Summary
Programmed death-1 receptor (PD-1) is expressed on T cells following TCR activation. Binding of this receptor to its cognate ligands, PD-L1 and PD-L2, down-regulates signals by the TCR, promoting T-cell anergy and apoptosis, thus leading to immune suppression. Here, we find that using an anti-PD-1 antibody (CT-011) with T-regulatory (Treg) cell depletion by low dose cyclophosphamide (CPM), combined with a tumor vaccine induces synergistic antigen-specific immune responses and reveals novel activities of each agent in this combination. This strategy leads to complete regression of established tumors in a significant percentage of treated animals, with survival prolongation. We show for the first time that combining CT-011 and CPM significantly increases the number of vaccine-induced tumor-infiltrating CD8+ T cells, with simultaneous decrease in infiltrating Treg cells. Interestingly, we find that CT-011 prolongs Treg inhibition induced by CPM, leading to a sustainable significant synergistic decrease of splenic and tumor-infiltrated T-regulatory cells. Surprisingly, we find that the anti-tumor effect elicited by the combination is dependant on both CD8+ and CD4+ T cell responses, although the antigen we used is a class I MHC-restricted peptide. Thus, we describe a novel and effective therapeutic approach by combining multiple strategies to target several tumor-mediated immune inhibitory mechanisms.
Keywords: PD-1, vaccine, cancer immunotherapy
Introduction
Immune suppression/evasion is one of the major impediments to the development of effective immune therapy for cancer. Programmed death-1 receptor (PD-1) is a member of the B7 family that is expressed on activated T cells and is found to play an important role in immune evasion. On binding its cognate ligands PD-L1 or PD-L2, PD-1 down-regulates signaling by the T-cell receptor (TCR), inducing T-cell anergy and apoptosis and thus leading to immune suppression [1–6]. Many human malignancies up regulate PD-L1, and this up regulation has been directly correlated with immune suppression and poor prognosis in several types of cancer [4, 7–11]. The PD-1/PDL-1 interaction leads to suppression and apoptosis of tumor-infiltrating effector lymphocytes in the tumor microenvironment [12, 13]. Furthermore, PD-L1 was found to be an anti-apoptotic receptor on tumor cells, functioning as an “immune shield” and protecting tumor cells from T cell cytotoxicity [14–16]. More recently, it was found that blocking the PD-1/PD-L1 interaction promotes antigen-specific cytotoxic T lymphocyte (CTL) proliferation by heightening CTL resistance to regulatory T cell inhibition, and limiting the inhibitory ability of regulatory T cells [17].
Regulatory T cells (Treg) are inhibitory CD4+ T-cells that are increased in cancer patients and can potentially form a barrier to eliciting effective immune response [17–22]. Not surprisingly, the inactivation or depletion of Treg cells has been actively pursued, in order to develop more potent anti-tumor immunotherapies. In several studies, antibodies against the CD25 cell surface marker have been used to examine the feasibility of enhancing anti-tumor responses through the inhibition of regulatory cell activity. Depletion of Treg cells by anti-CD25 antibodies has led to enhanced immunity in several tumor models [23–25]. One major obstacle for using this approach is that activated CD4+ and CD8+ cells also express CD25, and use of anti-CD25 antibodies might also affect these cells. Use of other cell markers, such as CTLA-4, may also be insufficient since it was previously demonstrated that Treg cells from CTLA-4 knockout mice maintain their suppressive function [26, 27].
Cyclophosphamide (CPM) has been used as a standard alkylating chemotherapeutic agent against certain solid tumors and lymphomas because of its direct cytotoxic effect and its inhibitory activity against actively dividing cells [28]. While high doses of CPM may lead to the depletion of immune cells, low doses of CPM have been shown to enhance immune responses and induce anti-tumor immune-mediated effects by reducing the number and function of Treg cells [27, 29–33].
Here we hypothesize that combining inhibition of Treg cells with strategies that block the PD-1/PDL-1 interaction and vaccine would result in a potent anti-tumor immunotherapeutic strategy. CT-011 is a novel humanized IgG1 kappa recombinant monoclonal anti-PD-1 antibody that has been shown to promote anti-tumor immunity in animal models and in a Phase I clinical trial in hematological malignancies [34].
We found that PD-1 blockade with low dose CPM, given in combination with vaccine, synergistically induces strong antigen-specific immune responses and increases CD8+ and CD4+FoxP3− T cell infiltration into the tumor, leading to a potent antitumor effect. Interestingly, we demonstrated that the efficacy of the combination relies not only on CD8+, but also on CD4+T cells. Furthermore, we found that the addition of CT-011 can enhance and prolong the effect of CPM induced Treg inhibition, simultaneously decreasing the levels of both tumor-infiltrated and splenic Tregs.
Thus, we showed for the first time that combining immune checkpoint inhibition (anti-PD-1) with Treg ablation (low dose CPM) in the setting of vaccine is a unique strategy that leads to an effective and clinically translatable approach for the treatment of established cancer.
Results
CT-011 and CPM synergistically promote tumor rejection when combined with peptide vaccine
In order to evaluate the antitumor efficacy of peptide vaccine in combination with anti-PD-1 treatment and Treg depletion with CPM, we used the TC-1 subcutaneous tumor model expressing HPV16 E7 antigen. We implanted a high number of tumor cells and chose a delayed treatment schedule to minimize the effect of vaccine and have more stringent conditions to test our treatment regimen.
Mice were implanted with 50,000 TC-1 tumor cells at day 0, and by day 7 established measurable tumors (~3–4mm in diameter) were treated with a single low dose of CPM or PBS followed by HPV16 E7 peptide vaccine or PBS in combination with CT-011 or IgG the next day. Two more doses of vaccine and CT-011 were given on days 15 and 22 after tumor implantation (Fig. 1A). Vaccine, CT-011 or CPM alone, as well as vaccine/CT-011, vaccine/CPM or CT-011/CPM treatments resulted in different levels of tumor growth inhibition, but none led to complete regression of tumors (Fig. 1B). On day 21 after tumor implantation, the last day when all mice from all groups were still alive, tumor volumes of mice treated with CT-011, E7 or CPM alone were smaller compared to non-treated mice (P<0.05, P<0.001 and P<0.001 respectively) (Fig. 1C). Notably, mice that received CPM, either alone or in combination with vaccine or CT-011, had smaller tumors and prolonged survival compared to other groups, but only the combination of anti-PD-1 antibody with CPM and vaccine resulted in complete tumor regression in 50% of mice and prolonged survival compared to all other treatments (Fig. 1B and 1D). These experiments demonstrate that targeting PD-1, combined with a single low dose of CPM enhances vaccine effect and allows the eradication of tumors even under stringent conditions.
Figure 1. Combination of vaccine, CT-011 and CPM provides potent antitumor immunity.
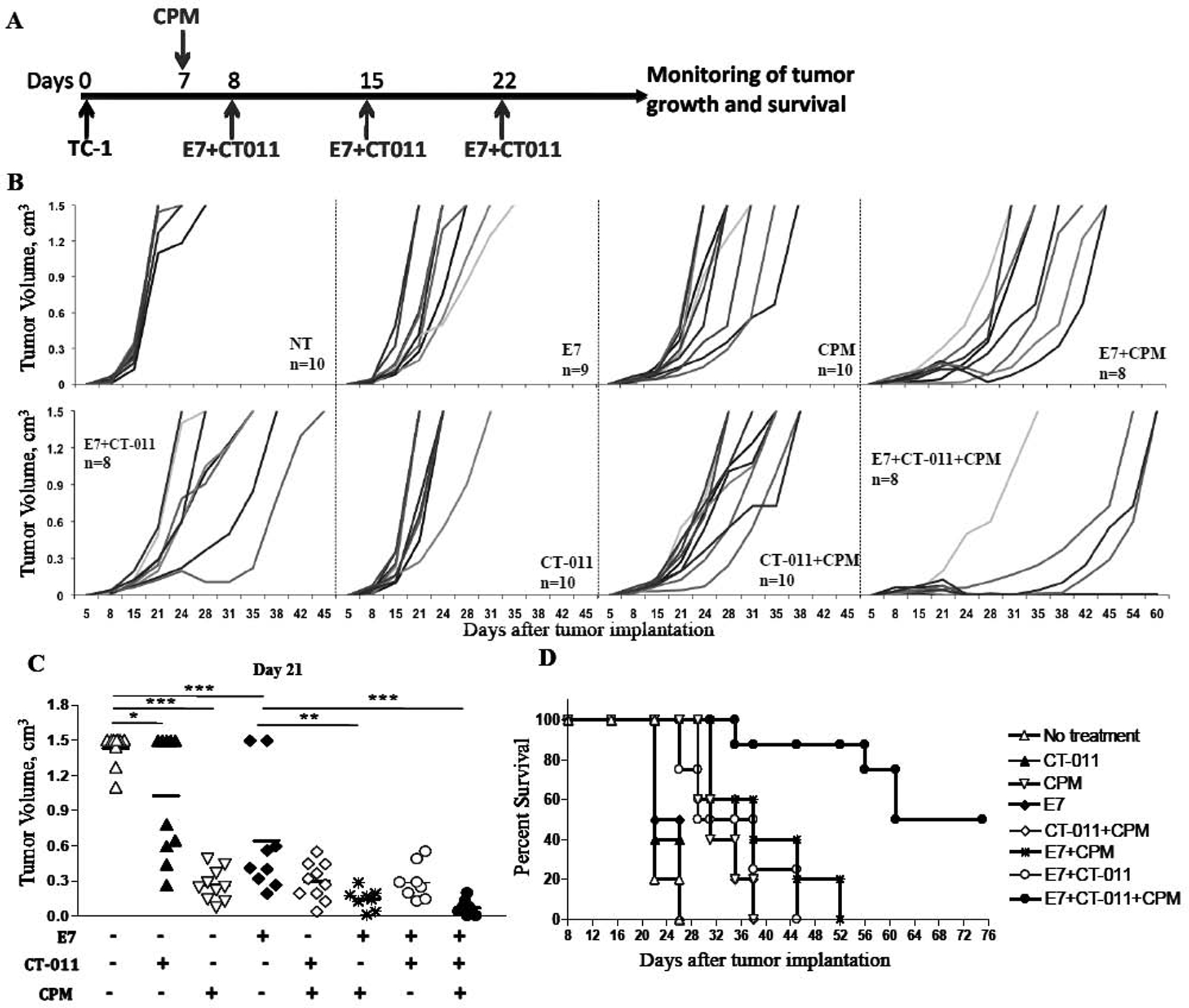
C57BL/6 mice (n = 8–10 per group) were injected s.c. in the right flank with 5×10e4 TC-1 cells. On day 7, CPM or PBS was injected. On day 8, 15 and 22 mice were injected with vaccine+CT-011, vaccine alone, CT-011 alone, PBS or IgG (A). Tumor sizes were measured periodically. (B) Plots represent tumor volumes of individual mice for each treatment. (C) Tumor volumes of individual mice on day 21, the last day when all mice from all groups were still alive. *P<0.05, **P<0.01 and ***P<0.001. (D) The Kaplan-Meier plot depicts overall survival. Similar results were obtained from two independent experiments.
CT-011 partially blocks tumor-induced suppression of Tconv cell proliferation in vitro
One known mechanism of action of anti-PD-1 antibodies in enhancing immune effect is by blocking the suppressive effect of the PD-1/PD-L1 interaction on T cell function, which has been shown to be beneficial in terms of elicitation of anti-tumor efficacy [35]. We found that PD-L1 is highly expressed on TC-1 cells (Fig. 2A). To test whether CT-011 is contributing to tumor response by blocking the effect of tumor on CD4+ T cells, we tested the ability of CT-011 antibody to block/inhibit the tumor-mediated suppression of T cell proliferation. We co-incubated TC-1 cells with TCR-stimulated CFSE-labeled CD4+CD25− Tconv cells in the presence and absence of CT-011 antibody and analyzed T cell proliferation. While at a 1:1 ratio TC-1 cells dramatically suppress proliferation of Tconv cells, CT-011 significantly recovers part of the ability of Tconv cells to proliferate (Fig. 2B and 2C) compared to isotype control antibody (P<0.001). Not surprisingly, when PD-L1-IgG protein was added instead of CT-011 into TC-1/Tconv cell co-culture, we observed further inhibition of Tconv cell proliferation, indicating the different mechanistic effects for CT-011 and PD-L1-IgG (Fig. 2B). These experiments demonstrate that blockade of PD-1/PD-L1 interaction with CT-011 partially overcomes one of the tumor-mediated inhibitory checkpoints.
Figure 2. CT-011 partially overcomes suppression of Tconv cell proliferation by PD-L1 expressing TC-1 tumor cells.
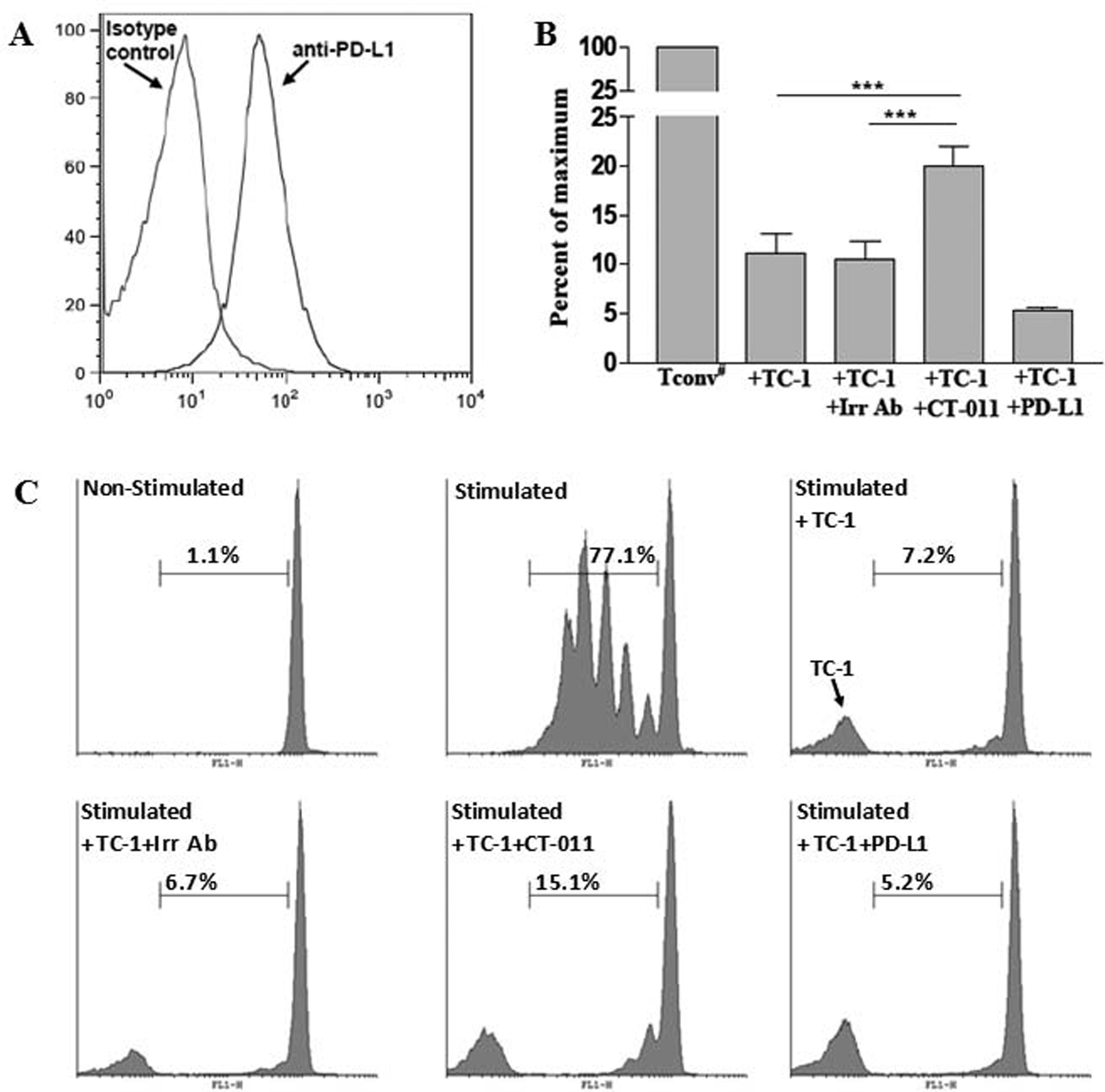
(A) TC-1 cells were stained with PE-labeled anti-PD-L1 antibody or isotype control antibody and analyzed on FACScan flow cytometer. (B) Purified CFSE labeled CD4+CD25− Tconv cells were stimulated with anti-CD3/anti-CD28 beads and co-incubated with TC-1 cells alone or in presence of CT-011, PD-L1-Ig or irrelevant IgG for 4 days. Proliferation percents were standardized based on the proliferation of Tconv cells in absence of TC-1 cells (100%). (C) Representative CFSE plots for non-stimulated and stimulated Tconv cells, as well as T conv cells coincubated with TC-1 cells alone or in presence of CT-011, PD-L1-Ig or irrelevant IgG. ***P<0.001. Similar results were obtained from two independent experiments.
The combination of CT-011 and CPM significantly enhances antigen-specific CD8+ T cell immune responses
To define the immune mechanisms of therapeutic effect of combining anti-PD-1 and CPM with HPV16 E7 antigen vaccine, we evaluated the antigen-specific IFNγ production and direct killing of target tumor cells by splenocytes from treated animals. Tumor-bearing mice were injected on day 7 with CPM or PBS, followed by vaccine or PBS and CT-011 or IgG injections on days 8 and 15. Six days after the second vaccine and CT-011 treatment, mice were sacrificed and production of IFNγ was analyzed. While vaccine alone, with CT-011 or with CPM induced comparable levels of IFNγ, vaccine combined with both CT-011 and CPM treatment significantly increased that level of IFNγ-producing cells compared to other treatments (P<0.001) (Fig. 3A). We observed the same pattern when we analyzed the direct killing of target TC-1 cells by freshly isolated splenocytes. The percent of activated caspase-3-positive target cells was significantly increased after co-incubation with splenocytes from mice treated with vaccine in combination with both CT-011 and CPM, compared to all other groups (P<0.001), at effector:target ratios of 50:1, 25:1 and 10:1 (Fig. 3B). Importantly, we observed a significant reverse correlation between tumor volume on day 21 and the number of IFNγ producing cells (R2= 0.8106, P<0.001) (Fig. 3C). No autoimmunity was detected in any of the treated mice. Thus, in these experiments we showed that combination of CT-011 and CPM significantly enhances vaccine-specific immune responses.
Figure 3. Vaccine/CT-011/CPM treatment induces strong E7-specific immune responses and decreases the level of splenic Treg cells.
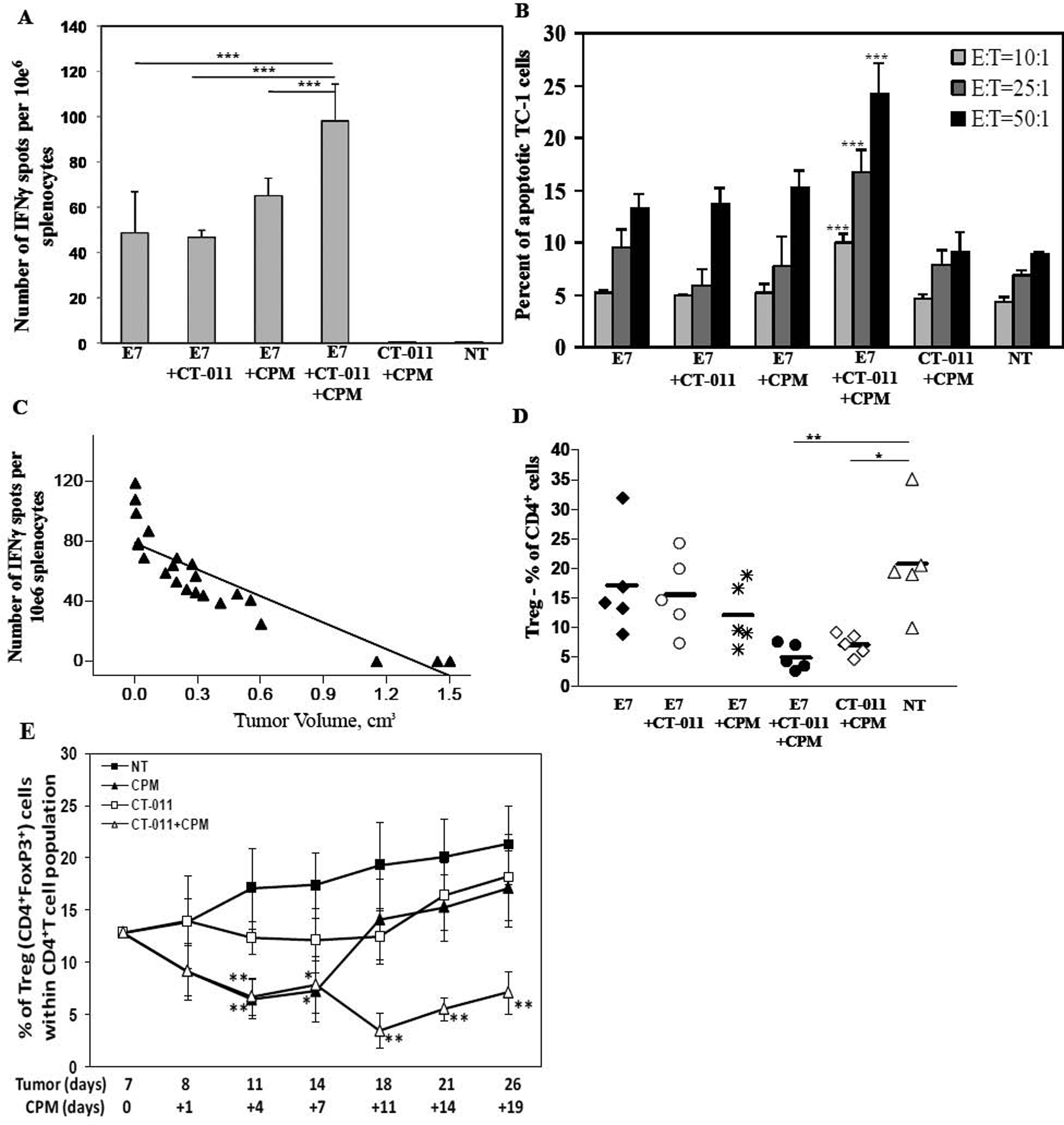
C57BL/6 mice (n=6 per group) were injected s.c. in the right flank with 5×10e4 TC-1 cells. On day 7, CPM or PBS was injected. On day 8 and 15 mice were injected with vaccine/CT-011, vaccine alone, CT-011 alone, PBS or IgG. On day 21 mice were sacrificed, IFNγ production in the presence or absence of E7 peptide was analyzed in single-cell suspension obtained from spleens. Values represent (A) number of spots from E7-re-stimulated culture minus that from irrelevant antigen re-stimulated culture (ranged 3–8 spots per million cells) ± SD. (B) Percentages of activated caspase-3 positive TC-1 cells after their co-incubation for 3h with freshly isolated splenocytes from experimental and control groups at effector:target ratios 50:1, 25:1 and 10:1 ± SD. (C) Number of spots plotted along with tumor volumes showing significant inverse correlation (P<0.01) with R2=0.8106. (D) The percentage of CD4+FoxP3+ cells (Treg cells) within CD4+ cell population of splenocytes from experimental and control groups. (E) Dynamics of splenic Treg level changes presented as the percentage of CD4+FoxP3+ cells within CD4+ cell population detected at different time-points. *P<0.05, **P<0.01 and ***P<0.001. Data shown are representative of two independent experiments.
CT-011 prolongs the suppressive effect of CPM on Treg cells
Since it is widely accepted that Treg cells potently inhibit immune response and comprise a major barrier to eliciting potent antitumor immunity [18], we analyzed the levels of CD4+FoxP3+ Treg cells in spleens of treated and control mice. Surprisingly, by day 21 after tumor implantation, a significant decrease of splenic Treg cell levels was observed only when mice were treated with CT-011 and CPM together. Neither combination of vaccine with CPM or with CT-011 show a significant decrease in splenic Treg levels on day 21 after tumor implantation (Fig. 3D), indicating that CT-011 and CPM exhibit synergistic effect in decreasing the level of Tregs. Importantly, no significant changes in total number of CD4+T cells were observed in treated animals compared to controls (data not shown). To further dissect the mechanism of this synergy, in a separate experiment we investigated the dynamics of splenic Treg level changes over time, after treatment with CPM, CT-011 or CPM/CT-011. It was previously reported that Treg cells nadir four days after CPM treatment to almost half of the level seen in untreated mice, and that they recover by day 10 to pretreatment level [27]. Similarly, we found that after treatment with CPM alone in tumor-bearing mice, the level of Treg cells is significantly decreased at day +4 after CPM treatment (days 11 and 14 after tumor implantation), and return to normal levels on day +11 of CPM (day 18 after tumor implantation) (Fig. 3E). Interestingly, we found that CT-011 alone does not affect the levels of Tregs in spleens. However, when CT-011 is given in combination with CPM it leads to a prolonged sustainable effect on Treg inhibition, with a synergistic effect at all time points analyzed up to day +19 of CPM treatment (day 26 after tumor implantation, Fig. 3E). Since non-treated mice did not survive longer than 26 days after tumor implantation, it was impossible to compare splenic Treg levels at later time-points.
Thus, in these experiments we showed that anti-PD-1 antibody given with low dose CPM maintains decreased levels of Treg cells in spleens of tumor-bearing mice.
Vaccine combination with both CT-011 and CPM increases CD8+/Treg and CD4+Foxp3−/Treg ratios within the tumors
After we showed that the combination of CT-011 and CPM with vaccine induces potent anti-tumor responses, we sought to dissect the effects of this therapy on the T-cell repertoire within the tumor. Mice were treated with CPM seven days after tumors were implanted and with HPV16 E7 peptide vaccine and CT-011 on days 8 and 15, with the appropriate controls. Mice were sacrificed on day 21 and tumor infiltration of CD8+, CD4+FoxP3− and CD4+FoxP3+ Treg cells was analyzed in tumor homogenates by flow cytometry. As expected, groups that received the E7 peptide vaccine showed a significant increase in tumor-infiltrated CD8+ cells (P<0.001) compared to control groups, and CD8+ T cell levels were comparable whether the vaccine was given alone or in combination with CT-011 or CPM. The group of mice that received the combination of anti-PD-1 antibody and CPM with E7 vaccine showed the highest significant increase in the number of tumor-infiltrated CD8+ cells (compared to vaccine alone (P<0.001), vaccine/CPM (P<0.001) or vaccine/CT-011 (P<0.05) groups) (Fig. 4A). Furthermore, we observed a significant increase in tumor infiltration of CD4+Foxp3− cells only when CPM was part of the vaccine treatment in either the vaccine/CPM or vaccine/CT-011/CPM groups compared to non-treated mice (P<0.05) (Fig. 4B). As with splenic Treg cells the combination of both CPM and CT-011 led to a significant decrease in the levels of tumor infiltrated CD4+FoxP3+ cells on day 21 after tumor implantation (Fig. 4C).
Figure 4. Three-component treatment affects tumor-infiltrated T cell profile.
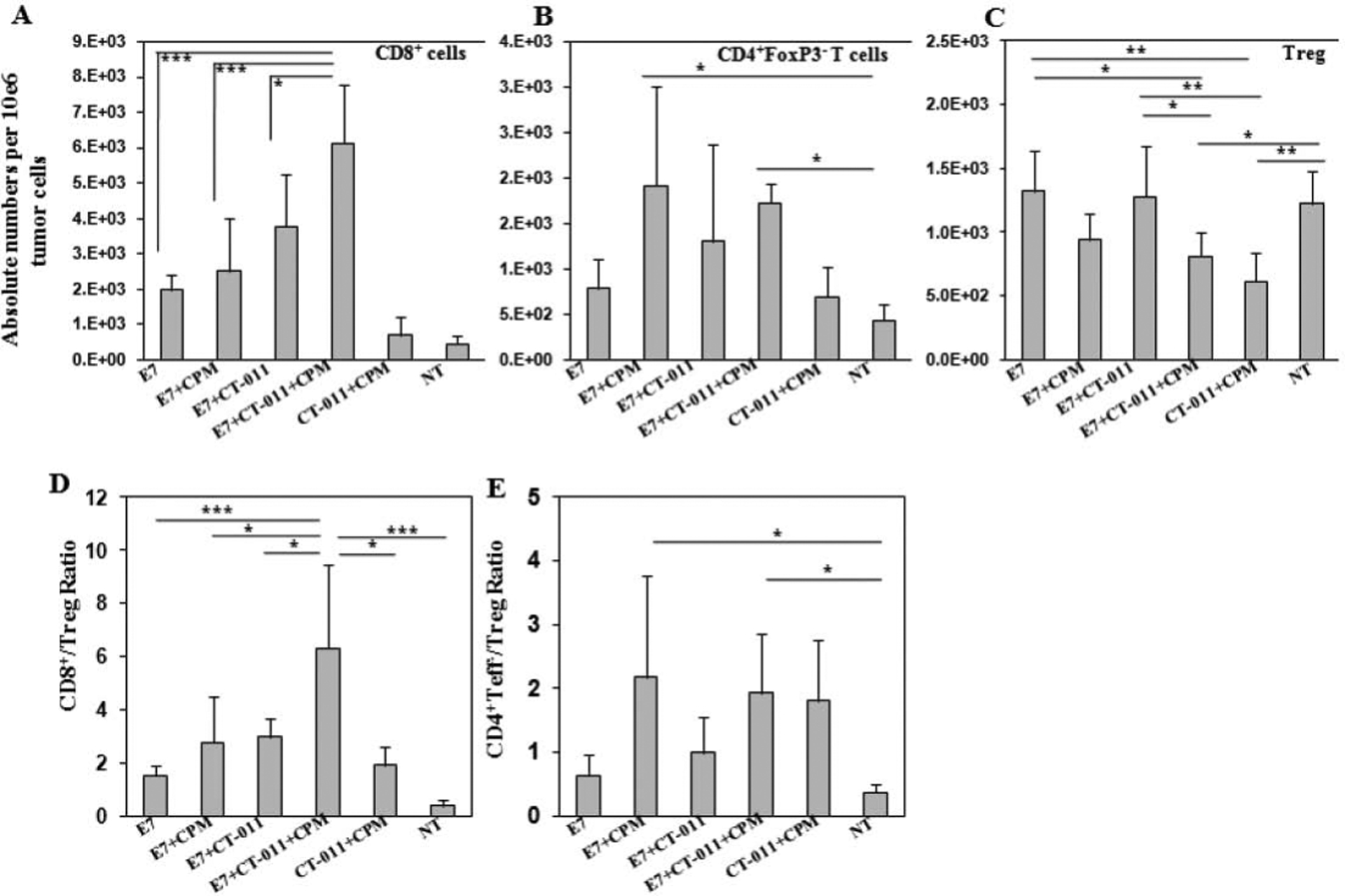
C57BL/6 mice (n=6 per group) were injected s.c. in the right flank with 5×10e4 TC-1 cells. On day 7, CPM or PBS was injected. On day 8 and 15 mice were injected with vaccine+CT-011, vaccine alone, CT-011 alone, PBS or IgG. On day 21 mice were sacrificed, and tumor infiltrated T cells were analyzed in tumor homogenates by flow cytometry. The absolute numbers of infiltrated cells were standardized per 10e6 of total tumor cells and presented as mean values ± SD for (A) CD8+, (B) CD4+FoxP3− and (C) CD4+FoxP3+ T cells. The mean values of CD8+ T cell ratios to Treg (D) and CD4+FoxP3− to Treg cells (E) ± SD are shown. *P<0.05, **P<0.01 and ***P<0.001. Similar results were obtained from two independent experiments.
Since tumor-infiltrated effector/suppressor cell ratios are well-established criteria that correlate with cancer prognosis [35–38], we calculated CD8+/Treg and CD4+Foxp3−/Treg ratios in tumor homogenates of treated and control mice. The CD8+/Treg ratio was significantly increased only when mice were treated with combination of vaccine, CT-011 and CPM (P<0.001 compared to vaccine alone and the non-treated group, and P<0.05 compared to two-component treatment groups) (Fig. 4D). The CD4+Foxp3−/Treg ratios were significantly increased (P<0.05) in mice treated with CPM, both vaccine/CPM and vaccine/CT-011/CPM compared to the non-treated group (Fig. 4E). These experiments demonstrate that the combination of CT-011 with vaccine and CPM simultaneously increases tumor-infiltrated CD8+ and CD4+non-Treg cells, decreases Treg cells, and thus significantly elevates the CD8+/Treg and CD4+Foxp3−/Treg ratios within the tumor.
Therapeutic efficacy of vaccine/CT-011/CPM treatment relies not only on CD8+ but also on CD4+ T cells
To further determine the immunologic mechanism of the response induced by combining anti-PD-1 with peptide vaccine and CPM, we next tested the role of different T cell subsets involved in anti-tumor efficacy of combinational treatment. Vaccine/CT-011/CPM treatment was conducted as described above, but in animals depleted of CD4+, CD8+ or both subsets of T cells. Control groups were either treated with vaccine/CT-011/CPM and IgG (the control for anti-CD4 and anti-CD8 mAb), or remained non-treated. Depletion of CD4+ and CD8+ T cells was confirmed using flow cytometry assay (data not shown). As expected, depletion of CD8+ cells, either alone or with CD4+ depletion completely abrogated the effect of treatment and resulted in tumor growth and survival rates similar to non-treated animals (Fig. 5A and B). Surprisingly however, CD4+ T cell depletion significantly decreased the efficacy of vaccine/CT-011/CPM treatment, resulting in higher tumor growth rate (P<0.001) (Fig. 5A) and decrease in survival, with no complete regression of tumor in any of the treated mice (Fig. 5B). These experiments suggest that the therapeutic efficacy of vaccine/CT011/CPM treatment requires not only CD8+ but also CD4+ T cells.
Figure 5. Vaccine/CT-011/CPM treatment efficacy relies on both CD8+ and CD4+ T cells.
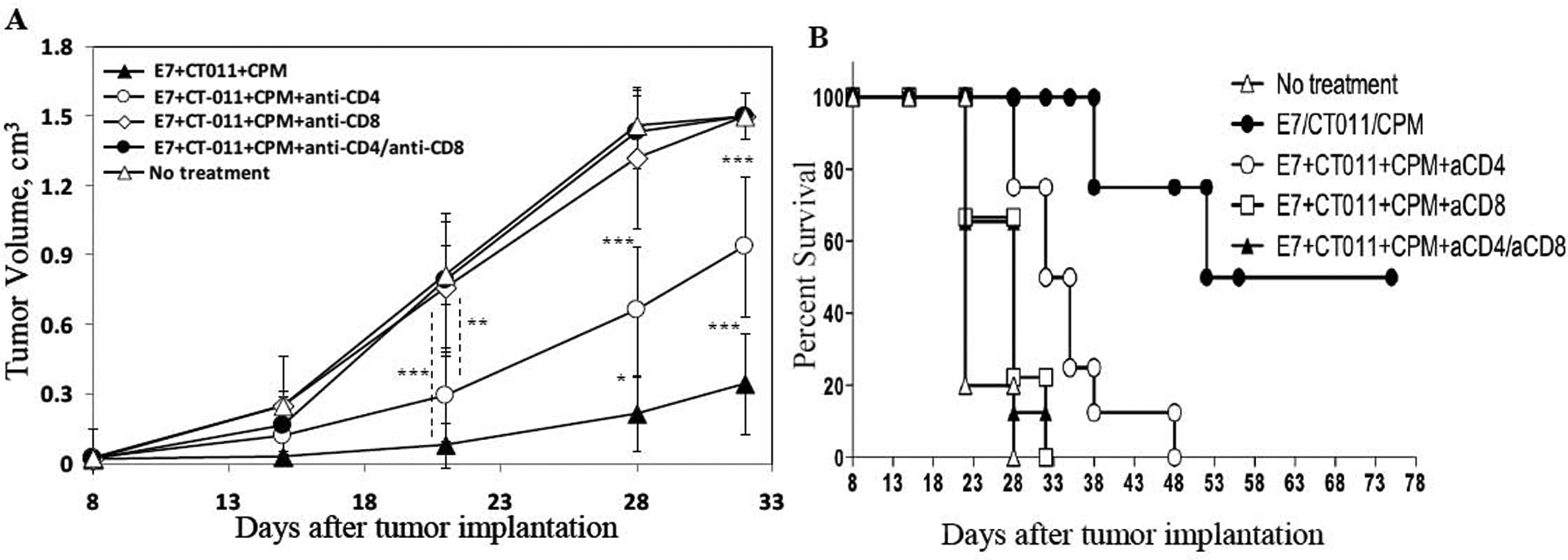
C57BL/6 mice (n = 6 per group) were injected s.c. in the right flank with 5×10e4 TC-1 cells. On day 7, CPM or PBS was injected. On day 8, 15 and 22 mice were injected with vaccine+CT-011, vaccine alone, CT-011 alone, PBS or IgG. Mice also were injected anti-CD4 mAb (300 μg/mouse) on days +5 and +17, and/or with anti-CD8 mAb (400 μg/mouse) on days +17 and +24, or with appropriate isotype control IgG. Tumor sizes were measured periodically. (A) Plots represent mean tumor volumes in mice for each treatment ± SD. (B) The Kaplan-Meier plot depicts overall survival. *P<0.05, **P<0.01 and ***P<0.001. Similar results were obtained from two independent experiments.
Discussion
There are several mechanisms by which tumors suppress the host immune response. One prominent mechanism is the expression of co-inhibitory molecules by tumor. Co-inhibitory molecules can lead to suppression and apoptosis of effector lymphocytes in the periphery and in the tumor microenvironment [12, 13]. PD-L1 is one of these molecules found to be up regulated in human malignancies, and has been directly correlated with immune suppression and poor prognosis in several types of cancer [4, 7–10, 39]. An inverse correlation has been found between PD-L1 expression on human tumor cells and the number of tumor-infiltrating CD8+T lymphocytes in cancer patients [4, 7–10, 39]. Blocking the PD-L1/PD-1 interaction has been found to enhance the efficacy of tumor antigen-specific CD8+T cells in the tumor microenvironment [4, 8, 12]. Another mechanism by which tumors inhibit anti-tumor immunity is through the induction of regulatory T cells. Tregs are inhibitory CD4+ T cells that are increased in cancer patients, both peripherally and in tumors, and can form a barrier to eliciting effective immune responses [17–22]. It has been shown that anti-tumor immunity is enhanced by depletion of Tregs with agents such as anti-CD25 and low dose CPM [23–25, 40–42]. Enhancing the therapeutic outcome of cancer vaccines would require a multi strategy approach to overcome different tumor-mediated inhibitory mechanism.
Here, we show that PD-1 blockade synergizes with Treg suppression by a single low dose of cyclophosphamide, leading to an enhanced therapeutic outcome of cancer vaccine.
Underlining the anti-tumor effect, we found, as expected, that vaccine alone was able to induce a specific CD8+ T cell immune response and increase CD8+ T cell infiltration into the tumor. However, while neither the addition of CT-011 or CPM alone was able to induce further increase in the CD8+ T cell response or increase in CD8+ T cell infiltration into the tumor, the combination of both with the vaccine demonstrated a significant increase in CD8+ T cell infiltration and antigen specific immune response.
A partially contributing factor to the increase of CD8+ within the tumor environment might be a blockade of the PD-1/PD-L1 interaction between tumor cells and T cells by CT-011, preventing induction of T cell inhibition and apoptosis. Our in vitro data showed that CT-011 is able to partially rescue the proliferation of tumor-suppressed CD4+T cells (Figure 2B). Interestingly, we didn’t observe similar rescue of proliferation for CD8+T cells (data not shown). One possible explanation for this difference might be the significantly lower expression of PD-1 on in vitro-stimulated CD8+ T cells compared to Tconv cells (data not shown).
Furthermore, we found that the CPM/CT-011 combination led to a significant decrease in both peripheral and tumor infiltrated Tregs, which may further enhance vaccine induced CD8+ immune response and tumor-infiltration. Low dose CPM is known to selectively ablate Treg cells, with the nadir at day 4, and recovery to pretreatment levels by day 10. We observed, as expected, that by day 14 after CPM treatment (day 21 after tumor implantation) there were no significant differences in the levels of splenic Treg cells in mice treated with CPM alone compared to untreated animals. However, interestingly, we found that while CT-011 alone does not affect the levels of Tregs in either the periphery or the tumor, the addition of CT-011 to CPM led to a sustainable effect on Treg inhibition within the spleens of treated animals, with a synergistic effect at all time points analyzed up to day +19 of CPM treatment (day 26 after tumor implantation, the last day non-treated mice were alive).
Surprisingly, we also found that the anti-tumor effect elicited by vaccine/CT-011/CPM treatment is abrogated by depletion not only of CD8+ but also CD4+T cells. This indicates that the anti-tumor effect is mediated not only by CD8+ T cells as predicted, since E7 peptide is a class I restricted peptide, but that CD4+ T cells also play a crucial rule in the mechanism of action of our treatment combination. We speculate that this can partially be explained through the effect of CD4+T helper cells leading to further activation of CD8+ T cells. Furthermore, the effect of CD4+ T cells may be enhanced in this combination due to: 1) the known effect of CPM on increasing CD4+ T helper like cells [43], and 2) the direct activating effect that anti-PD-1 antibody has on CD4+ T cells, as has been previously described [44].
In conclusion, here we describe a potent and clinically translatable novel therapeutic approach based on combining multiple approaches to target the immune inhibitory mechanisms of tumor, leading to enhancement of antigen specific immune responses. We combined vaccine with anti-PD-1 antibody to block the PD-1/PD-L1 interaction, and a single low dose of CPM to inhibit Tregs. We demonstrate that the combination of these strategies provides a synergistic outcome that is dependent on novel mechanisms that favorably alter the tumor microenvironment by affecting the balance between tumor mediated immune suppression and anti-tumor immunity. This represents a promising approach to enhancing cancer vaccines in clinical settings.
Material and Methods
Animals, cell lines, vaccine and other reagents
Female 6–8 weeks old C57/BL6 mice were purchased from NCI Frederick and housed under pathogen-free conditions. All procedures were carried out under the guidelines of the National Institutes of Health and in accordance with approved institutional animal protocols.
TC-1 cells stably transfected and expressing HPV 16 E6 and E7 antigens were obtained from ATCC. Cells were grown in RPMI 1640 supplemented with 10% FBS, 2mM L-glutamine, penicillin (100U/ml) and streptomycin (100ug/ml) at 37°C with 5% CO2.
The CT-011 humanized monoclonal antibody was obtained from CureTech Ltd (Israel) and was injected intravenously (i.v.) at a dose of 2.5mg/kg.
The 9-mer peptide from HPV16 E749–57, RAHYNIVTF, was obtained from Celltek Bioscience. E749–57 (100ug/mouse) was used as a model vaccine along with GM-CSF (5ug/mouse-Peprotech), anti-CD40 (20ug/mouse-BioLegend) and IFA (50ul/mouse-Sigma) in all studies (subcutaneous (s.c.) immunization), since anti-CD40 has been shown to synergize with GM-CSF to increase peptide vaccine efficacy [45]. Cyclophosphamide (CPM) was obtained from Baxter Healthcare Corporation and was injected intraperitonealy (i.p.) at a dose of 1 mg/mouse. PD-L1-IgG recombinant protein was purchased from R&D Systems and used for in vitro assays.
Immunization, tumor implantation and T cell depletion
In the therapeutic experiments, mice were implanted with 50,000 TC-1 cells/mouse s.c. into the right flank on day 0. Seven days later (day +7) when tumors are measurable (~3–4mm in diameter), mice from the appropriate groups were injected i.p with CPM (1mg/mouse). Twenty-four hours later (day +8) mice were injected s.c. with the vaccine (E7/GM-CSF/anti-CD40) and/or CT-011 (i.v. at 2.5mg/kg dose). Mice were vaccinated weekly for a total of 3 times. PBS was used in control mice instead of CPM and the same concentration of isotype control antibody (BD Biosciences) instead of CT-011. Tumors were measured every 3–4 days using a caliper and the tumor volume was calculated using the following formula: V=LxW2/2, where V is tumor volume, L is the length of tumor (longer diameter) and W is the width of the tumor (shorter diameter). For some experiments mice were monitored for tumor growth and survival. Mice were sacrificed when tumor reached 1.5cm3 volume or when they became moribund. For other experiments, mice were injected with CPM, followed by two treatments on days +8 and +15 and sacrificed on day +21 after tumor implantation (day 6 after second immunization), when spleens and tumors were isolated and analyzed for antigen-specific immunity, levels of Treg cells (tumor-bearing mice) and for tumor infiltrated immune cell profile study.
For T cell depletion experiments the same schedule was used, except, in addition to TC-1, vaccine and CT-011, mice also were injected with GK1.5 anti-CD4 mAb (BioXcell) on days +5 and +17 (300μg/mouse) and/or with 53.6.72 anti-CD8 mAb (BioXcell-400μg/mouse) on days +17 and +24 after tumor implantation. A total of 3 immunizations were performed and mice were monitored for tumor growth and survival.
Analysis of antigen-specific cellular immune responses
ELISPOT was used to detect production of IFNγ in E7-restimulated (10μg/ml) splenocyte cultures from vaccinated and control mice isolated on day 6 after the last immunization, as suggested by the manufacturer (BD Biosciences). Spots were counted using CTL Immunospot Analyzer (Cellular Technology), and the results were examined for differences between E7 re-stimulated and irrelevant peptide (hgp10025–33 – KVPRNQDWL- Celltek Bioscience) re-stimulated splenocyte cultures.
The flow cytometry assay was used to assess direct CTL activity in immunized mice as described [46]. Briefly, to test the effector cell function, freshly isolated splenocytes (effector cells) were mixed with target TC-1 cells labeled with CellTracker Green dye (Invitrogen) at E:T ratios of 50:1, 25:1, 10:1, and 0:1. After a 3 hour co-incubation, the E:T mixtures were washed, fixed, and permeabilized before staining with PE-labeled anti-caspase-3 Abs (BD Pharmingen). After incubation and washing, the number of activated caspase-3-positive apoptotic cells was detected in the CellTracker Green-positive target cells population, and then the percentage of apoptotic cells was calculated using CellQuest software.
Detection of tumor-infiltrated and splenic immune cell profiles
In some experiments, tumor tissue was harvested 6 days after the second vaccination, and processed using GentleMACS Dissociator (Militenyi Biotec) and solid tumor homogenization protocol, as suggested by the manufacturer. After washes, the number of tumor-infiltrated CD8+, CD4+FoxP3− and CD4+FoxP3+ cells were analyzed using flow cytometry assay and the following antibodies: FITC-labeled anti-mouse CD8, FITC-labeled anti-mouse CD4 (both from BD Biosciences) and PE-labeled anti-mouse FoxP3 mAb (eBiosciences), and appropriate FITC- and PE-labeled isotype control Ab (BD Biosciences).
The level of CD4+FoxP3+ cells (Treg cells) was also evaluated in spleens of tumor-bearing treated and control mice using the same flow cytometry assay.
PD-L1 expression by TC-1 cells
The expression of PD-L1 on the surface of TC-1 cells was detected by flow cytometry using anti-PD-L1 (CD274) mAb (eBiosciences). Briefly, confluent TC-1 cells were trypsinized, left for an hour on ice and stained with PE-labeled anti-mouse PD-L1 antibody for 30 min at 4°C. After washing, surface expression of PD-L1 on TC-1 cells was analyzed using FACScan flow cytometer and CellQuest software (BD Biosciences).
Suppression Assay
The ability of CT-011 antibody to inhibit the TC-1 tumor-mediated suppression of CD4+CD25− T cell proliferation was assessed by carboxyfluorescein diacetate, succinimidyl ester (CFSE)-based suppression assay. The CD4+CD25− T (Tconv) cells were purified from the spleens of naïve mice using the Militenyi Biotec MACS T cell purification kit as suggested by the manufacturer. Cells were labeled with 1μM CFSE dye as suggested by the manufacturer (Invitrogen), as suggested by the manufacturer . After washes, CFSE-labeled Tconv cells were stimulated with α-CD3 α-CD28 polystrene dynal beads (Invitrogen) and co-incubated with TC-1 cells at a 1:1 ratio for four days, alone or in the presence of 50μg/ml concentrations of CT-011 antibody, PD-L1-IgG protein or isotype control antibody. After washes, samples were evaluated for CFSE dye dilution using FACScan flow cytometer and CellQuest software (BD Biosciences).
Statistical analysis
All statistical parameters (average values, SD, significant differences between groups) were calculated using GraphPad Prism Software. Statistical significance between groups was determined by one-way ANOVA with Tukey’s multiple comparison post-test (P<0.05 was considered statistically significant).
Acknowledgments
We thank Daniel O’Mard, Ashley Reynolds and Gail McMullen from the NIH animal facility for their technical assistance with animal injections.
Abbreviations:
- CPM
cyclophosphamide
- PD-1
Programmed death-1 receptor
- PD-L1
Programmed death ligand 1
- Tconv
T conventional cell
Footnotes
Conflict of interest
R.R.Y. is an employee of CureTech Ltd, which provided CT-011. The remaining authors declare no competing financial interests.
References
- 1.Hachisuka T, Narikiyo M, Yamada Y, Ishikawa H, Ueno M, Uchida H, Yoriki R, Ohigashi Y, Miki K, Tamaki H, Mizuno T and Nakajima Y, High lymphatic vessel density correlates with overexpression of VEGF-C in gastric cancer. Oncol Rep 2005. 13: 733–737. [PubMed] [Google Scholar]
- 2.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T and Gajewski TF, PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res 2004. 64: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 3.Keir ME, Latchman YE, Freeman GJ and Sharpe AH, Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J Immunol 2005. 175: 7372–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H and Nishimura M, B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004. 10: 5094–5100. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki T and Honjo T, The PD-1-PD-L pathway in immunological tolerance. Trends Immunol 2006. 27: 195–201. [DOI] [PubMed] [Google Scholar]
- 6.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH and Freeman GJ, PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001. 2: 261–268. [DOI] [PubMed] [Google Scholar]
- 7.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M and Nakajima Y, Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005. 11: 2947–2953. [DOI] [PubMed] [Google Scholar]
- 8.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M and Nakajima Y, Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007. 13: 2151–2157. [DOI] [PubMed] [Google Scholar]
- 9.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC and Kwon ED, Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A 2004. 101: 17174–17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, Ajarim D, Al-Tweigeri T and Dermime S, The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006. 8: 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson RH and Kwon ED, Significance of B7-H1 overexpression in kidney cancer. Clin Genitourin Cancer 2006. 5: 206–211. [DOI] [PubMed] [Google Scholar]
- 12.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T and Fujii S, Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007. 104: 3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsushima F, Tanaka K, Otsuki N, Youngnak P, Iwai H, Omura K and Azuma M, Predominant expression of B7-H1 and its immunoregulatory roles in oral squamous cell carcinoma. Oral Oncol 2006. 42: 268–274. [DOI] [PubMed] [Google Scholar]
- 14.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR and Honjo T, Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000. 192: 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB and Riley JL, CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 2005. 25: 9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo SK, Seo HM, Jeong HY, Choi IW, Park YM, Yagita H, Chen L and Choi IH, Co-inhibitory role of T-cell-associated B7-H1 and B7-DC in the T-cell immune response. Immunol Lett 2006. 102: 222–228. [DOI] [PubMed] [Google Scholar]
- 17.Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H and Fujii H, Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 2003. 9: 4404–4408. [PubMed] [Google Scholar]
- 18.Gallimore A and Godkin A, Regulatory T cells and tumour immunity - observations in mice and men. Immunology 2008. 123: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikemoto T, Yamaguchi T, Morine Y, Imura S, Soejima Y, Fujii M, Maekawa Y, Yasutomo K and Shimada M, Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas 2006. 33: 386–390. [DOI] [PubMed] [Google Scholar]
- 20.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF and Korangy F, Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 2005. 65: 2457–2464. [DOI] [PubMed] [Google Scholar]
- 21.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P and Ferradini L, Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 2004. 173: 1444–1453. [DOI] [PubMed] [Google Scholar]
- 22.Raiter A, Rodionov G, Novogrodsky A and Hardy B, CD4+ T lymphocytes as a primary cellular target for BAT mAb stimulation. Int Immunol 2000. 12: 1623–1628. [DOI] [PubMed] [Google Scholar]
- 23.Shevach EM, Suppressor T cells: Rebirth, function and homeostasis. Curr Biol 2000. 10: R572–575. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J and Sakaguchi S, Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol 1998. 10: 1969–1980. [DOI] [PubMed] [Google Scholar]
- 25.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F and Sakaguchi S, Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol 1999. 162: 5317–5326. [PubMed] [Google Scholar]
- 26.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW and Sakaguchi S, Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med 2000. 192: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J and Sabzevari H, Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005. 105: 2862–2868. [DOI] [PubMed] [Google Scholar]
- 28.Sharabi A, Laronne-Bar-On A, Meshorer A and Haran-Ghera N, Chemoimmunotherapy Reduces the Progression of Multiple Myeloma in a Mouse Model. Cancer Prev Res (Phila Pa). [DOI] [PubMed] [Google Scholar]
- 29.Brode S and Cooke A, Immune-potentiating effects of the chemotherapeutic drug cyclophosphamide. Crit Rev Immunol 2008. 28: 109–126. [DOI] [PubMed] [Google Scholar]
- 30.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B and Martin F, CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004. 34: 336–344. [DOI] [PubMed] [Google Scholar]
- 31.Awwad M and North RJ, Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour. Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology 1988. 65: 87–92. [PMC free article] [PubMed] [Google Scholar]
- 32.Ikezawa Y, Nakazawa M, Tamura C, Takahashi K, Minami M and Ikezawa Z, Cyclophosphamide decreases the number, percentage and the function of CD25+ CD4+ regulatory T cells, which suppress induction of contact hypersensitivity. J Dermatol Sci 2005. 39: 105–112. [DOI] [PubMed] [Google Scholar]
- 33.North RJ, Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med 1982. 155: 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A and Nagler A, Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 2008. 14: 3044–3051. [DOI] [PubMed] [Google Scholar]
- 35.Curran MA, Montalvo W, Yagita H and Allison JP, PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 107: 4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C and Sun Y, Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 136: 1585–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biller BJ, Guth A, Burton JH and Dow SW, Decreased ratio of CD8+ T cells to regulatory T cells associated with decreased survival in dogs with osteosarcoma. J Vet Intern Med. 24: 1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O and Hashimoto K, Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 109: 744–752. [DOI] [PubMed] [Google Scholar]
- 39.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC and Kwon ED, Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006. 66: 3381–3385. [DOI] [PubMed] [Google Scholar]
- 40.Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R and Melief CJ, Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med 2001. 194: 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi S, Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004. 22: 531–562. [DOI] [PubMed] [Google Scholar]
- 42.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T and Nakayama E, Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 1999. 59: 3128–3133. [PubMed] [Google Scholar]
- 43.Ding ZC, Blazar BR, Mellor AL, Munn DH and Zhou G, Chemotherapy rescues tumor-driven aberrant CD4+ T-cell differentiation and restores an activated polyfunctional helper phenotype. Blood. 115: 2397–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.del Rio ML, Penuelas-Rivas G, Dominguez-Perles R, Ramirez P, Parrilla P and Rodriguez-Barbosa JI, Antibody-mediated signaling through PD-1 costimulates T cells and enhances CD28-dependent proliferation. Eur J Immunol 2005. 35: 3545–3560. [DOI] [PubMed] [Google Scholar]
- 45.Ahlers JD, Belyakov IM, Terabe M, Koka R, Donaldson DD, Thomas EK and Berzofsky JA, A push-pull approach to maximize vaccine efficacy: abrogating suppression with an IL-13 inhibitor while augmenting help with granulocyte/macrophage colony-stimulating factor and CD40L. Proc Natl Acad Sci U S A 2002. 99: 13020–13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghochikyan A, Mkrtichyan M, Loukinov D, Mamikonyan G, Pack SD, Movsesyan N, Ichim TE, Cribbs DH, Lobanenkov VV and Agadjanyan MG, Elicitation of T cell responses to histologically unrelated tumors by immunization with the novel cancer-testis antigen, brother of the regulator of imprinted sites. J Immunol 2007. 178: 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]


