Abstract
Clinical management of lymphoplasmacytic lymphoma (LPL)/Waldenström macroglobulinemia (WM) has changed considerably over recent years, reflected in the use of new therapeutic agents (purine analogs, monoclonal antibodies, thalidomide- and bortezomib-based therapies). No population-based studies and few randomized trials have been performed to assess survival in newly diagnosed LPL/WM. We performed a large population-based study in Sweden including 1,555 LPL/WM patients diagnosed from 1980 to 2005. Relative survival ratios (RSRs) and excess mortality rate ratios (EMRR) were computed as measures of survival. Survival of LPL/WM patients has improved significantly (P = 0.007) over time with 5-year RSR = 0.57 (95% confidence interval [CI] 0.46–0.68), 0.65 (0.57–0.73), 0.74 (0.68–0.80), 0.72 (0.66–0.77), and 0.78 (0.71–0.85) for patients diagnosed during the calendar periods 1980–1985, l986–1990, 1991–1995, 1996–2000, and 2001–2005, respectively. Improvement in 1- and 5-year relative survival was found in all age groups and for LPL and WM separately. Patients with WM had lower excess mortality compared to LPL (EMRR = 0.38; 95% CI 0.30–0.48). Older age at diagnosis was associated with a poorer survival (P < 0.001). Taken together, we found a significant improvement in survival in LPL/WM over time. Despite this progress, new effective agents with a more favourable toxicity profile are needed to further improve survival in LPL/WM, especially in the elderly.
Introduction
Lymphoplasmacytic lymphoma (LPL)/Waldenström macroglobulinemia (WM) is a rare chronic lymphoproliferative malignancy with a reported annual incidence rate of three to four cases per million people [1–3]. WM is a subset of LPL and defined as LPL with bone marrow involvement and a detectable monoclonal IgM spike in serum [1].
Clinical management of LPL/WM has changed considerably over recent years, reflected in the use of new therapeutic agents (such as purine analogs, monoclonal antibodies, thalidomide-, and bortezomib-based therapies), stratification of treatments, as well as improvement in supportive care measures [4–6]. Due to the rarity of LPL/WM, few large phase III randomized clinical trials have been performed to compare different treatment strategies and estimate overall survival. Median overall survival has varied in different series, ranging from 60 to 120 months [4,7–14]. Treatment of LPL/WM is mainly based on results from phase II trials and expert recommendations [5,6]. Clinical trials are associated with a varying degree of patient selection, especially in the elderly population. One study, including 345 symptomatic patients found no improvement in outcome over a 25-year period [15]. Population-based studies of trends in relative survival are a valuable complement to randomized clinical trials to determine the impact on survival of the introduction of new treatment strategies.
We have conducted a large population-based cohort study including 1,555 LPL/WM patients diagnosed in Sweden 1980–2005 (with follow-up through 2007). The aims of the study were to define LPL/WM survival patterns in the Swedish population and relate the findings to newly introduced therapeutic agents in the population.
Patients and Methods
Details of the study population have been described previously [16]. In brief, Sweden provides universal medical health care for the entire population, which is currently approximately 9.5 million people. In contrast to many other Western countries, patients with lymphoproliferative malignancies in Sweden are typically diagnosed, treated, and followed clinically by physicians at hospital-based hematology or oncology units.
Since 1958, all physicians, pathologists, and cytologists in Sweden are obliged by law to report each patient with cancer they diagnose or treat to the centralized nationwide Swedish Cancer Registry. In a recent validation study that focused on lymphoproliferative malignancies diagnosed from January 1, 1964, through December 31, 2003, we found the overall completeness and diagnostic accuracy of the registry to be greater than 90% [17]. For WM, the diagnostic accuracy was 93% but the completeness was 68%. To compensate for the lower completeness, we used parallel approaches to establish a representative Swedish LPL/WM cohort [16]. First, we identified all LPL/WM patients who were diagnosed from January 1, 1980, through December 31, 2005, in the nationwide Swedish Cancer Registry. Second, we retrieved information on patients with incident LPL/WM through our national network including all major hematology or oncology units in Sweden. By using these sources, we created a nationwide LPL/WM cohort including both symptomatic and asymptomatic patients.
Using the nationwide Cause of Death Registry, we obtained information on date of death for all LPL/WM who had died up to December 31, 2007. Information on the number of stem cell transplantations in LPL/WM patients reported from Swedish centers during the study period was obtained from the European Group for Blood and Marrow Transplantation (EBMT) register.
Approval was obtained from the Karolinska Institutional Review Board for this study. Informed consent was waived because we had no contact with study subjects. An exemption from Institutional Review Board review was obtained from the National Institutes of Health Office of Human Subjects Research because we used existing data without personal identifiers.
Statistical Analysis
Relative survival ratios (RSRs) were computed as measures of LPL/WM survival [18]. RSRs provide a measure of total excess mortality associated with a diagnosis of LPL/WM. 1-, 5-, 10-, and 15-year RSR can be interpreted as the proportion of patients who survived their LPL/WM at 1, 5, 10, and 15 years, respectively. Relative survival is defined as the observed survival in the group of patients (in which all deaths are considered events) divided by the expected survival of a comparable group from the general population, which is assumed to be free of the condition in question. Expected survival was estimated using the Ederer II method from Swedish population life-tables stratified by age, sex, and calendar period. 1-, 5-, 10-, and 15-year RSR were calculated for the calendar periods: 1980–1985, 1986–1990, 1991–1995, 1996–2000, and 2001–2005, and five age categories (<50, 50–59, 60–69, 70–79, and >80 years old). Poisson regression was used to model the effect of age at diagnosis, sex, calendar year at diagnosis, and subtype (LPL vs. WM) on the excess mortality rate ratio (EMRR). Time since diagnosis was used as the underlying time scale in all analyses. The first 2 years of follow-up were split into 1-year time bands whereas the remaining 8 years of follow-up were split into 2-year time bands prior to modeling. The estimates from this model are interpreted as EMRR; an EMRR of 1.5, for example, for males/females indicates that males experience a 50% higher excess mortality rate than females. Likelihood ratio tests were used to formally assess statistical significance of the variables used in the models (including possible interaction effects). The proportional hazards assumption was assessed by including interaction terms between the different variables considered and follow-up time. All calculations were performed using Stata (StataCorp 2009 Stata Statistical Software: Release 11, College Station, TX, StataCorp LP).
Results
A total of 1,555 patients with LPL/WM (755 LPL and 800 WM) were included in the study. The median age at diagnosis was 72 years (range 18–97 years). Males constituted 57.9% of the LPL/WM patients (Table I). The cancer registry was the major contributor of patients to the study after 1990, whereas the hospital-based cohort was the most common source before 1990. A total of six stem cell transplantations (five allogeneic and one autologous) for LPL/WM patients were reported to the EBMT register during the study period.
TABLE I.
Demographic Characteristics of LPL and WM Patients
| 1980–1985 | 1986–1990 | 1991–1995 | 1996–2000 | 2001–2005 | Total | |
|---|---|---|---|---|---|---|
| Total number of cases | 126 | 226 | 375 | 460 | 368 | 1,555 |
| Age, years (%) | ||||||
| Less than 50 | 6 (4.8) | 14 (6.2) | 25 (6.7) | 27 (5.9) | 20 (5.4) | 92 (5.9) |
| 50–59 | 14(11.1) | 28 (12.4) | 48 (12.8) | 55 (12.0) | 52 (14.1) | 197 (12.7) |
| 60–69 | 41 (32.5) | 51 (22.6) | 99 (26.4) | 101 (21.9) | 75 (20.4) | 367 (23.6) |
| 70–79 | 42 (33.3) | 86 (38.0) | 127 (33.8) | 156 (33.9) | 125 (34.0) | 536 (34.5) |
| 80 and above | 23 (18.3) | 47 (20.8) | 76 (20.3) | 121 (26.3) | 96 (26.1) | 363 (23.3) |
| Median age at diagnosis, years (range) | 70 (29–97) | 72 (18–91) | 71 (32–96) | 73 (21–95) | 73 (30–94) | 72 (18–97) |
| Gender (%) | ||||||
| Males | 71 (56.4) | 128 (56.6) | 219 (58.4) | 271 (58.9) | 212 (57.6) | 901 (57.9) |
| Females | 55 (43.6) | 98 (43.4) | 156 (41.6) | 189 (41.1) | 156 (42.4) | 654 (42.1) |
| WM subtype (%) | ||||||
| LPL | 49 (38.9) | 44 (19.5) | 231 (61.6) | 255 (55.4) | 176 (47.8) | 755 (48.6) |
| WM | 77 (61.1) | 182 (80.5) | 144 (38.4) | 205 (44.6) | 192 (52.2) | 800 (51.4) |
Abbreviations: LPL, lymphoplasmacytic lymphoma; WM, Waldenström macroglobulinemia.
Survival trends by calendar period
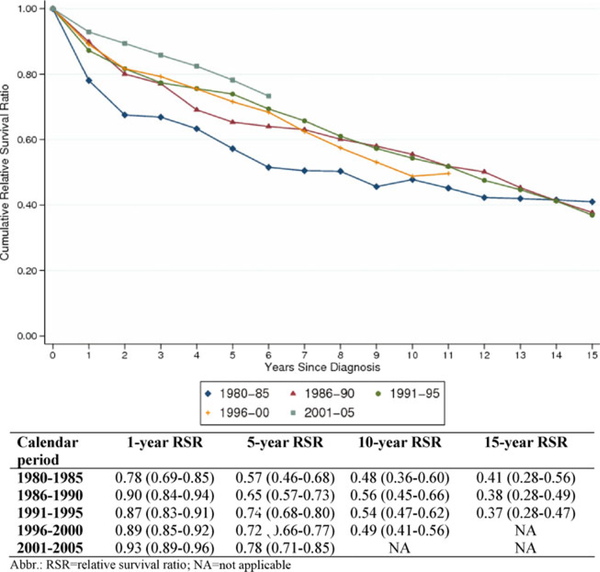
Cumulative RSR for all LPL/WM patients is shown in Fig. 1. 1- and 5-year RSR improved significantly for the complete cohort during the study period (Fig. 1). The 1-year RSR estimates were 0.78, 0.90, 0.87, 0.89, and 0.93, for the calendar periods 1980–1985, 1986–1990, 1991–1995, 1996–2000, and 2001–2005, respectively. The corresponding 5-year RSR estimates for the five calendar periods were 0.57, 0.65, 0.74, 0.72, and 0.78, respectively. The 10-year RSR estimates could only be computed for the first four calendar periods; the estimates were 0.48, 0.56, 0.54, and 0.49, respectively. Fifteen-year RSR estimates could only be computed for the first three periods and were 0.41, 0.38, and 0.37, respectively (Fig. 1). Patients diagnosed in the last (2001–2005) calendar period had a significantly lower excess mortality (EMRR = 0.67, 95% CI 0.47–0.96) compared to patients diagnosed 1991–1995 (Table II). There was some evidence that the effect of calendar period was not proportional throughout the 10 years of follow-up (P for interaction = 0.022).
Figure 1.
Cumulative relative survival of LPL/WM patients, stratified by calendar period at diagnosis. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
TABLE II.
Excess Mortality Ratios and 95% CI During the First 10 Years After LPL/WM Diagnosis, Stratified by Calendar Period at LPL/WM Diagnosis, Age Group, Sex, and Disease Entity
| EMRRa | 95 % CI | |
|---|---|---|
| Year of diagnosis | ||
| 1980–1985 | 1.72 | (1.21–2.44) |
| 1986–1990 | 1.38 | (0.99–1.93) |
| 1991–1995b | 1.00 | – |
| 1996–2000 | 1.01 | (0.78–1.31) |
| 2001–2005 | 0.67 | (0.47–0.96) |
| Age at diagnosis | ||
| < 50 years | 0.46 | (0.28–0.76) |
| 50–59 years | 0.77 | (0.55–1.08) |
| 60–69 yearsb | 1.00 | – |
| 70–79 years | 1.29 | (0.99–1.68) |
| >80 years | 2.25 | (1.68–3.03) |
| Sex | ||
| Maleb | 1.00 | – |
| Female | 0.79 | (0.64–0.97) |
| Subtype | ||
| LPLb | 1.00 | – |
| WM | 0.38 | (0.30–0.48) |
Abbreviations: EMRR, excess mortality rate ratio; LPL, lymphoplasmacytic lymphoma; WM, Waldenström macroglobulinemia.
Adjusted for calendar year, sex, age at diagnosis, follow-up time and subtype.
Baseline category.
Survival trends by age
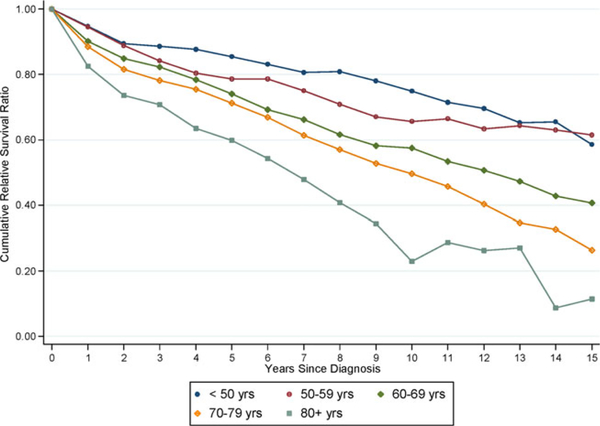
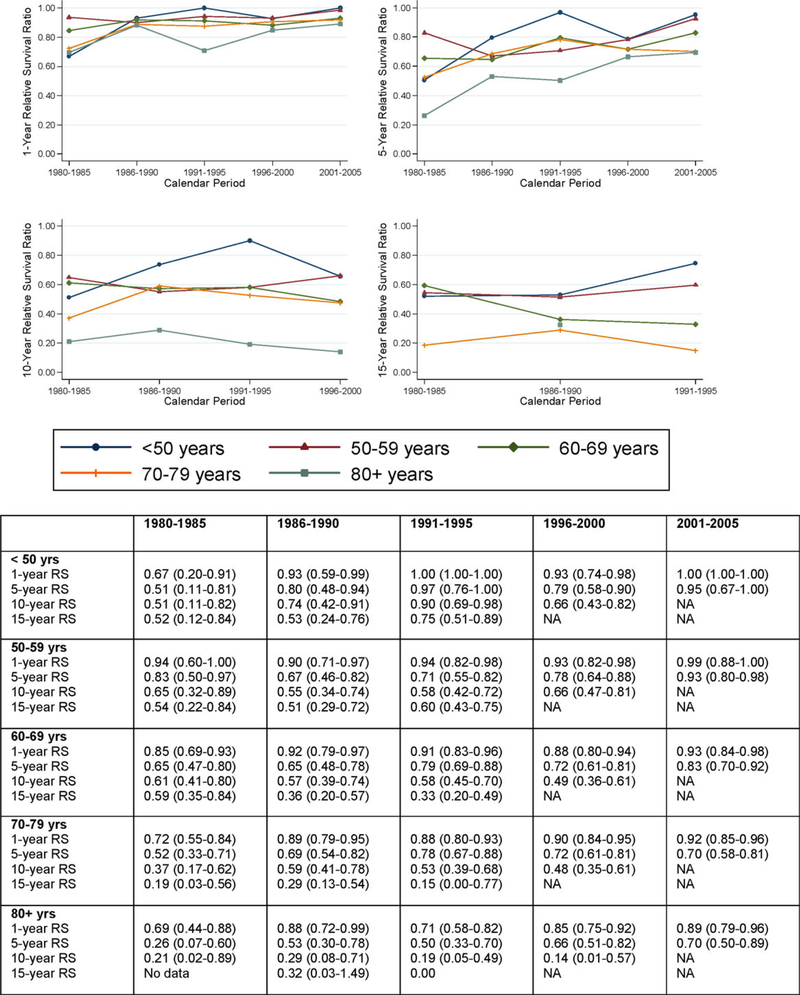
Older age at diagnosis was associated with a higher excess mortality (Table II and Figs. 2, 3). Patients diagnosed at an older age (>80 years) had a 2.25-fold (95% CI 1.68–3.03) higher excess mortality compared to patients diagnosed at 60–69 years (Table II). Patients diagnosed at younger age (<50 years) had a significantly lower excess mortality compared to patients diagnosed at 60–69 years (EMRR = 0.46; 95% CI 0.28–0.76). There was no evidence that the effect of age was modified by calendar time (P for interaction = 0.288), sex (P for interaction = 0.679), or subtype (P for interaction = 0.280). Improvement in 1- and 5-year RSR was observed in all age groups (Fig. 3), for example, in patients diagnosed at 80 years or older, an improvement in 5-year RSR was observed during the calendar periods, being 0.26, 0.53, 0.50, 0.66, and 0.70, respectively (Fig. 3).
Figure 2.
Cumulative relative survival of LPL/WM patients, stratified by age at diagnosis. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
1-, 5-, 10-, and 15-year RSRs of LPL/WM patients, by age at diagnosis and calendar period. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Survival trends by subtype
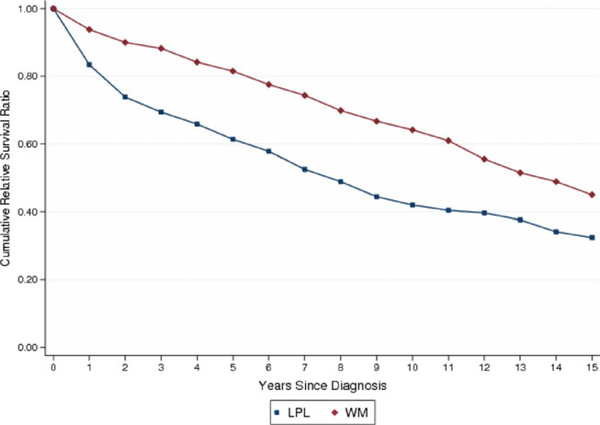
We found WM patients to have a significantly lower excess mortality rate compared to patients with LPL (EMRR = 0.38; 95% CI 0.30–0.48; Table II and Fig. 4). There was no evidence that the effect of subtype was modified by any other variable in the model (P for interaction with sex = 0.992, P for interaction with age = 0.280, P for interaction with calendar period = 0.218).
Figure 4.
Cumulative relative survival of LPL and WM patients. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Survival trends by sex
We found a significantly reduced (EMRR = 0.79, 95% CI 0.64–0.97) excess mortality rate for females compared to males (Table II). There was no evidence that the effect of sex (with females having better survival than males), differed by age at diagnosis (P for interaction = 0.679) or calendar period (P for interaction = 0.376).
Discussion
In this large study on survival among more than 1,500 LPL/WM patients, we show that survival has improved in recent years, with improvements observed for all age groups in both 1-year and 5-year relative survival. We found LPL/WM patients diagnosed after the year 2000 to have a lower excess mortality than patients diagnosed before. The underlying explanation for this is multifactorial but mainly involves introduction of new effective therapies as well as improvement in supportive care.
The introduction of combination therapy as well as novel therapeutic agents such as rituximab has probably contributed to the improvement in survival observed in our study. Initiation of therapy in early stage patients has not been shown to prolong survival, allowing asymptomatic patients to be followed clinically without treatment [3,8,10]. First line treatment of symptomatic WM patients has traditionally been based on single-agent therapy with alkylating agents (chlorambucil, cyclophosphamide) or later purine analogs (fludarabine, cladribine) [19–21]. These agents can be used in older individuals and have response rates of 30–80% depending on whether used as primary treatment or in relapsed disease. Very few complete remissions are observed. The impact of stem cell transplantations on improvement in survival in our study is limited due to the low number of these procedures performed in Sweden during the study period. Recently, the anti-CD20 antibody rituximab has been shown to have single agent activity and induce responses in almost half of WM patients [22]. There are, however, no data from prospective randomized studies to guide the choice between alkylating agents, nucleoside analogs, and rituximab for first line therapy of WM. During recent years, combination therapies with rituximab have resulted in good overall response rates but low complete response rates [23,24]. Newer therapies, including thalidomide and bortezomib have shown encouraging results; however, they were introduced during the last calendar period of this study and may have only a marginal effect on survival in this study [25–29]. Studies evaluating the effect of autologous stem cell transplantation in LPL/WM have been promising with low toxicities and high response rates [5,30–34]. However, randomized clinical trials are lacking. In a study from Germany, comparing WM patients treated in private oncology practices versus university hospital, a trend toward an improvement in survival was found in WM patients treated with a rituximab-containing first-line therapy [35]. However, our findings are in contrast to that reported by Kastritis et al. [15], who found no improvement in survival when comparing survival of 130 WM patients diagnosed 1985–1999 to 215 diagnosed after the year 2000. That study in contrast to ours, included only symptomatic patients, the follow-up for patients diagnosed after 2000 was only 39 months, and as pointed out by the authors, a longer follow-up is probably needed. In addition, the Greek study is not population-based and thus subject to referral/selection bias, as reflected in a much higher number of patients diagnosed during the last 10-year period (215 patients) compared to the 15 years before the year 2000 (130 patients). Taken together, the increase in available agents in the armamentarium in the treatment if LPL/WM patients have probably contributed to the improvement in survival LPL/WM patients in our study, during recent years.
An important consideration when interpreting our findings is the potential effect of lead time bias due to early LPL/WM detection. This issue is raised by a possible increase in the number of serum electrophoresis performed and change in diagnostic criteria over time. To help address this question, we recently conducted a nationwide validation study on WM cases (n = 91) diagnosed in Sweden in the period 1964–2003. In that study, there was 32% under-reporting of WM cases to the Swedish Cancer registry [17]. When we examined this in greater detail, we observed that under-reporting was constant over time and present in all calendar periods; it particularly occurred with elderly LPL/WM patients. Importantly, in this study, there was also a significant improvement in survival between the two last calendar periods (1996–2000 and 2001–2005). We recently analyzed medical charts from 137 patients with LPL/WM diagnosed at the Karolinska University Hospital. We found that the proportion of asymptomatic patients did not increase between the calendar period 1996–2000 and 2001–2005. Furthermore, when comparing the last two calendar periods, the incidence of LPL/WM has not increased. Since we observed an improvement in survival between these two periods, we do not consider lead-time bias as a major contributor to our findings. When taken together, we believe these data do not suggest that lead time bias is a major explanation for the improved survival we have observed in LPL/WM.
Clinical studies have shown age to be an important predictor of prognosis, with younger patients having a better survival [8]. The underlying factors are probably a combination of contributing factors such as comorbidity, more advanced disease at diagnosis, and the fact that elderly patients are not able to tolerate aggressive treatment. Importantly, even the oldest patient category improved their survival over time, indicating that despite having a poorer survival compared to younger patients, the improvement in management of elderly LPL/WM patients has also been beneficial for these patients. This has not been observed for example in population-based studies on multiple myeloma patients [36].
Compared to LPL patients, WM patients had a 62% lower excess mortality. The underlying explanations are probably multifactorial. Splenomegaly and lymphadenopathy are more frequently seen in LPL compared to WM patients. These are associated with anemia and low albumin, which are known to be associated with worse prognosis [8,14]. There may be a difference in the biology of these two entities, with LPL patients responding worse to treatment compared to WM patients. For instance, using fluorescent in situ hybridization analysis, LPL tumor cells have frequent t(9;14)(p13;q32) translocations, while WM tumor cells appear to be diploid or near diploid and often have deletions of 6q21 [37]. The differences in outcome for LPL and WM should be interpreted with some caution due to the lack of detailed clinical information. In this study, we combined the two entities based on previous studies that have shown that up to 90% of LPL patients have an M-protein thus fulfilling the criteria of WM [6]. As we have no information on whether electrophoresis was performed or if the patients had an M-protein it is possible that a substantial proportion of the LPL patients were reported by the pathologists as LPL although they had an M-protein and should thus have been classified as WM. This needs to be clarified in future studies.
We found a consistently better survival for women when adjusted for age and calendar period. Similar observations have been made in other hematologic malignancies, but the underlying mechanisms are unknown [36,38]. Possible explanations to this finding are different clinical stage at diagnosis, co-morbidity, and different distribution of prognostic factors among males and females.
Our study has several strengths, including its large size and high-quality data from Sweden in a stable population with access to standardized universal medical health care during the entire study period. Through our efforts at combining multiple sources, we believe we have assembled a cohort comprising almost all patients diagnosed with LPL/WM during the study period. Furthermore, the use of the nationwide register-based case-control design rules out recall bias and avoids the selection bias inherent in comparison of clinical trials from different time periods.
Limitations of our study include lack of clinical data, which does not allow risk assessment by stage, lack of information on whether patients were symptomatic or not, and absence of a systematic blinded validation of all LPL/WM diagnoses. Because of the large study size, we were not able to validate individual medical records. An inherent limitation of our study, which includes patients who were diagnosed with LPL/WM during a 25-year study period, is that diagnostic criteria have changed over time. However, in our large nationwide study on the ascertainment and diagnostic accuracy of lymphoproliferative malignancies that were diagnosed in Sweden, we found that the diagnostic accuracy for LPL/WM was 93% [17]. Furthermore, due to the underreporting of LPL/WM to the cancer registry, we used two sources for capturing cases. These were not evenly distributed through the study period which may bias our results. However, as the distribution was stable after 1990, we performed sensitivity analyses based only on LPL/WM patients diagnosed after 1990 and found virtually the same results. In addition, there was no evidence for confounding by source.
In conclusion, we observed a continuous improvement over time during a 25-year period in LPL/WM patients. Although the greatest improvement was observed from calendar periods 1980–1985 to 1986–1990, the improvement has continued into the last calendar period (2001–2005). We have no information on the treatment of individual patients but one can speculate that both better supportive care and introduction of more effective treatment regimens have contributed. Based on our improved understanding of the biology of monoclonal gammopathies, the paradigm for treatment has dramatically changed, and a number of novel therapeutic agents are now available. An increase in the use of novel agents and combination therapies is promising for LPL/WM patients and the effect on survival needs to be evaluated in future population-based studies. Indeed, the success of targeted therapy in multiple myeloma has led to the development and investigation of over 30 new compounds in multiple myeloma and in other plasma-cell dyscrasias including WM. Increased knowledge on risk stratifications and optimization of combination of new therapies is needed. Novel drug developments in phase I/II studies, in parallel with collaborative randomized phase III clinical trials, are warranted in this rare disease.
Acknowledgments
This research was supported by grants from the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the Cancer Society in Stockholm, and the Intramural Research Program of the NIH, NCI. The authors thank Ms. Shiva Ayobi, The National Board of Health and Welfare, Stockholm, Sweden; Ms. Susanne Dahllöf, Statistics Sweden, Orebro, Sweden; and Ms. Emily Steplowski, Information Management Services, Silver Spring, MD, for important efforts in the development of this database.
Footnotes
Conflict of Interest
The authors have no conflict of interests relevant to this paper.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 2.Kristinsson SY, Bjorkholm M, Goldin LR, et al. Risk of lymphoproliferative disorders among first-degree relatives of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia patients: A population-based study in Sweden. Blood 2008;112:3052–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waldenström J Incipient myelomatosis or ‘essential’ hyperglobulinemia with fibrinogenopenia: A new syndrome? Acta Med Scand 1944;117:216–247. [Google Scholar]
- 4.Morel P, Duhamel A, Gobbi P et al. International prognostic scoring system for Waldenstrom macroglobulinemia. Blood 2009;113:4163–4170. [DOI] [PubMed] [Google Scholar]
- 5.Dimopoulos MA, Gertz MA, Kastritis E, et al. Update on treatment recommendations from the Fourth International Workshop on Waldenstrom’s Macroglobulinemia. J Clin Oncol 2009;27:120–126. [DOI] [PubMed] [Google Scholar]
- 6.Treon SP How I treat Waldenstrom macroglobulinemia. Blood 2009;114: 2375–2385. [DOI] [PubMed] [Google Scholar]
- 7.Facon T, Brouillard M, Duhamel A, et al. Prognostic factors in Waldenstrom’s macroglobulinemia: A report of 167 cases. J Clin Oncol 1993;11:1553–1558. [DOI] [PubMed] [Google Scholar]
- 8.Morel P Monconduit M, Jacomy D, et al. Prognostic factors in Waldenström macroglobulinemia: a report on 232 patients with the description of a new scoring system and its validation on 253 other patients. Blood 2000;96:852–858. [PubMed] [Google Scholar]
- 9.Merlini G, Baldini L, Broglia C, et al. Prognostic factors in symptomatic Waldenstrom’s macroglobulinemia. Semin Oncol 2003;30:211–215. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Sanz R, Montoto S, Torrequebrada A, et al. Waldenstrom macroglobulinaemia: Presenting features and outcome in a series with 217 cases. Br J Haematol 2001;115:575–582. [DOI] [PubMed] [Google Scholar]
- 11.Dhodapkar MV, Jacobson JL, Gertz MA, et al. Prognostic factors and response to fludarabine therapy in Waldenstrom’s macroglobulinemia: An update of a US intergroup trial (SW0G S9003). Semin Oncol 2003;30:220–225. [DOI] [PubMed] [Google Scholar]
- 12.Dimopoulos MA, Panayiotidis P Moulopoulos LA, et al. Waldenstrom’s macroglobulinemia: Clinical features, complications, and management. J Clin Oncol 2000;18:214–226. [DOI] [PubMed] [Google Scholar]
- 13.Gobbi PG, Bettini R, Montecucco C, et al. Study of prognosis in Waldenstrom’s macroglobulinemia: A proposal for a simple binary classification with clinical and investigational utility. Blood 1994;83:2939–2945. [PubMed] [Google Scholar]
- 14.Bjorkholm M, Johansson E, Papamichael D, et al. Patterns of clinical presentation, treatment, and outcome in patients with Waldenstrom’s macroglobulinemia: A two-institution study. Semin Oncol 2003;30:226–230. [DOI] [PubMed] [Google Scholar]
- 15.Kastritis E, Kyrtsonis MC, Hatjiharissi E, et al. No significant improvement in the outcome of patients with Waldenstrom’s macroglobulinemia treated over the last 25 years. Am J Hematol 2011;86:479–483. [DOI] [PubMed] [Google Scholar]
- 16.Kristinsson SY, Koshiol J, Bjorkholm M, et al. Immune-related and inflammatory conditions and risk of lymphoplasmacytic lymphoma or Waldenstrom macroglobulinemia. J Natl Cancer Inst 2010;102:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turesson I, Linet MS, Bjorkholm M, et al. Ascertainment and diagnostic accuracy for hematopoietic lymphoproliferative malignancies in Sweden 1964–2003. Int J Cancer 2007;121:2260–2266. [DOI] [PubMed] [Google Scholar]
- 18.Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med 2006;260:103–117. [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, Alexanian R. Waldenstrom’s macroglobulinemia. Blood 1994;83:1452–1459. [PubMed] [Google Scholar]
- 20.Foran JM, Rohatiner AZ, Coiffier B, et al. Multicenter phase II study of fludarabine phosphate for patients with newly diagnosed lymphoplasmacytoid lymphoma, Waldenstrom’s macroglobulinemia, and mantle-cell lymphoma. J Clin Oncol 1999;17:546–553. [DOI] [PubMed] [Google Scholar]
- 21.Liu ES, Burian C, Miller WE, et al. Bolus administration of cladribine in the treatment of Waldenstrom macroglobulinaemia. Br J Haematol 1998;103:690–695. [DOI] [PubMed] [Google Scholar]
- 22.Dimopoulos MA, Zervas C, Zomas A, et al. Treatment of Waldenstrom’s macroglobulinemia with rituximab. J Clin Oncol 2002;20:2327–2333. [DOI] [PubMed] [Google Scholar]
- 23.Treon SP, Hunter Z, Barnagan AR. CHOP plus rituximab therapy in Waldenstrom’s macroglobulinemia. Clin Lymphoma 2005;5:273–277. [DOI] [PubMed] [Google Scholar]
- 24.Treon SP, Branagan AR, Ioakimidis L, et al. Long term outcomes to fludarabine and rituximab in Waldenstrom macroglobulinemia. Blood 2009;113: 3673–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Treatment of relapsed or refractory Waldenstrom’s macroglobulinemia with bortezomib. Haematologica 2005;90:1655–1658. [PubMed] [Google Scholar]
- 26.Chen CI, Kouroukis CT, White D, et al. Bortezomib is active in patients with untreated or relapsed Waldenstrom’s macroglobulinemia: A phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1570–1575. [DOI] [PubMed] [Google Scholar]
- 27.Dimopoulos MA, Zomas A, Viniou NA, et al. Treatment of Waldenstrom’s macroglobulinemia with thalidomide. J Clin Oncol 2001;19:3596–3601. [DOI] [PubMed] [Google Scholar]
- 28.Treon SP, Hatjiharissi E, Leleu X, et al. Novel agents in the treatment of Waldenstrom’s macroglobulinemia. Clin Lymphoma Myeloma 2007;7(suppl 5): S199–S206. [DOI] [PubMed] [Google Scholar]
- 29.Treon SP, Hunter ZR, Matous J, et al. Multicenter clinical trial of bortezomib in relapsed/refractory Waldenstrom’s macroglobulinemia: Results of WMCTG Trial 03–248. Clin Cancer Res 2007;13:3320–3325. [DOI] [PubMed] [Google Scholar]
- 30.Gilleece MH, Pearce R, Linch DC, et al. The outcome of haemopoietic stem cell transplantation in the treatment of lymphoplasmacytic lymphoma in the UK: A British Society Bone Marrow Transplantation study. Hematology 2008;13:119–127. [DOI] [PubMed] [Google Scholar]
- 31.Anagnostopoulos A, Hari PN, Perez WS, et al. Autologous or allogeneic stem cell transplantation in patients with Waldenstrom’s macroglobulinemia. Biol Blood Marrow Transplant 2006;12:845–854. [DOI] [PubMed] [Google Scholar]
- 32.Kyriakou C, Canals C, Cornelissen JJ, et al. Allogeneic stem-cell transplantation in patients with Waldenstrom macroglobulinemia: Report from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2010;28:4926–4934. [DOI] [PubMed] [Google Scholar]
- 33.Kyriakou C, Canals C, Sibon D, et al. High-dose therapy and autologous stem-cell transplantation in Waldenstrom macroglobulinemia: The Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol 2010;28:2227–2232. [DOI] [PubMed] [Google Scholar]
- 34.Garnier A, Robin M, Larosa F, et al. Allogeneic hematopoietic stem cell transplantation allows long-term complete remission and curability in high-risk Waldenstrom’s macroglobulinemia. Results of a retrospective analysis of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Haematologica 2010;95:950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hensel M, Brust J, Ploger C, et al. Excellent long-term survival of 170 patients with Waldenstrom’s macroglobulinemia treated in private oncology practices and a university hospital. Ann Hematol 2012. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36.Kristinsson SY, Landgren O, Dickman PW, et al. Patterns of survival in multiple myeloma: A population-based study of patients diagnosed in Sweden from 1973 to 2003. J Clin Oncol 2007;25:1993–1999. [DOI] [PubMed] [Google Scholar]
- 37.Schop RF, Kuehl WM, Van Wier SA, et al. Waldenstrom macroglobulinemia neoplastic cells lack immunoglobulin heavy chain locus translocations but have frequent 6q deletions. Blood 2002;100:2996–3001. [DOI] [PubMed] [Google Scholar]
- 38.Kristinsson SY, Dickman PW, Wilson WH, et al. Improved survival in chronic lymphocytic leukemia in the past decade: A population-based study including 11,179 patients diagnosed between 1973–2003 in Sweden. Haematologica 2009;94:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]