Abstract
Monoclonal gammopathy of undetermined significance (MGUS) was identified in 3.2% of 21 463 residents of Olmsted County, Minnesota, 50 years of age or older. The risk of progression to multiple myeloma, Waldenstrom’s macroglobulinemia, AL amyloidosis or a lymphoproliferative disorder is approximately 1% per year. Low-risk MGUS is characterized by having an M protein <15 g/l, IgG type and a normal free light chain (FLC) ratio. Patients should be followed with serum protein electrophoresis at six months and, if stable, can be followed every 2–3 years or when symptoms suggestive of a plasma cell malignancy arise. Patients with intermediate and high-risk MGUS should be followed in 6 months and then annually for life. The risk of smoldering (asymptomatic) multiple myeloma (SMM) progressing to multiple myeloma or a related disorder is 10% per year for the first 5 years, 3% per year for the next 5 years and 1–2% per year for the next 10 years. Testing should be done 2–3 months after the initial recognition of SMM. If the results are stable, the patient should be followed every 4–6 months for 1 year and, if stable, every 6–12 months.
Keywords: monoclonal gammopathy of undetermined significance, smoldering multiple myeloma, International Myeloma Working Group, MGUS
Monoclonal gammopathy of undetermined significance
Monoclonal gammopathy of undetermined significance (MGUS) is defined as a serum M protein <30 g/l, <10% clonal plasma cells (PCs) in the bone marrow (BM) and, most importantly, the absence of end-organ damage that can be attributed to the PC proliferative disorder (Table 1). End-organ damage is characterized by CRAB (hypercalcemia, renal insufficiency, anemia, bone lesions) related to the PC proliferative disorder.1
Table 1.
Diagnostic criteria for plasma cell disorders
| Disorder | Disease definition |
|---|---|
| Monoclonal gammopathy of undetermined significance | All three criteria must be met Serum monoclonal protein <3gm/dl Clonal bone marrow plasma cells <10%, and Absence of end-organ damage such as hypercalcemia, renal insufficiency, anemia, and bone lesions that can be attributed to the plasma cell proliferative disorder |
| Smoldering multiple myeloma (also referred to as asymptomatic multiple myeloma) | Both criteria must be met Serum monoclonal protein (IgG or IgA) ⩾3gm/dl and/or clonal bone marrow plasma cells ⩾10%, and Absence of end-organ damage such as lytic bone lesions, anemia, hypercalcemia, or renal failure that can be attributed to a plasma cell proliferative disorder |
| Multiple myeloma | All three criteria must be met except as noted Clonal bone marrow plasma cells ⩾10% Presence of serum and/or urinary monoclonal protein (except in patients with non-secretory multiple myeloma), and Evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically Hypercalcemia: serum calcium ⩾11.5 mg/dl or Renal insufficiency: serum creatinine >1.73 mmol/l) Anemia: normochromic, normocytic with a hemoglobin value of >2 g/dl below the lower limit of normal or a hemoglobin value < 10 g/dl Bone lesions: lytic lesions, severe osteopenia or pathological fractures |
Abbreviations: MGUS, Monoclonal gammopathy of undetermined significance; CRAB, hypercalcemia, renal insufficiency, anemia, and bone lesions.
Prevalence of MGUS
MGUS was identified in 3.2% of 21 463 residents of Olmsted County, Minnesota, 50 years of age or older.2 Age-adjusted rates were higher in men than in women (4.0 vs 2.7%). The prevalence of MGUS was 5.3% among persons 70 years of age or older and in 8.9% of men older than 85 years of age. The size of the M protein was <1.5 g/dl in 80% and ⩾ 2 g/dl in only 4.5%.2 The prevalence of MGUS in African Americans3,4 and Africans5 is approximately double that in Caucasians, whereas the prevalence in Japanese is lower than in Caucasians.6 The prevalence of MGUS in first degree relatives of patients with MGUS is increased suggesting a genetic factor.7,8 Only 21% of 70-year-old patients with MGUS have been recognized during routine clinical practice in a large population-based cohort.9 Fewer would likely be recognized outside Olmsted County because physicians might be less likely to order serum protein electrophoresis in everyday medical practice. At the time of its clinical recognition, the median duration of the patient’s MGUS is estimated to be 11 years.
Outcome of MGUS
A cohort of 241 patients with MGUS was followed up to 39 years (median, 13.7 years). Twenty-seven percent (n = 64) developed multiple myeloma (44), Waldenstrom’s macroglobulinemia (7), primary AL amyloidosis (AL) (8), or a lymphoproliferative disorder (LP) (5). The interval from recognition of MGUS to diagnosis of multiple myeloma or a related disorder ranged from 1 to 32 years (median 10.4 years). The overall risk of progression was 1.5% per year.10 In a cohort of 1384 patients living in Southeastern Minnesota, 115 (8%) developed multiple myeloma (n = 75), IgM lymphoma (19), AL amyloidosis (10), Waldenstrom’s macroglobulinemia (7), chronic lymphocytic leukemia (3) or plasmacytoma (1). The relative risk of progression compared with the SEER (Surveillance, Epidemiology, and End Results) population from Iowa was 25.0, 2.4, 8.4, 46.0, 0.9 and 8.5-fold, respectively. The relative risk of progression was 7.3-fold in these patients when compared with the white population of the SEER data. The cumulative probability of progression was 12% at 10 years, 25% at 20 years and 30% at 25 years. The risk of progression was approximately 1% per year.11
Predictors of risk of progression
Prediction of MGUS patients who will remain stable compared with those who progress is very difficult at the time of recognition of MGUS. However, the size of the M protein, type of M protein, number of BM PCs and the free light chain (FLC) ratio are helpful in identifying patients who are at a higher risk of progression. In addition, the physician should be aware that the presence of anemia, renal insufficiency or hypercalcemia may be unrelated to the presence of the M protein.
Size of M protein
The size of the M protein at the time of recognition of MGUS was the most important predictor of progression in 1384 patients with MGUS.11 The risk of progression to multiple myeloma or a related disorder 20 years after recognition of MGUS was 49% for those with an M-protein value of 25 g/l, compared with only 14% for patients with an initial M-protein value of 5 g/l or less. The risk of progression with an M-protein value of 15 g/l was almost twice that of a patient with an M-protein value of 5 g/l. The risk of progression with an M protein of 25 g/l was 4.6 times that of a patient with a 5 g/l spike. Rosinol12 emphasized that a progressive increase in size of the M protein during the first year of follow-up was the single most important risk factor for progression.
Type of immunoglobulin
Patients with IgM or IgA monoclonal protein had an increased risk of progression as compared with patients who had an IgG monoclonal protein in the 1384 patient cohort. Blade et al.13 also reported that patients with an IgA MGUS had a greater probability for progression to multiple myeloma.
Bone marrow plasma cells
Cesana et al.14 reported a series of 1104 patients with MGUS and found that those with more than 5% BM PCs had an increased risk of progression. In another series, the progression rate was 6.8% when the BM PCs were less than 10 and 37% for those in whom the PC level was 10–30%.15 At present, most would classify the patients with ⩾10% PCs as having smoldering multiple myeloma or symptomatic multiple myeloma.
Serum FLC ratio
One-third of 1148 patients with MGUS from Southeastern Minnesota had an abnormal FLC ratio at diagnosis. The risk of progression was significantly higher in patients with an abnormal FLC ratio than in those with a normal FLC ratio (hazard ratio 3.5). The FLC ratio was independent of the size and type of serum monoclonal protein.16
Rajkumar et al.16 developed a risk-stratification model for progression of MGUS. Patients with risk factors consisting of a serum M protein ⩾15 g/l, IgA or IgM MGUS and an abnormal serum FLC ratio had a risk of progression at 20 years of 58%; compared with 37% when two risk factors were present; 21% when one risk factor was present; and only 5% when none of the risk factors were present (Table 2).
Table 2.
Risk-stratification model to predict progression of monoclonal gammopathy of undetermined significance to myeloma or related disorders
| Risk group | No. of patients | Relative risk | Absolute risk of progression at 20 years (%) | Absolute risk of progression at 20 years accounting for death as a competing risk (%) |
|---|---|---|---|---|
| Low-risk (serum M protein <1.5 gm/dl, IgG subtype, normal FLC ratio (0.26–1.65) | 449 | 1 | 5 | 2 |
| Low-intermediate-risk (any 1 factor abnormal) | 420 | 5.4 | 21 | 10 |
| High-intermediate-risk (any two factors abnormal) | 226 | 10.1 | 37 | 18 |
| High-risk (all three factors abnormal) | 53 | 20.8 | 58 | 27 |
Abbreviation: MGUS, Monoclonal gammopathy of undetermined significance.
This table was originally published in Blood. Rajkumar SV et al., Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance (MGUS) Blood. 2005; 106;812–817. © the American Society of Hematology.
Flow cytometry and cytogenetics
In a study of 407 patients with MGUS and 93 with Smoldering (asymptomatic) multiple myeloma (SMM), Perez-Persona reported that a marked preponderance of aberrant PCs in the BM as assessed by flow cytometry showed a significantly higher risk of progression in both MGUS and multiple myeloma. The most important risk factors were the presence of ⩾95% aberrant PCs together with DNA aneuploidy. Using the two risk factors, they reported a progression-free survival of 2, 10 and 46% for the presence of none, one or two risk factors, respectively at 5 years in MGUS.17 The age, sex, presence of hepatosplenomegaly, values for hemoglobin, serum creatinine, serum albumin and presence or amount of a monoclonal urinary light chain are not predictors for progression. Although fluorescence in situ hybridization reveals almost the same number and type of abnormalities as in multiple myeloma, there is little evidence that this has a role in the progression of MGUS to multiple myeloma. The gene expression profile may be of benefit in predicting the risk of progression, but no convincing data exists at present.
Patient management
At first diagnosis, a complete history and physical examination should be done with emphasis on symptoms and findings that might suggest multiple myeloma or AL amyloidosis. A complete blood count, serum calcium and creatinine values and a qualitative test for urine protein should be performed. If proteinuria is found, electrophoresis and immunofixation is indicated. Serum protein electrophoresis should be repeated 3–6 months after recognition of MGUS to exclude multiple myeloma or Waldenstrom’s macroglobulinemia because the monoclonal protein is usually recognized by chance.
Low-risk MGUS
If the serum monoclonal protein is <15 g/l, IgG type and the FLC ratio is normal, the risk of eventual progression to myeloma or related malignancy is low. In this setting, a baseline BM examination or skeletal radiography is not routinely indicated if the clinical evaluation, complete blood count, serum creatinine and calcium values suggest MGUS. On the other hand, a BM is required if the patient has unexplained anemia, renal insufficiency, hypercalcemia, or bone lesions. Patients should be followed with serum protein electrophoresis in 6 months, and if stable can be followed every 2–3 years or when symptoms suggestive of a PC malignancy arise.
Intermediate and high risk MGUS
If a patient with apparent MGUS has a serum monoclonal protein >15 g/l, IgA or IgM protein type, or an abnormal FLC ratio, a BM aspirate and biopsy should be carried out at baseline to rule out underlying PC malignancy. As discussed earlier, a BM is always required if a patient with presumed MGUS has unexplained anemia, renal insufficiency, hypercalcemia, or bone lesions or a suspicion of AL amyloidosis. Both conventional cytogenetics and fluorescence in situ hybridization should be performed on the BM examination. If available, a PC labeling index and a search for circulating PCs in the peripheral blood using flow cytometry are useful.18 A computed tomographic scan of the abdomen should be done in the presence of an IgM monoclonal protein because asymptomatic retroperitoneal lymph nodes may be present. Lactate dehydrogenase, β−2-microglobulin, and C-reactive proteins should be determined if there is evidence of multiple myeloma or Waldenstrom’s macroglobulinemia. If the results of these tests are satisfactory, patients should be followed with serum protein electrophoresis and a complete blood count in 6 months and then annually for life. Treatment is not indicated unless it is part of a clinical trial.19 Patients must contact their physician if there is any change in their clinical condition.
Smoldering (asymptomatic) multiple myeloma
Smoldering (asymptomatic) multiple myeloma requires the presence of a monoclonal protein level of 30 g/l or more or a proportion of clonal PCs in the BM of 10% or more but no end-organ damage.1 It needs to be distinguished from MGUS because of a higher risk of progression to myeloma or related disorder; 10% per year for SMM versus 1% per year for MGUS.
Outcome
In a cohort of 276 patients fulfilling the criteria for SMM, 163 (59%) developed symptomatic multiple myeloma or AL amyloidosis during follow-up. The overall risk of progression was 10% per year for the first 5 years, approximately 3% per year for the next 5 years, and 1% per year for the last 10 years. The cumulative probability of progression to active multiple myeloma or AL amyloidosis was 51% at 5 years, 66% at 10 years, and 73% at 15 years. The median time to progression was 4.8 years.20
The number of patients with progression to symptomatic multiple myeloma was 522 times the number of persons without SMM who would be expected to have active disease, whereas the risk of AL amyloidosis was increased by a factor of 50-fold. Ninety-seven percent of those who progressed developed symptomatic multiple myeloma.
Risk factors for progression
The size of the serum monoclonal protein and the number of PCs in the BM are the most important factors for progression. Sex, hemoglobin level, type of serum heavy chain, serum albumin value, presence and type of urinary light chain, reduction in levels of uninvolved immunoglobulins and involvement of the interfatty marrow space were not significant risk factors for progression. The FLC (free light chain) ratio is an independent additional risk factor for progression.21
On the basis of the size of the monoclonal protein and number of BM PCs, a risk-stratification model was developed.20 If the patient had both ⩾10% PCs and ⩾30 g/l of monoclonal protein, the probability of progression at 15 years was 87%; if the patient had ⩾10% PCs and <30 g/l of monoclonal protein, the risk of progression was 70% at 15 years, whereas those patients with <10% PCs and ⩾30 g/l monoclonal protein had a progression risk of 39%. The median time to progression was 2 years, 8 years and 19 years, respectively in the above three groups. The type of serum heavy chain added significantly to the multivariate model containing the number of BM PCs and size of the serum M protein.
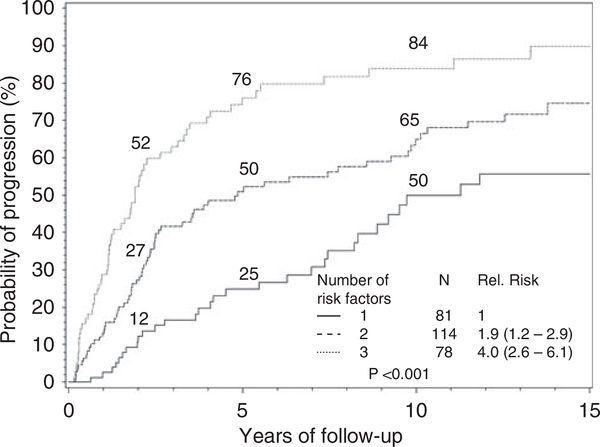
Dispenzieri et al.21 found that the serum free light chain assay provides additional prognostic information. An abnormal FLC ratio (defined as ≦0.125 or ⩾8) predicted for higher rates of progression, hazard ratio, 2.3; 95% CI, 1.6–3.2).21 A risk model was constructed, incorporating three risk factors: abnormal FLC ratio, BM PCs ⩾10%, and serum M protein ⩾3 g/dl. Patients with 1, 2 or 3 risk factors had 5-year progression rates of 25, 51 and 76%, respectively (Figure 1). Similar to what we have mentioned above for MGUS, the presence of > 95% aberrant PC from the total BM PC compartment detected by flow cytometry together with immunoparesis can discriminate three prognostic groups in SMM, with a progression risk of 5 years at 72, 46 and 4% if the patient has two, one or none of these risk factors.17
Figure 1.
Risk stratification for smoldering multiple myeloma. The model incorporates three risk factors: abnormal FLC ratio, bone marrow plasma cells ⩾10% and serum M protein ⩾3 g/dl. Patients with 1, 2 or 3 risk factors had 5-year progression rates of 25, 51 and 76%, respectively. Corresponding median times to progression are 10, 5.1 and 1.9 years, respectively.
Others have found that the presence of occult bone lesions on magnetic resonance imaging (MRI) increases the risk of progression in patients otherwise defined as having SMM.22 In a recent study, Wang and colleagues23 estimated risk of progression in 72 patients with SMM in whom an MRI of the spine was also performed. The median time to progression was significantly shorter with an abnormal MRI compared with normal MRI, 1.5 years versus 5 years.
Patient management
Serum protein electrophoresis, complete blood count, measurement of calcium and creatinine values and 24-h urine collection for electrophoresis and immunofixation should be performed at diagnosis and in 2–3 months after the initial recognition of SMM. A baseline BM biopsy and skeletal survey are mandatory. An MRI of the spine and pelvis is recommended because it can detect occult lesions and, if present, predict for a more rapid progression to symptomatic myeloma. If the results are stable, the studies should be repeated every 4–6 months for 1 year and, if stable, evaluation can be lengthened to every 6–12 months. A skeletal survey should be performed if there is evidence of progression in the above-mentioned routine studies.
Summary of IMWG recommendations for MGUS and SMM
Based on the discussion above, the summary recommendations that highlight the specific new recommendations by the IMWG panel are summarized in Table 3. As more data emerge we will reexamine these guidelines, particularly with regard to follow up and prophylactic strategies. Understanding the biology and mechanisms of progression of MGUS/SMM to myeloma will provide major insights toward the goal of finding a cure for multiple myeloma.
Table 3.
Summary of new IMWG recommendations on the management of MGUS and SMM
| New recommendation | Reason for change in recommendation |
|---|---|
| Definition of MGUS and SMM is based on proportion of clonal bone marrow plasma cells Patients with MGUS and SMM should be risk-stratified at diagnosis (see Table 2) to optimize counseling and follow up Follow up of MGUS is determined by the Risk-Stratification Model. Low-risk MGUS patients can be followed less frequently, either every 2–3 years or at time of progression. Preventive clinical trials need to be considered for patients with high risk smoldering myeloma |
The disease definition has been updated to reflect that clonality assessment is important in classification New risk stratification models give greater precision in estimating risk of progression in MGUS and SMM. In fact, a low-risk MGUS group with a 2% actual lifetime risk of MGUS progression can be identified by these models. This change is to minimize frequent testing for MGUS progression in patients at low risk of progression, and to emphasize that these patients should be followed for other health problems that are far more likely to affect survival compared with MGUS progression Patients with smoldering myeloma with FLC ratio ⩽0.125 or ⩾8 plus ⩾10% plasma cells in the marrow are at high risk of progression in the first 2 years following recognition. These patients should be considered candidates for chemoprevention trials. However, off-study, observation is still the standard even in this group. |
Abbreviations: MGUS, Monoclonal gammopathy of undetermined significance; SMM, smoldering (asymptomatic) multiple myeloma; IMWG, International Myeloma Working Group.
Appendix
International Myeloma Working Group
Rafat Abonour, Indiana University School of Medicine, Indianapolis, Indiana, USA
Ray Alexanian, MD Anderson, Houston, Texas, USA
Kenneth C Anderson, DFCI, Boston, Massachusetts, USA
Michael Attal, Purpan Hospital, Toulouse, France
Herve Avet-Loiseau, Institute de Biologie, Nantes, France
Ashraf Badros, University of Maryland, Baltimore, Maryland, USA
Bart Barlogie, MIRT UAMS Little Rock, Arkanas, USA
Dalsu Baris, National Cancer Institute, Bethesda, Maryland, USA
Regis Batille, Institute de Biologie, Nantes, France
Meral Beksac, Ankara University, Ankara, Turkey
Andrew Belch, Cross Cancer Institute, Alberta, Canada
Bill Bensinger, Fred Hutchinson Cancer Center, Seattle, Washington, USA
P Leif Bergsagel, Mayo Clinic Scottsdale, Scottsdale, Arizona, USA
Jenny Bird, Bristol Haematology and Oncology Center, Bristol, UK
Joan Bladé, Hospital Clinica, Barcelona, Spain
Mario Boccadoro, University of Torino, Torino, Italy
Michele Cavo, Universita di Bologna, Bologna, Italy
Asher Chanan-Khan, Roswell Park Cancer Institute, Buffalo, New York, USA
Wen Ming Chen, MM Research Center of Beijing, Beijing, China
Tony Child, Leeds General Hospital, Leeds, United Kingdom
James Chim, Department of Medicine, Queen Mary Hospital, Hong Kong
Wee-Joo Chng, National University Health System, Singapore
Ray Comenzo, Tufts Medical School, Boston, Massachusetts, USA
John Crowley, Cancer Research and Biostatistics, Seattle, Washington, USA
William Dalton, H Lee Moffitt, Tampa, Florida, USA
Faith Davies, Royal Marsden Hospital, London, England
Cármino de Souza, Univeridade de Campinas, Caminas, Brazil
Michel Delforge, University Hospital Gasthuisberg, Leuven, Belgium
Meletios Dimopoulos, University of Athens School of Medicine, Athens, Greece
Angela Dispenzieri, Mayo Clinic, Rochester, Minnesota, USA
Brian GM Durie, Cedars-Sinai Outpatient Cancer Center, Los Angeles, California, USA
Johannes Drach, University of Vienna, Vienna, Austria
Hermann Einsele, Universitätsklinik Würzburg, Würzburg, Germany
Theirry Facon, Centre Hospitalier Regional Universitaire de Lille, Lille, France
Dorotea Fantl, Socieded Argentinade Hematolgia, Buenos Aires, Argentina
Jean-Paul Fermand, Hopitaux de Paris, Paris, France
Rafael Fonseca, Mayo Clinic Arizona, Scottsdale, Arizona, USA
Gösta Gahrton, Karolinska Institute for Medicine, Huddinge, Sweden
Ramon Garcia-Sanz, University Hospital of Salamanca, Salamanca, Spain
Christina Gasparetto, Duke University Medical Center, Durham, North Carolina, USA
Morie Gertz, Mayo Clinic, Rochester, Minnesota, USA
John Gibson, Royal Prince Alfred Hospital, Sydney, Australia
Sergio Giralt, MD Anderson Cancer Center, Houston, Texas, USA
Hartmut Goldschmidt, University Hospital Heidelberg, Heidelberg, Germany
Philip Greipp, Mayo Clinic, Rochester, Minnesota, USA
Roman Hajek, Brno University, Brno, Czech Republic
Izhar Hardan, Tel Aviv University, Tel Aviv, Israel
Jean-Luc Harousseau, Institute de Biologie, Nantes, France
Hiroyuki Hata, Kumamoto University Hospital, Kumamoto, Japan
Yutaka Hattori, Keio University School of Medicine, Tokyo, Japan
Tom Heffner, Emory University, Atlanta, Georgia, USA
Joy Ho, Royal Prince Alfred Hospital, Sydney, Australia
Vania Hungria, Clinica San Germano, Sao Paolo, Brazil
Shinsuke Ida, Nagoya City University Medical School, Nagoya, Japan
Peter Jacobs, Constantiaberg Medi-Clinic, Plumstead, South Africa
Sundar Jagannath, St Vincent’s Comprehensive Cancer Center, New York, New York, USA
Hou Jian, Shanghai Chang Zheng Hospital, Shanghai, China
Douglas Joshua, Royal Prince Alfred Hospital, Sydney, Australia
Artur Jurczyszyn, The Myeloma Treatment Foundation, Poland
Michio Kawano, Yamaguchi University, Ube, Japan
Nicolaus Kröger, University Hospital Hamburg, Hamburg, Germany
Shaji Kumar, Department of Hematology, Mayo Clinic, Minnesota, USA
Robert A Kyle, Department of Laboratory Med. and Pathology, Mayo Clinic, Minnesota, USA
Juan Lahuerta, Grupo Espanol di Mieloma, Hospital Universitario, Madrid, Spain
Ola Landgren, National Cancer Institute, Bethesda, Maryland, USA
Jacob Laubach, Dana-Farber Cancer Institute, Boston, Massachusetts, USA
Jae Hoon Lee, Gachon University Gil Hospital, Incheon, Korea
Xavier LeLeu, Hospital Huriez, CHRU Lille, France
Suzanne Lentzsch, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
Henk Lokhorst, University Medical CenterUtrecht, Utrecht, The Netherlands
Sagar Lonial, Emory University Medical School, Atlanta, Georgia, USA
Heinz Ludwig, Wilhelminenspital Der Stat Wien, Vienna, Austria
Angelo Maiolino, Rua fonte da Saudade, Rio de Janeiro, Brazil
Maria Mateos, University of Salamanca, Salamanca, Spain
Jayesh Mehta, Northwestern University, Chicago, Illinois, USA
Ulf-Henrik Mellqvist, Sahlgrenska University Hospital, Gothenburg, Sweden
GiamPaolo Merlini, University of Pavia, Pavia, Italy
Joseph Mikhael, Mayo Clinic Arizona, Scottsdale, Arizona, USA
Angelina Rodriquez Morales, Bonco Metro Politano de Sangre, Caracas, Venezuela
Philippe Moreau, University Hospital, Nantes, France
Gareth Morgan, Royal Marsden Hospital, London, England
Nikhil Munshi, Diane Farber Cancer Institute, Boston, Massachusetts, USA
Ruben Niesvizky, Weill Medical College of Cornell University, New York, New York, USA
Amara Nouel, Hospital Rutz y Paez, Bolivar, Venezuela
Yana Novis, Hospital SírioLibanês, Bela Vista, Brazil
Robert Orlowski, MD Anderson Cancer Center, Houston, Texas, USA
Antonio Palumbo, Cathedra Ematologia, Torino, Italy
Santiago Pavlovsky, Fundaleu, Buenos Aires, Argentina
Linda Pilarski, University of Alberta, Alberta, Canada
Raymond Powles, Leukemia & Myeloma, Wimbledon, England
S Vincent Rajkumar, Mayo Clinic, Rochester, Minnesota, USA
Donna Reece, Princess Margaret Hospital, Toronto, Canada
Tony Reiman, Cross Cancer Institute, Alberta, Canada
Paul G Richardson, Dana Farber Cancer Institute, Boston, Massachusetts, USA
David Roodman, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania USA
Laura Rosinol, Hospital Clinic, Barcelona, Spain
Jesus San Miguel, University of Salamanca, Salamanca, Spain
Orhan Sezer, Universitätsklinik Wurzburg, Wurzburg, Germany
Jatin J Shah, MD Anderson Cancer Institute, Houston, Texas, USA
John Shaughnessy, MIRT UAMS, Little Rock, Arkansas, USA
Kazuyuki Shimizu, Nagoya City Midori General Hospital, Nagoya, Japan
Chaim Shustik, McGill University, Montreal, Canada
David Siegel, Hackensack, Cancer Center, Hackensack, New Jersey, USA
Seema Singhal, Northwestern University, Chicago, Illinois, USA
Pieter Sonneveld, Erasmus MC, Rotterdam, The Netherlands
Andrew Spencer, The Alfred Hospital, Melbourne, Australia
Edward Stadtmauer, University of Pennsylvania, Philadelphia, Pennsylvania, USA
Keith Stewart, Mayo Clinic Arizona, Scottsdale, Arizona, USA
Evangelos Terpos, University of Athens School of Medicine, Athens, Greece
Patrizia Tosi, Italian Cooperative Group, Istituto di Ematologia Seragnoli, Bologna, Italy
Guido Tricot, Huntsman Cancer Institute, Salt Lake City, Utah, USA
Ingemar Turesson, SKANE University Hospital, Malmo, Sweden
Karin Vanderkerken, Vrije University Brussels VUB, Brussels, Belgium
Brian Van Ness, University of Minnesota, Minneapolis, Minnesota, USA
Ivan Van Riet, Brussels Vrija University, Brussels, Belgium
Robert Vescio, Cedars-Sinai Cancer Center, Los Angeles, California, USA
David Vesole, Hackensack Cancer Center, Hackensack, New Jersey, USA
Anders Waage, University Hospital, Trondheim, Norway NSMG
Michael Wang, MD Anderson, Houston, Texas, USA
Donna Weber, MD Anderson, Houston, Texas, USA
Jan Westin, Sahlgrenska University Hospital, Gothenburg, Sweden
Keith Wheatley, University of Birmingham, Birmingham, United Kingdom
Dina B Yehuda, Department of Hematology, Hadassah University Hospital, Hadassah, Israel
Jeffrey Zonder, Karmanos Cancer Institute, Detroit, Michigan, USA.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003;121: 749–757. [PubMed] [Google Scholar]
- 2.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 2006;354: 1362–1369. [DOI] [PubMed] [Google Scholar]
- 3.Cohen HJ, Crawford J, Rao MK, Pieper CF, Currie MS. Racial differences in the prevalence of monoclonal gammopathy in a community-based sample of the elderly.[erratum appears in Am J Med 1998 Oct;105(4):362]. Am J Med 1998;104: 439–444. [DOI] [PubMed] [Google Scholar]
- 4.Singh J, Dudley AW Jr, Kulig KA. Increased incidence of monoclonal gammopathy of undetermined significance in blacks and its age-related differences with whites on the basis of a study of 397 men and one woman in a hospital setting. J Lab Clin Med 1990;116: 785–789. [PubMed] [Google Scholar]
- 5.Landgren O, Katzmann JA, Hsing AW, Pfeiffer RM, Kyle RA, Yeboah ED et al. Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin Proc 2007;82: 1468–1473. [DOI] [PubMed] [Google Scholar]
- 6.Iwanaga M, Tagawa M, Tsukasaki K, Kamihira S, Tomonaga M. Prevalence of monoclonal gammopathy of undetermined significance: study of 52,802 persons in Nagasaki City, Japan. Mayo Clin Proc 2007;82: 1474–1479. [DOI] [PubMed] [Google Scholar]
- 7.Landgren O, Kristinsson SY, Goldin LR, Caporaso NE, Blimark C, Mellqvist UH et al. Risk of plasma-cell and lymphoproliferative disorders among 14,621 first-degree relatives of 4,458 patients with monoclonal gammopathy of undetermined significance (MGUS) in Sweden. BLOOD 2009;114: 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vachon CM, Kyle RA, Therneau TM, Foreman BJ, Larson DR, Colby CL et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood 2009;114: 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyle RA, Therneau TM, Melton III LJ, Dispenzieri A, Larson D, Benson J et al. Monoclonal gammopathy of undetermined significance: estimated incidence and duration prior to recognition. Blood (Abstract) 2007;110: 79a. [Google Scholar]
- 10.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ III. Long-term follow-up of 241 patients with monoclonal gammopathy of undetermined significance: the original Mayo Clinic series 25 years later.[see comment]. Mayo Clinic Proc 2004;79: 859–866. [DOI] [PubMed] [Google Scholar]
- 11.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. [see comment]. New Engl J Med 2002;346: 564–569. [DOI] [PubMed] [Google Scholar]
- 12.Rosinol L, Cibeira MT, Montoto S, Rozman M, Esteve J, Filella X et al. Monoclonal gammopathy of undetermined significance: predictors of malignant transformation and recognition of an evolving type characterized by a progressive increase in M protein size. Mayo Clin Proc 2007; 82: 428–434. [DOI] [PubMed] [Google Scholar]
- 13.Blade J, Lopez-Guillermo A, Rozman C, Cervantes F, Salgado C, Aguilar JL et al. Malignant transformation and life expectancy in monoclonal gammopathy of undetermined significance. Br J Haematol 1992;81: 391–394. [DOI] [PubMed] [Google Scholar]
- 14.Cesana C, Klersy C, Barbarano L, Nosari AM, Crugnola M, Pungolino E et al. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. J Clin Oncol 2002; 20: 1625–1634. [DOI] [PubMed] [Google Scholar]
- 15.Baldini L, Guffanti A, Cesana BM, Colombi M, Chiorboli O, Damilano I et al. Role of different hematologic variables in defining the risk of malignant transformation in monoclonal gammopathy. Blood 1996;87: 912–918. [PubMed] [Google Scholar]
- 16.Rajkumar SV, Kyle RA, Therneau TM, Melton III LJ, Bradwell AR, Clark RJ et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood 2005;106: 812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Persona E, Vidriales MB, Mateo G, Garcia-Sanz R, Mateos MV, de Coca AG et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 2007; 110: 2586–2592. [DOI] [PubMed] [Google Scholar]
- 18.Nowakowski GS, Witzig TE, Dingli D, Tracz MJ, Gertz MA, Lacy MQ et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood 2005;106: 2276–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P. Clinically relevant end points and new drug approvals for myeloma. Leukemia 2008;22: 231–239. [DOI] [PubMed] [Google Scholar]
- 20.Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med 2007; 356: 2582–2590. [DOI] [PubMed] [Google Scholar]
- 21.Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood 2008;111: 785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma. Blood 2000;96: 2037–2044. [PubMed] [Google Scholar]
- 23.Wang M, Alexanian R, Delasalle K, Weber D. Abnormal MRI of spine is the dominant risk factor for early progression of asymptomatic multiple myeloma. Blood 2003;102: 687a (abstract). [Google Scholar]