Abstract
Background:
Infantile hemangioma is the most common tumor of infancy. Currently, propranolol is a preferred drug for treating hemangioma. The exact mechanism of action of propranolol is not known. In this study, we attempted to assess whether propranolol has any effect on vascular endothelial growth factor (VEGF) and tissue inhibitor of metalloproteinase-2 (TIMP-2) over a period of time, and if it is there, how long it affects it.
Materials and Methods:
Propranolol was administered in the dosage of 2–3 mg/kg. The first serum sample was collected before starting the propranolol treatment. Thereafter, samples were collected at monthly intervals up to a total of six samples. The samples were assessed for TIMP-2 and VEGF using enzyme-linked immunosorbent assay kit.
Results:
The duration of this study was from June 2016 to November 2017. The total number of patients in this study was 15. Thirteen patients responded to treatment. The mean age of patients was 7.1 months. The mean value of baseline VEGF was 0.234 ± 0.059 and that of TIMP-2 was 1.338 ± 0.679. As compared to baseline value, the P value was statistically not significant in any of sequential values. In category-wise analysis, apart from statistically significant value in the 6th month in excellent category and good response category in the 1st month, all other values did not reveal any significant change in VEGF analysis. The analysis of TIMP-2 revealed a significant change in the levels from Sample 2 to Sample 6 in the excellent response group; however, the levels did not show a specific trend either increasing or decreasing.
Conclusion:
Despite its beneficial action in regression of hemangioma, the exact mechanism is yet to be identified. The exact duration of treatment needs further evaluation.
KEYWORDS: Infantile hemangioma, propranolol, response, tissue inhibitors of metalloproteinases, vascular endothelial growth factor
INTRODUCTION
Infantile hemangioma (IH), the most common tumor of infancy, is vascular tumor characterized by rapid proliferation of endothelial cells during the first few months of postnatal life, followed by slow involution.[1] The usual tendency is to involute, but intervention is needed in up to 20% of cases.[2]
Stimulators of angiogenesis such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), mono cytochrome attractant protein-1, and insulin-like growth factor-2 are upregulated [3] in proliferating hemangioma. As the endothelial cells differentiate an influx of mast cells, various cells and tissue inhibitors of metalloproteinases (TIMPs) occur.[4] TIMP along with interferon and transforming growth factor produced by mast cells terminates the endothelial cell proliferation and passively induces the involution of senescence of endothelial cells. TIMPs (TIMP-1, TIMP-2, TIMP-3, and TIMP-4) are a family of intrinsic inhibitors of matrix metalloproteinases (MMPs).[5] Of these, TIMP-1 and TIMP-2 have been found to be increased during the involution phase of IH.[4,6,7] In current studies, this increase has been observed in the tissue samples.
With the serendipitously identified role of propranolol in the treatment of IH,[8] it has now become as the preferred drug for the treatment of both hemangioma and its complications such as ulceration. There have been studies suggesting the action of propranolol on the VEGF.[9,10,11,12,13] However, it has not specifically mentioned as to how long the treatment with propranolol affects the VEGF. However, the effect of propranolol on factor responsible for the involution of IH, TIMP-1 and TIMP-2, has not been evaluated in the literature. Besides, no study has evaluated the serum levels of TIMP in patients of IH.
In this study, we attempted to assess whether propranolol has any effect on serum levels of VEGF and TIMP-2 over a period of time, and if it is there, how long it affects it.
MATERIALS AND METHODS
This study was conducted in the pediatric surgery department of a medical university in collaboration with the department of pathology. It was a prospective cohort study. The study was approved by the hospital ethical committee. Inclusion criteria included all new patients of IH in need of treatment either due to cosmetic reasons or due to complications such as ulceration. The consent of patients' attendant was obtained. We followed the REporting recommendations for tumour MARKer (REMARK) prognostic studies protocol endorsed by the EQUATOR Network for conducting this study.
Before starting the propranolol treatment, the patients were assessed for the blood glucose and serum electrolyte levels. These levels were assessed at every monthly visit. Any history of asthma was ruled out on the basis of history. All patients were evaluated by two-dimensional echo and electrocardiogram for any cardiac problem.
Procedure
Propranolol was administered in the dosage of 1–2 mg/kg body weight, which was increased to 2–3 mg/kg body weight over a period of 3–5 days. During this period, the patients were admitted in ward for observation to look for and treat any unwanted complication, should it arise [Figure 1]. The patients were discharged after that and called at monthly visit for evaluation and sample collection. The first serum sample was collected just before starting the propranolol treatment. Thereafter, samples were collected at monthly intervals up to a total of six samples. Since enzyme-linked immunosorbent assay (ELISA) kit contained 96 wells, 15 patients were taken as the maximum number for final analysis.
Figure 1.
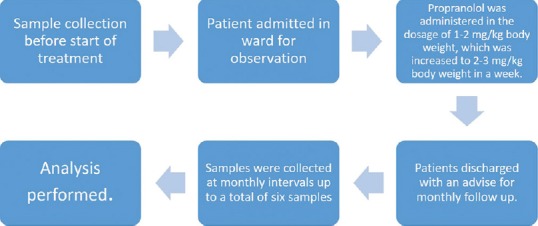
Management algorithm of patients of hemangioma
Sample evaluation
The samples were transported to the clinical biochemistry laboratory in the pathology department and stored in deep freezer at −20°C. The samples were assessed for the levels of TIMP-2 and VEGF using ELISA kit (RayBio® Human TIMP-2 ELISA Kit and RayBio® Human VEGF ELISA Kit, RayBiotech, Norcross, GA 30092, USA).
The procedure for evaluation of both TIMP-2 and VEGF is detailed below.
Reagent preparation
All samples were brought to 18°C–25°C temperature. Assay diluent B of kit was prepared in 1:5 dilution. Assay diluent A of kit was used for dilution of serum in 1:5 dilution. Calibrator (standard protein – Item C of kit) was prepared by spinning a vial of it and adding 640 μl of diluent A. Appropriate serial dilutions were made to establish the dilution range of the samples at which the concentration of the sample was in the linear range of the standard curve. Wash buffer was prepared in 1:20 dilution.
Detection antibody concentrate preparation was done by spinning the detection antibody vial of kit and addition of 100 μl of assay diluent B. Dose for ninety samples was calculated. Horseradish peroxidase (HRP)-streptavidin concentrate vial of kit was mixed gently and diluted 300 folds with assay diluent B.
Assay procedure
One hundred microliters of calibrator was added to wells and incubated for 2.5 h at room temperature. It was cleaned by wash buffer, followed by addition of 100 μl of antibody to each well and incubated for 1 h. Cleaning with wash buffer was repeated. Thereafter, 100 μl of streptavidin solution was added to each well and incubated for 45 min. Washing was again performed, which was followed by addition of 100 μl of tetramethylbenzidine substrate reagent to each well and incubated for 30 min. Finally, 50 μl of stop solution was added to each well, and reading was made immediately at 450 nm.
Clinical assessment
The clinical outcome was assessed as per the criteria mentioned before.[1] The regression was evaluated by cessation of growth, lightening of color, and flattening of surface. The cessation of growth was evaluated by measuring the lesion in two maximal dimensions perpendicular to each other, before and after the initiation of the treatment. The response was graded as:
Excellent – >75% regression without any significant scarring
Good – 50%–75% regression with or without scarring
Poor – 25%–50% regression with or without scarring
No response – <25% or no regression.
Responses 1 and 2 were taken as responded groups and 3 and 4 as nonresponding groups. Response 1 was placed in Category 1; Response 2 was placed in Category 2; Groups 3 and 4 were placed in Category 3 [Figure 2].
Figure 2.
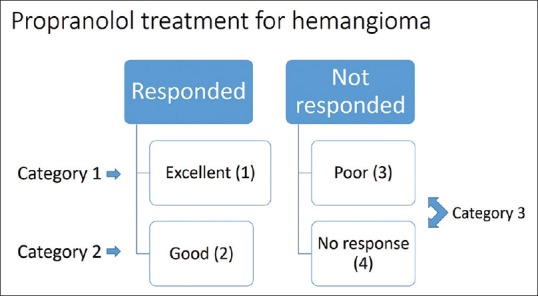
Flowchart showing placement of patients in various categories as per the response
Data analysis
All the details were entered into the Microsoft Excel datasheet. The result was analyzed using IBM SPSS Statistics for Windows, Version 16.0. (SPSS Inc., Chicago, IL, USA). The unit of measurement was picogram, and values are presented as mean ± standard deviation. Student's t-test was applied to compare results before and after the intervention at different intervals. One-way analysis of variance was used for category-wise analysis of response. P < 0.05 was taken as statistically significant.
RESULTS
The duration of this study was from June 2016 to November 2017. Of the 15 patients, which we evaluated, 11 were female and 4 were male. None of them had any cardiac problem. The mean age of patients was 7.1 months (range: 1–11 months). Thirteen patients responded to treatment (excellent – 4 and good – 9), [Figure 3].
Figure 3.
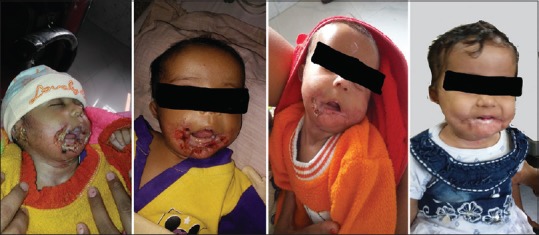
A patient of ulcerated hemangioma on propranolol treatment showing good response over a period of 6 months. the first frame is at 5 months, while the last one is at 11 months
The minimum size of hemangioma was 20 cm2. It was >100 cm2 in one patient. Hence, there was a wide variation in size. The involved parts included head and neck (9), upper limb (3), and back (3). There was ulceration in two patients. It was superficial hemangioma in 9 and mixed in 6 patients. There was no patient of deep hemangioma. The three patients who showed poor response were of mixed type [Table 1].
Table 1.
Demographic profile of patients of hemangioma
| Age (months) | Sex | Location | Type | Complication | Response |
|---|---|---|---|---|---|
| 8 | Female | Right cheek | Mixed | None | Good |
| 5 | Female | Left infraorbital | Superficial | None | Good |
| 7 | Male | Forehead | Superficial | None | Excellent |
| 8 | Male | Left supra-auricular | Mixed | None | Good |
| 5 | Female | Face | Superficial | Extensive ulceration | Good |
| 6 | Female | Scalp frontal region | Superficial | None | Excellent |
| 11 | Female | Right arm | Mixed | None | Poor |
| 1 | Female | Right temporal | Superficial | None | Excellent |
| 11 | Male | Back | Superficial | None | Excellent |
| 5 | Female | Right temporal and occipital region | Superficial | Ulceration | Good |
| 10 | Female | Back | Mixed | None | Good |
| 7 | Female | Right leg | Superficial | None | Good |
| 3 | Male | Left cheek | Superficial | None | Good |
| 9 | Female | Left thigh and scalp | Mixed | None | Good |
| 10 | Female | Back | Mixed | None | Poor |
The mean value of baseline VEGF was 0.234 ± 0.059 (range: 0.114–0.291). The sequential monthly values were 0.211 ± 0.075 (range: 0.102–0.375), 0.227 ± 0.056 (range: 0.16–0.325), 0.210 ± 0.068 (range: 0.134–0.359), 0.205 ± 0.045 (range: 0.146–0.283), and 0.207 ± 0.066 (range: 0.137–0.341), respectively. Overall comparison of all patients showed that visible changes were observed in levels of VEGF. However, as compared to baseline value, the P value was statistically not significant in any of these sequential values (P > 0.05).
The mean value of baseline TIMP-2 was 1.338 ± 0.679 (range: 0.999–3.135). The sequential monthly values were 1.015 ± 0.313 (range: 0.149–1.26), 1.174 ± 0.091 (range: 1.047–1.303), 1.170 ± 0.138 (range: 1.017–1.449), 1.088 ± 0.115 (range: 0.929–1.295), and 1.155 ± 0.095 (range: 1.006–1.316), respectively. Like the VEGF values, visible changes were observed in levels of TIMP-2. However, as compared to baseline value, the P value was statistically not significant in any of these sequential values (P > 0.05).
Category-wise analysis was also done within the groups. The mean value of VEGF and TIMP-2 at different intervals was compared with baseline mean value for all the three categories. As regard to VEGF analysis, apart from statistically significant value in the 6th month in excellent category and good response category in the 1st month, all other values did not reveal any significant change. The analysis of TIMP-2 revealed a significant change in the levels from Sample 2 to Sample 6 in the excellent response group. No other group or sample revealed a significant change [Tables 2–5]. However, it is to be noted that despite a significant change in levels of TIMP-2 levels in the excellent group, the levels did not show a specific trend either increasing or decreasing. Hence, a definite correlation could not be established.
Table 2.
Serum levels of vascular endothelial growth factor in various response groups
| Category (number of patients) | Baseline sample | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
|---|---|---|---|---|---|---|
| Excellent response (4) | 0.297±0.034 | 0.278±0.0 62 | 0.258±0.0 36 | 0.246±0.0 26 | 0.167±0.0 52 | 0.150±0.0 38 |
| Good response (9) | 0.242±0.046 | 0.180±0.046 | 0.199±0.038 | 0.208±0.080 | 0.202±0.050 | 0.218±0.073 |
| Poor response (2) | 0.173±0.083 | 0.285±0.127 | 0.308±0.025 | 0.197±0.041 | 0.231±0.018 | 0.196±0.054 |
| Total (15) | 0.234±0.059 | 0.211±0.075 | 0.227±0.056 | 0.210±0.068 | 0.205±0.045 | 0.207±0.066 |
Values are expressed as Mean±SD. SD: Standard deviation
Table 5.
Analysis of variance for tissue inhibitor of metalloproteinase-2 levels in response groups
| Category (number of patients) | Baseline | Baseline and Sample 2 | Baseline and Sample 3 | Baseline and Sample 4 | Baseline and Sample 5 | Baseline and Sample 6 |
|---|---|---|---|---|---|---|
| Excellent response (4) | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | |
| Good response (9) | 0.838 | 0.574 | 0.782 | 0.783 | 0.641 | |
| Poor response (2) | 0.519 | 0.698 | 0.685 | 0.578 | 0.094 |
In excellent response group, there has been a significant change in the levels from Sample 2 to Sample 6. No other group or sample revealed a significant change
Table 3.
Analysis of variance for vascular endothelial growth factor levels in response groups
| Category (number of patients) | Baseline | Baseline and Sample 2 | Baseline and Sample 3 | Baseline and Sample 4 | Baseline and Sample 5 | Baseline and Sample 6 |
|---|---|---|---|---|---|---|
| Excellent response (4) | 0.610 | 0.166 | 0.055 | 0.006 | 0.001 | |
| Good response (9) | 0.027 | 0.081 | 0.349 | 0.145 | 0.476 | |
| Poor response (2) | 0.406 | 0.159 | 0.749 | 0.436 | 0.774 |
Apart from statistically significant value in the 6th month in excellent category and good response category in the 1st month, all other values did not reveal any significant change
Table 4.
Serum levels of tissue inhibitor of metalloproteinase-2 in various response groups
| Category (number of patients) | Baseline sample | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
|---|---|---|---|---|---|---|
| Excellent response (4) | 3.135±0.0 56 | 0.149±0.086 | 1.047±0.772 | 1.017±0.226 | 1.002±0.034 | 1.006±0.428 |
| Good response (9) | 1.136±0.304 | 1.111±0.088 | 1.205±0.086 | 1.170±0.094 | 1.101±0.125 | 1.193±0.083 |
| Poor response (2) | 1.146±0.022 | 1.113±0.056 | 1.129±0.049 | 1.243±0.291 | 1.085±0.129 | 1.095±0.009 |
| Total (15) | 1.338±0.679 | 1.015±0.313 | 1.174±0.091 | 1.170±0.138 | 1.088±0.115 | 1.155±0.095 |
Values are expressed as Mean±SD. SD: Standard deviation
DISCUSSION
Propranolol is a well-known nonselective beta-blocker. After its incidental discovery in 2008,[8] it has become the standard treatment for IH all around the world. Despite its continuous use, the exact mechanism of action of propranolol is not clear. Based on studies, it has been suggested that endothelial cells in proliferating IH are arrested in an early developmental stage of vascular differentiation.[3] Since angiogenic factors such as VEGF, bFGF, and others are raised during proliferation phase of IH,[6,10,14,15] researchers have focused their attention on the possibility of VEGF being the target for action of propranolol.
In a study of 22 patients, VEGF evaluation was conducted at 1 and 3 months, respectively. The authors observed a statistically significant decrease in VEGF levels at 1-month interval. The decrease continued at 3 months, although it was not statistically significant.[9] In another study comprising 97 patients, the authors demonstrated a statistically significant decrease in the levels of not only VEGF but also that of bFGF and MMP after 2 months of propranolol treatment.[10] In contrast to these studies, the statistically significant decrease in VEGF levels was not noted at 1 month in a study of 35 patients.[13] However, the decrease was found to be statistically significant after 2 months of treatment with propranolol. Finally, there has been a study where decrease in VEGF levels was statistically significant at both 1- and 2-month intervals.[12]
From all of the above-discussed studies, it is evident that VEGF does decrease significantly after treatment with propranolol. However, it may occur at either 1- or 2-month interval or both. The current study differs from these by the fact that VEGF was measured sequentially for 6 months. We observed that at no point of time, the changes in the VEGF levels were statistically significant. In our study, when category-wise analysis was performed, it is to be noticed that there were statistically significant changes in the VEGF levels only at the 6th month and not at one or two months. Hence, to assume that propranolol affects VEGF on the basis of randomly taken two samples does not appear tenable. Propranolol not affecting VEGF was also demonstrated in a study of fifty patients.[16] Although there was a declining trend, there was no statistically significant decrease after 3 months of continuous therapy. Besides, this study contradicted the correlation of IH size and VEGF levels, which was found by Chen et al.[9]
Since TIMP-1 and TIMP-2 levels increase during the involution phase,[3,4,6,7,17] this decreases the activity of MMP, which is upgraded in the proliferative phase of IH. For this reason, we wished to assess whether propranolol, apart on a possible role in halting the proliferation phase, has a role in promoting or enhancing involution of IH. At the inception of this study, there was no previous work, which has evaluated the effect of propranolol on TIMP. While we assessed the TIMP-2, there may be a question as to why we did not go for TIMP-1 analysis. First, as literature points that both TIMP-1 and TIMP-2 are increased during involution, any of them may be assessed. Second, there has been extensive analysis, which points out that both TIMP-1 and TIMP-2 in serum are major serum factors that play important roles in inducing the TIMP-1 gene and causing secretion of TIMP-1 protein from Gin-1 cells.[5] Besides these, cost limitation did not allow us to evaluate both of them. We did not go for the tissue analysis, as almost all of our patients responded to propranolol, and it was not ethically possible to obtain samples from them.
In our study, we could not find any statistically significant change in the levels of TIMP-2 over a period of 6 months. As mentioned in the results, despite a significant change in its levels in the excellent response category, a definite trend could not be established. Hence, it appears that propranolol does not affect its action by promoting involution. In a very recent experimental study in human monocytes, it has been observed that propranolol inhibits MMP-9; however, its effect on TIMP-1 or TIMP-2 could not be confirmed.[18] As discussed above, propranolol has an effect on MMP.
Since there was no statistically significant change in the levels of VEGF and TIMP-2 over a period of 6 months, the rationale for a 6-month duration of propranolol treatment [19] could not be substantiated. It appears to be a recommendation, which has no definite proof. In fact, a very recent study suggests that treatment with propranolol may be continued beyond 6 months.[20]
The limitation of this study is small sample size. Cost constraints did not permit us to go beyond 15 patients. However, we feel that ninety samples may be adequate for analysis. We were not able to analyze tissue samples, where all of the studies have found that TIMP-1 or TIMP-2 is increased during involution. Besides, not studying TIMP-1 levels for certain reasons as mentioned earlier also limited our analysis. However, we feel that a new domain in the form of serum analysis is opened for further researches.
An important concern was the age of patients. In Western world, patients present early, and early initiation of treatment may be possible. In our setup, patients present to a specialized center at a later age (7 months in this study). This is due to multiple factors such as lack of information, economic reasons, and treatment taken from nearby centers who manage the condition inappropriately. We are unable to deal with delayed presentation. Still, as mentioned in the literature that 1st year is the proliferative phase in the natural cycle of IH,[4,21,22] we believe that there is always a possibility of response to propranolol and evaluation of the serum levels of VEGF and TIMP (TIMP-2 in our study).
CONCLUSION
On the basis of serum levels VEGF and TIMP-2, we did not find concrete evidence that propranolol acts by affecting serum VEGF or TIMP-2. Since the changes in serum levels did not change with the duration of treatment, especially for VEGF, the arbitrary limit of 6 months may need to be redefined. Further studies in this regard may substantiate our efforts.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The study has been funded by a grant from Research Cell, King George's Medical University, Lucknow, Uttar Pradesh, India.
REFERENCES
- 1.Pandey A, Gangopadhyay AN, Gopal SC, Kumar V, Sharma SP, Gupta DK, et al. Twenty years' experience of steroids in infantile hemangioma – A developing country's perspective. J Pediatr Surg. 2009;44:688–94. doi: 10.1016/j.jpedsurg.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 2.Hasan Q, Tan ST, Gush J, Peters SG, Davis PF. Steroid therapy of a proliferating hemangioma: Histochemical and molecular changes. Pediatrics. 2000;105:117–20. doi: 10.1542/peds.105.1.117. [DOI] [PubMed] [Google Scholar]
- 3.Dadras SS, North PE, Bertoncini J, Mihm MC, Detmar M. Infantile hemangiomas are arrested in an early developmental vascular differentiation state. Mod Pathol. 2004;17:1068–79. doi: 10.1038/modpathol.3800153. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Mulliken JB, Kozakewich HP, Rogers RA, Folkman J, Ezekowitz RA, et al. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93:2357–64. doi: 10.1172/JCI117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo XK, Zhao WQ, Kondo C, Shimojo N, Yamashita K, Aoki T, et al. Tissue inhibitors of metalloproteinases-1 (TIMP-1) and -2(TIMP-2) are major serum factors that stimulate the TIMP-1 gene in human gingival fibroblasts. Biochim Biophys Acta. 2006;1763:296–304. doi: 10.1016/j.bbamcr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Przewratil P, Sitkiewicz A, Andrzejewska E. Local serum levels of vascular endothelial growth factor in infantile hemangioma: Intriguing mechanism of endothelial growth. Cytokine. 2010;49:141–7. doi: 10.1016/j.cyto.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Blanke K, Dähnert I, Salameh A. Role of connexins in infantile hemangiomas. Front Pharmacol. 2013;4:41. doi: 10.3389/fphar.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–51. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 9.Chen XD, Ma G, Huang JL, Chen H, Jin YB, Ye XX, et al. Serum-level changes of vascular endothelial growth factor in children with infantile hemangioma after oral propranolol therapy. Pediatr Dermatol. 2013;30:549–53. doi: 10.1111/pde.12192. [DOI] [PubMed] [Google Scholar]
- 10.Wu S, Wang B, Chen L, Xiong S, Zhuang F, Huang X, et al. Clinical efficacy of propranolol in the treatment of hemangioma and changes in serum VEGF, bFGF and MMP-9. Exp Ther Med. 2015;10:1079–83. doi: 10.3892/etm.2015.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bin L, Manli C, Jie L, Xiaopeng Y, Zhaoquan L, Zhongcheng G, et al. [Expression of serum and urinary vascular endothelial growth factor-A and epidermal growth factor-like domain 7 in proliferating hemangioma treated with propranolol] Hua Xi Kou Qiang Yi Xue Za Zhi. 2014;32:441–5. doi: 10.7518/hxkq.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao ZF, Lü RR, Huo R, Fu HB, Xu GQ. The change of serum vascular endothelial growth factor and matrix metalloproteinases-9 in proliferative hemangioma treated with propranolol. Zhonghua Zheng Xing Wai Ke Za Zhi. 2011;27:359–61. [PubMed] [Google Scholar]
- 13.Yuan WL, Jin ZL, Wei JJ, Liu ZY, Xue L, Wang XK, et al. Propranolol given orally for proliferating infantile haemangiomas: Analysis of efficacy and serological changes in vascular endothelial growth factor and endothelial nitric oxide synthase in 35 patients. Br J Oral Maxillofac Surg. 2013;51:656–61. doi: 10.1016/j.bjoms.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Kleinman ME, Greives MR, Churgin SS, Blechman KM, Chang EI, Ceradini DJ, et al. Hypoxia-induced mediators of stem/progenitor cell trafficking are increased in children with hemangioma. Arterioscler Thromb Vasc Biol. 2007;27:2664–70. doi: 10.1161/ATVBAHA.107.150284. [DOI] [PubMed] [Google Scholar]
- 15.Przewratil P, Sitkiewicz A, Wyka K, Andrzejewska E. Serum levels of vascular endothelial growth factor and basic fibroblastic growth factor in children with hemangiomas and vascular malformations – Preliminary report. Pediatr Dermatol. 2009;26:399–404. doi: 10.1111/j.1525-1470.2009.00910.x. [DOI] [PubMed] [Google Scholar]
- 16.Przewratil P, Kobos J, Wnęk A, Szemraj J, Wyrzykowski D, Chrzanowska B, et al. Serum and tissue profile of VEGF and its receptors VGFR1/R2 in children with infantile hemangiomas on systemic propranolol treatment. Immunol Lett. 2016;175:44–9. doi: 10.1016/j.imlet.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Vergani V, Garofalo A, Bani MR, Borsotti P, Parker MP, Drudis T, et al. Inhibition of matrix metalloproteinases by over-expression of tissue inhibitor of metalloproteinase-2 inhibits the growth of experimental hemangiomas. Int J Cancer. 2001;91:241–7. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1035>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 18.Yin X, Zhou L, Han F, Han J, Zhang Y, Sun Z, et al. Beta-adrenoceptor activation by norepinephrine enhances lipopolysaccharide-induced matrix metalloproteinase-9 expression through the ERK/JNK-c-Fos pathway in human THP-1 cells. J Atheroscler Thromb. 2017;24:55–67. doi: 10.5551/jat.35204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leaute-Labreze C. Propranolol and beta-blockers in the medical management of infantile hemangioma. In: Matttassi R, Loose DA, Vaghi M, editors. Hemangioma and Vascular Malformations. 2nd ed. London, UK: Springer-Verlag; 2015. pp. 97–102. [Google Scholar]
- 20.Baselga E, Dembowska-Baginska B, Przewratil P, González-Enseñat MA, Wyrzykowski D, Torrelo A, et al. Efficacy of propranolol between 6 and 12 months of age in high-risk infantile hemangioma. Pediatrics. 2018;142:pii: e20173866. doi: 10.1542/peds.2017-3866. [DOI] [PubMed] [Google Scholar]
- 21.Kulungowski AM, Fishman SJ. Vascular anomalies. In: Coran AG, Adzick NS, Krummel TM, Laberge J, Shamberger RC, Caldmone AA, editors. Pediatric Surgery. 7th ed. Philadelphia PA: Elsevier Saunders; 2012. pp. 97–102. [Google Scholar]
- 22.Limaye N, Vikkula M. Molecular and genetic aspects of hemangioma and vascular malformation. In: Matttassi R, Loose DA, Vaghi M, editors. Hemangioma and Vascular Malformations. 2nd ed. London UK: Springer-Verlag; 2015. pp. 97–102. [Google Scholar]


