Abstract
Background
Cardiovascular complications, such as diabetic cardiomyopathy (DCM), are the leading cause of death in diabetic patients. Shengmai Powder (SMP) was found to have cardioprotective effects.
Material/Methods
Based on the systematic pharmacological methodology, this research determined the genes of DCM and the known targets of SMP, predicted potential compounds and targets of SMP, constructed networks for DCM and SMP, and performed network analysis.
Results
Five network were constructed: (1) the DCM gene PPI network; (2) the Compound-compound target network of SMP; (3) the SMP-DCM PPI network; (4) the Compound-known target network of SMP; (5) and the SMP known target-DCM PPI network. Several DCM and treatment related targets, clusters, signaling pathways, and biological processes were found.
Conclusions
SMP is able to regulate glycometabolism-related, lipid metabolism-related, inflammatory response-related, oxidative stress-related signaling pathways, and biological processes and targets, which suggests that SMP may have a therapeutic effect on DCM.
MeSH Keywords: Diabetic Cardiomyopathies; Herbal Medicine; Medicine, Chinese Traditional
Background
Diabetic cardiomyopathy (DCM) is defined as ventricular dysfunction without coronary artery disease (CAD) and hypertension [1]. The number of new cases of DCM increases in proportion to newly diagnosed cases of diabetes mellitus (DM) worldwide [2]. As a serious metabolic disease, diabetes affects about 5% of the world’s people. Epidemiological data show that the number of people with diabetes is expected to increase dramatically, to 592 million by 2035, which will lead to huge medical and economic burdens [2,3]. More importantly, more than 1 billion people worldwide are overweight (BMI >25 and <29.9) or obese (BMI >30) [4]. These factors contribute to the DM epidemic.
Cardiovascular complications, mainly ischemic heart disease, are the leading cause of death in diabetic patients. The early changes in the structure and function of DCM are mainly congestive heart failure with diastolic dysfunction; in the later stage, the heart failure caused by extensive myocardial necrosis and systolic dysfunction leads to cardiogenic shock [4–7]. The potential molecular mechanisms of DCM may include: (1) Increased oxidation of fatty acids and lipotoxicity lead to mitochondrial dysfunction; (2) Mitochondrial dysfunction and endoplasmic reticulum stress promote apoptosis; (3) Oxidative stress, increased AGE signaling, and inflammation may promote the expression of profibrotic genes or apoptosis [7–9].
At present, there are many treatment strategies for DCM, including blood sugar control, β-adrenergic blocker, blood lipid regulation, exercise, PPARα agonists, antioxidants, antagonistic renin-angiotensin-aldosterone system (RAAS), and calcium upregulation [5,7,10]. With the concept of comprehensive treatment of DCM, alternative medicine is gradually becoming an important part of DCM treatment, which significantly improves the clinical symptoms of DCM. Traditional Chinese Medicine (TCM), as an important supplement and complementary form of alternative medicine, has a long history of application in DCM treatment and is a promising strategy that has been widely used in the treatment of DCM. Shengmai Powder (SMP) is an herbal TCM formula. It was originally recorded in “Yi Xue Qi Yuan”, whose author was Yuansu Zhang of the Jin Dynasty. It consists of Renshen (Panax ginseng, Araliaceae), Maidong (Ophiopogon japonicas, Liliaceae), and Wuweizi (Schisandra chinensis, Schisandraceae). The details of this formula are recorded in the Chinese Pharmacopoeia [11]. SMP is traditionally used for patient with ischemic diseases, diabetes, and heart failure. A systematic review of Shengmai Injection (SMI) showed that Western medicine combined with SMI can improve its therapeutic effect on chronic heart failure [11]. However, higher-quality randomized controlled trials are needed to increase the strength of evidence [11]. In addition, although the safety of SMI should be carefully monitored during the trial [11], the safety of SMP for oral use is high.
Recently, more and more evidence has shown the protective effect of SMP on heart and blood vessels. Zhao et al. (2016) pointed out that SMP attenuates cardiac hypertrophy and fibrosis through the TGF-β-dependent pathway [12]. Liu et al. (2018) reported that SMP can improve cell apoptosis and promote angiogenesis in rats with myocardial ischemia-reperfusion injury [13]. Zhan et al. (2015) found that SMP can regulate myocardial energy metabolism through multiple metabolic pathways (such as stimulating glucose metabolism and inhibiting fatty acid metabolism) to improve cardiac efficiency [14]. Ni et al. (2011) also found that SMP can inhibit myocardial fibrosis in diabetic cardiomyopathy mice and significantly delay the formation of diabetic cardiomyopathy in hyperglycemia rat models [15]. However, the pharmacological mechanisms underlying the effect of SMP on DCM have not yet been fully elucidated. Since the characteristics of systemic pharmacology are compatible with the “multi-target – multi-biological process – multi-pathway” characteristics of TCM, the present study explored the mechanism underlying the effect of SMP on DCM by using a systematic pharmacological methodology.
Material and Methods
Materials collection
SMP compounds collection
This research utilized the Traditional Chinese Medicine (TCM) Database@Taiwan [16] (http://tcm.cmu.edu.tw/zh-tw/) and the Traditional Chinese Medicine Systems Pharmacology Database [17] (TcmSPTM, http://lsp.nwsuaf.edu.cn) to determine the compounds present in SMP.
The TCM Database@Taiwan is a comprehensive TCM database and a Traditional Chinese Medicine Systems Pharmacology Database [17], while TCMSP is a unique system pharmacology platform designed for Chinese herbal medicines [18].
Potential compounds prediction
To predict the potential compounds of SMP, 3 ADME-related models – oral bioavailability (OB), Caco-2 permeability, and drug-likeness (DL) – were used in this research [18,19]. The standard was OB ≥30%, Caco-2 >–0.4, and DL ≥0.18. Any compounds meeting this standard were selected for subsequent research, while all others were excluded. The details of OB, Coca-4, and DL, and the methodology of acquisition, were described in our previous work [18,19].
After the prediction, we discovered many potential compounds of SMP: alexandrin, aposiopolamine, arachidonate, beta-sitosterol, celabenzine, chrysanthemaxanthin, dianthramine, diop, frutinone A, fumarine, ginsenoside Rg5, ginsenoside Rh4, girinimbin, gomisin B, inermin, kaempferol, panaxadiol, stigmasterol, suchilactone, malkangunin, deoxyharringtonine, angeloylgomisin O, gomisin A, gomisin G, gomisin R, longikaurin A, schizandrer B, and wuweizisu C. The details of these compounds are described in Supplementary Table 1.
Supplements of potential compounds
Because the predictive ability of biological models is limited, some of the important compounds may be missing. To avoid this, we searched many references and selected oral compounds with pharmacological activity as supplement compounds [20].
After the discovery of supplements potential compounds [21], we found several supplements potential compounds: 5,7,2,4-tetrahydroxy-8-methoxy-6-methyl homoisoflavanone (1243677-84-0), 5,7,4-trihydroxy-6-methyl homoisoflavanone (1243677-86-2), 5,7-dihydroxy-4-methoxy-6-methyl homoisoflavanone (212201-10-0), 6-formylisoophiopogonanone A, desmethylisophiopogonone B, methylophiopogonanone A, methylophiopogonanone B, methylophiopogonone A, ophiopogonanone A, ophiopogonanone E, ophiopogonin D, and ophiopogonin D′. The details of these compounds are described in Supplementary Table 2.
Compound targets and known targets for SMP
The structures of potential compounds were acquired from SciFinder (http://scifinder.cas.org). The PharmMapper (http://lilab.ecust.edu.cn/pharmmapper/) was used to predict the compound targets [22]. The known targets were obtained from the TCMSP [17].
Protein name correction
The UniProtKB (http://www.uniprot.org/) was used for the correction of protein names and the collection of official symbols, with the species limited to “Homo sapiens”. The details are described in Supplementary Tables 3 and 4.
DCM genes
The OMIM database and Genecards were used to collect the DCM-related disease genes and targets [18,19]. The OMIM database (http://omim.org/) is the database that catalogs all known diseases with a genetic component [23]. Genecards (http://www.genecards.org) is “a database about genes, their products and biomedical applications maintained by Israel’s Weizmann Institute of science” [18,19]. The details of DCM genes are shown in Supplementary Table 5.
Protein–protein interaction data
The String database (http://string-db.org/) and the IntAct database (http://www.ebi.ac.uk/intact/) were utilized to obtain data on protein-protein interaction (PPI). While searching the String database, the species was limited to “Homo sapiens” [18,19,24,25] with confidence score ≥0.4. The node interaction type was default.
Network construction
Network construction method
Cytoscape 3.4.0 software was used for the network visualization and network analysis [26] (http://cytoscape.org/). Several networks were constructed: (1) DCM gene PPI network; (2) Compound-compound target network of SMP; (3) SMP-DCM PPI network; (4) Compound-known target network of SMP; and (5) SMP known target-DCM PPI network.
Cluster
The definition the acquisition methods of clusters were described in our previous works [18,19]. The clusters of each network were obtained by analyzing the corresponding networks by MCODE, a plug-in of Cytoscape [27,28] with default value (Degree Cutoff=2, Node Density Cutoff=0.1, Node Score Cutoff=0.2, K-Core=2, and Max. Depth=100).
Enrichment analysis
DAVID ver 6.8 (https://david-d.ncifcrf.gov) was applied for Gene Ontology (GO) enrichment analysis and pathway enrichment analysis [29]. The P value in the biological processes and signaling pathways is the modified Fisher exact P value, EASE score [29], in which a lower score indicated greater enrichment.
Results and Discussion
Diabetic cardiomyopathy network analysis
Diabetic cardiomyopathy network
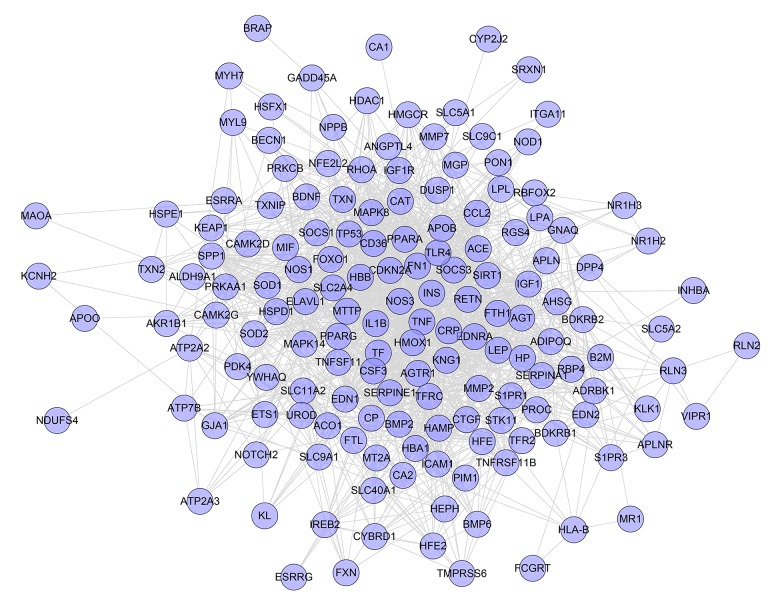
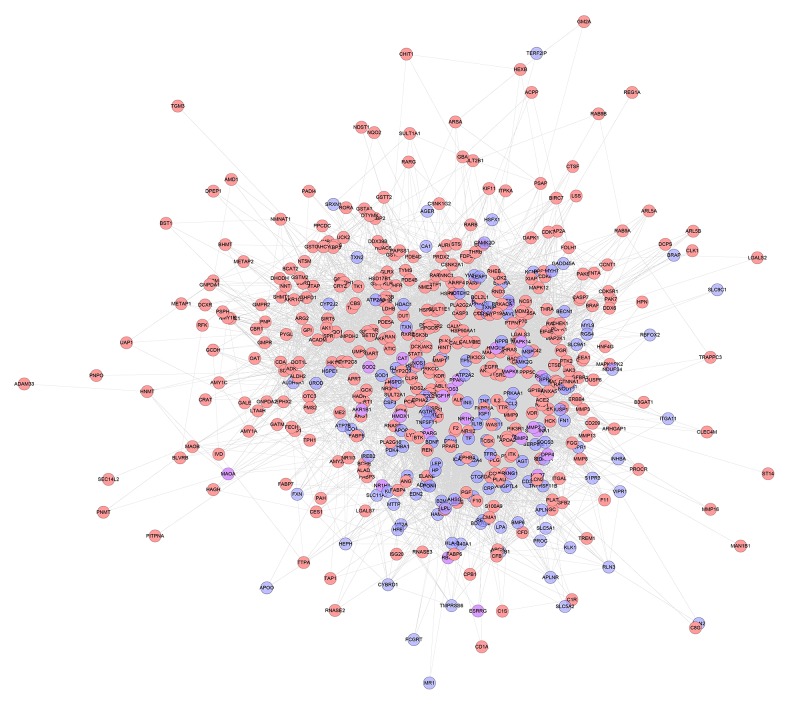
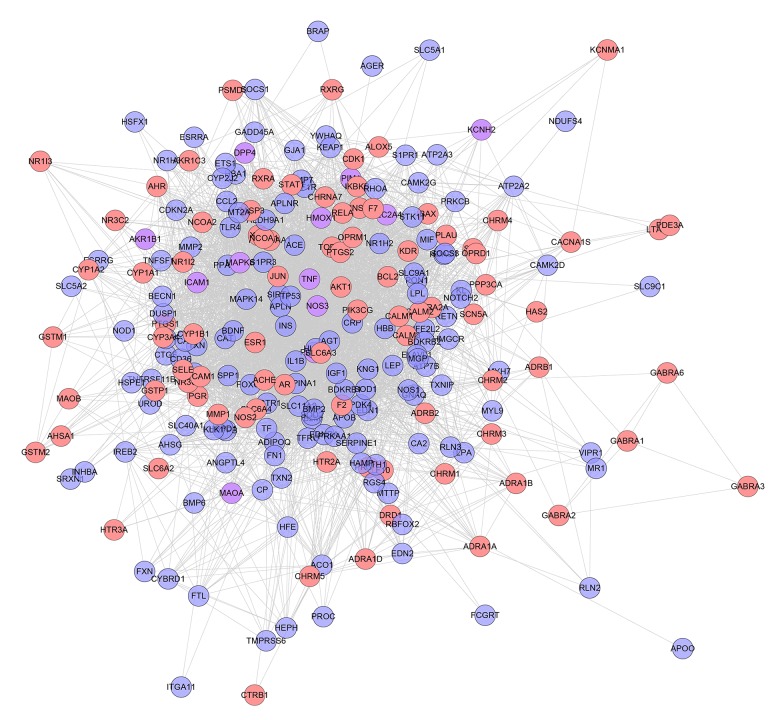
One hundred and fifty-seven genes were input into the String database to collect the PPI information so as to build this work. It contains 157 nodes and 1502 edges (Figure 1). Several genes are thought to be the key gene because they have higher degrees: INS (88 edges), TNF (73 edges), TP53 (64 edges), NOS3 (61 edges), MAPK8 (58 edges), IGF1 (57 edges), CRP (55 edges), IL1B (51 edges), TF (50 edges), EDN1(50 edges), and PPARG (50 edges). These genes may be the key or central genes in diabetic cardiomyopathy.
Figure 1.
Diabetic cardiomyopathy network.
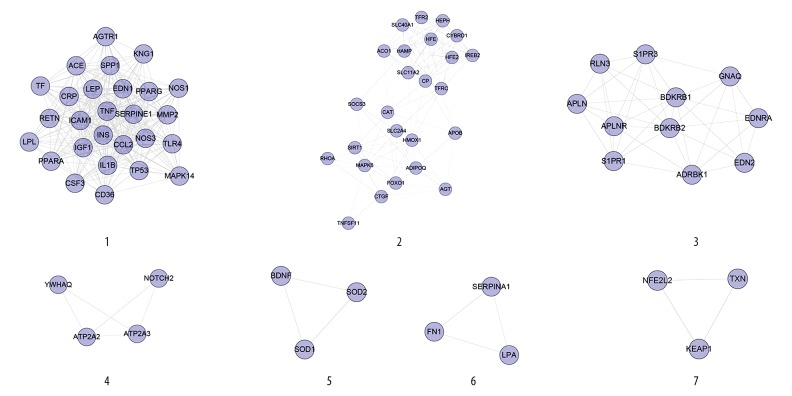
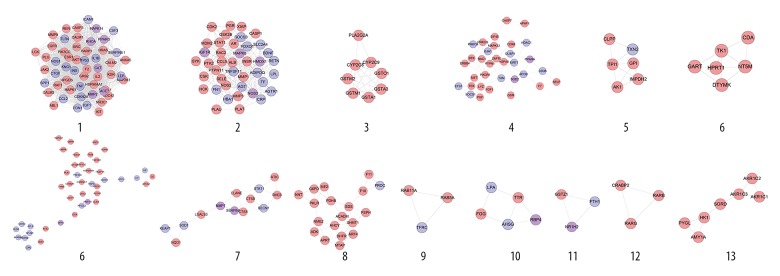
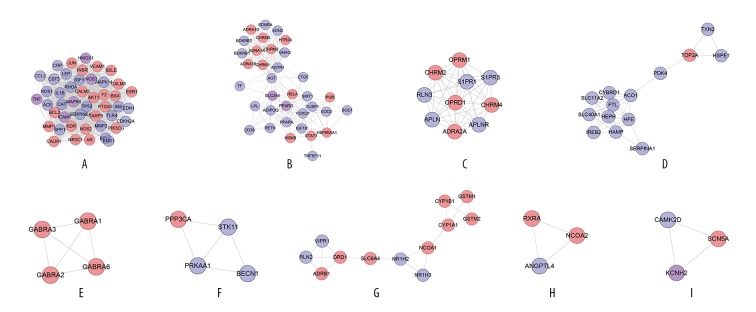
Clusters of diabetic cardiomyopathy network
Network analysis produced 7 clusters (Table 1, Figure 2). The genes of each cluster were entered into DAVID for GO enrichment analysis, which yielded several biological processes. Cluster 6 did not return any DCM-related biological processes.
Table 1.
Cluster of diabetic cardiomyopathy network.
| Cluster | Score | Nodes | Edges | Genes |
|---|---|---|---|---|
| 1 | 21.385 | 27 | 278 | AGTR1, NOS1, CD36, TNF, IL1B, MMP2, CSF3, SERPINE1, TLR4, TF, SPP1, IGF1, PPARA, TP53, ACE, CRP, RETN, INS, EDN1, ICAM1, CCL2, PPARG, LPL, LEP, MAPK14, KNG1, NOS3 |
| 2 | 9.417 | 25 | 113 | RHOA, SOCS3, CP, SLC11A2, SLC40A1, CYBRD1, IREB2, TFRC, HEPH, TNFSF11, ACO1, SLC2A4, CAT, FOXO1, SIRT1, AGT, HFE, HFE2, HAMP, APOB, MAPK8, HMOX1, ADIPOQ, CTGF, TFR2 |
| 3 | 7.6 | 11 | 38 | APLN, APLNR, ADRBK1, GNAQ, S1PR3, BDKRB1, RLN3, BDKRB2, S1PR1, EDNRA, EDN2 |
| 4 | 3.333 | 4 | 5 | YWHAQ, ATP2A3, ATP2A2, NOTCH2 |
| 5 | 3 | 3 | 3 | BDNF, SOD2, SOD1 |
| 6 | 3 | 3 | 3 | FN1, LPA, SERPINA1 |
| 7 | 3 | 3 | 3 | NFE2L2, TXN, KEAP1 |
Figure 2.
Cluster of diabetic cardiomyopathy network.
Cluster 1 contained glycometabolism-related, lipid metabolism-related, cardiovascular disease-related (e.g., blood pressure, blood lipids, and foam cells), inflammatory-related (e.g., inflammatory factors and interleukins), and oxidative stress-related biological processes. Cluster 2 is mainly related to iron metabolism and oxidative stress. Cluster 3 is associated with vasomotor regulation. Cluster 4 is related to calcium ion homeostasis. Clusters 5 and 7 contain several oxidative stress-related biological processes. The details of each cluster and biological process are described in Supplementary Table 6.
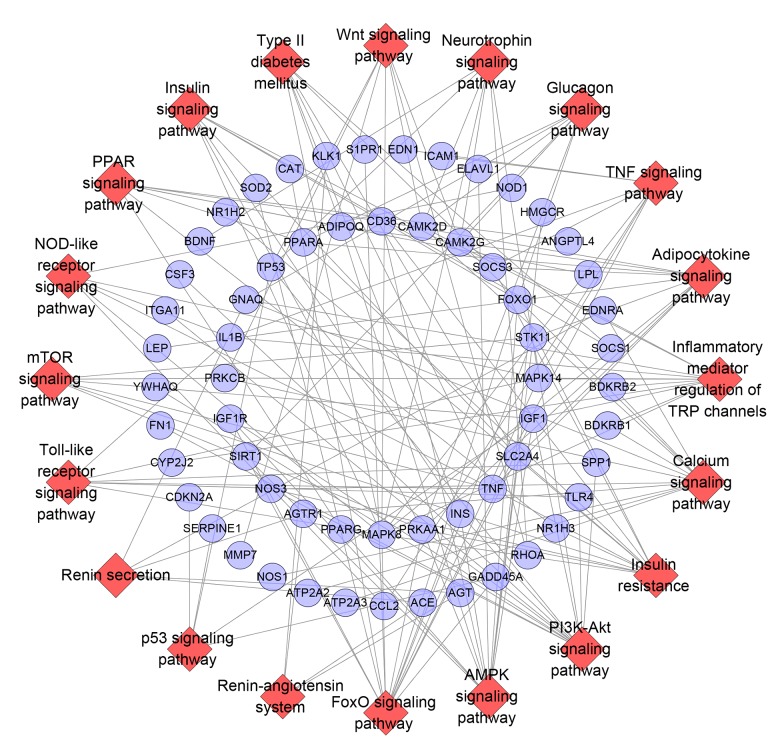
Pathway of diabetic cardiomyopathy network
All DCM genes were entered into DAVID for pathway enrichment analysis, showing 29 DCM-related pathways (Figure 3). The FoxO signaling pathway contained 14 genes, the PI3K-Akt signaling pathway and AMPK signaling pathway included 13 genes, the insulin resistance and calcium signaling pathway had 12 genes, and the inflammatory mediator regulation of TRP channels contained 11 genes (Supplementary Table 7)
Figure 3.
Pathway of diabetic cardiomyopathy network (Blue circle stands for breast cancer gene. Red diamond stands for pathway).
The above network analysis of DCM genes in the PPI network showed that glycometabolism-related, lipid metabolism-related, cardiovascular disease-related, inflammatory-related, and oxidative stress-related biological processes are involved in the development of DCM. Recent research also shows that targeted administration of drugs interfering with these biological processes can benefit some patients with DCM [7,8]. FoxO, PI3K-Akt, AMPK signaling pathway, and insulin resistance are the core pathways that mediate cardiomyocyte metabolic disorders, coronary microangiopathy, myocardial interstitial fibrosis, and cardiac autonomic neuropathy in DCM [30,31]. These pathways have many DCM-related genes and may be the key pathways in development of DCM. Early intervention in these signaling pathways may be a strategy for future treatment and prevention of DCM.
Cardiovascular complications of diabetes have important effects on the life expectancy and quality of life of T2DM patients. DCM is mainly characterized by diastolic dysfunction, myocardial fibrosis, and cardiac hypertrophy [32], which are associated with reactive oxygen species (ROS) production, inflammation, and mitochondrial dysfunction [33]. Abnormal signaling pathways in the metabolism of hearts of diabetic patients include insulin signaling, AMPK signaling, and β-adrenergic signaling. Some studies have shown that knocking out cardiac insulin receptors will reduce glucose uptake in cardiomyocytes, increase oxidative stress in the heart, and reduce mitochondrial function and cardiac function [34–36]. The AMPK signaling pathway is involved in a variety of cellular processes, including regulation of glycolysis and fatty acid oxidation, initiation of autophagy, and synthesis of lipids, glycogen, and proteins [37]. Many studies have shown that metformin, which is an AMPK activator, can improve cardiac outcome in T1DM and T2DM rodent models [38]. The relationship between β-adrenergic signaling and insulin resistance is becoming the focus of DCM research [39]. Some findings suggest a complex relationship between β-adrenergic and insulin signaling pathways in diabetic hearts, and each pathway plays an indispensable role in the development of cardiac pathology [40,41]. In addition, diabetes-related systemic disorders of glucose, insulin, and fatty acids promote changes in cardiac metabolic characteristics and changes in key cardiac signaling pathways [30].
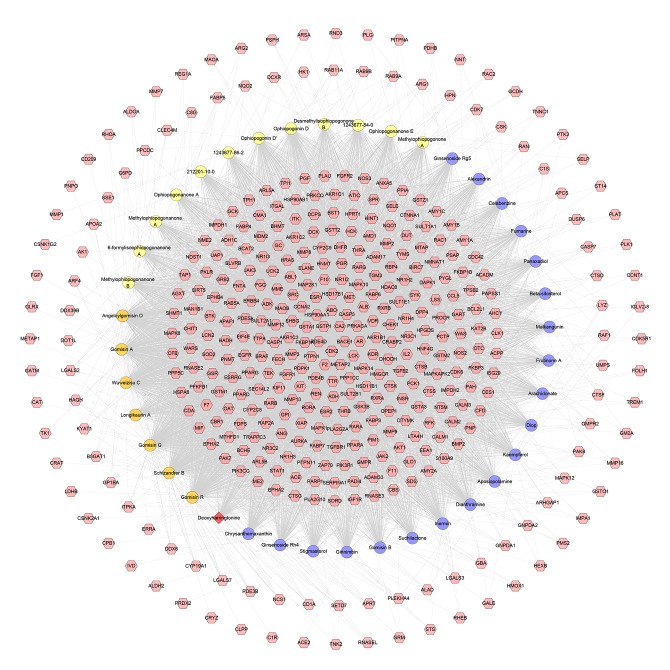
Compound–compound target network
This network consists of 440 nodes (400 compound target nodes and 40 compound nodes) and 6361 edges. The relationship among compounds and targets can be observed through this network. For example, the targets CDK2, BACE1, CA2, GSTP1, PDE4D, ABO, HSP90AA1, GSTA1, FKBP1A, PTPN1, F2, METAP2, and LCK are regulated by all compounds. These targets are in the center of the network, and may be the most important ones for SMP (Figure 4).
Figure 4.
Compound-compound target network of SMP consist of 400 compound targets and 40 compounds (Pink hexagon stand for compound targets; The circles with different colors stand for compounds of different herbs: orange circle – Schisandra chinensis, Schisandraceae, yellow circle – Ophiopogon japonicas, Liliaceae, blue circle – Panax ginseng, Araliaceae. Red diamond stands for common compound of Schisandra chinensis, Schisandraceae and Panax ginseng, Araliaceae).
SMP-DCM network analysis
SMP-DCM network
The Integrated DCM network and SMP network and the SMP-DCM network were assessed. This network contains 519 nodes and 8293 edges. There are 363 nodes that are compound targets, 26 nodes are DCM-compound targets, and 130 nodes are DCM genes (Figure 5).
Figure 5.
SMP-DCM network (Pink, blue, purple circle stand for compound target, DCM genes, DCM-compound target, resp.).
Clusters of SMP-DCM network
Fourteen clusters were collected after being analyzed by MCODE. There are some DCM-related genes in clusters (Blue circles). These genes may be the key genes of SMP involved in treating DCM (Table 2, Figure 6).
Table 2.
Cluster of SMP-DCM network.
| Cluster | Score | Nodes | Edges | Targets and genes |
|---|---|---|---|---|
| 1 | 40.981 | 54 | 1086 | ICAM1, SERPINE1, IGF1, JAK2, CALM1, AKT1, CALM2, CALM3, GRB2, EDN1, TP53, LCK, MAPK14, CDKN2A, CAT, KDR, CTGF, IL1B, KIT, CCL2, NOS1, ANXA5, REN, RHOA, SPP1, IL2, NR3C1, INS, PIK3CG, HRAS, LEP, ABL1, TNF, TLR4, CASP3, CSF3, ESR1, EGFR, MMP2, MAPK1, SRC, ACE, HSP90AA1, MAP2K1, KNG1, HPGDS, CDC42, BCL2L1, MMP9, F2, PARP1, PLG, RAF1, PPARG |
| 2 | 13.25 | 41 | 265 | PTK2, ADIPOQ, LPL, CDK2, BDNF, AR, CRP, TNFSF11, PLAT, SOCS3, XIAP, ALB, INSR, HBA1, PTPN11, SLC2A4, MMP3, AGT, PGR, RAC2, IGF1R, SYK, CSK, AGTR1, MAPK8, STAT1, NOS2, FN1, MMP1, MDM2, CASP1, GSK3B, HMOX1, NOS3, TF, HCK, FOXO1, SELE, CCL5, RETN, PLAU |
| 3 | 6.571 | 8 | 23 | GSTO1, CYP2C9, CYP2C8, GSTM1, PLA2G2A, GSTA3, GSTA1, GSTM2 |
| 4 | 4.375 | 33 | 70 | MET, FGF1, F7, SIRT1, PRKCQ, MAPK12, PGF, SOCS1, RAC1, CDK6, EIF4E, CD36, GSR, PIK3R1, HDAC1, ZAP70, SOD2, BTK, TXN, GJA1, APAF1, CASP7, PRKCB, PTPN1, SELP, PPARA, HSPA8, ETS1, DUSP1, APOB, ERBB4, TEK, LYZ |
| 5 | 4 | 6 | 10 | TXN2, CLPP, GPI, IMPDH2, AK1, TPI1 |
| 6 | 4 | 6 | 10 | GART, HPRT1, CDA, NT5M, TK1, DTYMK |
| 7 | 3.512 | 42 | 72 | ACE2, PDE5A, RLN3, APLN, PLK1, GLRX, NFE2L2, GMPR, GC, CTNNA1, GSTP1, CSNK2A1, RND3, S1PR3, DPP4, HSP90AB1, APOA2, HSPD1, CRYZ, GMPR2, YWHAQ, PDPK1, FKBP1A, TGFBR1, HSPE1, GP1BA, EDN2, GCK, BDKRB2, CMA1, BDKRB1, AKR1B1, PPP1CC, CP, PAK6, APLNR, TYMS, ACO1, ALDH2, HP, PRDX2, IMPDH1 |
| 8 | 3.5 | 13 | 21 | NQO1, STK11, SOD1, ELANE, BECN1, KEAP1, SERPINA1, MMP7, LGALS3, ATIC, CTSS, CTSB, RHEB |
| 9 | 3.222 | 19 | 29 | ADK, ME2, NNT, PDHB, PKLR, PROC, AHCY, G6PD, ARF4, F11, APRT, DHFR, SHMT1, PSPH, F10, SDS, MTAP, NME2, ACADM |
| 10 | 3 | 3 | 3 | RAB5A, TFRC, RAB11A |
| 11 | 3 | 5 | 6 | LPA, RBP4, AHSG, FGG, TTR |
| 12 | 3 | 3 | 3 | GSTZ1, FTH1, NR1H2 |
| 13 | 3 | 3 | 3 | RARG, CRABP2, RARB |
| 14 | 2.667 | 7 | 8 | SORD, AKR1C2, AKR1C3, AKR1C1, AMY1A, HK1, PYGL |
Figure 6.
Cluster of SMP-DCM network (Pink, blue, purple circle stands for compound target, DCM genes and compound-DCM target, resp.).
These clusters, except for cluster 12, were subjected to GO enrichment analysis and several biological processes were obtained. Clusters 6, 9, 10, 11, 13, and 14 did not contain DCM-related biological processes. Cluster 1 contains glycometabolism-related, lipid metabolism-related, cardiovascular disease-related (e.g., blood pressure, blood lipids, and foam cells), inflammatory-related (e.g., inflammatory factors and interleukins), and oxidative stress-related biological processes. Cluster 2 contains vasomotor regulation-associated, cardiomyocyte apoptosis-related, and inflammatory-related biological processes. Cluster 3 mainly contains anti-oxidation processes. Clusters 4 and 7 are mainly related to anti-cardiomyocyte apoptosis and anti-oxidation. Cluster 5 is associated with glucose metabolism. Cluster 8 involves apoptosis and oxidative stress-related biological processes. The details of each cluster and biological process are described in Supplementary Table 8.
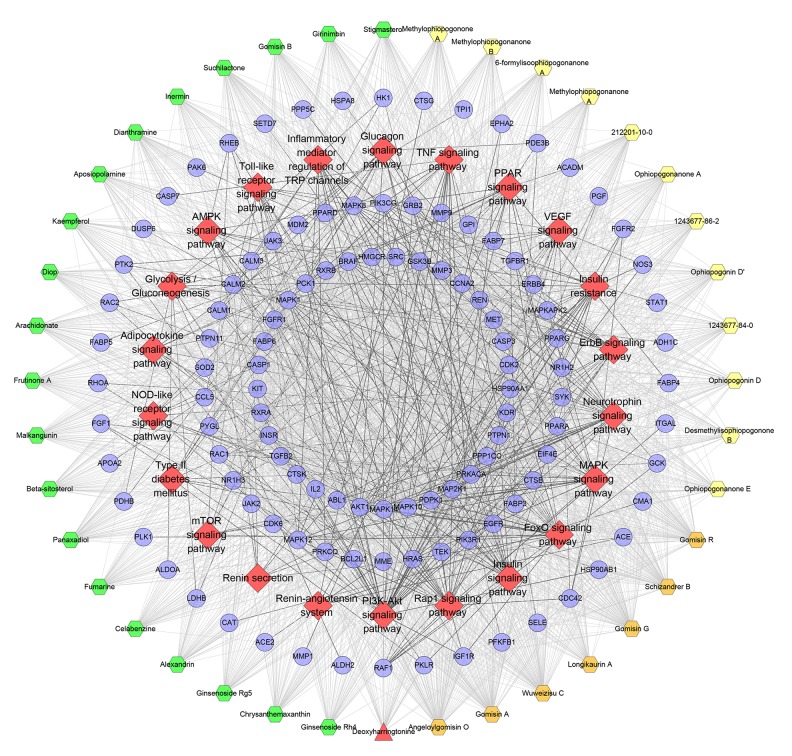
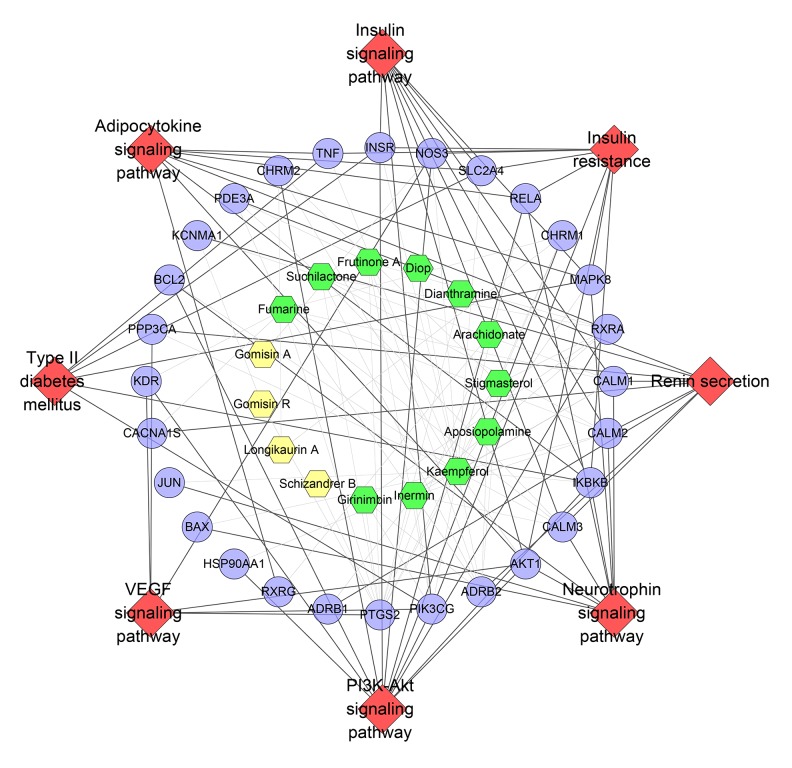
Pathway of SMP-DCM network
By importing all targets into DAVID, we found 22 DCM-related pathways (Figure 7). The PI3K-Akt signaling pathway has the most genes (39 genes), the Rap1 signaling pathway has 31 genes, the insulin signaling pathway has 27 genes, and the MAPK signaling pathway has 26 genes. The targets near the center are regulated by more compounds. These pathways and targets may be the key pathways by which SMP is active in treating DCM. The details are described in Supplementary Table 9.
Figure 7.
Pathway o of SMP-DCM network (Blue circle stands for compound target. Red diamond stands for pathway. The hexagons with different colors stand for compounds of different herbs: orange hexagon – Schisandra chinensis, Schisandraceae, yellow hexagon – Ophiopogon japonicas, Liliaceae, blue hexagon – Panax ginseng, Araliaceae. Red triangle stands for common compound of Schisandra chinensis, Schisandraceae and Panax ginseng, Araliaceae).
As mentioned above, abnormalities of insulin signaling, AMPK signaling, and β-adrenergic signaling play a crucial role in the development of DCM, which was also found in pathways of the SMP-DCM network that SMP can regulate the signaling pathways associated with these 3 pathways. Atherosclerosis also contributes to the development of DCM and is considered to be the main cause of heart failure in diabetic patients. GO enrichment revealed many atherosclerosis-related biological processes and pathways (e.g., signaling pathways and biological processes that regulate insulin resistance, blood pressure, and blood lipids), such as GO: 0008217, GO: 0010744, GO: 0032869, GO: 0045907, GO: 0050995, GO: 0035924, GO: 0045909, hsa04370, hsa04931, hsa04668, hsa04924, and hsa04920, suggesting that SMP may have a therapeutic effect on atherosclerosis.
In addition, in the pharmacological experimental studies of these compounds, it was also found that they can affect DCM-related genes, targets, biological processes, and signaling pathways. Zhang et al. found that, in a Wistar rat model of myocardial injury induced by doxorubicin, ginsenoside Rg1, ophiopogonin D, ophiopogonin D′, and schisandrin reduced the risk of myocardial fibrosis and inhibited cardiac remodeling by downregulating IL-6 and TNF-α and inhibiting MMPs and COL-IV overexpression [42]. Qian et al. found that ophiopogonin D can inhibit the enzymatic activity of catalase, HO-1, and caspases and block activation of the NF-kappaB and ERK signaling pathways in an endothelial injury model induced by H(2)O(2) [43]. Huang et al. showed that ophiopogonin D can exert anti-oxidative and anti-apoptotic effects by activating the PI3K/Akt/eNOS signaling pathway and inhibiting the NF-κB signaling pathway to regulate cardiac function, reduce lactate dehydrogenase and creatine kinase production, and improve damaged myocardial structures [44]. Liu et. al found that kaempferol can attenuate the cardiac fibrosis, hypertrophy, and dysfunction induced by AngII in mice [45]. Another study showed that kaempferol can inhibit inflammatory responses and oxidative stress in rats with DCM [46].
Apoptosis is another important factor in the pathology of diabetes, which can lead to loss of myocardial cells and decreased myocardial contractility, and ultimately lead to cardiac remodeling [47]. Recent studies have shown that cardiomyocyte apoptosis is increased in the hearts of diabetic patients and animals [48–50], the degree of which is similar to the severity of diabetic cardiomyopathy [50]. The cardiomyocyte apoptosis in DCM is initiated by the intrinsic pathway (mitochondria-mediated pathway) and the exogenous pathway (a death receptor-mediated pathway) [51–53]. The activation of caspase-8 in the exogenous pathway or cytochrome C-activated caspase-9 in the endogenous pathway can activate the final performer caspase-3 and lead to apoptosis [54]. This study shows that SMP can regulate the endogenous or exogenous apoptotic pathway or factors in the Bcl-2 family to delay the development of DCM, including GO: 0043066 and GO: 0010667.
Altered myocardial insulin signaling may be one of the important mechanisms for the development of DCM [55]. There have been many studies on the relationship between insulin resistance and heart failure, showing, for example, that there is a complex interaction between the Akt signaling pathway and the insulin signaling pathway, in which chronic activation accelerates ventricular remodeling [56–58]. Human studies have shown that insulin receptor (IR)-mediated signaling is enhanced in heart failure [58], while hyperinsulinemia directly impairs cardiac function by inhibiting cardiomyocyte adrenergic signaling, which involves the myocardial contraction-required cAMP/PKA activity and substrate phosphorylation [59]. As a member of the MAPK signaling pathway family, the ERK1/2 signaling pathway plays a crucial role in accelerating the development of DCM. Many studies have shown that activation of ERK1/2 promotes oxidative stress [60], inflammation, cardiac hypertrophic fibrosis [61–63], myocardial dysfunction [64,65], ventricular remodeling, and apoptosis [66] in the hearts of DCM patients, which eventually lead to heart failure. The present study shows that SMP can prevent ERK1/2 activation in cardiomyocytes by targeted regulation of the MAPK signaling pathway and ultimately preventing DCM.
From a macroscopic perspective, metabolic disorders in T2DM patients can also cause a series of pathophysiological changes, including the above molecular pathological changes. The hyperglycemia and hyperlipidemia in T2DM patients increase the production of inflammatory substances TNF-α, IL-1 β, and IL-6 by increasing ROS production or activation of the NF-κB signaling pathway [67,68] in the myocardium of diabetics, which leads to oxidative stress, endothelial dysfunction, damaged cell calcium treatment, mitochondrial dysfunction, metabolic disorders, and extracellular matrix remodeling, which ultimately results in DCM [69,70]. Hyperinsulinemia may also negatively regulate the activation of Akt/endothelial nitric oxide synthase (eNOS); and the endothelial dysfunction caused by it mainly includes 2 parts: (1) increased oxidized low-density lipoprotein, endothelin-1, angiotensin II, and oxidative stress; and (2) reduction of insulin or growth factors in endothelial cells [71]. These may be the main factors leading to coronary dysfunction in diabetes. Fortunately, our study shows that SMP can regulate signaling pathways and biological processes related to blood lipids (e.g., GO: 0050995, GO: 0045444, and hsa04920), blood glucose (e.g., GO: 0006006, GO: 0046326, GO: 0045725, hsa04922, and hsa00010), and NO metabolism (e.g., GO: 0045429, GO: 0050999, GO: 0051000, GO: 0051770, and hsa04621).
In the energy stress adaptation of diabetic cardiomyocytes, reactive oxygen species promote the development of DCM. Antioxidant treatment strategies have been found to have some cardiac benefits in diabetic animal models [72]. Recent evidence shows that supplementation with endogenous antioxidants facilitates the treatment of DCM [72], including conventional antioxidants (SOD isoforms, catalase, GPx, Trx, and vitamins C and E), and novel therapies (representing drug coenzyme Q10). Coenzyme Q10 attenuated diastolic dysfunction, cardiomyocyte hypertrophy, and cardiac fibrosis in a type 2 diabetes db/db mouse model [73], and targeted improvement of upregulation of reactive oxygen species after hyperglycemia can prevent DCM in type 1 diabetic mice [74].
In summary, SMP is able to regulate a variety of signaling pathways and biological processes related to glycometabolism, lipid metabolism, inflammatory, and oxidative stress. This suggests that SMP can prevent DCM, but further research is needed.
Compound-known target-DCM network analysis
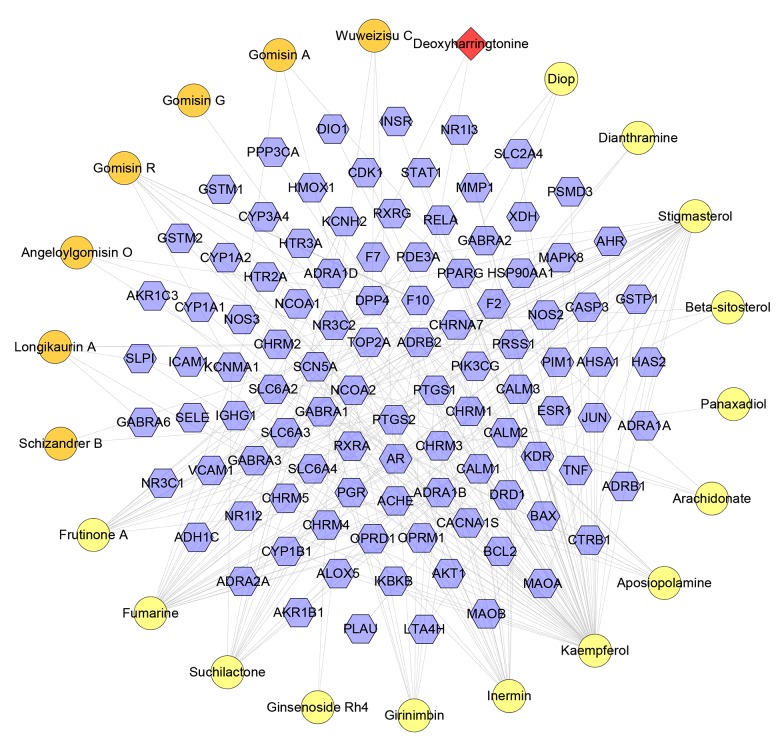
Compound-known target network
This network has 124 nodes (22 compound nodes and 102 compound target nodes) and 288 edges. This network is the verification of the previous network, and some of the relationships discovered in the previous network can be found in this network (Figure 8).
Figure 8.
Compound-known target network (Blue hexagon stand for known target. The representative of red, orange circle, and purple and red diamond is the same as Figure 4).
SMP known target-DCM network
The SMP known target-DCM network has 242 nodes and 3354 edges (Figure 9). This network is also the verification of the previous network, while some of the relationships discovered in the previous network can be found in this network.
Figure 9.
SMP known target-DCM network (Pink, blue, purple circle stand for known target, DCM genes, DCM-compound target, resp.).
Cluster and pathway of SMP known target-DCM network
Network analysis found 9 clusters (Table 3, Figure 10). The genes of each cluster were entered into DAVID to undergo GO enrichment analysis, which yielded several biological processes. Clusters 3, 5, and 8 did not return DCM-related biological processes.
Table 3.
Cluster of SMP known target-DCM network.
| Cluster | Score | Nodes | Edges | Targets and genes |
|---|---|---|---|---|
| 1 | 36.533 | 46 | 822 | IL1B, CCL2, NOS2, HMOX1, NOS3, NOS1, AKT1, RHOA, BCL2, SPP1, BAX, JUN, CASP3, MMP1, SELE, VCAM1, ICAM1, INS, SERPINE1, AR, IGF1, BDNF, CRP, LEP, INSR, PTGS2, TNF, TLR4, NR3C1, F2, CSF3, PIK3CG, EDN1, TP53, MMP2, ACE, MAPK14, MAPK8, CALM1, CALM2, KNG1, CALM3, CDKN2A, CAT, KDR, ESR1 |
| 2 | 10.824 | 35 | 184 | PPARA, HSP90AA1, TF, FOXO1, RELA, IKBKB, DUSP1, RETN, STAT1, PPARG, LPL, PGR, ADIPOQ, CHRM3, CHRM1, TNFSF11, SIRT1, CD36, ADRA1A, CHRM5, SLC2A4, AGT, HTR2A, AGTR1, IGF1R, SOD2, ADRA1B, ADRA1D, EDNRA, SOD1, EDN2, BDKRB2, CTGF, BDKRB1, GNAQ |
| 3 | 10 | 10 | 45 | CHRM4, OPRD1, CHRM2, RLN3, APLN, S1PR1, OPRM1, ADRA2A, S1PR3, APLNR |
| 4 | 6.154 | 14 | 40 | HAMP, SERPINA1, FTL, SLC11A2, TXN2, ACO1, IREB2, HEPH, CYBRD1, PDK4, TOP2A, HSPE1, HFE, SLC40A1 |
| 5 | 4 | 4 | 6 | GABRA1, GABRA2, GABRA3, GABRA6 |
| 6 | 3.333 | 4 | 5 | PRKAA1, STK11, BECN1, PPP3CA |
| 7 | 3.273 | 12 | 18 | NCOA1, SLC6A4, GSTM1, GSTM2, RLN2, NR1H2, CYP1A1, NR1H3, CYP1B1, VIPR1, DRD1, ADRB1 |
| 8 | 3 | 3 | 3 | NCOA2, RXRA, ANGPTL4 |
| 9 | 3 | 3 | 3 | KCNH2, SCN5A, CAMK2D |
Figure 10.
Cluster of SMP known target-DCM network (Pink, blue, purple circle stands for known target, DCM genes and known-DCM target, resp.).
Clusters 1 and 2 contain glycometabolism-related, lipid metabolism-related, cardiovascular disease-related (e.g., blood pressure, blood lipids, and foam cells), inflammatory-related (e.g., inflammatory factors and interleukins), and oxidative stress-related biological processes. Cluster 4 is mainly involved in iron metabolism. Cluster 6 is mainly related to glucose metabolism and apoptosis. Cluster 7 involves several lipid metabolism-related biological processes. Cluster 9 is related to vasomotor regulation. These results are shown in Supplementary Table 10.
We found 8 DCM-related pathways, which are identical to the pathways in Figures 3 and 7, demonstrating that these pathways may be key pathways for DCM development (Figure 11, Supplementary Table 11).
Figure 11.
Pathway o of SMP known target-DCM network (Blue circle stands for known target. Red diamond stands for pathway. Yellow, green hexagon stand for Schisandra chinensis, Schisandraceae, Panax ginseng, Araliaceae, rep.).
In this compound-known target network, we found some signaling pathways and biological processes that have appeared in the previous network, such as hsa04924, hsa04920, hsa04931, GO: 0055117, and GO: 0051000. Several clusters are also similar in functions. This somewhat verifies the reliability of the predicted target network. These results show that SMP can regulate DCM-related genes and targets through its compounds, thereby further regulating DCM-related biological processes and signaling pathways. This may be the mechanism by which SMP intervenes in DCM. However, because this method failed to directly reflect the impact of compound content on biological processes, more research is required to test the effects of SMP and compounds in relation to DCM and to confirm the pharmacological and molecular mechanisms involved.
In the current strategy of treating DCM, it can be found that the strategies for protecting the myocardium are regulating energy metabolism and improving myocardial fibrosis and anti-inflammatory and anti-oxidative stress, in which regulation of myocardial energy metabolism has become an important strategy for the treatment of DCM and preventing its progression to late events such as heart failure [75]. The present study shows the important role of SMP in regulating cellular metabolism, especially in energy metabolism. At present, trimetazidine is the main drug used to improve myocardial metabolism in clinical practice, the mechanism of which is to selectively inhibit the activity of long-chain 3-ketoacyl-CoA thiolase, thereby improving the energy metabolism of cardiomyocytes [76,77]. Early administration of trimetazidine can inhibit myocardial fibrosis and against oxidative stress, thereby improving DCM [78], and piperazine and ranolazine, as regulators of free fatty acid metabolism, can also improve myocardial metabolism and mitochondrial function [79,80].
To further validate the network constructed by the small-molecule drug virtual prediction database and its core important pathways and important core biological processes, we searched the experimental evidence of SMP intervention in DCM in PubMed. Tian et al. found that SMP alleviated DCM by improving mitochondrial lipid metabolism disorder [81]. Zhao et al. found that SMP attenuates cardiac hypertrophy and fibrosis through a TGF-β-dependent pathway, thereby exerting cardioprotective effects on DCM [12]. Ni et al. found that SMP can inhibit myocardial fibrosis in a DCM rat model [15]. We contacted the authors of these 3 studies by email, in which Tian et al. replied to us and were willing to share their data to verify our research.
Experiments show that upregulation of SIRT1/AMPK/PGC1 signaling may contribute to the cardioprotective effects of SMP [12,15,81]. Western blot analysis showed a decrease in SIRT1 and p-AMPK (Thr172) protein levels in diabetic hearts, accompanied by an increase in acetylated PGC-1α levels (acetylated lysine sites at 110 KDa), although expression of PGC-1α was not altered [82–84]. SMP significantly enhanced the protein levels of SIRT1 and p-AMPKα and decreased the expression of acetylated PGC-1α. SMP also restores NRF1 and TFAM levels [12,15,81]. These results are shown in Table 4.
Table 4.
SIRT1/AMPK/PGC-1α signaling pathway (mean±SEM). Data from reference [82–84].
| P-AMPK/AMPK | SIRT1/beta-actin | Acetylated PGC-1α | PGC-1α/beta-actin | |
|---|---|---|---|---|
| Non-DM | 1.0±0.09 | 1.0±0.09 | 1.0±0.09 | 1.0±0.25 |
| db/db | 0.49±0.01** | 0.75±0.09** | 1.7±0.09** | 0.68±0.09 |
| db/db±SL | 0.07±0.01# | 0.95±0.10# | 1.25±0.04# | 0.98±0.02 |
Tip:
P<0.05,
P<0.01 vs. non-DM group;
P<0.05,
P<0.01 vs. db/db group.
Conclusions
SMP can regulate glycometabolism-related, lipid metabolism-related, inflammatory response-related, and oxidative stress-related signaling pathways, as well as biological processes and targets, which suggest that SMP may have a therapeutic effect on DCM.
Footnotes
Source of support: This work is supported by the National Natural Science Foundation of China (No. 81603512 & No. 81874429), the postgraduate scientific research innovation project of Hunan Province of China (No. CX20190536, No. CX20190591, No. CX2018B465 and No. CX2017B428), Natural Science Foundation of Hunan Province of China (No. 2018JJ2289), Innovation project for graduate students of Hunan University of Chinese Medicine (No. 2018CX05 and No. 2018CX25), the postgraduate scientific research innovation project of Guangdong Yifang Pharmaceutical Co., Ltd. (No. yf201708), and the Digital Chinese medicine innovation research platform project (No. 49021001003005)
Supplementary Data
Supplementary Table 1. The details of each compound.
Supplementary Table 2. The details of each compound.
Supplementary Table 3. Compound targets for each compounds.
Supplementary Table 4. Known targets for each compounds.
Supplementary Table 5. Diabetic heart disease Ggenes.
Supplementary Table 6. Enrichment analysis of clusters based on Gene Ontology (GO) annotation.
Supplementary Table 7. Pathway enrichment analysis.
Supplementary Table 8. Enrichment analysis of clusters based on Gene Ontology (GO) annotation.
Supplementary Table 9. Pathway enrichment analysis.
Supplementary Table 10. Enrichment analysis of clusters based on Gene Ontology (GO) annotation.
Supplementary Table 11. Pathway enrichment analysis.
Supplementary/raw data available from the corresponding author on request.
Conflict of Interest
None.
References
- 1.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144–53. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–66. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association. Diabetes statistics. 2011. Available from www.diabetes.org/diabetes-basics/diabetes-statistics/
- 4.Anand SS, Yusuf S. Stemming the global tsunami of cardiovascular disease. Lancet. 2011;377:529–32. doi: 10.1016/S0140-6736(10)62346-X. [DOI] [PubMed] [Google Scholar]
- 5.Isfort M, Stevens SC, Schaffer S, et al. Metabolic dysfunction in diabetic cardiomyopathy. Heart Fail Rev. 2014;19:35–48. doi: 10.1007/s10741-013-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 7.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–71. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord. 2010;11:31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao J, Bledsoe G, Yin H, Chao L. The tissue kallikrein-kinin system protects against cardiovascular and renal diseases and ischemic stroke independently of blood pressure reduction. Biol Chem. 2006;387:665–75. doi: 10.1515/BC.2006.085. [DOI] [PubMed] [Google Scholar]
- 10.Sivasankar D, George M, Sriram DK. Novel approaches in the treatment of diabetic cardiomyopathy. Biomed Pharmacother. 2018;106:1039–45. doi: 10.1016/j.biopha.2018.07.051. [DOI] [PubMed] [Google Scholar]
- 11.Wang KH, Wu JR, Zhang D, et al. Comparative efficacy of Chinese herbal injections for treating chronic heart failure: A network meta-analysis. BMC Complement Altern Med. 2018;31(1):41. doi: 10.1186/s12906-018-2090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Cao TT, Tian J, et al. Shengmai san ameliorates myocardial dysfunction and fibrosis in diabetic db/db mice. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/4621235. 4621235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Tan W, Yang F, et al. Shengmai injection reduces apoptosis and enhances angiogenesis after myocardial ischaemia and reperfusion injury in rats. Biomed Pharmacother. 2018;104:629–36. doi: 10.1016/j.biopha.2018.04.180. [DOI] [PubMed] [Google Scholar]
- 14.Zhan S, Fan X, Zhang F, et al. A proteomic study of Shengmai injection’s mechanism on preventing cardiac ischemia-reperfusion injury via energy metabolism modulation. Mol Biosyst. 2015;11:540–48. doi: 10.1039/c4mb00161c. [DOI] [PubMed] [Google Scholar]
- 15.Ni Q, Wang J, Li EQ, et al. Study on the protective effect of shengmai san (see text) on the myocardium in the type 2 diabetic cardiomyopathy model rat. J Tradit Chin Med. 2011;31:209–19. doi: 10.1016/s0254-6272(11)60044-7. [DOI] [PubMed] [Google Scholar]
- 16.Chen F, Chang C, Hwang S, et al. Chinese herbal prescriptions for osteoarthritis in Taiwan: Analysis of national health insurance dataset. BMC Complement Altern Med. 2014;14(1):91. doi: 10.1186/1472-6882-14-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ru J, Li P, Wang J, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6(1):13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang K, Zeng L, Ge J. Exploring the pharmacological mechanism of Danzhi Xiaoyao powder on ER-positive breast cancer by a network pharmacology approach. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/5059743. 5059743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng L, Yang K, Ge J. Uncovering the pharmacological mechanism of astragalus salvia compound on pregnancy-induced hypertension syndrome by a network pharmacology approach. Sci Rep. 2018;7:16849. doi: 10.1038/s41598-017-17139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metodiewa D, Kochman A, Karolczak S. Evidence for antiradical and antioxidant properties of four biologically active N,N-diethylaminoethyl ethers of flavanone oximes: A comparison with natural polyphenolic flavonoid (rutin) action. Biochem Mol Biol Int. 1997;41:1067–75. doi: 10.1080/15216549700202141. [DOI] [PubMed] [Google Scholar]
- 21.Wang YH, Qiu C, Wang DW, et al. Identification of multiple constituents in the traditional Chinese medicine formula Sheng-Mai San and rat plasma after oral administration by HPLC-DAD-MS/MS. J Pharm Biomed Anal. 2011;54:1110–27. doi: 10.1016/j.jpba.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Ouyang S, Yu B, et al. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010;38:W609–14. doi: 10.1093/nar/gkq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamosh A, Scott AF, Amberger JS, et al. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33:D514–17. doi: 10.1093/nar/gki033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–52. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orchard S, Ammari M, Aranda B, et al. The MIntAct project – IntAct as a common curation platform for 11 molecular interaction databases. Nucleic Acids Res. 2014;42:D358–63. doi: 10.1093/nar/gkt1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Missiuro PV, Liu K, Zou L, et al. Information flow analysis of interactome networks. PLoS Comput Biol. 2009;5:e1000350. doi: 10.1371/journal.pcbi.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. 2003;4:2. doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barabási AL, Gulbahce N, Loscalzo J. Network medicine: A network-based approach to human disease. Nat Rev Genet. 2011;12:56–68. doi: 10.1038/nrg2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 30.Varma U, Koutsifeli P, Benson VL, et al. Molecular mechanisms of cardiac pathology in diabetes – Experimental insights. Biochim Biophys Acta Mol Basis Dis. 2018;1864(5 Pt B):1949–59. doi: 10.1016/j.bbadis.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 31.Frati G, Schirone L, Chimenti I, et al. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc Res. 2017;113(4):378–88. doi: 10.1093/cvr/cvx011. [DOI] [PubMed] [Google Scholar]
- 32.Liu JE, Palmieri V, Roman MJ, et al. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: The Strong Heart Study. J Am Coll Cardiol. 2001;37:1943–49. doi: 10.1016/s0735-1097(01)01230-x. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Zhao D, Ren J, Yang J. Endoplasmic reticulum stress and protein quality control in diabetic cardiomyopathy. Biochim Biophys Acta. 2015;1852:209–18. doi: 10.1016/j.bbadis.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Belke DD, Betuing S, Tuttle MJ, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–39. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boudina S, Bugger H, Sena S, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–83. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bugger H, Riehle C, Jaishy B, et al. Genetic loss of insulin receptors worsens cardiac efficiency in diabetes. J Mol Cell Cardiol. 2012;52:1019–26. doi: 10.1016/j.yjmcc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bairwa SC, Parajuli N, Dyck JR. The role of AMPK in cardiomyocyte health and survival. Biochim Biophys Acta. 2016;1862:2199–210. doi: 10.1016/j.bbadis.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Verma S, McNeill JH. Metformin improves cardiac function in isolated streptozotocin-diabetic rat hearts. Am J Phys. 1994;266:714–19. doi: 10.1152/ajpheart.1994.266.2.H714. [DOI] [PubMed] [Google Scholar]
- 39.Mangmool S, Denkaew T, Phosri S, et al. Nishida Sustained beta AR stimulation mediates cardiac insulin resistance in a PKA-dependent manner. Mol Endocrinol. 2016;30:118–32. doi: 10.1210/me.2015-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu Q, Wang YK. Xiang Insulin and beta adrenergic receptor signaling: Crosstalk in heart Trends Endocrinol. Metab. 2017;28:416–27. doi: 10.1016/j.tem.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mangmool S, Denkaew T, Parichatikanond W, Kurose H. Kurose beta-Adrenergic receptor and insulin resistance in the heart. Biomol Ther. 2017;25:44–56. doi: 10.4062/biomolther.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang K, Zhang J, Wang X, et al. Cardioprotection of Sheng Mai Yin a classic formula on adriamycin induced myocardial injury in Wistar rats. Phytomedicine. 2018;38:1–11. doi: 10.1016/j.phymed.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Qian J, Jiang F, Wang B, et al. Ophiopogonin D prevents H2O2-induced injury in primary human umbilical vein endothelial cells. J Ethnopharmacol. 2010;128(2):438–45. doi: 10.1016/j.jep.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Huang X, Wang Y, Wang Y, et al. Ophiopogonin D reduces myocardial ischemia-reperfusion injury via upregulating CYP2J3/EETs in rats. Cell Physiol Biochem. 2018;49(4):1646–58. doi: 10.1159/000493500. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Gao L, Guo S, et al. Kaempferol alleviates angiotensin II-induced cardiac dysfunction and interstitial fibrosis in mice. Cell Physiol Biochem. 2017;43(6):2253–63. doi: 10.1159/000484304. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Qian J, Wang L, et al. Kaempferol attenuates hyperglycemia-induced cardiac injuries by inhibiting inflammatory responses and oxidative stress. Endocrine. 2018;60(1):83–94. doi: 10.1007/s12020-018-1525-4. [DOI] [PubMed] [Google Scholar]
- 47.Cai L, Wang Y, Zhou G, et al. Attenuation by metallothionein of early cardiac cell death via suppression of mitochondrial oxidative stress results in a prevention of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;48:1688–97. doi: 10.1016/j.jacc.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 48.Frustaci A, Kajstura J, Chimenti C, et al. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–32. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 49.Fiordaliso F, Bianchi R, Staszewsky L, et al. Antioxidant treatment attenuates hyperglycemia-induced cardiomyocyte death in rats. J Mol Cell Cardiol. 2004;37:959–68. doi: 10.1016/j.yjmcc.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh S, Pulinilkunnil T, Yuen G, et al. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am J Physiol Heart Circ Physiol. 2005;289:768–76. doi: 10.1152/ajpheart.00038.2005. [DOI] [PubMed] [Google Scholar]
- 51.Abdel-Hamid AA, Firgany Ael D. Atorvastatin alleviates experimental diabetic cardiomyopathy by suppressing apoptosis and oxidative stress. J Mol Histol. 2015;46(4–5):337–45. doi: 10.1007/s10735-015-9625-4. [DOI] [PubMed] [Google Scholar]
- 52.Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: A brief review. Cardiovasc Toxicol. 2001;1:181–93. doi: 10.1385/ct:1:3:181. [DOI] [PubMed] [Google Scholar]
- 53.Amin AH, El-Missiry MA, Othman AI. Melatonin ameliorates metabolic risk factors, modulates apoptotic proteins, and protects the rat heart against diabetes-induced apoptosis. Eur J Pharmacol. 2015;747:166–73. doi: 10.1016/j.ejphar.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Budihardjo I, Oliver H, Lutter M, et al. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–90. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 55.Abel ED, O’Shea KM, Ramasamy R. Insulin resistance: Metabolic mechanisms and consequences in the heart. Arterioscler Thromb Vasc Biol. 2012;32:2068–76. doi: 10.1161/ATVBAHA.111.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shimizu I, Minamino T, Toko H, et al. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J Clin Invest. 2010;120:1506–14. doi: 10.1172/JCI40096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wende AR, O’Neill BT, Bugger H, et al. Enhanced cardiac Akt/protein kinase B signaling contributes to pathological cardiac hypertrophy in part by impairing mitochondrial function via transcriptional repression of mitochondrion-targeted nuclear genes. Mol Cell Biol. 2015;35:831–46. doi: 10.1128/MCB.01109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cook SA, Varela-Carver A, Mongillo M, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–11. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q, Liu Y, Fu Q, et al. Inhibiting insulin-mediated β2AR activation prevents diabetes-associated cardiac dysfunction. Circulation. 2017;135:73–88. doi: 10.1161/CIRCULATIONAHA.116.022281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka K, Honda M, Takabatake T. Redox regulation of mapk pathways and cardiac hypertrophy in adult rat cardiac myocyte. J Am Coll Cardiol. 2001;37:676–85. doi: 10.1016/s0735-1097(00)01123-2. [DOI] [PubMed] [Google Scholar]
- 61.Wang S, Luo M, Zhang Z, et al. Zinc deficiency exacerbates while zinc supplement attenuates cardiac hypertrophy in high-fat diet-induced obese mice through modulating p38 MAPK-dependent signaling. Toxicol Lett. 2016;258:134–46. doi: 10.1016/j.toxlet.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 62.Wei WY, Ma ZG, Xu SC, et al. Pioglitazone protected against cardiac hypertrophy via inhibiting AKT/GSK3B and MAPK signaling pathways. PPAR Res. 2016;2015 doi: 10.1155/2016/9174190. 9174190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang N, Yang Z, Yuan Y, et al. Naringenin attenuates pressure overload-induced cardiac hypertrophy. Exp Ther Med. 2015;10:2206–12. doi: 10.3892/etm.2015.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallo S, Sala V, Gatti S, Crepaldi T. Cellular and molecular mechanisms of HGF/MET in the cardiovascular system. Clin Sci. 2015;129:1173–93. doi: 10.1042/CS20150502. [DOI] [PubMed] [Google Scholar]
- 65.Javadov S, Jang S, Agostini B. Crosstalk between mitogen-activated protein kinases and mitochondria in cardiac diseases: Therapeutic perspectives. Pharmacol Ther. 2014;144:202–25. doi: 10.1016/j.pharmthera.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao Y, Li R, Ma Y, et al. α-Lipoic acid increases tolerance of cardiomyoblasts to glucose/glucose oxidase-induced injury via ROS-dependent ERK1/2 activation. Biochim Biophys Acta. 2012;1823:920–29. doi: 10.1016/j.bbamcr.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 67.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–31. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Z, Zhuang X, Xie C, et al. Exogenous hydrogen sulfide attenuates high glucose-induced cardiotoxicity by inhibiting NLRP3 Inflammasome activation by suppressing TLR4/NF-κB pathway in H9c2 cells. Cell Physiol Biochem. 2016;40:1578–90. doi: 10.1159/000453208. [DOI] [PubMed] [Google Scholar]
- 69.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 70.Miki T, Yuda S, Kouzu H, Miura T. Diabetic cardiomyopathy: Pathophysiology and clinical features. Heart Fail Rev. 2013;18:149–66. doi: 10.1007/s10741-012-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bulhak AA, Jung C, Ostenson CG, et al. PPAR-alpha activation protects the type 2 diabetic myocardium against ischemia-reperfusion injury: Involvement of the PI3-Kinase/Akt and NO pathway. Am J Physiol Heart Circ Physiol. 2009;296:719–27. doi: 10.1152/ajpheart.00394.2008. [DOI] [PubMed] [Google Scholar]
- 72.Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: Mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014;142(3):375–415. doi: 10.1016/j.pharmthera.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Huynh K, Kiriazis H, Du XJ, et al. Coenzyme Q(10) attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia. 2012;55(5):1544–53. doi: 10.1007/s00125-012-2495-3. [DOI] [PubMed] [Google Scholar]
- 74.Huynh K, Kiriazis H, Du XJ, et al. Targeting the upregulation of reactive oxygen species subsequent to hyperglycemia prevents type 1 diabetic cardiomyopathy in mice. Free Radic Biol Med. 2013;60:307–17. doi: 10.1016/j.freeradbiomed.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 75.Fukushima A, Milner K, Gupta A, Lopaschuk GD. Myocardial energy substrate metabolism in heart failure: From pathways to therapeutic targets. Curr Pharm Des. 2015;21(25):3654–64. doi: 10.2174/1381612821666150710150445. [DOI] [PubMed] [Google Scholar]
- 76.Lopatin YM, Rosano GM, Fragasso G, et al. Rationale and benefits of trimetazidine by acting on cardiac metabolism in heart failure. Int J Cardiol. 2016;203:909–15. doi: 10.1016/j.ijcard.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 77.Guarini G, Huqi A, Morrone D, et al. Trimetazidine and other metabolic modifiers. Eur Cardiol. 2018;13(2):104–11. doi: 10.15420/ecr.2018.15.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zou H, Zhu XX, Ding YH, et al. Trimetazidine in conditions other than coronary disease, old drug, new tricks? Int J Cardiol. 2017;234:1–6. doi: 10.1016/j.ijcard.2017.02.083. [DOI] [PubMed] [Google Scholar]
- 79.Horowitz JD, Chirkov YY, Kennedy JA, Sverdlov AL. Modulation of myocardial metabolism: An emerging therapeutic principle. Curr Opin Cardiol. 2010;25(4):329–34. doi: 10.1097/HCO.0b013e328339f191. [DOI] [PubMed] [Google Scholar]
- 80.Steggall A, Mordi IR, Lang CC. Targeting metabolic modulation and mitochondrial dysfunction in the treatment of heart failure. Diseases. 2017;5(2) doi: 10.3390/diseases5020014. pii: E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tian J, Tang W, Xu M, et al. Shengmai san alleviates diabetic cardiomyopathy through improvement of mitochondrial lipid metabolic disorder. Cell Physiol Biochem. 2018;50(5):1726–39. doi: 10.1159/000494791. [DOI] [PubMed] [Google Scholar]