Abstract
Background
The aim of this study was to explore the effects of NADPH oxidase 5 (NOX5) in high glucose-stimulated human glomerular mesangial cells (HMCs).
Material/Methods
Cells were cultured under normal glucose (NG) or high glucose (HG) conditions. Then, NOX5 siRNA was transfected into HG-treated HMCs. A Cell Counting Kit-8 assay, colony formation assay and 5-ethynyl-20-deoxyuridine (EDU) incorporation assay were applied to measure cell proliferative ability. In addition, the levels of oxidative stress factors including reactive oxygen species (ROS), malonaldehyde (MDA), NADPH, superoxide dismutase (SOD), and glutathione peroxidase (GSH-PX), inflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-1β, and monocyte chemoattractant protein-1 (MCP-1) in HMCs were detected by kits. Moreover, the expression of TLR4/NF-κB signaling and extracellular matrix (ECM) associated genes were evaluated by western blotting.
Results
The results revealed that the NOX5 was overexpressed in HG-treated HMCs. Silencing of NOX5 decreased proliferation of HMCs induced by HG. And NOX5 silencing alleviated the production of MDA and NADPH accompanied by an increase of SOD and GSH-PX levels. Additionally, the contents of TNF-α, IL-6, IL-1β, and MCP-1 were reduced after transfection with NOX5 siRNA. Furthermore, silencing of NOX5 deceased the expression of collagen I, collagen IV, TGF-β1, and fibronectin induced by HG stimulation. TLR4, MyD88, and phospho-NF-κB p65 expression were downregulated notably in NOX5 silencing group.
Conclusions
Taken together, these findings demonstrated that inhibition of NOX5 attenuated HG-induced HMCs oxidative stress, inflammation, and ECM accumulation, suggesting that NOX5 may serve as a potential therapeutic target for diabetic nephropathy (DN) treatment.
MeSH Keywords: Cell Proliferation, Inflammation, Mesangial Cells, Oxidative Stress
Background
Diabetic nephropathy (DN) is a major microvascular complication of diabetes that is one of the leading causes of end-stage kidney disease, which is also primarily attributed to the increasing prevalence of type 2 diabetes [1,2]. A previous study reported that DN accounts for more than 30% of complications globally in patients with diabetes [3]; DN has thus attracted much attention from a variety of researchers.
DN is characterized by the excessive production and accumulation of extracellular matrix (ECM) components in kidney glomeruli, which ultimately can lead to glomerulosclerosis [4]. It has been well reported that glomerular mesangial cells (MCs) play significant roles in maintaining the integrity of the glomeruli and are responsible for the accumulation of ECM [5]. Previous studies have demonstrated that high glucose (HG)-induced MCs proliferation and ECM accumulation are the most vital pathological changes in the DN, which could further worsen DN with the prolonged activation of oxidative stress and inflammatory response in the progression of DN [6–8]. Numerous studies unveiled that multiple agents and intracellular regulators contributed to the pathophysiology of oxidative stress, inflammation, and ECM accumulation [9,10]. Therefore, regulation of these 3 factors would be an important approach to protect MCs against high glucose-induced injury, and might improve our understanding of the pathogenesis and therapies methods in DN.
Increasing evidence suggests that NADPH oxidase 5 (NOX5) plays a significant role in human DN [11]. Emerging evidence supports the notion that NOX5 could aggravate renal injury in a DN mouse model [12]. NOX5-derived reactive oxygen species (ROS) in podocytes can promote renal inflammation by regulating the Toll-like receptor (TLR) pathway [13]. In addition, the expression of NOX5 is increased in damaged proximal tubular epithelial cells [14]. However, the role of NOX5 in HG-induced human mesangial cells (HMCs) remains to be elucidated. In this study, we aimed to investigate the effects of NOX5 on oxidative stress, inflammation, and ECM production in HMCs stimulated by HG.
Material and Methods
Cell culture and treatment
HMCs was obtained from the Shanghai Academy of Life Sciences (Shanghai, China) and cultured in Dulbecco’s Modified Eagle Medium (DMEM; Thermo Fisher Scientific, MA, USA) containing 10% fetal bovine serum (FBS; Gibco, USA) at 37°C in a humidified 5% CO2/95% air atmosphere. Cells in the control group were maintained in DMEM supplemented with normal glucose (NG, 5.5 mM glucose). Cells in the osmotic pressure control (OP) were cultured in medium with 5.5 mM glucose plus 22.5 mM mannitol. And cells in HG group were exposed to medium containing 25 mM glucose. After 24-hour incubation, the following experiments were carried out.
Cell transfection
Cells were plated in 6-well plates (1×106 cells/well) before transfection. When cells reached 80% confluence, the HMCs were transfected with NOX5 siRNA or negative control siRNA (siRNA-NC) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. At 24 hours post-transfection, cells were collected, and successful transfections were measured by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting.
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8) obtained from Shanghai Yi Sheng Biotechnology Co., Ltd. (Shanghai, China) was employed to assess cell proliferation. After transfection, cells were incubated under NG or HG conditions for 24 hours. Then, 10 μL CCK-8 solution was added to each well. Following incubation at 37°C for 1 hour, the optical density (OD) was detected at 450 nm using a micro-plate reader.
Colony formation assay
HMCs cells were seeded into 6-well plates (500 cells/per well) and cultured at 37°C in a humidified 5% CO2/95% air atmosphere for 7 to 12 days. The cells were fixed and stained with 0.2% crystal violet. ImageJ software was used to determine colony numbers.
EDU (5-ethynyl-20-deoxyuridine) incorporation assay
An EDU assay kit (Ribobio, China) was applied to examine the proliferation of HMCs in the light of the manufacturer’s protocol. In brief, cells were incubated with 10 μM EDU and then fixed in 4% paraformaldehyde. After EDU staining, cell nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI). The images of EDU-positive cells were observed and captured randomly in 5 fields under a confocal fluorescence microscope (Nikon, Tokyo, Japan).
Measurement of inflammatory cytokines levels
Cells were stimulated with medium containing NG or HG for 24 hours after transfection, and the levels of inflammatory cytokines secretion including tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, IL-1β, and monocyte chemoattractant protein-1 (MCP-1) in the culture supernatant of HMCs were detected by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocols. The ELISA kits were purchased from Shanghai Xitang Biotechnology Co., Ltd. (Shanghai, China).
Measurement of intracellular ROS levels
After cells were incubated with medium containing NG or HG for 24 hours, the levels of intracellular ROS levels were detected by using 2′, 7′-dichlorofluorescein diacetate (DCF-DA) which serves as a fluorescent probe. In brief, cells were plated in 24-well plates and then stained with 10 μM DCF-DA and incubated in the dark for 30 minutes at room temperature. Then, cells were washed with phosphate-buffered saline (PBS) for 3 times. The fluorescence of DCF in HMCs was observed using a multi-detection microplate reader.
Assessment of malonaldehyde (MDA), NADPH, superoxide dismutase (SOD), and glutathione peroxidase (GSH-PX) levels
After HMCs were treated with HG for 24 hours, the culture supernatant in each sample was collected. The content of MDA and NADPH production as well as the activity of SOD and GSH-PX were evaluated using the related assay kits in accordance with the manufacturer’s instructions. The kits for MDA, SOD, and GSH-PX were the products of Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The kit for NADPH was obtained from Beyotime (Shanghai, China).
Immunofluorescence assay
Cells were seeded in plates which contained glass coverslips. Then, cells were fixed with 4% paraformaldehyde for 20 minutes. Subsequently, cells were permeabilized using 0.1% Triton X-100 in Tris-buffered saline (TBS) for 10 minutes. Cells were then blocked in 3% bovine serum albumin (BSA) for 1 hour, followed by incubation with anti-TGF-β1 (Cell Signaling Technology, Inc.; Boston, MA, USA) overnight. Following cells were incubated with DyLight™ 488-conjugated secondary antibodies (Thermo Scientific) for 1 hour. Nucleus were stained with DAPI (Sigma-Aldrich) for 5 minutes. A confocal laser scanning microscope (Olympus FV1000) was used to observe fluorescence intensities.
RT-qPCR analysis
Total RNA was extracted from HMCs using the TRIzol reagent kit (Invitrogen). A RevertAid First Strand cDNA Synthesis kit (Thermo Fermentas, USA) was employed to synthesize cDNA. RT-qPCR was performed on an ABI7500 instrument (Applied Biosystems). The primer sequences used in this study was as follows:
NOX5, forward, 5′-CTATTGGACTCACCTGTCCTACC-3′, and
reverse 5′-GGAAAAACAAGATTCCAGGCAC-3′;
GAPDH, forward, 5′-ACAACTTTGGTATCGTGGAAGG-3′, and
reverse 5′-GCCATCACGCCACAGTTTC-3′.
The gene expression was quantified by the method of 2−ΔΔCq.
Western blot analysis
Total proteins in HMCs were extracted by using RIPA Lysis Buffer (Beyotime, Shanghai, China). Proteins concentration was detected by Bradford assay (Bio-Rad) following the manufacturer’s instructions. Protein samples of 40 μg per lane were loaded on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred onto polyvinylidene fluoride (PVDF) membranes. Membranes were subsequently immersed into 5% nonfat milk, and then incubated with primary antibodies. All blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (A0216, Beyotime, Shanghai, China) for 1 hour and then visualized using an enhanced chemiluminescence (ECL) reagent. ImageJ software was used to analyze the results. GAPDH was used as internal control. Anti-NOX5, anti-fibronectin (FN), anti-collagen I and anti-collagen IV antibodies were the products of Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-TLR4, anti-MyD88, anti-phospho-NF-κB p65 (p-NF-κB p65), anti-NF-κB p65, anti-TGF-β1, anti-TGF-β2, anti-p-Smad2, anti-p-Smad3, anti-Smad2, anti-Smad3 and anti-GAPDH antibodies were obtained from Cell Signaling Technology, Inc. (Boston, MA, USA).
Statistical analysis
All experiments were repeated for at least 3 times in the present study and results were presented as mean±standard deviation (SD). Statistical analysis was performed by SPSS software 19.0 (SPSS Inc., Chicago, IL, USA). Quantitative data between 2 groups was conducted using Student’s t-test, and the comparisons among multiple groups were performed using one-way analysis of variance (ANOVA) followed by Turkey’s post hoc test. A value of P<0.05 was considered statistically significant.
Results
The expression of NOX5 was upregulated in HG-treated HMCs
To explore the role of NOX5 in DN, HG medium was employed to culture HMCs in the current study. Then, the expression of NOX5 was determined by western blotting. As presented in Figure 1A and 1B, the level of NOX5 was increased notably after HG-treatment. In addition, the expression of NOX5 had no significant change between the NC and OS groups but upregulated in the high glucose medium, suggesting that there was little effect of osmotic pressure on the expression of NOX5 after induction with HG (Figure 1C, 1D). Subsequently, siRNA-NOX5-1 and siRNA-NOX5-2 were transfected into HMCs. The effect of NOX5 gene silencing was measured by western blotting and RT-qPCR, respectively. We found that the expression of NOX5 was decreased significantly following transfected with siRNA-NOX5, and siRNA-NOX5-1 exhibited the better inhibitory effect (Figure 1E, 1F). Therefore, we chose siRNA-NOX5-2 to perform the further investigation. Our finding indicated that HG-treatment promoted the expression of NOX5 in HMCs.
Figure 1.
The expression of NOX5 was upregulated in HG-treated HMCs. The expression of NOX5 was measured by (A) western blotting and (B) RT-qPCR in HG induced HMCs. ## P<0.01 versus NG. The expression of NOX5 was detected by (C) western blotting and (D) RT-qPCR after incubation with medium containing OP or HG. ### P<0.001 versus OP. The expression of NOX5 was determined by (E) western blotting and (F) RT-qPCR after transfection with siRNA-NOX5-1 or siRNA-NOX5-2. ## P<0.01, ### P<0.001 versus HG+siRNA-NC. NOX5 – NADPH oxidase 5; HG – high glucose; OP – osmotic pressure control; HMCs – human glomerular mesangial cells.
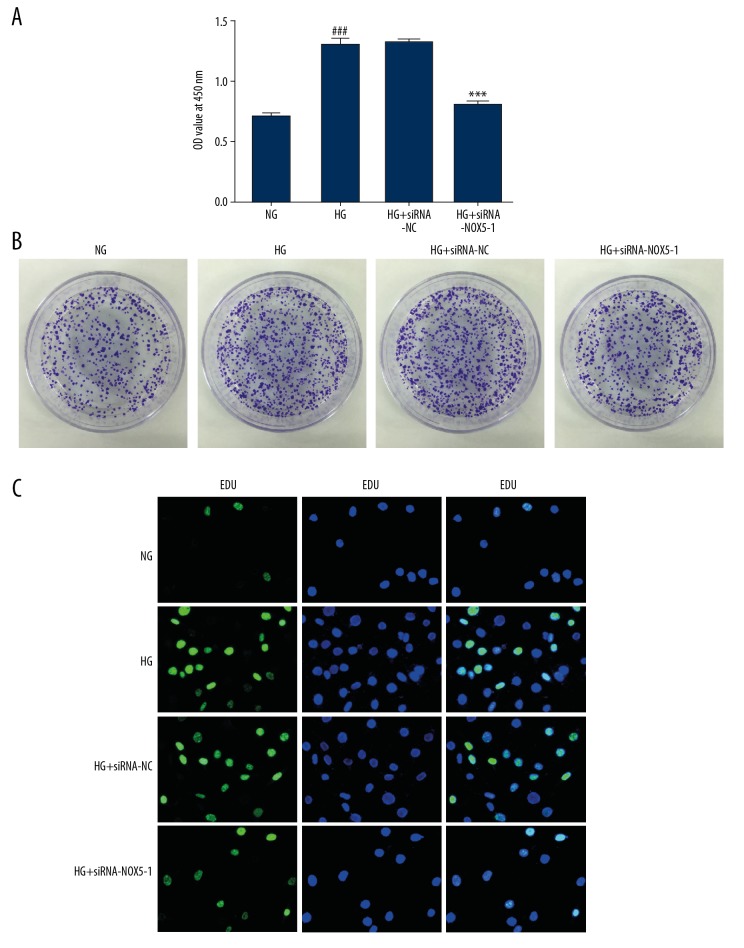
Inhibition of NOX5 suppressed proliferation of HMCs exposed to HG
After transfection, cells were incubated under NG or HG condition for 24 hours. To investigate the effect of NOX5 silencing on proliferation of HMCs in HG medium, a CCK-8 kit, colony formation assay, and EDU assay were applied to test cell proliferation. As shown in Figure 2A and 2B, HG culture promoted proliferation of HMCs compared with the NG group. Concurrently, a similar result was observed from the EDU incorporation assay (Figure 2C). Following transfected with siRNA-NOX5-1, cell proliferation was reduced obviously, suggesting that inhibition of NOX5 suppressed proliferation of HMCs in HG medium.
Figure 2.
NOX5 silencing inhibited proliferation of HMCs induced by HG. Proliferation of HMCs were evaluated by (A) CCK-8 assay, (B) colony formation assay and (C) EDU incorporation assay, respectively. ### P<0.001 versus NG; *** P<0.001 versus HG+siRNA-NC. NOX5 – NADPH oxidase 5; HG – high glucose; HMCs – human glomerular mesangial cells; CCK-8 – Cell Counting Kit-8 assay; EDU – 5-ethynyl-20-deoxyuridine.
Inhibition of NOX5 decreased HG-induced oxidative stress in HMCs
Oxidative stress associated markers were measured by kits in our study to explore the effect of NOX5 silencing on ROS in HMCs induced by HG. As exhibited in Figure 3A and 3B, the content of ROS detected by DCF-DA was increased significantly following HMCs being treated with HG alone in comparison with NG group. Concurrently, obvious increase of MDA and NADPH levels accompanied with a decrease in SOD and GSH-PX were observed in the HG treatment HMCs compared with the NG group (Figure 3C–3F). After silencing of NOX5, the contents of ROS, MDA, and NADPH were reduced notably, coupled with an increase in SOD and GAPDH. These findings indicated that NOX5 silencing inhibited oxidative stress in HMCs induced HG.
Figure 3.
Inhibition of NOX5 reduced HG-induced oxidative stress in HMCs. (A) The expression of ROS was assessed by ROS-DCF-DA assay. (B) Relative quantitative analysis of ROS. The concentration of (C) MDA and (D) NADPH as well as the enzymatic activities of (E) SOD and (F) GSH-PX were measured by kits. ### P<0.001 versus NG; ** P<0.05, *** P<0.001 versus HG+siRNA-NC. NOX5 – NADPH oxidase 5; HG – high glucose; HMCs – human glomerular mesangial cells; ROS – reactive oxygen species; MDA – malonaldehyde; SOD – superoxide dismutase; GSH-PX – glutathione peroxidase.
Inhibition of NOX5 restrained HG-induced inflammatory cytokines production in HMCs
Cells were stimulated with medium containing NG or HG for 24 hours after transfection, and then the levels of inflammation-associated cytokines were determined to evaluate the role of NOX5 inhibition on inflammatory response. An obvious increasing trend of TNF-α, IL-6, IL-1β, and MCP-1 was found in the HG group compared with the NG group (Figure 4A–4D). Following transfected with siRNA-NOX5-1, the contents of the aforementioned inflammatory cytokines were attenuated markedly. Concurrently, it has been reported that NOX5 can promote renal inflammation by regulating TLR pathway in podocytes [13], therefore, the levels of key proteins in TLR4/NF-κB signaling pathway were assessed by western blot in our current study. As shown in Figure 5, HG stimulation upregulated the expression of TLR4, MyD88, and p-NF-κB p65, whereas NOX5 silencing downregulated the expression of the aforementioned proteins in comparison with the NG group. Overall, these data suggested that inhibition of NOX5 restrained HG-induced inflammatory cytokines production through suppressing TLR4/NF-κB signaling pathway.
Figure 4.
Silencing of NOX5 attenuated the levels of inflammation associated cytokines in culture supernatant of HMCs induced by HG. The levels of (A) TNF-α, (B) IL-6, (C) IL-1β, and (D) MCP-1 were assessed by ELISA kits. ### P<0.001 versus NG; *** P<0.001 versus HG+siRNA-NC. NOX5 – NADPH oxidase 5; HG – high glucose; HMCs – human glomerular mesangial cells; TNF-α – tumor necrosis factor-alpha; IL-6 – interleukin (IL)-6; MCP-1 – monocyte chemoattractant protein-1; ELISA – enzyme-linked immunosorbent assay.
Figure 5.
NOX5 silencing inhibited the expression of key proteins in TLR4/NF-κB signaling pathway in HMCs induced by HG. The expression of TLR4, MyD88, p-NF-κB p65 and NF-κB p65 were determined by western blotting. ### P<0.001 versus NG; ** P<0.01 versus HG+siRNA-NC. NOX5 – NADPH oxidase 5; HG – high glucose; HMCs – human glomerular mesangial cells; TLR4 – toll-like receptor 4; p-NF-κB p65 – phosphor-NF-κB p65.
Inhibition of NOX5 reduced HG-induced ECM in HMCs
The expression of fibrosis components, including TGF-β1, TGF-β2, collagen I, collagen IV, and FN, were measured by western blotting. As presented in Figure 6, HG stimulation increased the expression of TGF-β1 while NOX5 silencing remarkably decreased this increase compare with the NG group, which was detected by immunofluorescence assay. Meanwhile, transfection with siRNA-NOX5-1 downregulated the expression of TGF-β2, collagen I, collagen IV, and FN which were upregulated by HG induction (Figure 7). And the expression of p-Smad2 and p-Smad3 presented similar results. Our results indicated that NOX5 silencing reduced HG-induced ECM in HMCs.
Figure 6.
Inhibition of NOX5 decreased the expression of TGF-β1 in HMCs induced by HG. The expression of NOX5 was detected by immunofluorescence assay. NOX5 – NADPH oxidase 5; HG – high glucose; HMCs – human glomerular mesangial cells; TGF-β1 – transforming growth factor-β1.
Figure 7.
NOX5 silencing reduced HG-induced ECM in HMCs. The expression of TGF-β1, TGF-β2, collagen I, collagen IV, FN, p-Smad2, and p-Smad3 were evaluated by western blotting. ### P<0.001 versus NG; * P<0.05, ** P<0.01 versus HG+siRNA-NC. NOX5 – NADPH oxidase 5; HG – high glucose; HMCs – human glomerular mesangial cells; ECM – extracellular matrix; TGF-β1 – transforming growth factor-β1; FN – fibronectin; p-Smad2 – phospho-Smad2.
Discussion
The data presented in the present study, for the first time, provided the evidence that NOX5 silencing alleviated HG-stimulated HMCs oxidative stress, inflammation, and ECM accumulation, suggesting that NOX5 might serve as a potential therapeutic target for diabetic nephropathy treatment.
A growing body of evidence suggests that glomerular MCs are a kind of major renal resident cells, and that they are highly associated with the occurrence and development of DN [15]. Increased proliferation of MCs is considered as a dominate factor in the pathophysiologic mechanism implicated in the DN [16]. It has been well reported that HG stimulation enhanced the proliferation of MCs and promoted renal interstitial fibrosis eventually [17]. Additionally, HG was confirmed to induce proliferation of MCs [18,19]. Emerging evidence supports that increased expression of NOX5 in kidneys of patients with diabetes compared with control kidneys [20]. And NOX5 could aggravate renal injury in mouse model of DN [12]. In addition, a previous study showed that NOX5 could accelerate proliferation and fibrosis in human hepatic stellate cells [21]. In line with the previous study, our findings suggested that HG treatment significantly promoted proliferation of MCs. And NOX5 silencing inhibited HG-stimulated MCs proliferation. The aforementioned results demonstrated that silencing of NOX5 mitigated DN in vitro via suppressing MCs proliferation.
Excessive oxidative stress and inflammation serve as crucial pathogenic factors in the development of DN [22,23]. It has been well documented that HG treatment induced the production of oxides and inflammatory cytokines in MCs [24]. Mounting evidence supported that antioxidant and anti-inflammatory contribute to the treatment of DN [25]. Importantly, it has been reported that NOX5, a source of ROS, could increase oxidative stress in human renal proximal tubule cells and promote renal podocyte inflammation [13,14]. In agreement with the results of the aforementioned previous studies, we observed that HG stimulation increased the contents of ROS, MDA, and NADPH in MCs, accompanied by a decrease in SOD and GSH-PX, whereas silencing of NOX5 alleviated the increase of oxidative stress. In addition, NOX5 silencing attenuated the levels of inflammatory cytokines induced by HG in MCs. Emerging evidence shows that NOX5 could promote renal inflammation through activation of TLR pathway [13]. Moreover, NOX5-S regulated acid-induced cyclo-oxygenase-2 expression by activating NF-κB signaling in Barrett’s esophageal adenocarcinoma cells [26]. In our study, HG treatment increased the expression of the TLR4/NF-κB signaling pathway, which was in accordance with the previous findings [9,27]. Following transfection with siRNA-NOX5-1, marked inhibition of TLR4/NF-κB signaling proteins expression was observed. These findings suggested that inhibition of NOX5 decreased HG-induced oxidative stress and inflammatory responses in HMCs by inactivation of the TLR4/NF-κB signaling pathway.
Accumulating evidence has shown that the excessive accumulation of ECM components in mesangium is closely related to the development of DN [28]. ECM consists of a variety of extracellular molecules, and collagens are the richest members in it. TGF-β1 is highly expressed in DN renal tissue and serves as a pivotal marker of renal fibrosis [29,30]. And TGF-β1 could upregulate the expression of FN, which is a critical molecule of the ECM and is primarily synthesized during DN [31]. Numerous studies have unveiled that HG might enhance the secretion of collagen I, collagen IV, and FN as well upregulate the expression of TGF-β1 in MCs [32,33]. An obvious decreasing trend of TGF-β1, TGF-β2, collagen I, collagen IV, and FN expression were found following NOX5 silencing in HG-stimulated MCs in our study. In addition, the expression of p-Smad2 and p-Smand3 were downregulated remarkably following NOX5 silencing. These findings suggested that inhibition of NOX5 is able to reduce HG-induced ECM in HMCs.
Conclusions
Taken together, our study demonstrated that NOX5 silencing suppressed HG-induced HMCs oxidative stress, inflammation, and ECM accumulation, suggesting that NOX5 might become a sensible and potential therapeutic target for the treatment of diabetic nephropathy. Specific inhibition of signaling pathway and in vivo animal experiments are limitation of this study, which warrants further investigation in the future to understand the effects of NOX5 in DN.
Acknowledgments
All authors would like to thank Jing Feng for her help during materials collection.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124:2333–40. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badal SS, Danesh FR. New insights into molecular mechanisms of diabetic kidney disease. Am J Kidney Dis. 2014;63:S63–83. doi: 10.1053/j.ajkd.2013.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim A. Diabetic nephropathy – complications and treatment. Int J Nephrol Renovasc Dis. 2014;7:361–81. doi: 10.2147/IJNRD.S40172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhaskaragoud G, Geetha V, Sharanappa T, et al. Hypolipidemic and antioxidant properties of oryzanol concentrate in reducing diabetic nephropathy via SREBP1 downregulation rather than beta-oxidation. Mol Nutr Food Res. 2018;62:e1700511. doi: 10.1002/mnfr.201700511. [DOI] [PubMed] [Google Scholar]
- 5.Diab El-Harakeh M, Njeim R, Youssef A, et al. Novel triazine-based pyrimidines suppress glomerular mesangial cells proliferation and matrix protein accumulation through a ROS-dependent mechanism in the diabetic milieu. Bioorg Med Chem Lett. 2019;29:1580–85. doi: 10.1016/j.bmcl.2019.04.052. [DOI] [PubMed] [Google Scholar]
- 6.Ichinose K, Kawasaki E, Eguchi K. Recent advancement of understanding pathogenesis of type 1 diabetes and potential relevance to diabetic nephropathy. Am J Nephrol. 2007;27:554–64. doi: 10.1159/000107758. [DOI] [PubMed] [Google Scholar]
- 7.Liu Z, Han Y, Zhao F, et al. Nobiletin suppresses high-glucose-induced inflammation and ECM accumulation in human mesangial cells through STAT3/NF-kappaB pathway. J Cell Biochem. 2019;120:3467–73. doi: 10.1002/jcb.27621. [DOI] [PubMed] [Google Scholar]
- 8.Sheu ML, Shen CC, Jheng JR, Chiang CK. Activation of PI3K in response to high glucose leads to regulation of SOCS-3 and STAT1/3 signals and induction of glomerular mesangial extracellular matrix formation. Oncotarget. 2017;8:16925–38. doi: 10.18632/oncotarget.14808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, Zhu X, Sun Z, Ma Y. Astilbin inhibits high glucose-induced inflammation and extracellular matrix accumulation by suppressing the TLR4/MyD88/NF-kappaB pathway in rat glomerular mesangial cells. Front Pharmacol. 2018;9:1187. doi: 10.3389/fphar.2018.01187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Ren L, Wang C, et al. Effect of chenodeoxycholic acid on fibrosis, inflammation, and oxidative stress in kidney in high-fructose-fed Wistar rats. Kidney Blood Press Res. 2012;36:85–97. doi: 10.1159/000341485. [DOI] [PubMed] [Google Scholar]
- 11.Holterman CE, Thibodeau JF, Kennedy CR. NADPH oxidase 5 and renal disease. Curr Opin Nephrol Hypertens. 2015;24:81–87. doi: 10.1097/MNH.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 12.Jha JC, Dai AZ, Cooper ME, et al. NADPH oxidase Nox5 aggravates renal injury in Akita mouse model of diabetic nephropathy. Diabetes. 2018;67(Suppl 1) [Google Scholar]
- 13.Holterman CE, Boisvert NC, Thibodeau JF, et al. Podocyte NADPH oxidase 5 promotes renal inflammation regulated by the Toll-like receptor pathway. Antioxid Redox Signal. 2019;30:1817–30. doi: 10.1089/ars.2017.7402. [DOI] [PubMed] [Google Scholar]
- 14.Yu P, Han W, Villar VA, et al. Unique role of NADPH oxidase 5 in oxidative stress in human renal proximal tubule cells. Redox Biol. 2014;2:570–79. doi: 10.1016/j.redox.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin L, Zhang R, Yang S, et al. Knockdown of ANGPTL-4 inhibits inflammatory response and extracellular matrix accumulation in glomerular mesangial cells cultured under high glucose condition. Artif Cells Nanomed Biotechnol. 2019;47:3368–73. doi: 10.1080/21691401.2019.1649274. [DOI] [PubMed] [Google Scholar]
- 16.Xu JL, Gan XX, Ni J, et al. SND p102 promotes extracellular matrix accumulation and cell proliferation in rat glomerular mesangial cells via the AT1R/ERK/Smad3 pathway. Acta Pharmacol Sin. 2018;39:1513–21. doi: 10.1038/aps.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu XM, Gao YB, Cui FQ, Zhang N. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open. 2016;5:484–91. doi: 10.1242/bio.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Shi L, Han Z, Liu B. Follistatin-like 3 suppresses cell proliferation and fibronectin expression via p38MAPK pathway in rat mesangial cells cultured under high glucose. Int J Clin Exp Med. 2015;8:15214–21. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Fang H, Dong B, et al. Effects of free anthraquinones extract from the rhubarb on cell proliferation and accumulation of extracellular matrix in high glucose cultured-mesangial cells. Korean J Physiol Pharmacol. 2015;19:485–89. doi: 10.4196/kjpp.2015.19.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holterman CE, Thibodeau JF, Towaij C, et al. Nephropathy and elevated BP in mice with podocyte-specific NADPH oxidase 5 expression. J Am Soc Nephrol. 2014;25:784–97. doi: 10.1681/ASN.2013040371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andueza A, Garde N, Garcia-Garzon A, et al. NADPH oxidase 5 promotes proliferation and fibrosis in human hepatic stellate cells. Free Radic Biol Med. 2018;126:15–26. doi: 10.1016/j.freeradbiomed.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Han Q, Zhu H, Chen X, Liu Z. Non-genetic mechanisms of diabetic nephropathy. Front Med. 2017;11:319–32. doi: 10.1007/s11684-017-0569-9. [DOI] [PubMed] [Google Scholar]
- 23.Duran-Salgado MB, Rubio-Guerra AF. Diabetic nephropathy and inflammation. World J Diabetes. 2014;5:393–98. doi: 10.4239/wjd.v5.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai J, Wang Y, Zhu X, Shi J. Eriodictyol inhibits high glucose-induced extracellular matrix accumulation, oxidative stress, and inflammation in human glomerular mesangial cells. Phytother Res. 2019;33(10):2775–82. doi: 10.1002/ptr.6463. [DOI] [PubMed] [Google Scholar]
- 25.Parveen A, Jin M, Kim SY. Bioactive phytochemicals that regulate the cellular processes involved in diabetic nephropathy. Phytomedicine. 2018;39:146–59. doi: 10.1016/j.phymed.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Si J, Fu X, Behar J, et al. NADPH oxidase NOX5-S mediates acid-induced cyclooxygenase-2 expression via activation of NF-kappaB in Barrett’s esophageal adenocarcinoma cells. J Biol Chem. 2007;282:16244–55. doi: 10.1074/jbc.M700297200. [DOI] [PubMed] [Google Scholar]
- 27.Sun Z, Ma Y, Chen F, et al. Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-kappaB/NLRP3 inflammasome pathway. Chem Biol Interact. 2018;293:11–19. doi: 10.1016/j.cbi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Wang Y, Chen B, Shi J. Silencing of PAQR3 suppresses extracellular matrix accumulation in high glucose-stimulated human glomerular mesangial cells via PI3K/AKT signaling pathway. Eur J Pharmacol. 2018;832:50–55. doi: 10.1016/j.ejphar.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 29.Lan HY. Diverse roles of TGF-beta/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7:1056–67. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Wang Y, Yan R, et al. Oxymatrine inhibits renal tubular EMT induced by high glucose via upregulation of SnoN and inhibition of TGF-beta1/Smad signaling pathway. PLoS One. 2016;11:e0151986. doi: 10.1371/journal.pone.0151986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang S, Gao C, Long Y, et al. Maresin 1 mitigates high glucose-induced mouse glomerular mesangial cell injury by inhibiting inflammation and fibrosis. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/2438247. 2438247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R, Li J, Huang T, Wang X. Danggui buxue tang suppresses high glucose-induced proliferation and extracellular matrix accumulation of mesangial cells via inhibiting lncRNA PVT1. Am J Transl Res. 2017;9:3732–40. [PMC free article] [PubMed] [Google Scholar]
- 33.Chen P, Shi X, Xu X, et al. Liraglutide ameliorates early renal injury by the activation of renal FoxO1 in a type 2 diabetic kidney disease rat model. Diabetes Res Clin Pract. 2018;137:173–82. doi: 10.1016/j.diabres.2017.09.006. [DOI] [PubMed] [Google Scholar]