not receive analgesia while in the hospital, admission and physical examination times were still included. For animals that did not receive a second evaluation, data from the first evaluation (pain and sedation scale scores) were still included.
Cases were categorized according to final diagnosis (with the exception of cases presented for euthanasia). Animals were excluded from the study if any of the following criteria were met: presence of sedation at first evaluation (score > 6/12), requirement for immediate treatment or aggressive behavior.
Statistical analysis
Data were entered in an electronic spreadsheet and analyses were performed using commercial software (Prism 6.07; GraphPad Software, La Jolla, California, USA). Non-parametric tests were applied after assessment of data distribution with a D’Agostino-Pearson omnibus normality test. Descriptive statistics of all cases are presented using proportions for categorical variables or median (range) for continuous variables. Comparisons of scores between painful and non-painful animals (classified according to the CMPS-SF analgesic intervention threshold) were performed using a Mann-Whitney test. A Spearman “r” correlation coefficient was calculated between the CMPS-SF and CCAPS scores. The data supporting the study results are available in an electronic repository (17). Values of P < 0.05 were considered significant.
Results
During the study period, 118 eligible dogs were admitted to the emergency department. Of these, 109 dogs completed the first evaluation for pain and sedation, with 4 dogs excluded for not receiving an initial pain evaluation and 5 dogs excluded for having sedation scale scores exceeding the predetermined threshold. Demographic data are presented in Table 1. Bichon breeds (n = 8), Labrador retrievers (n = 8), mixed breeds (n = 8), and poodles (n = 7) were the most frequently encountered breeds.
Table 1.
Demographic data for dogs included in the study.
| Parameters | Painful dogs (n = 40) | Non-painful dogs (n = 69) | All dogs (n = 109) |
|---|---|---|---|
| Age (years) | 5.5 [0.2 to 16.1] | 6.6 [0.1 to 16.2] | 6.2 [0.1 to 16.2] |
| Body mass (kg) | 10.3 [0.7 to 55.0] | 21.6 [1.8 to 70.0] | 17.4 [0.7 to 70.0] |
| BCS (/9) | 5 [2 to 7] | 5 [1 to 9] | 5 [1 to 9] |
| Gender | |||
| Female spay (%) | 11 (28) | 31 (45) | 42 (39) |
| Female intact (%) | 6 (15) | 8 (12) | 14 (13) |
| Male castrated (%) | 16 (40) | 22 (32) | 38 (35) |
| Male intact (%) | 7 (18) | 8 (12) | 15 (14) |
BCS — body condition score. Data are median [range] or raw data (gender distribution).
A complete data set (first and second evaluations completed) was collected from 95 dogs (14 dogs did not receive a second evaluation: 9 euthanized, 1 in surgery, 3 sent home before evaluation could be performed, 1 aggressive).
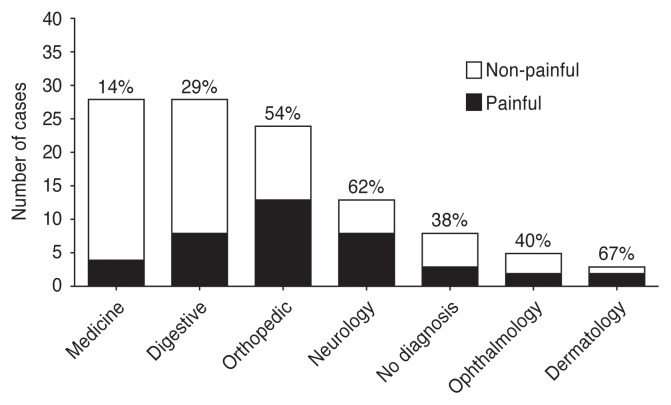
Approximately 1/3 of the dogs were identified as painful at the first evaluation following admission (40/109, 36.7%). More than half of the dogs presenting with dermatologic (67%), neurologic (62%), or orthopedic/trauma (54%) problems were painful (Figure 1) with the most common diagnoses being intervertebral disc disease (n = 6), bone fracture (n = 4), and skin lacerations (n = 4). Three of the 40 painful dogs were presented for euthanasia (CMPS-SF pain scores 8, 7, 7) and did not receive any analgesic treatment or a second evaluation, and 1 dog underwent surgery before the second evaluation could be performed. The remaining 36 painful dogs had second evaluations completed. Approximately half of these (19/36, 53%) received an analgesic treatment in the clinic, while 11/36 (31%) received an analgesic treatment for home administration and 6/36 (17%) did not receive an analgesic treatment (1 received an intramuscular corticosteroid injection). The most frequent treatments administered with the intention of providing pain relief were opioids, non-steroidal anti-inflammatory drugs, alpha-2 adrenergic receptor agonists, and gabapentin (Table 2).
Figure 1.
Diagnostic categories after examination of painful (black bars, n = 40) and non-painful (clear bars, n = 69) dogs admitted to the emergency department. Dogs with a pain scale score of ≥ 5/20 were identified as painful. No diagnosis includes the dogs that were euthanized. The percentage above each column represents the proportion of painful animals.
Table 2.
Treatments administered to painful animals (n = 37) admitted to the emergency department.
| Drugs | Number of cases | Administration route | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| IV | IM | SC | PO | VRI | Topical | |||
| Opioids | hydromorphone | 8 | 5 | 3 | ||||
| tramadol | 7 | 7 | ||||||
| butorphanol | 6 | 3 | 3 | |||||
| buprenorphine | 3 | 1 | 1 | 1 | ||||
| remifentanil | 1 | 1 | ||||||
| NSAID | meloxicam | 9 | 2 | 2 | 8 | |||
| robenacoxib | 1 | 1 | ||||||
| Alpha-2 | dexmedetomidine | 8 | 8 | |||||
| Cyclohexamine | ketamine | 1 | 1 | |||||
| Gabapentinoid | gabapentin | 6 | 6 | |||||
| Other | 4 | 4 | ||||||
Number of cases represents all treatments administered so that some animals are represented more than once, that is receiving a combination of agents or the same agent by different routes (e.g., meloxicam IV in the clinic, followed by an oral prescription). Other treatments were: ophthalmologic (proparacaine hydrochloride) and dermatologic (dexamethasone/neomycin/polymyxin-B or diethanolamine fusidate/framycetin sulphate/nystatin/prednisolone) treatments. IV — intravenous; IM — intramuscular; SC — subcutaneous; PO — per os (sublingual for buprenorphine); VRI — variable rate infusion; NSAID — non-steroidal anti-inflammatory drug; Alpha-2 — alpha-2 adrenergic receptor agonist.
Between the first and second evaluation, CMPS-SF pain scale scores decreased in 79% (n = 15/19) of dogs that received an analgesic within the clinic and 63% (n = 12/19) had scores decrease below the analgesic intervention threshold at the time of the second evaluation (Table 3). A small number of dogs (3/19) had an increase in CMPS-SF pain score over the course of the evaluations and 1 dog had no change in pain score. Sedation levels did not differ significantly between painful [1 (0, 6)] and non-painful [1 (0, 6)] animals at the first evaluation [P > 0.99, 95% confidence interval (CI): 0 to 0], but painful animals that received analgesia had significantly higher sedation scores [3 (0, 8)] compared to non-painful animals [1 (0, 5)] at the second evaluation (P = 0.0003, 95% CI: 1 to 4).
Table 3.
Treatment response [First Glasgow Composite Measure Pain Scale-Short Form (CMPS-SF) score — second CMPS-SF score] in dogs presented to the emergency service.
| Painfula dogs: in-clinic pain treatment (n = 19) (%) | Painful dogs: no pain treatment in clinicb (n = 17) (%) | Non-painful dogs (n = 59) (%) | |
|---|---|---|---|
| Number of dogs with decrease in pain score (%) | 15 (79) | 10 (59) | 12 (20) |
| Median [range] decrease in pain score | 4 [1 to 13] | 2 [1 to 3] | 2 [1 to 4] |
| Number of dogs with pain score decreasing below intervention threshold | 12 (63) | 3 (18) | — |
| Number of dogs with increase in pain score (%) | 3 (16) | 2 (12) | 15 (25) |
| Median [range] increase in pain score | 1 [1 to 5] | 3 [2 to 4] | 2 [1 to 6] |
| Number of dogs with pain score increasing above intervention threshold | — | — | 2 (3) |
| Number of dogs with no change in pain score (%) | 1 (5) | 5 (29) | 35 (54) |
Only dogs in which full evaluations were done (both pain assessments completed, n = 95) are presented. Data are median [range].
Classified according to the CMPS-SF analgesic intervention threshold (≥ 5/20).
Includes animals that did not receive any pain-relieving treatment and animals that were prescribed analgesics for administration at home.
Of the 17 painful dogs that did not receive analgesia during their time in the clinic, 10 had a decrease and 2 had an increase in CMPS-SF pain score between the first and second evaluations. Of those which had a reduction in pain score, 3 decreased below the analgesic intervention threshold (Table 3).
One quarter of dogs (15/59) initially classified as non-painful showed an increase in GCMPS-SF pain score, with scores exceeding the intervention threshold in 2 dogs (Table 3).
Colorado Canine Acute Pain Scale
The CCAPS and CMPS-SF scores were positively correlated at both the first [r = 0.81 (0.73 to 0.87), P < 0.0001] and the second [r = 0.69 (0.57 to 0.79), P < 0.0001] evaluations. However, the evolution (change between first and second evaluations) of CCAPS scores differed from that of CMPS-SF scores. Of the 25 painful dogs that had a reduction in CMPS-SF score at the second evaluation, 14 were evaluated as having no change in score according to the CCAPS scale (Table 4). Similarly, 5 dogs identified as having an increase in CMPS-SF score were identified as showing no change in CCAPS scale (Table 4). Similar discrepancies between the CMPS-SF and CCAPS were observed for dogs initially identified as non-painful (Table 5).
Table 4.
Glasgow Composite Measures Pain Scale — short form (CMPS-SF) pain scores evolution (change between first and second evaluations) and the corresponding Colorado Canine Acute Pain Scale (CCAPS) evolution for painful dogs (n = 36; CMPS-SF ≥ 5/20) admitted to the emergency service.
| CMPS-SF evolution | CCAPS evolution |
|---|---|
| Increased (n = 5) | 0 increased |
| 1 decreased | |
| 4 unchanged | |
| Decreased (n = 25) | 2 increased |
| 9 decreased | |
| 14 unchanged | |
| Unchanged (n = 6) | 0 increased |
| 0 decreased | |
| 6 unchanged |
Table 5.
Glasgow Composite Measures Pain Scale — short form (CMPS-SF) pain scores evolution (change between first and second evaluations) and the corresponding Colorado Canine Acute Pain Scale (CCAPS) for the non-painful animals (n = 59; CMPS-SF < 5/20) admitted to the emergency department.
| CMPS-SF evolution | CCAPS evolution |
|---|---|
| Increased (n = 15) | 2 increased |
| 0 decreased | |
| 13 unchanged | |
| Decreased (n = 12) | 0 increased |
| 2 decreased | |
| 10 unchanged | |
| Unchanged (n = 32) | 0 increased |
| 3 decreased | |
| 29 unchanged |
Patient handling time data
For the time period from admission to examination, data from 3 painful dogs were unavailable (2 dogs euthanized, 1 time not recorded) to give a sample size of 37 dogs. For the same period, in the non-painful dogs, data from 6 dogs were unavailable (4 animals euthanized, 2 times not recorded) to give a sample size of 63 dogs.
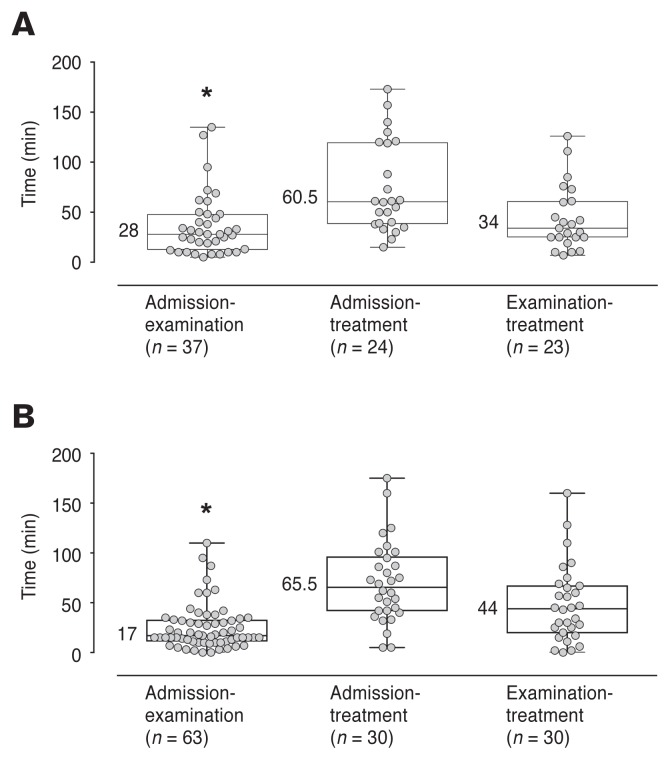
The overall time, from admission to treatment, did not differ between painful and non-painful groups (P = 0.96, 95% CI: –23 to 22, Figure 2A, B). The time from admission to examination by a veterinarian was significantly shorter in the non-painful than the painful animals (P = 0.04 95% CI: 0 to 16, Figure 2A, B), but there was no difference from the time of examination to time of treatment (P = 0.73, 95% CI: –14 to 20, Figure 2A, B). There was no significant difference in the number of active cases currently managed by the attending veterinarian between painful and non-painful dogs (P = 0.08, 95% CI: –1 to 0). The median time between the first and second evaluation by the investigator was 88 (18 to 270) min. The number of dogs evaluated within 30 min of initial treatment was 8/95. None of these dogs were in the painful group.
Figure 2.
Time elapsed for dogs which were presented to the emergency department and assessed as painful (A) or non-painful (B) between admission and examination by a veterinarian, between admission and initial treatment, and between examination and initial treatment. Box plots show the median, interquartile range (box limits), and range (whiskers). Numbers indicate median time (min). The number of dogs decreased for both admission to treatment and examination to treatment since not all dogs received a treatment in clinics. Asterisks (*) represent a significant difference between painful and non-painful groups (P = 0.04).
Discussion
In the present study, approximately 1/3 of dogs presenting to the emergency service exceeded the intervention threshold on the CMPS-SF (≥ 5/20) and were classified as painful. This is similar to the findings of 2 previous studies in a veterinary teaching hospital, in which the proportions of dogs identified as painful were 20% (outpatient population) and 56% (emergency service population) (12,18). A direct comparison between studies, particularly with regard to changes in level of pain, is limited by the substantial differences in the pain assessment scales that were used. The current study used a published validated pain scale that includes an analgesic intervention threshold to identify pain and guide clinical decision-making. In contrast to the scale used by Wiese et al (12), the CMPS-SF was developed using psychometric principles (10,19) in a mixed hospital population. This approach, applied to a clinical population of dogs with diverse sources of pain, encompasses an established process of item selection, questionnaire construction, testing for validity, reliability, and sensitivity, and derivation of an analgesic intervention threshold in a clinical population (10,11) Therefore, it is likely that the CMPS-SF confers greater accuracy and reliability in pain assessment. Unfortunately, the CMPS-SF was not available at the time of the previous study. Notwithstanding this difference, the causes of pain reported here shared similarities with those reported by Wiese et al (12) for dogs presenting to an emergency service.
A validated pain scale confers the ability to accurately and precisely track changes in pain levels over time, allowing evaluation of pain control. In most cases, the provision of analgesia in the clinic was effective in decreasing pain to a level below the intervention threshold. This rapid efficacy as a result of providing in-clinic pain relief was highlighted in dogs in which pain was identified using the CMPS-SF but analgesia was not provided during the study observation period. A smaller proportion of these dogs had pain scores fall below the intervention threshold while a larger proportion remained painful. The dynamic nature of disease and pain is highlighted by the number of dogs in which pain scores increased between evaluations. This observation emphasizes the importance of regular pain assessment in patients, including those that have been given analgesics.
Encouragingly, despite structured pain assessment (with a pain scale) not being routinely performed on this population at this veterinary teaching hospital, the majority of painful dogs received analgesic treatment, either in the hospital or with at home medication. These findings demonstrate a significantly higher percentage of analgesic therapy than previously reported in the veterinary literature, with approximately 66% use of analgesics in the emergency department (12) and 61% in the perioperative period for elective surgical procedures (20). Nevertheless, it is a concern that a small number of dogs were not given analgesia despite the presence of identifiable pain. This may reflect a lack of adequate recognition of pain or a reluctance to provide analgesia, both of which have been previously identified as barriers to analgesic therapy, with multiple underlying causes, including: lack of pain scores being incorporated into triage assessment, knowledge and attitude towards pain and the potential for gender or cultural bias, difficulty in assessing pain in this population, perceived side effects with analgesic usage, and concerns with the masking of clinical signs (3–5,7,21). Importantly, many clinical and experimental studies have noted that the deleterious effects of pain outweigh perceived or possible adverse effects from the use of analgesic medications (22–25). Pain assessment and management can no longer be perceived as of secondary importance in the acute care setting. The prevalence of painful conditions reported here and in the literature underlines the value and importance of pain assessment and management (12,18). It should be a goal to provide effective analgesia to all painful patients presenting to an emergency service.
A secondary aim of this study was to compare the performance of the CCAPS alongside the CMPS-SF. The differences in the progression of pain identified using these scales points to weaknesses in evaluating and managing pain using an unvalidated pain scale. Additionally, the existence of an analgesic intervention threshold for the CMPS-SF provides a valuable guide for analgesic management in the use of this scale. While the scale developers do not claim that the CCAPS is validated, its ready availability and apparent simplicity make its use appealing (21). It is likely, though currently unconfirmed, that the limited number of scale items and combination of different behaviors within items adversely affect its discriminatory capacity and reliability (26).
Available validated pain assessment scales in dogs and cats include evaluation of behavioral responses and human-animal interaction. Therefore, the presence of sedation or behaviors affecting interaction (e.g., aggression) are potential confounding factors in the application of these scales (10,11,27–30). Sedation as a potential confounding factor was accounted for during development of the CMPS-SF by not including dogs that were sedated or otherwise recovering from the effects of anesthesia in the study population (11). In contrast to feline studies, the impact of sedation or behaviors affecting interaction with observers on pain assessment in a clinical canine population is unknown (27–29). The concurrent application of a sedation scale in this study limited the effects of this potential confounder at the first evaluation; however, the threshold to identify sedation was selected based on an investigator’s (DJSP) experience and has not been formally derived. The greater level of sedation observed in dogs following analgesic administration is predictable, as an effect of the agents used, and this may have impeded a behavioral response during pain assessment.
The period from admission to treatment encompasses early steps in the patient care pathway (admission at reception, initial evaluation and triage, examination by a clinician, and initiation of diagnosis and treatment) and reflects any delay in providing analgesia to a painful patient. The absence of significant difference in this period between dogs identified as painful and non-painful is a concern. The initial triage of all emergency admissions, occurring immediately following admission, represents a time when pain could be evaluated early; however, triage is performed by more junior staff or students and pain evaluation may not have been sufficiently emphasized in their assessment of the animal. A similar pattern was apparent following examination by a veterinarian, when the time to treatment in painful dogs did not differ from that for non-painful dogs. Surprisingly, the time from admission to examination was significantly longer in the painful group. The reasons for this are unclear and require further investigation. This is also an area of concern in human medicine, particularly in the emergency department, where adequate and timely pain management remains a challenge despite policies to meet time targets for analgesic administration (31,32). These delays indicate student and veterinary awareness of pain assessment and management should be a target for education (21).
Limitations of the study include a lack of blinding and variable assessment time for the second evaluation. The investigators were not blinded to treatment during the second pain and sedation evaluation. With the limited number of study personnel and the desire to not unduly influence case management by the ER service, it was necessary for the observers to collect treatment information. Therefore, though a risk of bias is present, this was limited to the second evaluation and did not affect the results of the first pain and sedation evaluation (and consequent estimate of pain prevalence) or the time data. Furthermore, the CMPS-SF was developed to assess pain and guide analgesic treatments in a clinical setting, such as that encountered here. Ideally, the timing of the second evaluation should have been tailored to the predicted onset of action of the analgesics administered. This was not controlled to maximize case collection and this limitation has minimal impact on the data as most cases in which the second evaluation was performed within 30 min of the first did not receive any analgesics. No conclusions can be drawn about pain in seriously ill dogs as this population was excluded from the study.
Recognition and assessment of pain is an integral part of veterinary clinical practice. This has been emphasized more in the last 20 to 25 years due to client expectations for pain relief in their pets, increased public awareness of issues surrounding the use of animals in biomedical research, and the veterinary clinical communities prioritizing the importance of pain control in clinical practice (2,3,33). In the present study, a significant percentage (36%) of the dogs presenting to the emergency service had signs consistent with pain above an interventional threshold. Regardless of the important implications of animal welfare and the veterinarian’s responsibility to relieve animal suffering (15), the prompt recognition and treatment of acute pain can influence many physiological factors: acute neurohormonal changes, production of inflammatory cytokines, reduction of systemic stress, improving hemodynamic stability (22,23), prevention of postoperative complications (24,34) and prevention of chronic pain syndromes (35,36). Given the prevalence of painful conditions presenting to an emergency service analgesic therapy should be an integral aspect of therapy.
The timely identification and treatment of pain is a challenge in veterinary emergency medicine. Applying a validated pain assessment scale should form an integral part of patient triage and pain management. The use of analgesics remains suboptimal, suggesting that focused training in pain assessment and analgesic use is warranted.
Acknowledgments
The authors thank Dr. Guy Beauchamp for statistical support. DSJP receives funding from the Natural Sciences and Engineering Research Council of Canada (Discovery Grant 424022-2013) and the Fondation J-Louis Lévesque. Funders had no role in study design, data collection and analysis, or decision to publish. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Tompkins DA, Hobelmann JG, Compton P. Providing chronic pain management in the “Fifth Vital Sign” era: Historical and treatment perspectives on a modern-day medical dilemma. Drug Alcohol Depend. 2017;173:S11–S21. doi: 10.1016/j.drugalcdep.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beswick A, Dewey C, Johnson R, Dowsett-Cooper J, Niel L. Survey of Ontario veterinarians’ knowledge and attitudes on pain in dogs and cats in 2012. Can Vet J. 2016;57:1274–1280. [PMC free article] [PubMed] [Google Scholar]
- 3.Hugonnard M, Leblond A, Keroack S, Cadoré JL, Troncy E. Attitudes and concerns of French veterinarians towards pain and analgesia in dogs and cats. Vet Anaesth Analg. 2004;31:154–163. doi: 10.1111/j.1467-2987.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 4.Hewson CJ, Dohoo IR, Lemke KA. Factors affecting the use of post-incisional analgesics in dogs and cats by Canadian veterinarians in 2001. Can Vet J. 2006;47:453–459. [PMC free article] [PubMed] [Google Scholar]
- 5.Joubert KE. The use of analgesic drugs by South African veterinarians. J S Afr Vet Assoc. 2001;72:57–60. doi: 10.4102/jsava.v72i1.613. [DOI] [PubMed] [Google Scholar]
- 6.Lascelles BDX, Capner CA, Waterman-Pearson AE. Current British veterinary attitudes to perioperative analgesia for cats and small mammals. Vet Rec. 1999;145:601–604. doi: 10.1136/vr.145.4.95. [DOI] [PubMed] [Google Scholar]
- 7.Watson AD, Nicholson A, Church DB, Pearson MR. Use of anti-inflammatory and analgesic drugs in dogs and cats. Aust Vet J. 1996;74:203–210. doi: 10.1111/j.1751-0813.1996.tb15405.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams VM, Lascelles BDX, Robson MC. Current attitudes to, and use of, peri-operative analgesia in dogs and cats by veterinarians in New Zealand. N Z Vet J. 2005;53:193–202. doi: 10.1080/00480169.2005.36504. [DOI] [PubMed] [Google Scholar]
- 9.Joubert KE. Anaesthesia and analgesia for dogs and cats in South Africa undergoing sterilisation and with osteoarthritis — An update from 2000. J S Afr Vet Assoc. 2006;77:224–228. doi: 10.4102/jsava.v77i4.383. [DOI] [PubMed] [Google Scholar]
- 10.Morton CM, Reid J, Scott EM, Holton LL, Nolan AM. Application of a scaling model to establish and validate an interval level pain scale for assessment of acute pain in dogs. Am J Vet Res. 2005;66:2154–2166. doi: 10.2460/ajvr.2005.66.2154. [DOI] [PubMed] [Google Scholar]
- 11.Reid J, Nolan AM, Hughes JML, Lascelles D, Pawson P, Scott EM. Development of the short-form Glasgow Composite Measure Pain Scale (CMPS-SF) and derivation of an analgesic intervention score. Anim Welf. 2007;16:97–104. [Google Scholar]
- 12.Wiese AJ, Muir WW, Wittum TE. Characteristics of pain and response to analgesic treatment in dogs and cats examined at a veterinary teaching hospital emergency service. J Am Vet Med Assoc. 2005;226:2004–2009. doi: 10.2460/javma.2005.226.2004. [DOI] [PubMed] [Google Scholar]
- 13.Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: An integrative overview of research on mechanisms. J Pain. 2017;18:359.e1–359.e38. doi: 10.1016/j.jpain.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Martell-Moran NK, Solano M, Townsend HG. Pain and adverse behavior in declawed cats. J Feline Med Surg. 2017;20:280–288. doi: 10.1177/1098612X17705044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellyer P, Rodan I, Brunt J, Downing R. AAHA/AAFP pain management guidelines for dogs and cats. J Feline Med Surg. 2007;9:466–480. doi: 10.1016/j.jfms.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner MC, Hecker KG, Pang DSJ. Sedation levels in dogs: A validation study. BMC Vet Res. 2017;13:1–8. doi: 10.1186/s12917-017-1027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang DSJ. ER pain prevalence in dogs in an academic teaching hospital. Harvard dataverse V1. 2018 [Google Scholar]
- 18.Muir WW, Wiese AJ, Wittum TE. Prevalence and characteristics of pain in dogs and cats examined as outpatients at a veterinary teaching hospital. J Am Vet Med Assoc. 2004;224:1459–1463. doi: 10.2460/javma.2004.224.1459. [DOI] [PubMed] [Google Scholar]
- 19.Holton L, Pawson P, Nolan A, Reid J, Scott EM. Development of a behaviour-based scale to measure acute pain in dogs. Vet Rec. 2001;148:525–531. doi: 10.1136/vr.148.17.525. [DOI] [PubMed] [Google Scholar]
- 20.Reimann J, Dewey C, Bateman SW, Kerr C, Johnson R. Perioperative analgesic use by Ontario veterinarians, 2012. Can Vet J. 2017;58:149–156. [PMC free article] [PubMed] [Google Scholar]
- 21.Mich PM, Hellyer PW, Kogan L, Schoenfeld-Tacher R. Effects of a pilot training program on veterinary students’ pain knowledge, attitude, and assessment skills. J Vet Med Educ. 2010;37:358–368. doi: 10.3138/jvme.37.4.358. [DOI] [PubMed] [Google Scholar]
- 22.Haskins SC, Copland VS, Patz JD. The cardiopulmonary effects of oxymorphone in hypovolemic dogs. J Vet Emerg Crit Care. 1991;1:32–38. [Google Scholar]
- 23.Foëx BA, Kirkman E, Little RA. Injury (nociceptive afferent nerve stimulation) modifies the hemodynamic and metabolic responses to hemorrhage in immature swine. Crit Care Med. 2004;32:740–746. doi: 10.1097/01.ccm.0000117320.69308.e5. [DOI] [PubMed] [Google Scholar]
- 24.Sinatra R. Causes and consequences of inadequate management of acute pain. Pain Med. 2010;11:1859–1871. doi: 10.1111/j.1526-4637.2010.00983.x. [DOI] [PubMed] [Google Scholar]
- 25.Siddall PJ, Cousins MJ. Persistent pain as a disease entity: Implications for clinical management. Anesth Analg. 2004;99:510–520. doi: 10.1213/01.ANE.0000133383.17666.3A. [DOI] [PubMed] [Google Scholar]
- 26.Streiner DL, Norman GR, Cairney J. Health Measurement Scales: A Practical Guide to their Development and Use. 5th ed. New York, New York: Oxford University Press; 2015. [Google Scholar]
- 27.Buisman M, Wagner MC, Hasiuk MM, Prebble M, Law L, Pang DS. Effects of ketamine and alfaxalone on application of a feline pain assessment scale. J Feline Med Surg. 2016;18:643–651. doi: 10.1177/1098612X15591590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buisman M, Hasiuk MMM, Gunn M, Pang DSJ. The influence of demeanor on scores from two validated feline pain assessment scales during the perioperative period. Vet Anaesth Analg. 2017;44:646–655. doi: 10.1016/j.vaa.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong T, Wagner MC, Cheema J, Pang DS. Assessing analgesia equivalence and appetite following alfaxalone- or ketamine-based injectable anesthesia for feline castration as an example of enhanced recovery after surgery. J Feline Med Surg. 2018;20:73–82. doi: 10.1177/1098612X17693517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calvo G, Holden E, Reid J, et al. Development of a behaviour-based measurement tool with defined intervention level for assessing acute pain in cats. J Small Anim Pract. 2014;55:622–629. doi: 10.1111/jsap.12280. [DOI] [PubMed] [Google Scholar]
- 31.Patrick PA, Rosenthal BM, Iezzi CA, Brand DA. Timely pain management in the emergency department. J Emerg Med. 2015;48:267–273. doi: 10.1016/j.jemermed.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Chang HL, Jung JH, Kwak YH, et al. Quality improvement activity for improving pain management in acute extremity injuries in the emergency department. Clin Exp Emerg Med. 2018;5:51–59. doi: 10.15441/ceem.17.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen BD. Assessment of pain in dogs: Veterinary clinical studies. ILAR J. 2003;44:197–205. doi: 10.1093/ilar.44.3.197. [DOI] [PubMed] [Google Scholar]
- 34.Dunwoody CJ, Krenzischek DA, Pasero C, Rathmell JP, Polomano RC. Assessment, physiological monitoring, and consequences of inadequately treated acute pain. J Perianesth Nurs. 2008;23:S15–S27. doi: 10.1016/j.jopan.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Pergolizzi JV, Jr, Raffa RB, Taylor R., Jr Review Article treating acute pain in light of the chronification of pain. Pain Manag Nurs. 2014;15:380–390. doi: 10.1016/j.pmn.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Cousins MJ. Persistent pain: A disease entity. J Pain Symptom Manag. 2007;33:S4–S10. [Google Scholar]