Abstract
Background:
The human body contains numerous long-lived proteins which deteriorate with age, typically by racemisation, deamidation, crosslinking and truncation. Previously we elucidated one reaction responsible for age-related crosslinking, the spontaneous formation of dehydroalanine (DHA) intermediates from phosphoserine and cysteine. This resulted in non-disulphide covalent crosslinks. This paper outlines a novel posttranslational modification (PTM) in human proteins, which involves the addition of dehydroalanylglycine (DHAGly) to Lys residues.
Methods:
Human lens digests were examined by mass spectrometry for the presence of (DHA)Gly (+144.0535 Da) adducts to Lys residues. Peptide model studies were undertaken to understand the mechanism of formation.
Results:
In the lens, this PTM was detected at 18 lysine sites in 7 proteins. Using model peptides, a pathway for its formation was found to involve initial formation of the glutathione degradation product, γ-Glu(DHA)Gly from oxidised glutathione (GSSG). Once the Lys adduct formed, the Glu residue was lost in a hydrolytic mechanism apparently catalysed by the ε-amino group of the Lys.
Conclusions:
This discovery suggests that within cells, the functional groups of amino acids in proteins may be susceptible to modification by reactive metabolites derived from GSSG.
General Significance:
Our finding demonstrates a novel + 144.0535 Da PTM arising from the breakdown of oxidised glutathione.
Keywords: Post-translational modifications, Aging, Dehydroalanine, Crosslinking, Glutathione, Human aging, Protein crosslinks, glutathione, Long-lived proteins
Introduction:
The human body contains numerous long-lived proteins, that are present in tissues such the brain, eye, heart, skin and muscle [1]. Due to their longevity, long-lived proteins undergo a range of spontaneous age-related modifications including racemisation/isomerisation, deamidation, crosslinking and peptide bond cleavage [2–5]. In previous studies we showed that breakdown of phosphoSer and Cys residues by β-elimination resulted in the formation of the reactive intermediate dehydroalanine (DHA) in human lens proteins [5]. Since DHA is an electrophile, we observed the formation of covalent non-disulphide crosslinks of crystallins and crystallin-GSH modifications via thioether and lysinoalanine linkages.
In the lens, the tripeptide glutathione (γ-glutamyl-cyteinylglycine) is the major antioxidant, present at some of the highest levels in human tissue in concentrations between 2–20mM [6]. As the lens is avascular, and contains no organelles in mature fiber cells, GSH levels in the lens are maintained by synthesis and recycling of glutathione disulphide (GSSG) in the metabolically-active outer part of the lens. This requires GSH to diffuse freely into the centre of the lens and for GSSG to diffuse back out. At middle age, when a barrier to GSH and GSSG diffusion develops in the normal human lens [7], GSH is no longer able to circulate freely and GSSG becomes trapped in the nucleus. Recent experiments showed that disulphide bond formation was a prerequisite for DHA formation in peptides via β-elimination [8]. On that basis, we hypothesized that GSSG may also break down to form γ-glutamyldehydroalanylglycine (γ-Glu(DHA)Gly) in cells. If this were true, it may lead to crosslinking of γ-Glu(DHA)Gly with protein sulfhydryl and amino groups. As part of an ongoing study of crosslinking in the human lens, evidence for this process was investigated using mass spectrometry and model peptide studies. During this investigation, evidence for modification by(DHA)Gly was found on both lysine and cysteine residues.
Materials and Methods
Digestion of human lens protein and LC-MS/MS analysis
Frozen human lenses were obtained from NDRI (Philadelphia, PA). Detailed information about the donors is listed in Supplemental Table 1. Human lenses were homogenized in homogenizing buffer (25 mM Tris, 150 mM NaCl, 5 mM EDTA, 1 mM PMSF, pH 7.4). The samples were fractionated as Water soluble fraction (WSF), Urea Soluble fraction (USF) and urea insoluble fraction (UIF) as described previously [9]. WSF, USF and UIF from 22, 53 and 50 year-old lenses and UIF from 37 and 58 year-old lenses were analyzed independently. Proteins in each fraction were reduced and alkylated and digested as previously described (5). Tryptic peptides from 22 year-old, 53 year-old and 50 year-old lenses were separated by one-dimensional HPLC using a fused silica capillary column (200 mm × 100 μm) packed with Phenomenex Jupiter resin (3 μm mean particle size, 300 Å pore size) coupled with an Easy-nLC system (ThermoFisher Scientific, San Jose, CA). A 70-minute gradient was performed, consisting of the following: 0–60 min, 2–45% ACN (0.1% formic acid); 60–70 min, 45–95% ACN (0.1% formic acid) balanced with 0.1% formic acid. The eluate was directly infused into a Q Exactive instrument (ThermoFisher Scientific, San Jose, CA) equipped with a nanoelectrospray ionization source. The data-dependent instrument method consisted of MS1 acquisition (R=70,000), using an MS AGC target value of 1e6, followed by up to 15 MS/MS scans (R=17,500) of the most abundant ions detected in the preceding MS scan. The MS2 AGC target value was set to 2e5 ions, with a maximum ion time of 200ms, and a 5% underfill ratio, and intensity threshold of 5e4. HCD collision energy was set to 27, dynamic exclusion was set to 5s, and peptide match and isotope exclusion were enabled. The digests from UIF samples from 37 year-old and 58 year-old lenses were analyzed by multidimensional protein identification technology as described previously (5). The eluate from the analytical column was directly electrosprayed into a Velos Orbitrap mass spectrometer (ThermoFisher Scientific, San Jose, CA). The instrument was operated in a 15-step data dependent mode with one precursor scan event (Orbitrap scan) to identify the top 14 most abundant ions in each MS scan, which were subsequently selected for fragmentation in an MS/MS scan in the ion trap. Dynamic exclusion (repeat count 2, exclusion list size 300, and exclusion duration 60s) was enabled to allow detection of low abundance ions.
Data Analysis:
Tandem mass spectra were analyzed using a suite of custom-developed bioinformatics tools. All MS/MS spectra were converted to mzML files by Scansifter, a tool under development at Vanderbilt University Medical Center and searched on a 2500 node Linux cluster supercomputer using a custom version of the TagRecon algorithm against a concatenated forward and reversed (decoy) Uniprot human database (Nov 14, 2016). [10]. Trypsin specificity was used with a maximum of two missed cleavage sites. A variable modification of carbamidomethylation of cysteine, oxidation of methionine and deamination of asparagine was used. Variable modifications of +144.0535 Da ((DHA)Gly) and +273.0961 Da ((γ-Glu(DHA)Gly) on lysine and cysteine residues were searched. The search results were filtered by IDPicker by controlling protein FDR to less than 1%. All of modified peptides reported in this paper were manually verified.
Preparation of γ-Glu(DHA)Gly and phenylethylamine crosslink
γ-Glu(DHA)Gly was prepared as described by Sokolovsk et al. with minor modifications [11].GSH (5mg/mL) was dissolved in 50mM citrate buffer pH 5.5. A 1:1 molar ratio of 1-fluoro-2,4-dinitrobenzene (Sigma-Aldrich) was added and placed on a shaker for 1h. The resultant GSH dinitrobenzene adduct was purified by HPLC and freeze dried. γ-Glu(DHA)Gly was generated by dissolving the dinitrobenzene adduct in 0.5M NaOH and incubating at room temperature for 1 hour in the presence of O2. γ-Glu(DHA)Gly was purified by HPLC and freeze dried and stored at −20°C.
To generate the γ-Glu(DHA)Gly phenylethylamine (PE) crosslink, γ-Glu(DHA)Gly (1mg/mL) was dissolved in 0.5M NaOH together with a 5 molar excess of PE. Samples were incubated at 60°C for 2hs. The γ-Glu(DHA)Gly PE crosslink was purified by HPLC and its structure confirmed by MS/MS.
GSH Model studies
The γ-Glu(DHA)Gly PE crosslink and S-Me-GSH (Sigma Aldrich) were incubated separately in 100mM phosphate pH 7.0 at 37°C with a drop of chloroform added to prevent bacterial growth. Aliquots were taken daily and the loss of gamma Glu and formation of (DHA)Gly PE and S-Me-CysGly monitored by RP-HPLC (Aeris Peptide 2.6u XB-C18, Phenomenex, Torrance, CA). A 31 minute gradient was used, consisting of the following: 0–3 min, 0% B; 3–15 min, 40% B; 15–20 min, 80% B; 20–25 min, 80% B; 25–31, 0% B (Solvent A 1% ACN, 0.1% TFA; Solvent B 100 ACN, 0.1% TFA). Elution was monitored at 216nm. (DHA)Gly PE crosslink and S-Me-CysGly were freeze-dried and analyzed using a LTQ mass spectrometer (ThermoFisher Scientific, San Jose, CA).
CysGly disulphide (Sigma Aldrich) was incubated in 100mM phosphate pH 7.0 at 37°C with a drop of chloroform. Aliquots were taken daily and (DHA)Gly was monitored by HPLC and mass spectrometry.
Results:
Identification of GSH adducts in the human lens
Since disulphide bond formation was found to be a prerequisite for DHA formation from Cys residues in proteins [8], it was hypothesized that GSSG may also break down to form γ-glutamyldehydroalanylglycine (γ-Glu(DHA)Gly) in the lens. If this reactive intermediate added to protein Cys residues, the thioether product would be indistinguishable from that formed by the addition of GSH to a DHA residue on the protein. This approach would therefore not permit an unambiguous evidence of γ-Glu(DHA)Gly formation in cells. On the other hand addition of γ-Glu(DHA)Gly to Lys residues would form a structure that could form only via this intermediate.
Preliminary studies showed that when N-acetyl Lys was incubated with γ-Glu(DHA)Gly, the major product detected was N-acetyl Lys - (DHA)Gly, where the Glu residue of the tripeptide had been lost. This finding was consistent with other studies where the γ-glutamyl bond of GSH has been shown to be labile [12].
Human lens membrane fraction digests were therefore examined by mass spectrometry for the presence of γ-Glu(DHA)Gly (+273.0961 m/z) and (DHA)Gly (+144.0535 m/z) adducts to Lys residues. No evidence of γ-Glu(DHA)Gly crosslinks to Lys were detected, however the addition of +144.0535 (DHA)Gly at a number of Lys sites was observed, and this was confirmed by tandem mass spectrometry (see Supplementary materials). A representative tandem mass spectrum highlighting the addition of (DHA)Gly to Lys 14 in γS crystallin is shown in Fig. 1.
Fig. 1.
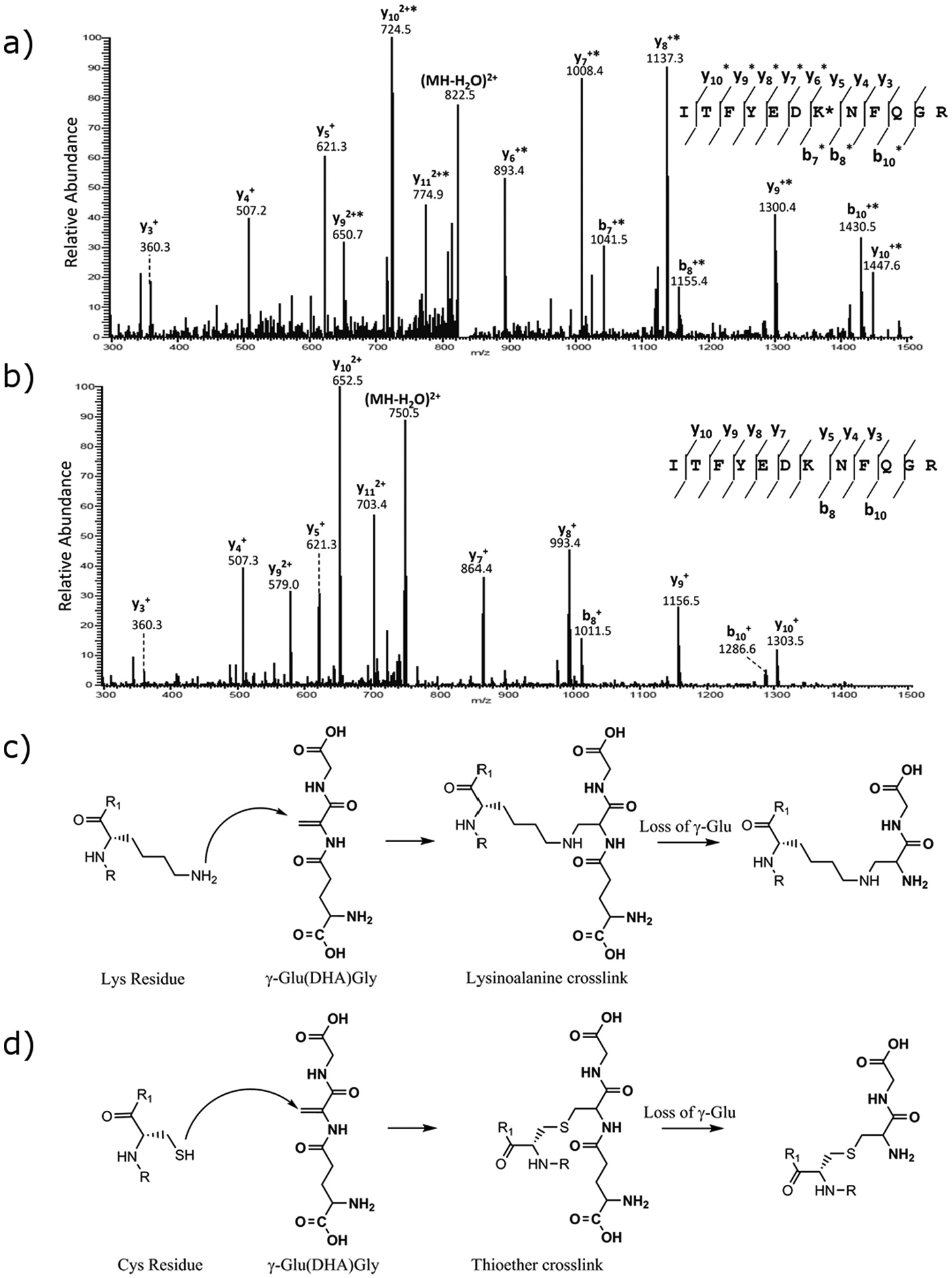
a) MS/MS spectrum of human lens γS-crystallin peptide containing residues 8–19 confirming the +144 Da adduct on Lys14. b) MS/MS spectrum of human lens γS-crystallin peptide containing residues 8–19 unmodified. c) A scheme illustrating formation of (DHA)Gly adduct via a lysinoalanine crosslink. d) A scheme illustrating formation of (DHA)Gly adduct via a thioether crosslink.
In total, 379 proteins were identified in three lenses analyzed by 1D-LC-MS/MS on a Q Exactive instrument and these proteins are listed in the Supplemental Table 3. 834 proteins were identified in the MudPIT analysis of UIF of two normal lenses and these proteins are listed in Supplemental Table 4. In total 18 sites on 7 lens proteins (γS-crystallin, βB1-crystallin, βB2-crystallin, βA3-crystallin, βA4-crystallin, αA-crystallin, αB-crystallin) were found that were modified by (DHA)Gly on Lys residues (Table 1a). Of particular interest, γS-crystallin was modified at 5 of a possible 10 Lys residues. When mapped onto the crystal structure of γS-crystallin (see Fig 2), it was apparent that most adducts were observed in the unstructured regions of the protein. γ-Glu(DHA)Gly and (DHA)Gly modification was also detected on Cys residues on 6 sites in 6 lens proteins (Table 1b).
Table 1a:
Lys residues of lens proteins that are modified by (DHA)Gly.
| Protein | Site | Peptide | [MH]+exp | [MH]+Cal | Error (ppm) |
|---|---|---|---|---|---|
| γS-Crystallin | K7 | TGTK*ITFYEDKNFQGR | 2049.0013 | 2049.0036 | 1.14 |
| K14 | ITFYEDK*NFQGR | 1661.7863 | 1661.7918 | 3.31 | |
| K95 | AVHLPSGGQYK*IQIFEK | 2059.0943 | 2059.0971 | 1.35 | |
| K144 | QYLLDK*KEYR | 1499.7803 | 1499.7853 | 3.34 | |
| K159 | K*PIDWGAASPAVQSFR | 1873.9523 | 1873.9555 | 1.70 | |
| βB1-Crystallin | K118 | GEMFILEK*GEYPR | 1712.8263 | 1712.8312 | 2.86 |
| K187 | VGSVK*VSSGTWVGYQYPGYR | 2334.1473 | 2334.1513 | 1.71 | |
| βB2-Crystallin | K120 | IILYENPNFTGK*K | 1680.8893 | 1680.8956 | 3.75 |
| βA4-Crystallin | K118 | LTIFEQENFLGK*K | 1710.8993 | 1710.9061 | 3.97 |
| βA3-Crystallin | K44 | ITIYDQENFQGK*R | 1755.8583 | 1755.8660 | 4.37 |
| K131 | MTIFEK*ENFIGR | 1628.8023 | 1628.8101 | 4.79 | |
| αB-Crystallin | K90 | HFSPEELK*VK | 1357.7063 | 1357.7111 | 3.53 |
| K92 | VK*VLGDVIEVHGK | 1536.8683 | 1536.8744 | 3.96 | |
| K174 | EEKPAVTAAPK*K | 1412.7674 | 1412.7744 | 4.95 | |
| αA-Crystallin | K70 | SDRDK*FVIFLDVK | 1725.9143 | 1725.9170 | 1.55 |
| K78 | FVIFLDVK*HFSPEDLTVK | 2278.2063 | 2278.2118 | 2.41 | |
| K88 | HFSPEDLTVK*VQDDFVEIHGK | 2584.2623 | 2584.2678 | 2.12 | |
| K99 | VQDDFVEIHGK*HNER | 1966.9303 | 1966.9366 | 3.19 |
Sites were detected by searching tryptic digests of human lenses by LC-MS/MS for sites of missed cleavage that contained + 144.0535 Da on lysine residues.
Corresponds to site of modification.
Fig. 2.
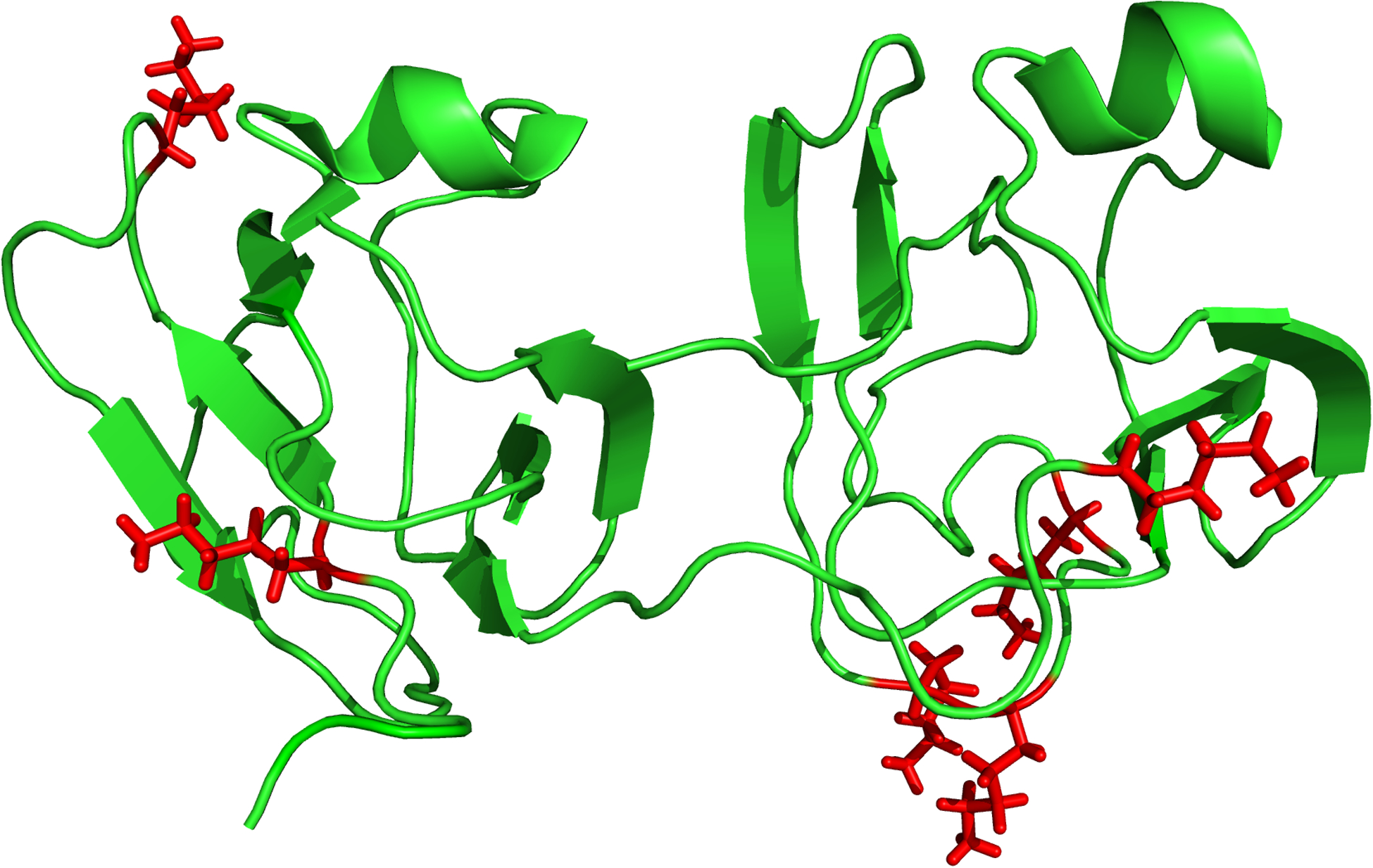
The structure of human gamma S crystallin derived from NMR data, where residue Gly18 was mutated to Val (PDBe 2M3U). Four of the five sites of attachment of DHAGly to Lys that are highlighted (red), occur within unstructured regions with the other site located at the boundary of a structured and unstructured region
Table 1b:
Cys residues of lens proteins that are modified by (DHA)Gly.
| Protein | Site | Peptide | [MH]+exp | [MH]+Cal | Error (ppm) |
|---|---|---|---|---|---|
| βB1-Crystallin | C80 | RAEFSGEC*SNLADR | 1698.7533 | 1698.7500 | 1.95 |
| βB2-Crystallin | C67 | AGSVLVQAGPWVGYEQANC*KGEQFVFEK | 3185.5373 | 3185.5361 | 0.38 |
| βA4-Crystallin | C5 | Ac-TLQC*TK | 879.4243 | 879.4241 | 0.186 |
| βA3-Crystallin | FC*GQQFILER | 1384.6659 | 1384.6678 | 1.34 | |
| γ-crystallin C | C80 | SCC*LIPQTVSHR | 1544.7291 | 1544.7308 | 1.07 |
| Phakinin | C65 | APGVYVGTAPSGC*IGGLGAR | 1946.9732 | 1946.9753 | 1.10 |
Sites were detected by searching tryptic digests of human lenses by LC-MS/MS for sites of missed cleavage that contained + 144.0535 Da on cysteine residues.
Corresponds to site of modification. It should be noted that +273.0961 Da modification corresponding to γ-Glu(DHA)Gly were also observed as these sites.
GSH model studies
To investigate why only (DHA)Gly Lys adducts, and not γ-Glu(DHA)Gly adducts, were detected in the lens, peptide model studies were undertaken. Previous studies have shown the γ-glutamyl bond of GSH to be labile under physiological conditions [5, 12]. The effect of a thioether crosslink compared to a lysinoalanine crosslink on the stability of this bond was examined, S-Me-GSH and γ-Glu(DHA)Gly crosslinked to phenylethylamine, (a lysine mimic), were incubated at physiological pH, and the loss of the Glu was monitored by HPLC. As seen in Fig 3, the effect of the amine linkage on the stability of γ-glutamyl bond was clear; after two days of incubation at 37°C pH 7.0, more than 2% of the bond had been cleaved. By comparison, cleavage of the γ-glutamyl bond in S-Me-GSH was less than 0.1%. In effect, the N-linked γ-Glu(DHA)Gly was ~20 fold less stable than the corresponding S-linked species.
Fig. 3.
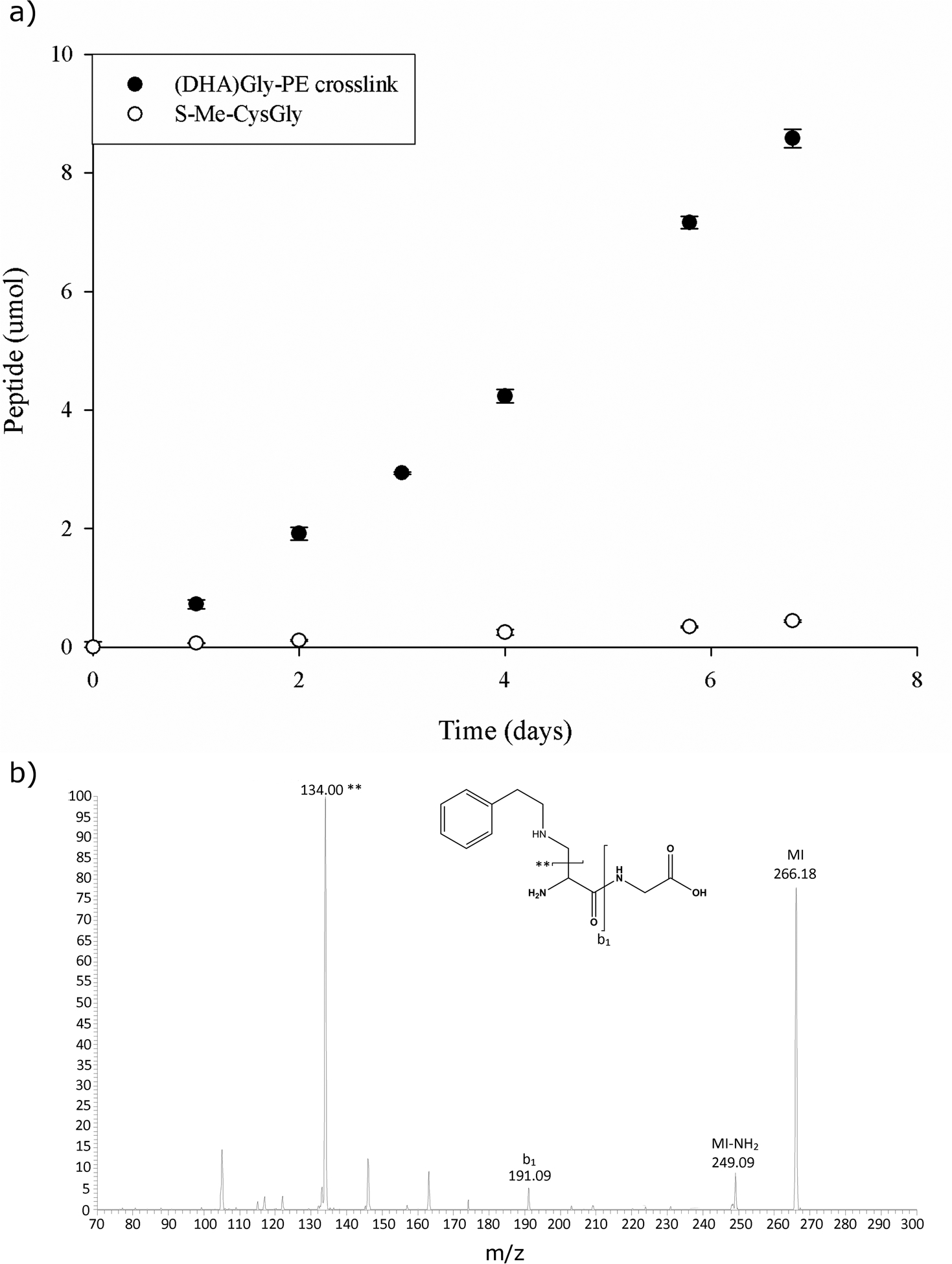
Cleavage of the γ-glutamyl bond in N- and S-linked glutathione adducts under physiological conditions. a) Loss of Glu from S-Me-GSH (open circle) and loss of Glu from γ-Glu(DHA)Gly PE crosslink (closed circle) both incubated at pH 7.0, 37°C. The loss of Glu was monitored at 216nm by HLPC. b) Mass spectrum of the (DHA)Gly - PE product generated after incubation of γ-Glu(DHA)Gly - PE. MI = molecular ion.
Other potential sources of (DHA)Gly
Literature data [11, 12] show that γ-Glu(DHA)Gly can be formed readily by incubation of GSSG at alkaline pHs. The possibility that the (DHA)Gly adduct could be generated directly from CysGly disulphide at neutral pH was also examined. CysGly disulphide was incubated at pH 7 at both 37°C and 60°C, however no (DHA)Gly was detected by HPLC. In a second series of experiments, attempts were made to synthesize (DHA)Gly using the same conditions used to produce γ-Glu(DHA)Gly from GSSG (see Methods section) and the mixture was incubated with a 10-fold excess of PE. No PE crosslink was detected even after two days of incubation under basic conditions in 0.5M NaOH at 60°C. Increased time of incubation, higher temperatures and more basic conditions did not lead to detectable crosslinking. These findings are in agreement with published data showing the instability of an N-terminal DHA and its conversion to pyruvylGlycine [13]. On the basis of these data the (DHA)Gly adduct in proteins must form via breakdown of an initial γ-Glu(DHA)Gly adduct.
Discussion:
This paper describes the novel modification of proteins by the addition of (DHA)Gly to lysine residues. Through the use of model peptides, it was demonstrated that this + 144 Da modification could arise by the breakdown of glutathione. A mechanism is illustrated in Fig. 4. We propose that GSSG undergoes β-elimination forming γ-Glu(DHA)Gly. This reactive dehydroalanine intermediate can potentially form a crosslink with any free amino or sulfhydryl group, such as the side chains of Lys or Cys residues. Due to the lability of the γ-glutamyl bond [12], following the formation of the γ-Glu(DHA)Gly adduct, the Glu can then be lost via a hydrolytic mechanism probably catalysed by the ε-amino group of the Lys similar to the mechanism observed previously [14, 15]. The γ-Glu linkage can also be cleaved in the thioether adduct with Cys, although this occurs approximately 20 times more slowly.
Fig. 4.
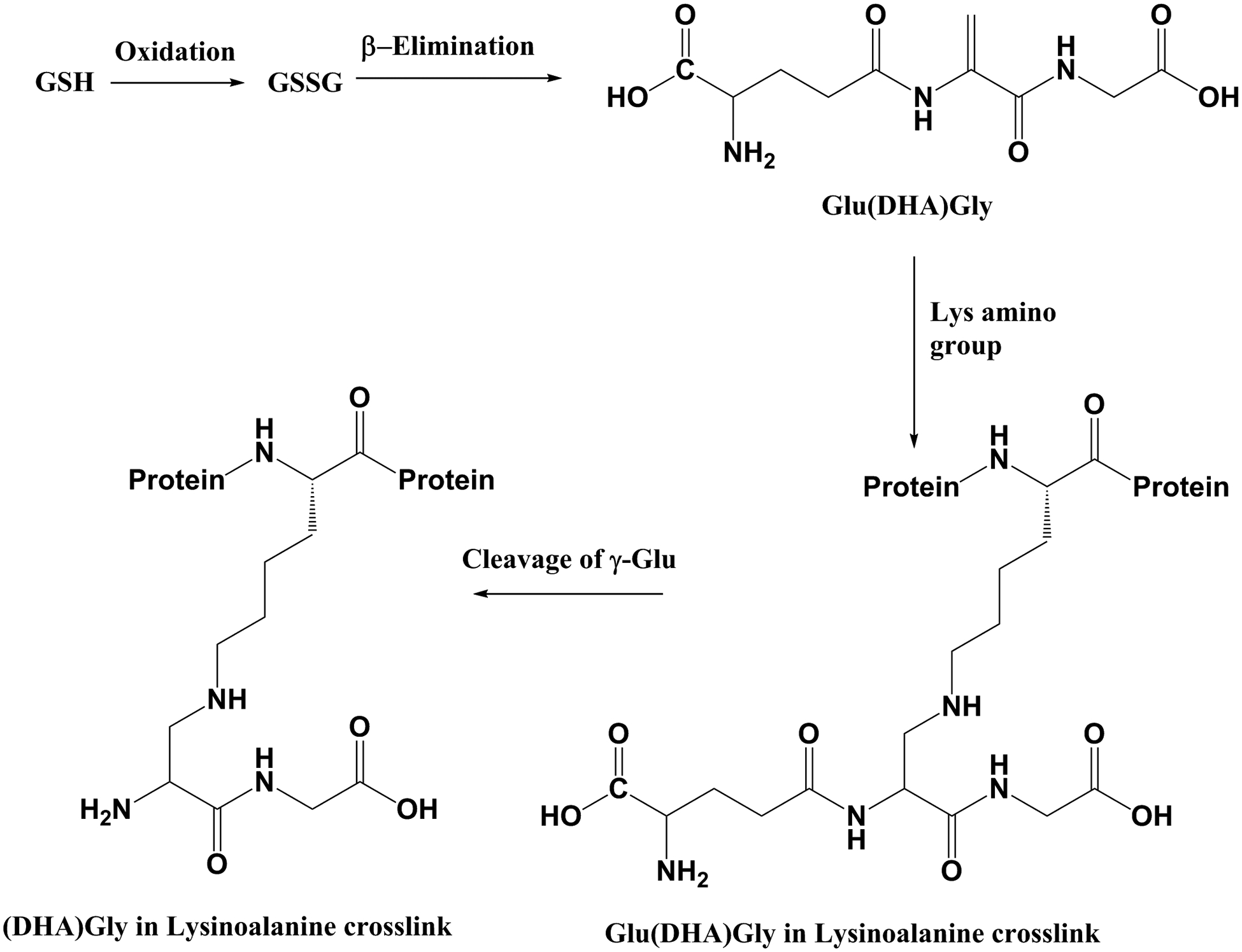
A proposed scheme illustrating the breakdown of GSSG and formation of lysinoalanine crosslinks.
Proteomic investigation revealed 18 sites of Lys modification in 7 human lens proteins (Table 1a). Modifications on the same sites were repeatedly detected in multiple lenses and the results also suggest an age-related increase of modification, however more lenses would need to analysed to confirm this. In the case of the most highly modified protein, γS-crystallin, four of the five modified Lys residues appeared to be localised in unstructured regions. Other spontaneous age-related PTMs, such as deamidation, which involve intramolecular reactions [16], occur predominantly in unstructured regions of proteins [17,18], although some sites of deamidation have recently been detected in structured regions [16,19]. Our data on glutathione modification imply that functional groups of amino acids in unstructured regions may be more susceptible to PTM by reactive small molecules although solvent accessibility is also likely to be a contributory factor. The effect of the formation of the (DHA)Gly crosslink to Lys would be two-fold: firstly, loss of a charged amino acid could affect protein-protein interactions and protein packing in the cell. Human lens function is reliant on ordered protein to focus light onto the retina. It could be envisioned that alterations in both charge and the conformational impact following the addition of (DHA)Gly could have consequences for lens transparency. In addition, structural changes due to the addition of a dipeptide may lead to unfolding of the protein as well as the generation of a site that may be resistant to proteolysis.
Previous studies have shown that γ-Glu(DHA)Gly can be formed via a β-elimination reaction of GSSG in alkali [20,21] and from similar reactions involving the GSH adducts of certain nucleophilic drugs, such as Busulfan[22]. Our current study suggests that GSSG can undergo β-elimination under physiological conditions resulting in the formation of γ-Glu(DHA)Gly and ultimately a lysinoalanine crosslink with proteins. Free sulfhydryl groups, such as cysteine residues, react more rapidly with DHA than do amino groups [8] and several (DHA)Gly modified Cys residues were detected in this study (Table 1b). In all likelihood, if γ-Glu(DHA)Gly forms within cells it will preferentially crosslink with Cys residues and such thioethers have been reported [5, 8, 23]. Unfortunately, the thioether adduct that forms will be indistinguishable from that which is formed by reaction of GSH with a Cys-derived DHA residue of the particular protein. The discovery of the Lys adduct shows that formation of γ-Glu(DHA)Gly in cells should be considered as a potential reactive intermediate.
Whilst we cannot prove that the Lys (DHA)Gly adduct described in this paper arises due to the β-elimination of GSSG, it is a probable route. GSSG is present in the human lens especially in older lenses where transport of GSH and re-reduction of GSSG is disrupted by the lens barrier [7]. Oxidation is a characteristic feature of age-related nuclear cataract and typically GSH is low, or absent, in the centre of such cataract lenses and levels of GSSG are high [24].
Free CysGly is present in the lens [6], however model experiments using CysGly or oxidised CysGly failed to generate detectable (DHA)Gly or the lysinoalanine crosslink, whereas GSSG under the same conditions formed the lysinoalanine crosslink readily. It is known that (DHA)Gly is unstable and decomposes readily to pyruvate derivatives [13]. It is also possible that γ-Glu(DHA)Gly can form in cells via β-elimination of disulphide-linked glutathionated proteins since γ-GluCysGly disulphides have been recorded in adult human lens proteins [25].
Based on the results of this study and previous studies, [5, 8] once a DHA group forms there are several possible outcomes. Reaction with a free sulfhydryl group forming a thioether, such as with GSH or protein Cys, will be favoured. Our proteomic data revealed 6 sites of protein modification where Cys residues had potentially been modified by (DHA)Gly (Table 1b). If sulfhydryl groups are in short supply, then lysinoalanine crosslinks can form. These findings demonstrate the potential of GSH as to act as a reactive intermediate leading to the formation of irreversible covalent crosslinks.
Supplementary Material
Funding information:
Funding for this study was provided by National Institutes of Health by grants R01 EY024258 and P30 EY008126.
Abbreviations
- DHA
Dehydroalanine
- γ-Glu(DHA)Gly
γ-glutamyldehydroalanylglycine
- GSSG
oxidised glutathione
- GSH
glutathione
References
- [1].Truscott RJW, Schey KL, Friedrich MG, Old Proteins in Man: A Field in its Infancy, Trends in Biochemical Sciences, 41 (2016) 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Friedrich MG, Lam J, Truscott RJW, Degradation of an Old Human Protein: Age-Dependent Cleavage of γS-Crystallin Generates a Peptide That Binds To Cell Membranes, Journal of Biological Chemistry, 287 (2012) 39012–39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hooi MYS, Raftery MJ, Truscott RJW, Racemization of Two Proteins over Our Lifespan: Deamidation of Asparagine 76 in γS Crystallin Is Greater in Cataract than in Normal Lenses across the Age Range, Investigative Ophthalmology & Visual Science, 53 (2012) 3554–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA, David LL, Age-Related Changes in Human Crystallins Determined from Comparative Analysis of Post-translational Modifications in Young and Aged Lens: Does Deamidation Contribute to Crystallin Insolubility?, Journal of Proteome Research, 5 (2006) 2554–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang Z, Lyons B, Truscott RJW, Schey KL, Human protein aging: modification and crosslinking through dehydroalanine and dehydrobutyrine intermediates, Aging Cell, 13 (2014) 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Giblin FJ, Glutathione: A Vital Lens Antioxidant, Journal of Ocular Pharmacology and Therapeutics, 16 (2000) 121–135. [DOI] [PubMed] [Google Scholar]
- [7].Sweeney MHJ, Truscott RJW, An Impediment to Glutathione Diffusion in Older Normal Human Lenses: a Possible Precondition for Nuclear Cataract, Experimental Eye Research, 67 (1998) 587–595. [DOI] [PubMed] [Google Scholar]
- [8].Friedrich MG, Wang Z, Oakley AJ, Schey KL, Truscott RJW, Hotspots of Age-Related Protein Degradation. The Importance of Neighbouring Residues for the Formation of Non-Disulfide Crosslinks derived from Cysteine., Biochemical Journal, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang Z, Obidike JE, Schey KL, Posttranslational Modifications of the Bovine Lens Beaded Filament Proteins Filensin and CP49, Investigative Ophthalmology & Visual Science, 51 (2010) 1565–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dasari S, Chambers MC, Slebos RJ, Zimmerman LJ, Ham A-JL, Tabb DL, TagRecon: High-Throughput Mutation Identification through Sequence Tagging, Journal of Proteome Research, 9 (2010) 1716–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sokolovsky M, Sadeh T, Patchornik A, Nonenzymatic cleavages of peptide chains at the cysteine and serine residues through their conversion to dehydroalanine (DHAL). II. The specific chemical cleavage of cysteinyl peptides, J Am Chem Soc, 86 (1964) 1212–1217. [Google Scholar]
- [12].Deshmukh M, Kutscher H, Stein S, Sinko P, Nonenzymatic, Self-Elimination Degradation Mechanism of Glutathione, Chemistry & Biodiversity, 6 (2009) 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Recsei PA, Huynh QK, Snell EE, Conversion of prohistidine decarboxylase to histidine decarboxylase: peptide chain cleavage by nonhydrolytic serinolysis, Proc Natl Acad Sci U S A, 80 (1983) 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lyons B, Friedrich M, Raftery M, Truscott R, Amyloid Plaque in the Human Brain Can Decompose from Aβ(1–40/1–42) by Spontaneous Nonenzymatic Processes, Analytical Chemistry, 88 (2016) 2675–2684. [DOI] [PubMed] [Google Scholar]
- [15].Fife TH, Singh R, Bembi R, Intramolecular General Base Catalyzed Ester Hydrolysis. The Hydrolysis of 2-Aminobenzoate Esters, The Journal of Organic Chemistry, 67 (2002) 3179–3183. [DOI] [PubMed] [Google Scholar]
- [16].Geiger T, Clarke S, Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to proetein degradation, J Biol Chem 262 (1987) 785–794. [PubMed] [Google Scholar]
- [17].Hooi MYS, Raftery MJ, Truscott RJW, Age-dependent deamidation of glutamine residues in human γS crystallin: deamidation and unstructured regions, Protein Sci 21 (2012) 1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wearne s.j., Creighton TE, Effect of protein conformation on rate of deamidation: ribonuclease A, Proteins, 5 (1989) 8–12. [DOI] [PubMed] [Google Scholar]
- [19].Lyon YA, Sabbah GM, Julian RR, Identification of sequence similarities among isomerizatin hotspots in crystallin proteins, J. Proteome Res, 16 (2017) 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Asquith RS, Carthew P, The preparation and subsequent identification of a dehydroalanyl peptide from alkali-treated oxidised glutathione, Biochim Biophys Acta, 285 (1972) 346–351. [DOI] [PubMed] [Google Scholar]
- [21].Jones AJ, Helmerhorst E, Stokes GB, The formation of dehydroalanine residues in alkali-treated insulin and oxidized glutathione. A nuclear-magnetic-resonance study, Biochem J, 211 (1983) 499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Younis IR, Elliott M, Peer CJ, Cooper AJL, Pinto JT, Konat GW, Kraszpulski M, Petros WP, Callery PS, Dehydroalanine analog of glutathione: an electrophilic busulfan metabolite that binds to human glutathione S-transferase A1–1, J Pharmacol Exp Ther, 327 (2008) 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Linetsky M, LeGrand RD, Glutahtionylation of lens proteins through the formation of thioether bond, Mol Cell Biochem, 272 (2005) 133–144. [DOI] [PubMed] [Google Scholar]
- [24].Truscott RJW, Augusten RC, The state of sulphydryl groups in normal and cataractous human lenses, Exp Eye Res, 25 (1977) 139–148. [DOI] [PubMed] [Google Scholar]
- [25].Harding JJ, Free and protein-bound glutathione in normal and cataractous human lenses, Biochem J, 117 (1970) 957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


